Expanded Properties and Applications of Porous Flame-Retardant Polymers Containing Graphene and Its Derivatives
Abstract
1. Introduction
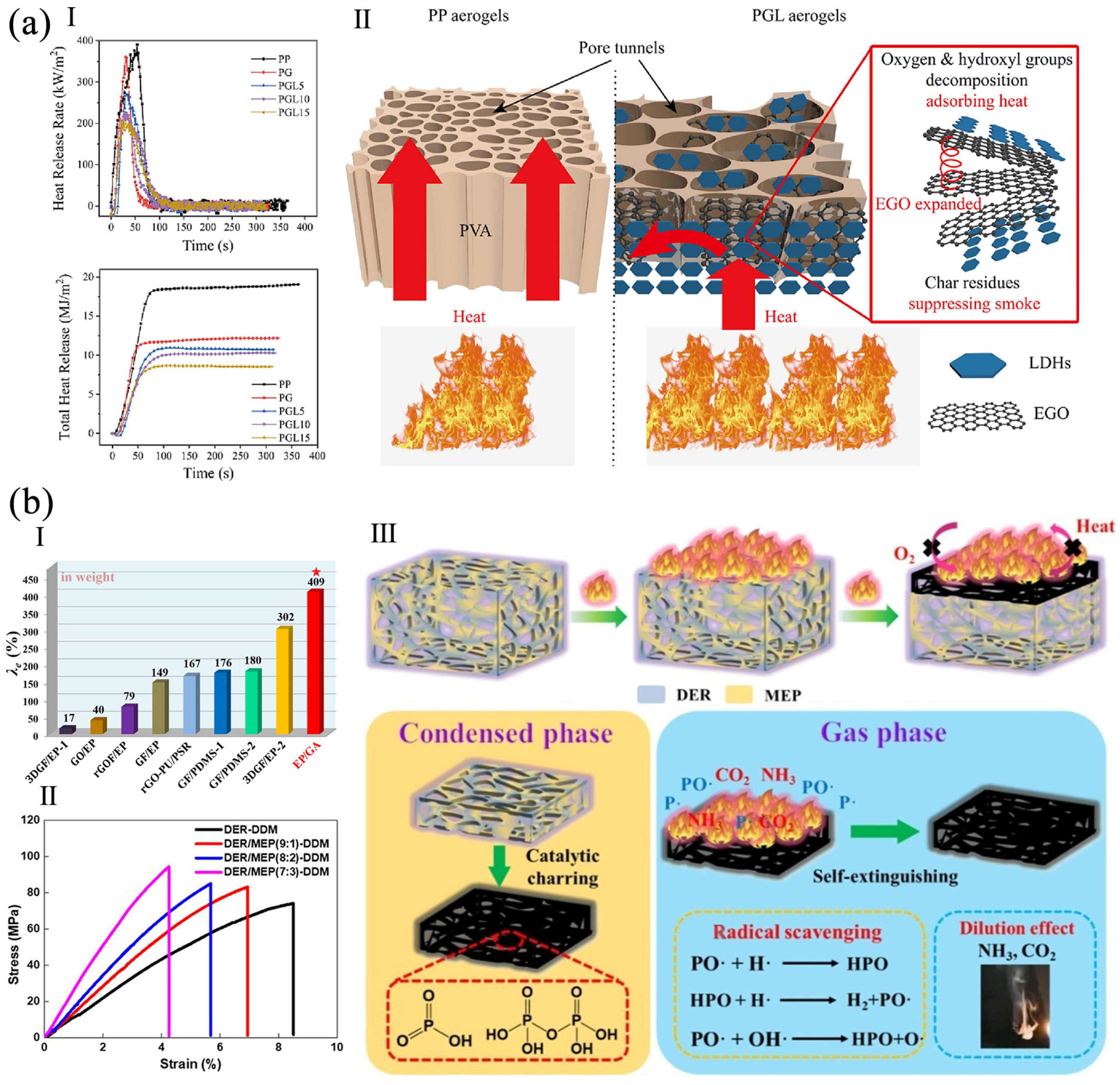
2. Flame Retardant Porous Materials Containing Graphene and Its Derivatives
3. Expanded Applications of Porous Flame-Retardant Materials Containing Graphene
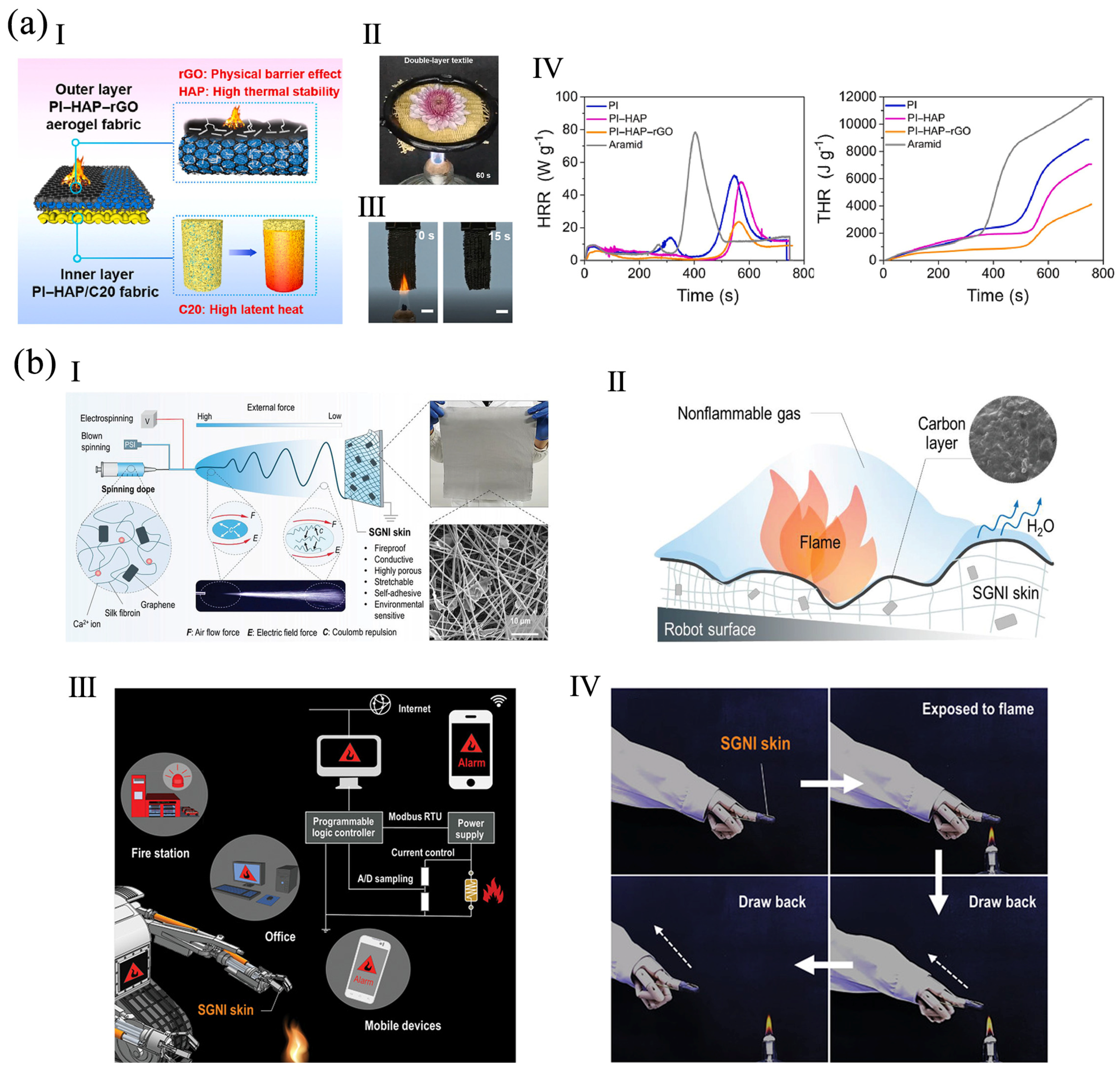
3.1. Firefighter Clothing
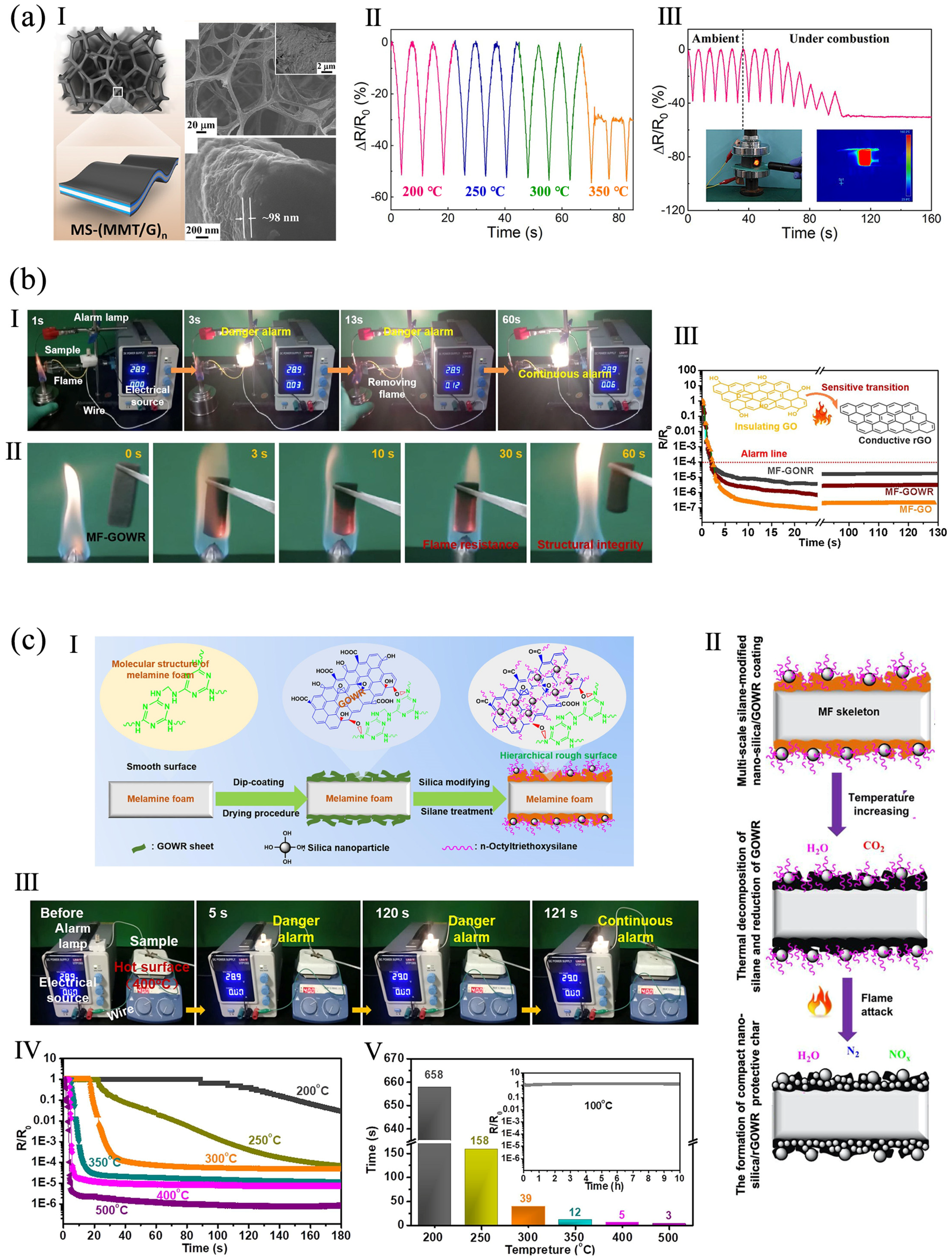
3.2. Fire Alarm Sensor
3.3. Flexible Electronic Skin
3.4. Solar Energy Storage
3.5. Energy-Efficient Building
3.6. Stealth Material
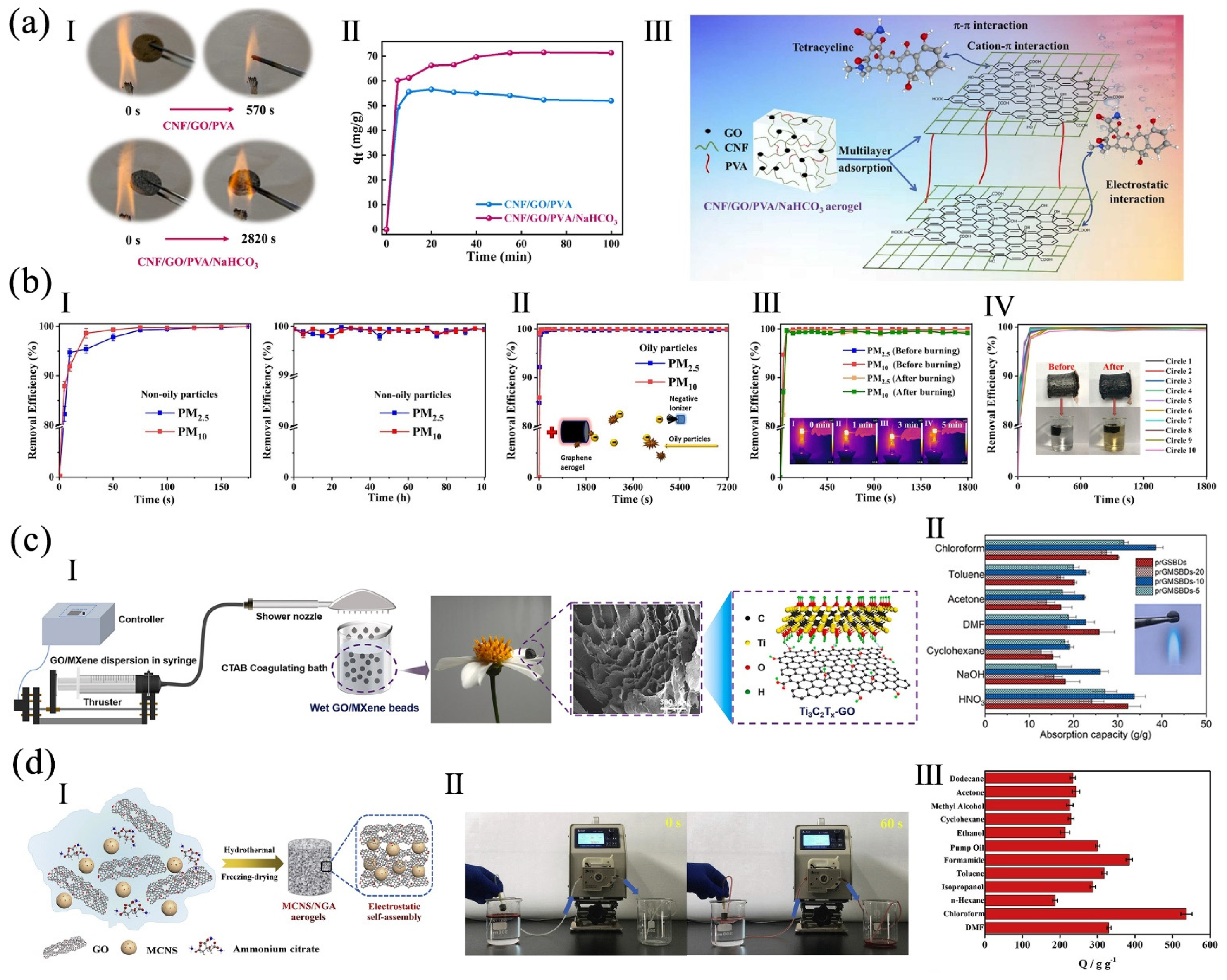
3.7. Separation
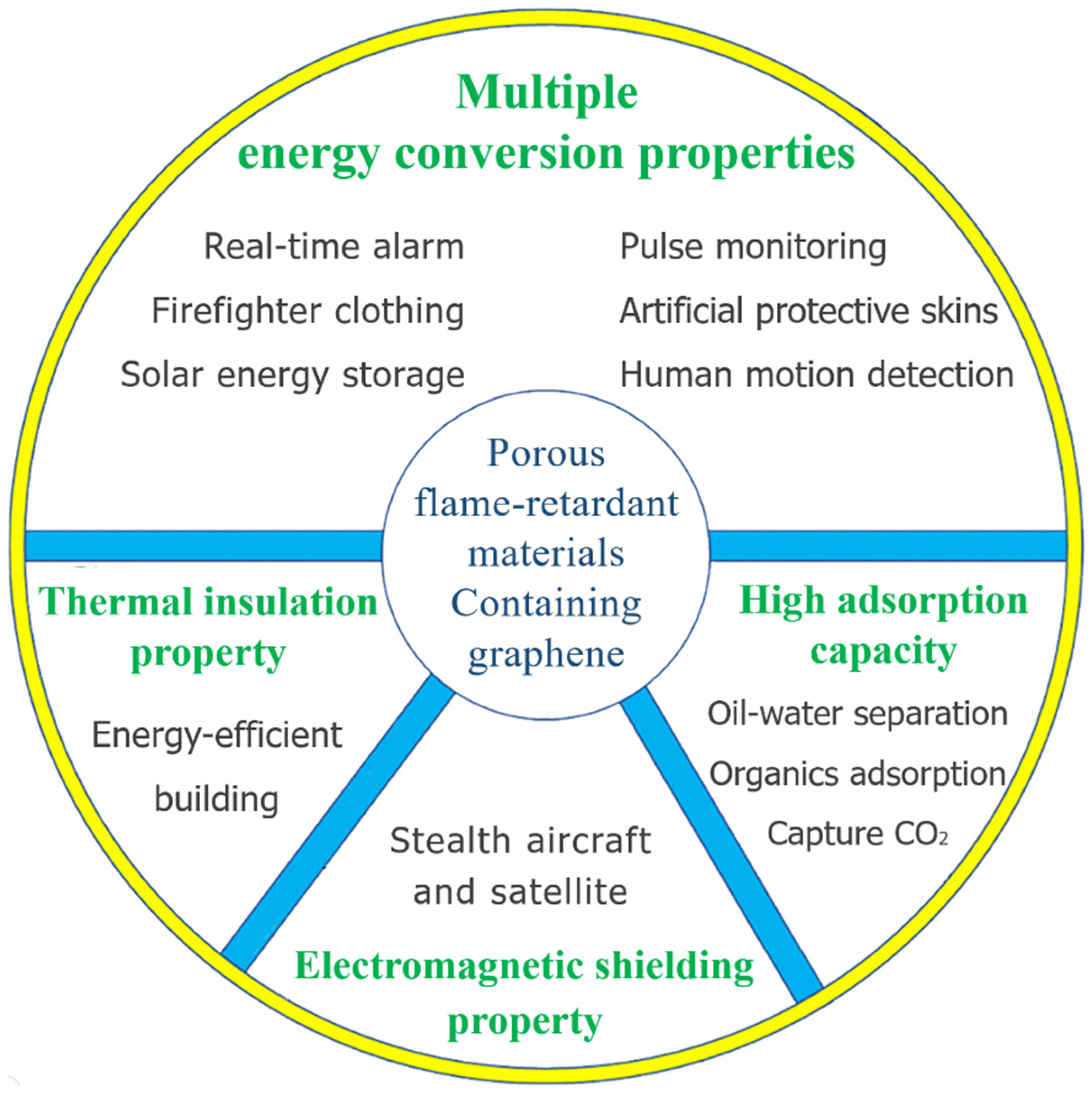
4. Improved Properties of Porous Flame-Retardant Materials Containing Graphene
4.1. Multiple Energy Conversion Properties
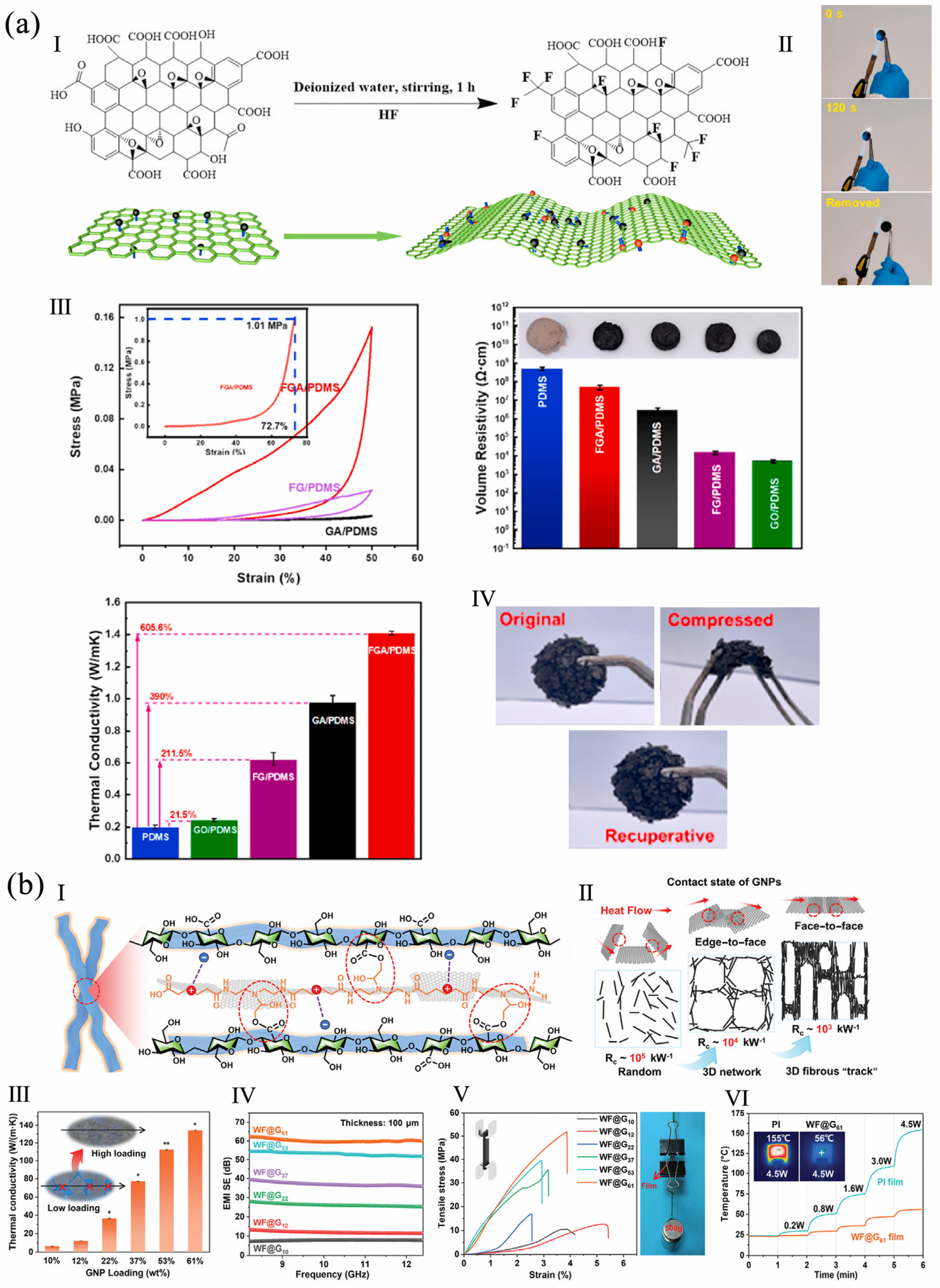
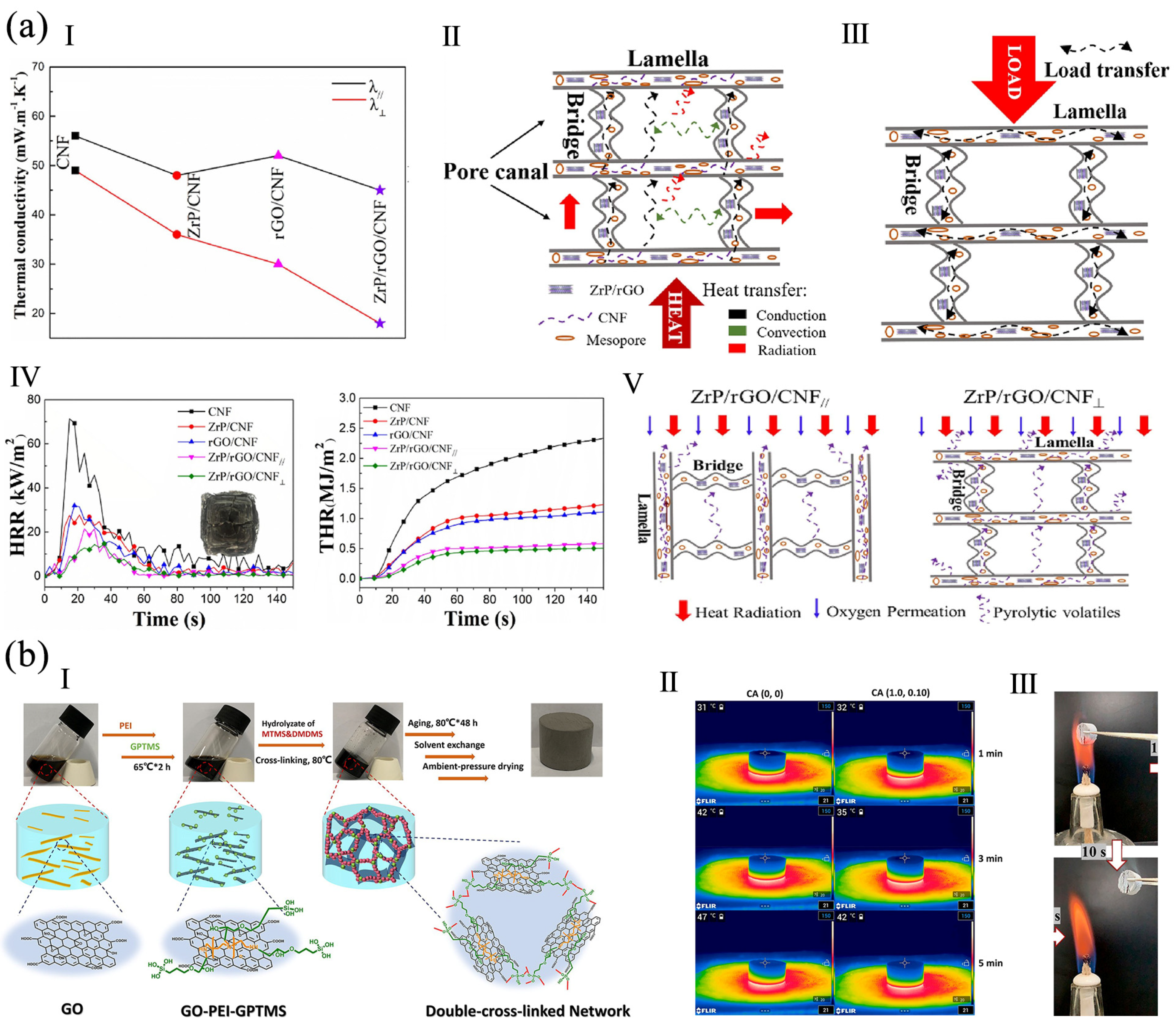
4.2. Thermal Insulation Property
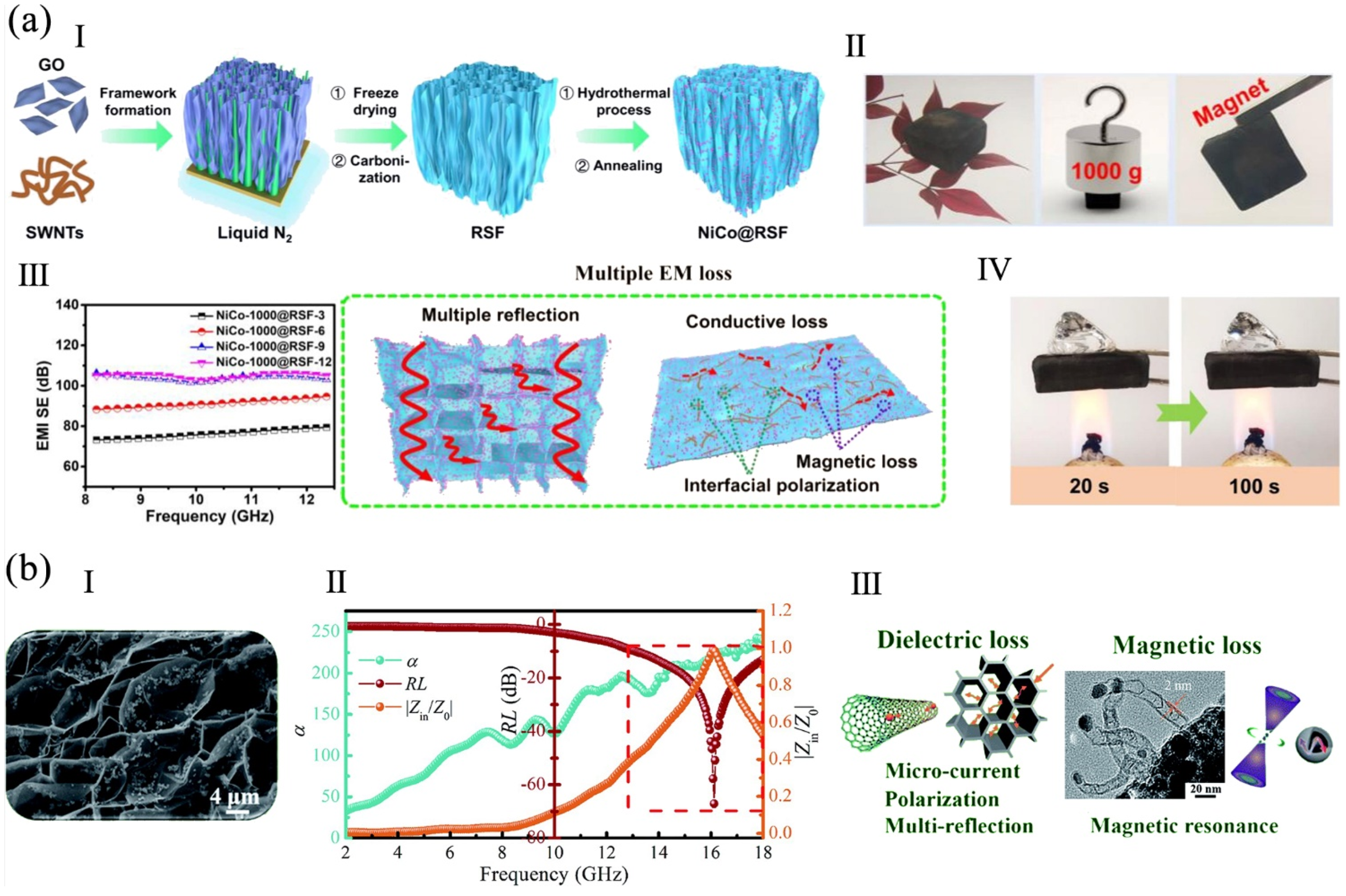
4.3. Electromagnetic Shielding Property
4.4. High Adsorption Capacity
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Yao, Y.; Jin, S.; Ma, X.; Yu, R.; Zou, H.; Wang, H.; Lv, X.; Shu, Q. Graphene-containing flexible polyurethane porous composites with improved electromagnetic shielding and flame retardancy. Compos. Sci. Technol. 2020, 200, 108457. [Google Scholar] [CrossRef]
- Song, Z.; Li, W.; Bao, Y.; Kong, H.; Gan, S.; Wang, W.; Liu, Z.; Ma, Y.; Han, D.; Niu, L. Space-Confined Graphene Films for Pressure-Sensing Applications. ACS Appl. Nano Mater. 2020, 3, 1731–1740. [Google Scholar] [CrossRef]
- Liu, S.; Yan, H.; Fang, Z.; Wang, H. Effect of graphene nanosheets on morphology, thermal stability and flame retardancy of epoxy resin. Compos. Sci. Technol. 2014, 90, 40–47. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Li, Q.; Wei, J.; Li, X.; Zhang, Y.; Liu, J. In situ formation of phosphorus-doped porous graphene via laser induction. Rsc Adv. 2020, 10, 23953–23958. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-T.; Lin, H.; Yang, T.; Jia, B. Structured graphene metamaterial selective absorbers for high efficiency and omnidirectional solar thermal energy conversion. Nat. Commun. 2020, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Dong, H.; Kou, Y.; Yang, H.; Liu, H.; Li, Y.; Shi, Q. Flexible graphene aerogel-based phase change film for solar-thermal energy conversion and storage in personal thermal management applications. Chem. Eng. J. 2021, 419, 129637. [Google Scholar] [CrossRef]
- Ayazi, H.; Akhavan, O.; Raoufi, M.; Varshochian, R.; Hosseini Motlagh, N.S.; Atyabi, F. Graphene aerogel nanoparticles for in-situ loading/pH sensitive releasing anticancer drugs. Colloids Surf. B Biointerfaces 2020, 186, 110712. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, M.; Zhang, D.; Gao, C.; Liu, W.; Yang, L.; Huang, F.; Qin, S. New construction strategies of carbon-based composites for flexible electromagnetic interference shielding films. Polym. Compos. 2024, 45, 5804–5826. [Google Scholar] [CrossRef]
- Ren, L.; Hui, K.N.; Hui, K.S.; Liu, Y.; Qi, X.; Zhong, J.; Du, Y.; Yang, J. 3D hierarchical porous graphene aerogel with tunable meso-pores on graphene nanosheets for high-performance energy storage. Sci. Rep. 2015, 5, 14229. [Google Scholar] [CrossRef]
- Shi, X.R.; Yang, L.F.; Tian, L.; Shen, J.P.; Li, Z.Q.; Pei, C.H. Laser ignited combustion of mesoporous nitrocellulose/graphene oxide composite aerogel with strong photo-thermal sensitivity and high flame propagation rate. J. Appl. Phys. 2021, 130, 225105. [Google Scholar] [CrossRef]
- Ran, J.; Girardi, L.; Drazic, G.; Wang, Z.; Agnoli, S.; Xia, H.; Granozzi, G. The Effect of the 3D Nanoarchitecture and Ni-Promotion on the Hydrogen Evolution Reaction in MoS2/Reduced GO Aerogel Hybrid Microspheres Produced by a Simple One-Pot Electrospraying Procedure. Small 2022, 18, 2105694. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, A.; Yudaev, P.; Orlov, A.; Loban, O.; Lukashov, N.; Chistyakov, E. Aryloxyphosphazene-Modified and Graphite-Filled Epoxy Compositions with Reduced Flammability and Electrically Conductive Properties. J. Compos. Sci. 2023, 7, 417. [Google Scholar] [CrossRef]
- Yang, W.-J.; Wei, C.-X.; Yuen, A.C.Y.; Lin, B.; Yeoh, G.H.; Lu, H.-D.; Yang, W. Fire-retarded nanocomposite aerogels for multifunctional applications: A review. Compos. Part B Eng. 2022, 237, 109866. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, Y.; Hu, J.; Wang, H. Flexible, Strong, Multifunctional Graphene Oxide/Silica-Based Composite Aerogels via a Double-Cross-Linked Network Approach. ACS Appl. Mater. Interfaces 2020, 12, 47854–47864. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.C.; Marques, P.A.A.P.; Vicente, R.; Godinho, L.; Duarte, I. Hybrid Structures Made of Polyurethane/Graphene Nanocomposite Foams Embedded within Aluminum Open-Cell Foam. Metals 2020, 10, 768. [Google Scholar] [CrossRef]
- De la Cruz, L.G.; Abt, T.; Leon, N.; Wang, L.; Sanchez-Soto, M. Ice-Template Crosslinked PVA Aerogels Modified with Tannic Acid and Sodium Alginate. Gels 2022, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Cui, Y.; Qin, L.; Yang, Y.; Zhao, Z.; Wang, X.; Liu, X. A novel robust adsorbent for efficient oil/water separation: Magnetic carbon nanospheres/graphene composite aerogel. J. Hazard. Mater. 2020, 392, 122499. [Google Scholar] [CrossRef]
- Xue, T.; Fan, W.; Zhang, X.; Zhao, X.; Yang, F.; Liu, T. Layered double hydroxide/graphene oxide synergistically enhanced polyimide aerogels for thermal insulation and fire-retardancy. Compos. Part B Eng. 2021, 219, 108963. [Google Scholar] [CrossRef]
- Idumah, C.I.; Ezika, A.C.; Okpechi, V.U. Emerging trends in polymer aerogel nanoarchitectures, surfaces, interfaces and applications. Surf. Interfaces 2021, 25, 101258. [Google Scholar] [CrossRef]
- Li, L.; Yuan, X.; Zhai, H.; Zhang, Y.; Wei, Q.; Xu, Y.; Wang, G. Flexible and Ultrathin Graphene/Aramid Nanofiber Carbonizing Films with Nacre-like Structures for Heat-Conducting Electromagnetic Wave Shielding/Absorption. ACS Appl. Mater. Interfaces 2023, 15, 15872–15883. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, A.; Chen, Y.; Wang, L.; Li, M.; Yang, H.; Hou, Y. Surface modification of core-shell structured ZIF-67@Cobalt coordination compound to improve the fire safety of biomass aerogel insulation materials. Chem. Eng. J. 2022, 430, 132809. [Google Scholar] [CrossRef]
- Zhou, S.; Apostolopoulou-Kalkavoura, V.; da Costa, M.V.T.; Bergstrom, L.; Stromme, M.; Xu, C. Elastic Aerogels of Cellulose Nanofibers@Metal-Organic Frameworks for Thermal Insulation and Fire Retardancy. Nano-Micro Lett. 2020, 12, 9. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Huang, H.; Pan, R.; Wu, C.; Yan, X.; Wang, H.; Pan, Y.; Hong, C.; Zhang, X. Multiscale, elastic, and low-density carbon fibre/siliconoxycarbide-phenolic interpenetrating aerogel nanocomposite for ablative thermal protection. Compos. Part B Eng. 2022, 245, 110212. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Guo, W.; Niu, H.; Song, L.; Hu, Y. Eco-friendly thermally insulating cellulose aerogels with exceptional flame retardancy, mechanical property and thermal stability. J. Taiwan Inst. Chem. Eng. 2022, 131, 104159. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Xia, Y.; Zhao, J.; Yi, L.-F.; Song, Y.-J.; Wu, H.; Guo, S.-Y.; Zhao, L.-J.; Wu, J.-R. Flame-retardant and Self-healing Biomass Aerogels Based on Electrostatic Assembly. Chin. J. Polym. Sci. 2020, 38, 1294–1304. [Google Scholar] [CrossRef]
- Stanly, S.; John, H. Uncarbonized crosslinked PVA-modified MMT/reduced graphene hybrid aerogel for efficient carbon dioxide adsorption at low pressure. J. Polym. Res. 2021, 28, 280. [Google Scholar] [CrossRef]
- Liu, S.; Chevali, V.S.; Xu, Z.; Hui, D.; Wang, H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos. Part B Eng. 2018, 136, 197–214. [Google Scholar] [CrossRef]
- Attia, N.F.; Elashery, S.E.A.; Zakria, A.M.; Eltaweil, A.S.; Oh, H. Recent advances in graphene sheets as new generation of flame retardant materials. Mater. Sci. Eng. B 2021, 274, 115460. [Google Scholar] [CrossRef]
- Szeluga, U.; Pusz, S.; Kumanek, B.; Olszowska, K.; Kobyliukh, A.; Trzebicka, B. Effect of graphene filler structure on electrical, thermal, mechanical, and fire retardant properties of epoxy-graphene nanocomposites—A review. Crit. Rev. Solid State Mater. Sci. 2021, 46, 152–187. [Google Scholar] [CrossRef]
- Li, Z.; Hu, M.; Shen, K.; Liu, Q.; Li, M.; Chen, Z.; Cheng, X.; Wu, X. Tuning thermal stability and fire hazards of hydrophobic silica aerogels via doping reduced graphene oxide. J. Non-Cryst. Solids 2024, 625, 122747. [Google Scholar] [CrossRef]
- Nabipour, H.; Nie, S.; Wang, X.; Song, L.; Hu, Y. Zeolitic imidazolate framework-8/polyvinyl alcohol hybrid aerogels with excellent flame retardancy. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105720. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; Xiang, Z.; Lu, W. Hollow CuS microflowers anchored porous carbon composites as lightweight and broadband microwave absorber with flame-retardant and thermal stealth functions. Carbon 2021, 184, 514–525. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, L.; Han, J.-M.; Li, H. Molding fabrication of copper azide/porous graphene with high electrostatic safety by self-assembly of graphene oxide. Nanotechnology 2021, 32, 385704. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Korobeinichev, O.; Shaklein, A.; Trubachev, S.; Karpov, A.; Paletsky, A.; Chernov, A.; Sosnin, E.; Shmakov, A. The Influence of Flame Retardants on Combustion of Glass Fiber-Reinforced Epoxy Resin. Polymers 2022, 14, 3379. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Wang, H. Superior flame retardancy of epoxy resin by the combined addition of graphene nanosheets and DOPO. Rsc Adv. 2016, 6, 5288–5295. [Google Scholar] [CrossRef]
- Chun, S.; Bang, S.; Jang, S.; Kim, D.W.; Lee, S.; Park, W.; Pang, C. A Hierarchical 3D Graphene Nanocomposite Foam for Extremely Tough, Non-Wettable, and Elastic Conductor. Adv. Mater. Interfaces 2020, 7, 2000354. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Lei, Q.; Fang, X.; Xie, H.; Yu, W. Tightly-packed fluorinated graphene aerogel/polydimethylsiloxane composite with excellent thermal management properties. Compos. Sci. Technol. 2022, 220, 109302. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Gao, Q.; Wang, Z.; Ye, N.; Li, J.; Lu, Y. Nerve-Fiber-Inspired Construction of 3D Graphene “Tracks” Supported by Wood Fibers for Multifunctional Biocomposite with Metal-Level Thermal Conductivity. Adv. Funct. Mater. 2023, 33, 2213274. [Google Scholar] [CrossRef]
- Shahzadi, K.; Ge, X.; Sun, Y.; Chen, S.; Jiang, Y. Fire retardant cellulose aerogel with improved strength and hydrophobic surface by one-pot method. J. Appl. Polym. Sci. 2021, 138, 50224. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Wang, S.; Chen, B.; Feng, Y.; Pei, Y.; Yan, Y.; Tang, L.; Qiu, H.; Wu, L. Exceptionally flame-retardant flexible polyurethane foam composites: Synergistic effect of the silicone resin/graphene oxide coating. Front. Chem. Sci. Eng. 2021, 15, 969–983. [Google Scholar] [CrossRef]
- Chen, S.; Lin, D.; Li, H.; Lai, X.; Zeng, X. Facile fabrication of superhydrophobic, flame-retardant and conductive polyurethane sponge via dip-coating. Mater. Lett. 2021, 287, 129307. [Google Scholar] [CrossRef]
- UL-94; Tests for Flammability of Plastic Materials for Parts in Devices and Appliances. Underwriters Laboratories: Northbrook, IL, USA, 2023.
- Hong, X.; Zheng, Y.; Shi, Y.; Zheng, W.; Lin, F.; Xiong, L. A facile strategy for constructing lightweight, fire safety and compression resistance poly(vinylalcohol) aerogels with highly-efficient expansible graphene oxide/layered double hydroxides hybrid synergistic flame retardant. J. Colloid Interface Sci. 2023, 650, 686–700. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Liu, T.; Ou, R.; Lin, J.; Puglia, D.; Xu, P.; Wang, Q.; Dong, W.; Du, M.; et al. Design of Intrinsically Flame-Retardant Vanillin-Based Epoxy Resin for Thermal-Conductive Epoxy/Graphene Aerogel Composites. ACS Appl. Mater. Interfaces 2021, 13, 59341–59351. [Google Scholar] [CrossRef]
- Xue, B.; Yang, S.; Qin, R.; Deng, S.; Niu, M.; Zhang, L. Effect of a graphene-APP composite aerogel coating on the polyester fabric for outstanding flammability. Prog. Org. Coat. 2022, 172, 107130. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Xue, T.; Tian, J.; Fan, W.; Liu, T. Flame-retardant and thermal-protective polyimide-hydroxyapatite aerogel fiber-based composite textile for firefighting clothing. Compos. Part B Eng. 2023, 248, 110377. [Google Scholar] [CrossRef]
- He, H.; Liu, J.; Wang, Y.; Zhao, Y.; Qin, Y.; Zhu, Z.; Yu, Z.; Wang, J. An Ultralight Self-Powered Fire Alarm e-Textile Based on Conductive Aerogel Fiber with Repeatable Temperature Monitoring Performance Used in Firefighting Clothing. ACS Nano 2022, 16, 2953–2967. [Google Scholar] [CrossRef]
- Li, H.; Hu, C.; He, Y.; Sun, Z.; Yin, Z.; Tang, D. Emerging surface strategies for porous materials-based phase change composites. Matter 2022, 5, 3225–3259. [Google Scholar] [CrossRef]
- Yin, L.; Xie, D.; Gong, K.; Shi, C.; Qian, X.; Zhou, K. Phase Change Materials Encapsulated in Coral-Inspired Organic-Inorganic Aerogels for Flame-Retardant and Thermal Energy Storage. ACS Appl. Nano Mater. 2023, 6, 8752–8762. [Google Scholar] [CrossRef]
- Cao, C.-F.; Liu, W.-J.; Xu, H.; Yu, K.-X.; Gong, L.-X.; Guo, B.-F.; Li, Y.-T.; Feng, X.-L.; Lv, L.-Y.; Pan, H.-T.; et al. Temperature-induced resistance transition behaviors of melamine sponge composites wrapped with different graphene oxide derivatives. J. Mater. Sci. Technol. 2021, 85, 194–204. [Google Scholar] [CrossRef]
- Cao, C.; Yuan, B. Thermally induced fire early warning aerogel with efficient thermal isolation and flame-retardant properties. Polym. Adv. Technol. 2021, 32, 2159–2168. [Google Scholar] [CrossRef]
- Luo, Z.; Ning, H.; Zhou, X.; Yuan, B. Efficient flame-retardant biomass aerogel endowed with graphene oxide interconnected networks for ultrasensitive fire warning. Mater. Lett. 2022, 318, 132237. [Google Scholar] [CrossRef]
- Zuo, B.; Yuan, B. Flame-retardant cellulose nanofiber aerogel modified with graphene oxide and sodium montmorillonite and its fire-alarm application. Polym. Adv. Technol. 2021, 32, 1877–1887. [Google Scholar] [CrossRef]
- Singh, S.B.; Dastgheib, S.A. Physicochemical transformation of graphene oxide during heat treatment at 110–200 °C. Carbon Trends 2023, 10, 100251. [Google Scholar] [CrossRef]
- Sánchez-Trujillo, D.J.; Osorio-Maldonado, L.V.; Prías-Barragán, J.J. Temperature dependence of electrical conductivity and variable hopping range mechanism on graphene oxide films. Sci. Rep. 2023, 13, 4810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Bongiorno, A. Origin of the Chemical and Kinetic Stability of Graphene Oxide. Sci. Rep. 2013, 3, 2484. [Google Scholar] [CrossRef] [PubMed]
- Świniarski, M.; Wróblewska, A.; Dużyńska, A.; Zdrojek, M.; Judek, J. Kinetics of the thermal reduction process in graphene oxide thin films from in-situ transport measurements. Mater. Res. Express 2021, 8, 015601. [Google Scholar] [CrossRef]
- Li, S.-L.; Wang, J.; Zhao, H.-B.; Cheng, J.-B.; Zhang, A.-N.; Wang, T.; Cao, M.; Fu, T.; Wang, Y.-Z. Ultralight Biomass Aerogels with Multifunctionality and Superelasticity Under Extreme Conditions. ACS Appl. Mater. Interfaces 2021, 13, 59231–59242. [Google Scholar] [CrossRef]
- Liu, Z.; Wan, K.; Zhu, T.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Superelastic, Fatigue-Resistant, and Flame-Retardant Spongy Conductor for Human Motion Detection against a Harsh High-Temperature Condition. ACS Appl. Mater. Interfaces 2021, 13, 7580–7591. [Google Scholar] [CrossRef]
- Huang, L.; Guo, H.; Long, C.; Fu, J.; Zeng, W.; Xiong, Y.; Wei, N.; Guo, X.; Xu, C.; Fang, P. Regenerated Silk Fibroin-Modified Soft Graphene Aerogels for Supercapacitive Stress Sensors. J. Electrochem. Soc. 2021, 168, 117511. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; Zhang, L.; El Azab, I.H.; Chen, Y.; Huang, M.; Huang, J.; Sheng, X. Flame-retardant and leakage-proof phase change composites based on MXene/polyimide aerogels toward solar thermal energy harvesting. Adv. Compos. Hybrid Mater. 2022, 5, 1253–1267. [Google Scholar] [CrossRef]
- Lin, K.; Cheng, Y.; Lu, J.; He, M.; Zhou, K. Graphene Aerogels Embedded with Boron Nitride Nanoparticles for Solar Energy Storage and Flame-Retardant Materials. ACS Appl. Nano Mater. 2023, 6, 21270–21281. [Google Scholar] [CrossRef]
- He, M.; Xie, D.; Yin, L.; Gong, K.; Zhou, K. Influences of reduction temperature on energy storage performance of paraffin wax/graphene aerogel composite phase change materials. Mater. Today Commun. 2023, 34, 105288. [Google Scholar] [CrossRef]
- Lee, J.; Han, H.; Noh, D.; Lee, J.; Lim, D.D.; Park, J.; Gu, G.X.; Choi, W. Multiscale Porous Architecture Consisting of Graphene Aerogels and Metastructures Enabling Robust Thermal and Mechanical Functionalities of Phase Change Materials. Adv. Funct. Mater. 2024, 2405625. [Google Scholar] [CrossRef]
- He, M.; Lu, J.; Shi, C.; Qian, X.; Yin, L.; Gong, K.; Zhou, K. Multi-Stimuli-Responsive Aerogels Composed of Ag Nanoparticle-Coated Graphene Nanosheets for Energy Storage and Conversion. Acs. Appl. Nano Mater. 2023, 6, 16503–16514. [Google Scholar] [CrossRef]
- Mu, B.; Li, M. Fabrication and thermal properties of tetradecanol/graphene aerogel form-stable composite phase change materials. Sci. Rep. 2018, 8, 8878. [Google Scholar] [CrossRef]
- Tao, Z.; He, W.; Xu, X.; Fan, J.; Zhang, Z.; Yang, Z.; Liu, Y.; Ma, H.; Qian, M.; Yang, M. Three-Dimensional Macroporous rGO-Aerogel-Based Composite Phase-Change Materials with High Thermal Storage Capacity and Enhanced Thermal Conductivity. Materials 2023, 16, 4878. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Song, Y.S. Graphene Aerogel-Supported Phase-Change Material for Pyroelectric Energy Harvesting: Structural Modification and Form Stability Analysis. Energy Technol. 2023, 11, 2201108. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, K.; Min, X.; Xiao, J.; Xu, Z.; Huang, Z.; Liu, Y.g.; Wu, X.; Fang, M. Graphene aerogel stabilized phase change material for thermal energy storage. Case Stud. Therm. Eng. 2022, 40, 102497. [Google Scholar] [CrossRef]
- Kong, X.; Nie, R.; Yuan, J. A review of shape stabilized aerogel-based phase change materials for preparation, classification and applications. Energy Built Environ. 2023. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Han, S.; Yang, R.; Min, P.; Yu, Z.-Z. High-quality graphene aerogels for thermally conductive phase change composites with excellent shape stability. J. Mater. Chem. A 2018, 6, 5880–5886. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Yang, Z.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Mechanically Robust and Flame-Retardant Silicon Aerogel Elastomers for Thermal Insulation and Efficient Solar Steam Generation. Acs Omega 2020, 5, 8638–8646. [Google Scholar] [CrossRef]
- Li, H.; Hou, L.; Liu, Y.; Yao, Z.; Liang, L.; Tian, D.; Liu, C.; Xue, J.; Zhan, L.; Liu, Y.; et al. Balanced Thermal Insulation, Flame-Retardant and Mechanical Properties of PU Foam Constructed via Cost-Effective EG/APP/SA Ternary Synergistic Modification. Polymers 2024, 16, 330. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Peng, H.; Yu, B.; Zhou, K.; Pan, H.; Zhang, L.; Li, M.; Liu, M.; Tian, A.; Fu, S. Biomimetic structural cellulose nanofiber aerogels with exceptional mechanical, flame-retardant and thermal-insulating properties. Chem. Eng. J. 2020, 389, 124449. [Google Scholar] [CrossRef]
- Wang, T.; Long, M.-C.; Zhao, H.-B.; An, W.-L.; Xu, S.; Deng, C.; Wang, Y.-Z. Temperature-Responsive Intumescent Chemistry toward Fire Resistance and Super Thermal Insulation under Extremely Harsh Conditions. Chem. Mater. 2021, 33, 6018–6028. [Google Scholar] [CrossRef]
- Guerrero-Alburquerque, N.; Zhao, S.; Adilien, N.; Koebel, M.M.; Lattuada, M.; Malfait, W.J. Strong, Machinable, and Insulating Chitosan-Urea Aerogels: Toward Ambient Pressure Drying of Biopolymer Aerogel Monoliths. ACS Appl. Mater. Inter. 2020, 12, 22037–22049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Chen, Z.-Y.; Tang, Y.-H.; Li, Y.-T.; Ma, D.; Zhang, G.-D.; Boukherroub, R.; Cao, C.-F.; Gong, L.-X.; Song, P.; et al. Silicone/graphene oxide co-cross-linked aerogels with wide-temperature mechanical flexibility, super-hydrophobicity and flame resistance for exceptional thermal insulation and oil/water separation. J. Mater. Sci. Technol. 2022, 114, 131–142. [Google Scholar] [CrossRef]
- Ma, W.; Liu, X.; Qiu, Z.; Cai, Z.; Diao, J.; Huang, Y. Hydrophobic and flame-retardant multifunctional foam for enhanced thermal insulation and broadband microwave absorption via a triple-continuous network of RGO/MWCNT-melamine composite. Carbon 2022, 196, 913–922. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; He, X.; Xiang, Z.; Heinze, T.; Qi, H. Lignocellulose nanofibril/gelatin/MXene composite aerogel with fire-warning properties for enhanced electromagnetic interference shielding performance. Chem. Eng. J. 2022, 431, 133907. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; Jiang, Y.; Song, P.; Wang, H. Lightweight high-performance carbon-polymer nanocomposites for electromagnetic interference shielding. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106376. [Google Scholar]
- Shu, R.; Xu, J.; Nie, L.; Shi, J. Facile construction of three-dimensional porous netlike reduced graphene oxide/zinc oxide composite aerogels as the lightweight, flame retardant, compression resilience and high-performance electromagnetic wave absorbers. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107068. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Zhao, Z.; Hu, H.; Li, B.; Zhu, C.; Zhang, X.; Chen, Y. Lightweight, Fire-Retardant, and Anti-Compressed Honeycombed-Like Carbon Aerogels for Thermal Management and High-Efficiency Electromagnetic Absorbing Properties. Small 2021, 17, 2102032. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Zhu, X.; Dong, Y.; Zhang, X.; Shi, Y.; Lu, W. Enhanced electromagnetic wave absorption of magnetic Co nanoparticles/CNTs/EG porous composites with waterproof, flame-retardant and thermal management functions. J. Mater. Chem. A 2021, 9, 17538–17552. [Google Scholar] [CrossRef]
- Li, Y.-R.; Li, Y.-M.; Chen, B.B.; Hu, W.-J.; Wang, D.-Y. Highly efficient electromagnetic wave absorption Fe-MOF-rGO based composites with enhanced flame retardancy. J. Alloys Compd. 2022, 918, 165516. [Google Scholar] [CrossRef]
- Fu, H.; Chen, L.; Liu, D.; Zhang, Y.; Cao, Y.; Wu, C.; Yong, Z.; Yu, Y.; Li, Q. Multifunctional NiCo@RGO/SWNTs foam with oriented pore structure for excellent electromagnetic interference shielding. Chem. Eng. J. 2023, 454, 140324. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Tan, J.; Ma, C.; Luo, S.; Li, W.; Liu, S. Hierarchical porous graphene oxide/carbon foam nanocomposites derived from larch for enhanced CO2 capture and energy storage performance. J. CO2 Util. 2021, 52, 101666. [Google Scholar] [CrossRef]
- Mao, M.; Xu, H.; Guo, K.-Y.; Zhang, J.-W.; Xia, Q.-Q.; Zhang, G.-D.; Zhao, L.; Gao, J.-F.; Tang, L.-C. Mechanically flexible, super-hydrophobic and flame-retardant hybrid nano-silica/graphene oxide wide ribbon decorated sponges for efficient oil/water separation and fire warning response. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106191. [Google Scholar] [CrossRef]
- Cao, M.; Li, S.-L.; Cheng, J.-B.; Zhang, A.-N.; Wang, Y.-Z.; Zhao, H.-B. Fully bio-based, low fire-hazard and superelastic aerogel without hazardous cross-linkers for excellent thermal insulation and oil clean-up absorption. J. Hazard. Mater. 2021, 403, 123977. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Liu, X.; Su, Y.; Wang, J.; Fan, T.; Ning, X.; Ramakrishn, S. Ultralight and multifunctional PVDF/SiO2@GO nanofibrous aerogel for efficient harsh environmental oil-water separation and crude oil absorption. Carbon 2022, 193, 77–87. [Google Scholar] [CrossRef]
- Nabipour, H.; Nie, S.; Wang, X.; Song, L.; Hu, Y. Highly flame retardant zeolitic imidazole framework-8@cellulose composite aerogels as absorption materials for organic pollutants. Cellulose 2020, 27, 2237–2251. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Li, L.; Qiu, S.; He, W.; Xu, W.; Mai, Y.; Guo, F. Phytic acid assisted preparation of high-performance supercapacitor electrodes from noncarbonizable polyvinylpyrrolidone. J. Power Sources 2020, 448, 227402. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Q.; Ren, J.; Chen, W.; Pei, Y.; Kaplan, D.L.; Ling, S. Electro-Blown Spun Silk/Graphene Nanoionotronic Skin for Multifunctional Fire Protection and Alarm. Adv. Mater. 2021, 33, 2102500. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Liu, L.; Cao, Y.; Zhang, L.; Sheng, X.; Chen, Y. Biomass modified boron nitride/polyimide hybrid aerogel supported phase change composites with superior energy storage capacity and improved flame retardancy for solar-thermal energy storage. Sol. Energy 2022, 242, 287–297. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Jiang, H.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D.; Wu, H.; Mei, Y. Flame-retardant and form-stable phase change composites based on MXene with high thermostability and thermal conductivity for thermal energy storage. Chem. Eng. J. 2021, 420, 130466. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Wei, J.; Li, X.; Li, N.; Liu, J. An Intelligent Fire-Protection Coating Based on Ammonium Polyphosphate/Epoxy Composites and Laser-Induced Graphene. Polymers 2021, 13, 984. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, C.H.; Yang, C.-M. Binder-free silicon anodes wrapped in multiple graphene shells for high-performance lithium-ion batteries. J. Power Sources 2021, 486, 229350. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Wang, T.; Han, S.; Zhang, X.; Wang, J.; Sun, G. Polyurethane Foam with High-Efficiency Flame Retardant, Heat Insulation, and Sound Absorption Modified By Phosphorus-Containing Graphene Oxide. ACS Appl. Polym. Mater. 2024, 6, 1878–1890. [Google Scholar] [CrossRef]
- Wi, S.; Berardi, U.; Di Loreto, S.; Kim, S. Microstructure and thermal characterization of aerogel-graphite polyurethane spray-foam composite for high efficiency thermal energy utilization. J. Hazard. Mater. 2020, 397, 122656. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Z.; Su, D. Lightweight and large-scale rGO reinforced SiBCN aerogels with hierarchical cellular structures exposed to high-temperature environments. J. Mater. Sci. Technol. 2024, 179, 145–154. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, W.; Fang, Y.; Cheng, M.; Wang, S. Investigation of novel expandable polystyrene/alumina aerogel composite thermal insulation material. Energy 2023, 284, 129238. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wu, L.; Wang, X. Metal-graphene-synergized melamine aerogel with robust elasticity and flame-retardancy for thermal-insulated-packaging industry. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106195. [Google Scholar] [CrossRef]
- Chen, W.; Liu, S.; Dong, Y.; Zhou, X.; Zhou, F. Poly (m-phenylene isophthalamide)/graphene composite aerogels with enhanced compressive shape stability for thermal insulation. J. Sol-Gel Sci. Technol. 2020, 96, 370–381. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Zhang, J.; Zhang, Y.; Gu, J. Lightweight and robust rGO/sugarcane derived hybrid carbon foams with outstanding EMI shielding performance. J. Mater. Sci. Technol. 2020, 52, 119–126. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, M.; Liu, C.; Fu, L.; Lv, Y.; Feng, Y.; Huang, P.; Yang, F.; Song, P.; Liu, M. Lightweight, amphipathic and fire-resistant prGO/MXene spherical beads for rapid elimination of hazardous chemicals. J. Hazard. Mater. 2022, 423, 127069. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Ha, L.Q.; Van, L.C.T.; Huynh, P.T.B.; Nguyen, D.M.; Nguyen, V.P.; Tran, T.H.; Hoang, D. Antibiotics tetracycline adsorption and flame-retardant capacity of eco-friendly aerogel-based nanocellulose, graphene oxide, polyvinyl alcohol, and sodium bicarbonate. J. Environ. Chem. Eng. 2023, 11, 109523. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, J.; Mao, J.; Bao, Z.; Chen, Z.; Lai, Y. Charged graphene aerogel filter enabled superior particulate matter removal efficiency in harsh environment. Chem. Eng. J. 2020, 395, 125086. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; He, M.; Qin, Q.; Liu, W.; Liao, L.; Qin, S. Expanded Properties and Applications of Porous Flame-Retardant Polymers Containing Graphene and Its Derivatives. Polymers 2024, 16, 2053. https://doi.org/10.3390/polym16142053
Liu S, He M, Qin Q, Liu W, Liao L, Qin S. Expanded Properties and Applications of Porous Flame-Retardant Polymers Containing Graphene and Its Derivatives. Polymers. 2024; 16(14):2053. https://doi.org/10.3390/polym16142053
Chicago/Turabian StyleLiu, Shan, Min He, Qingdong Qin, Wei Liu, Longfeng Liao, and Shuhao Qin. 2024. "Expanded Properties and Applications of Porous Flame-Retardant Polymers Containing Graphene and Its Derivatives" Polymers 16, no. 14: 2053. https://doi.org/10.3390/polym16142053
APA StyleLiu, S., He, M., Qin, Q., Liu, W., Liao, L., & Qin, S. (2024). Expanded Properties and Applications of Porous Flame-Retardant Polymers Containing Graphene and Its Derivatives. Polymers, 16(14), 2053. https://doi.org/10.3390/polym16142053







