Protective Encapsulation of a Bioactive Compound in Starch–Polyethylene Glycol-Modified Microparticles: Degradation Analysis with Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microparticules
2.3. Characterization
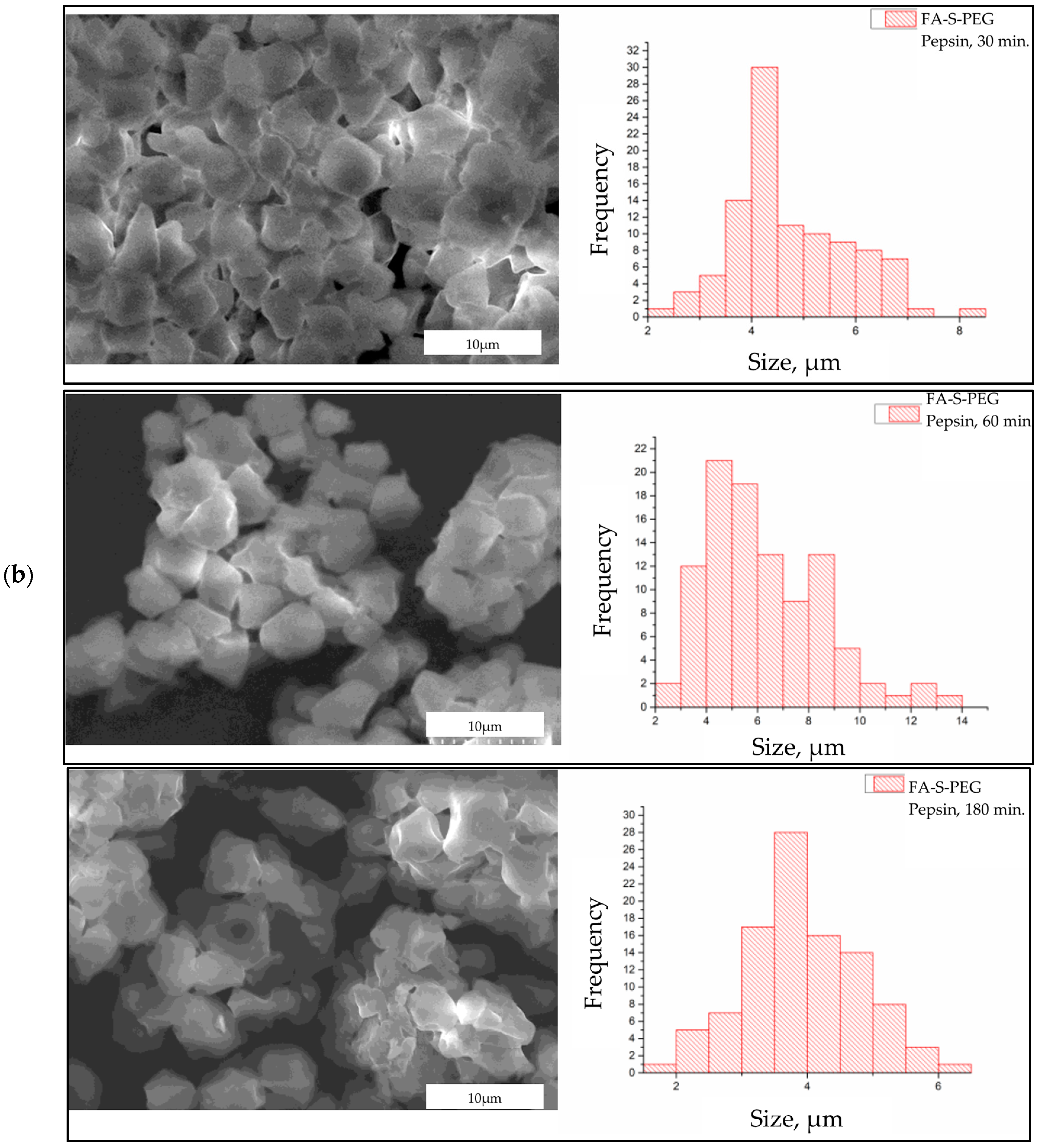
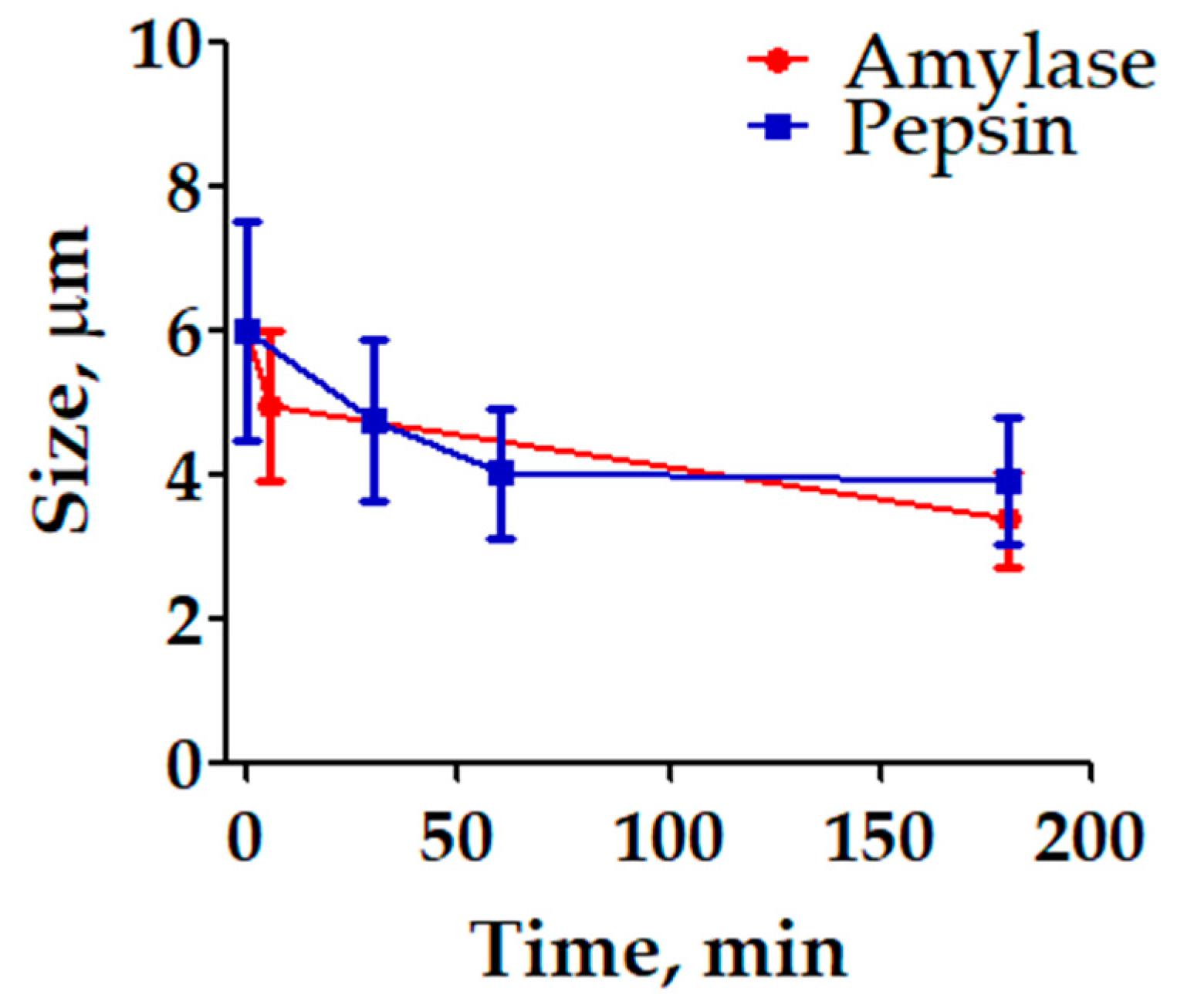
2.4. Enzimatic Degradation of FA-S-PEG in Physiological Simulated Conditions
2.5. Statistical Analysis
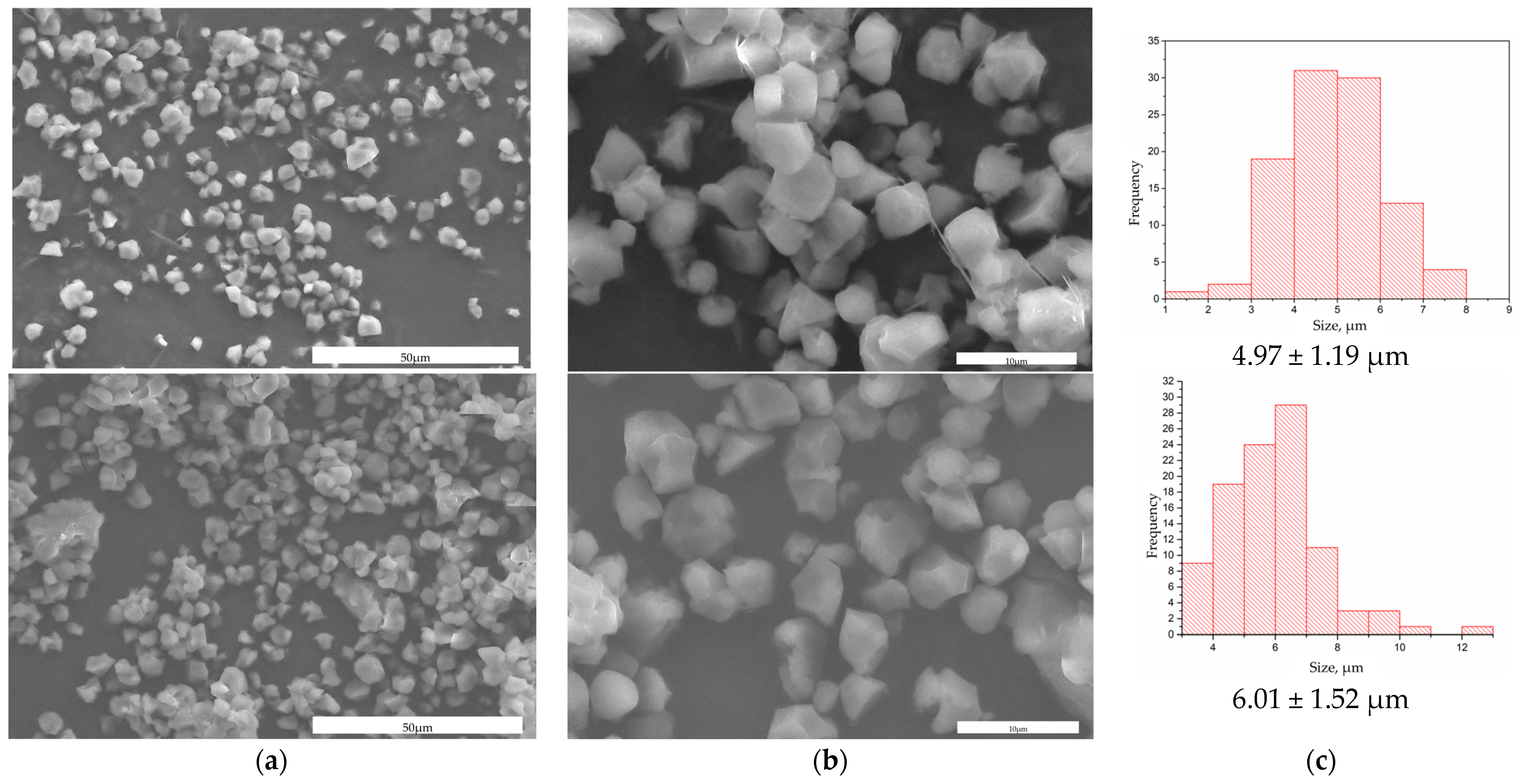
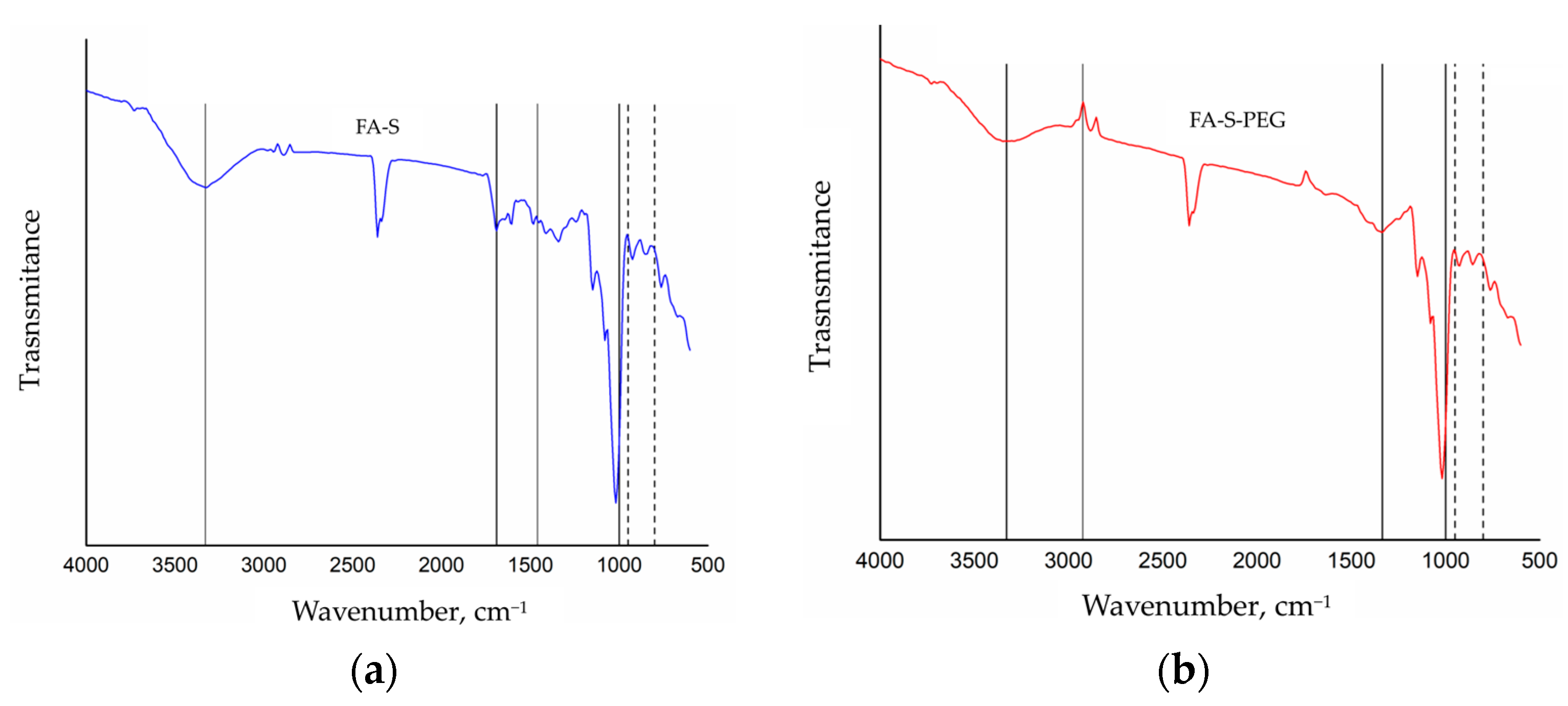
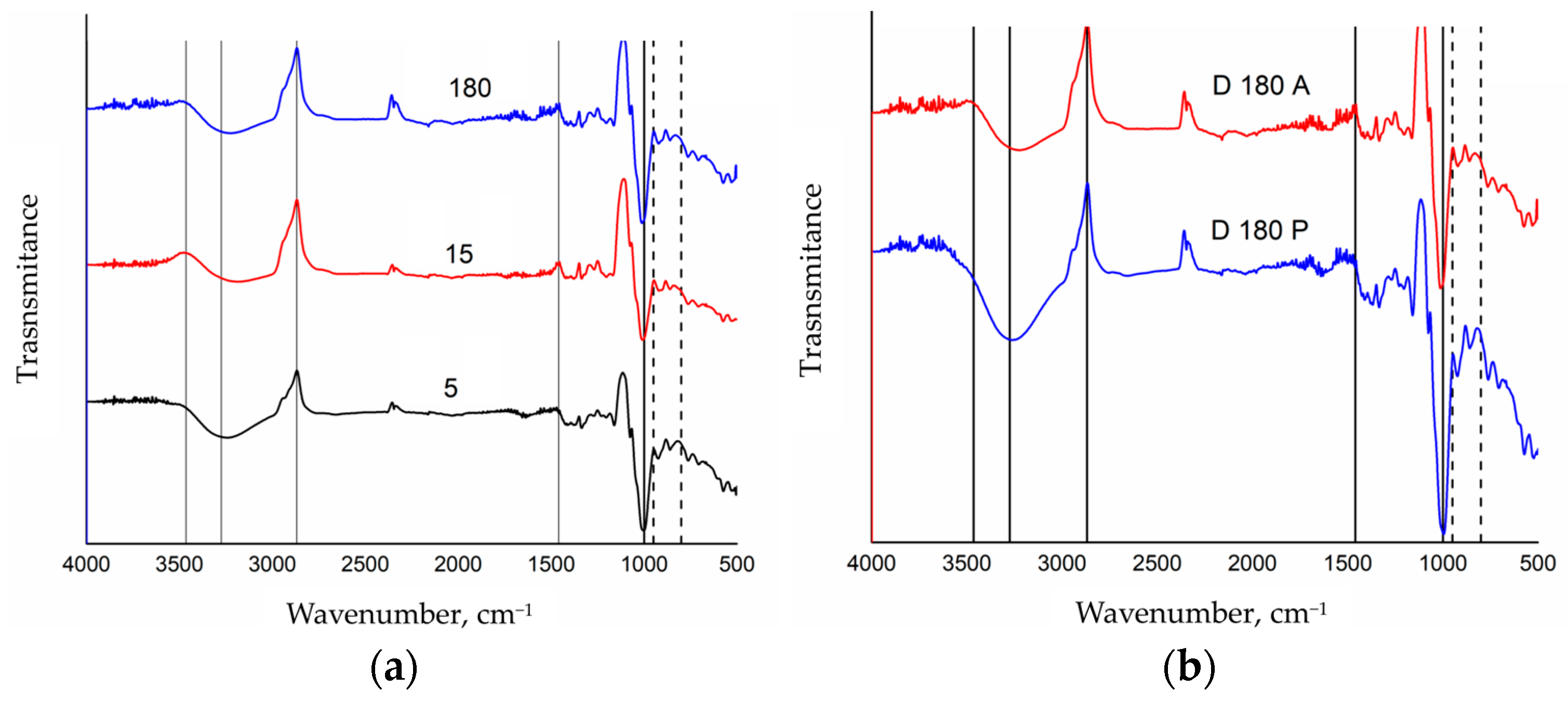
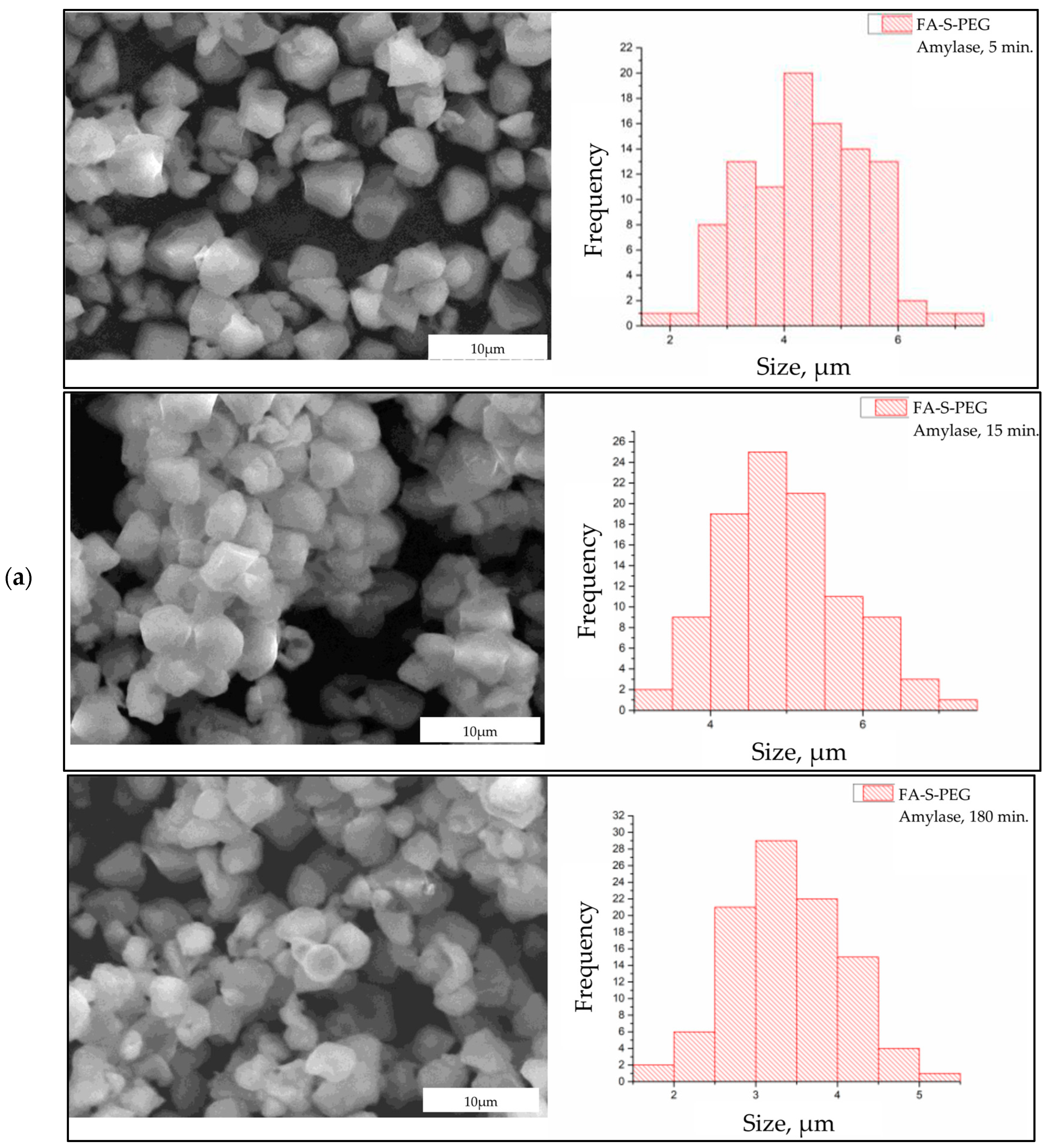
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Wang, S.; Zhang, C.; Ma, M.; Yan, B.; Hu, X.; Shao, T.; Piao, Y.; Jin, L.; Gao, J. Microstructure Formation and Characterization of Long-Acting Injectable Microspheres: The Gateway to Fully Controlled Drug Release Pattern. Int. J. Nanomed. 2024, 19, 1571–1595. [Google Scholar] [CrossRef] [PubMed]
- Kupikowska-Stobba, B.; Lewińska, D. Polymer Microcapsules and Microbeads as Cell Carriers for in Vivo Biomedical Applications. Biomater. Sci. 2020, 8, 1536–1574. [Google Scholar] [CrossRef] [PubMed]
- Bhujel, R.; Maharjan, R.; Kim, N.A.; Jeong, S.H. Practical Quality Attributes of Polymeric Microparticles with Current Understanding and Future Perspectives. J. Drug Deliv. Sci. Technol. 2021, 64, 102608. [Google Scholar] [CrossRef]
- Ma, G.; Yue, H. Advances in Uniform Polymer Microspheres and Microcapsules: Preparation and Biomedical Applications. Chin. J. Chem. 2020, 38, 911–923. [Google Scholar] [CrossRef]
- Roacho-Pérez, J.A.; Rodríguez-Aguillón, K.O.; Gallardo-Blanco, H.L.; Velazco-Campos, M.R.; Sosa-Cruz, K.V.; García-Casillas, P.E.; Rojas-Patlán, L.; Sánchez-Domínguez, M.; Rivas-Estilla, A.M.; Gómez-Flores, V.; et al. A Full Set of In Vitro Assays in Chitosan/Tween 80 Microspheres Loaded with Magnetite Nanoparticles. Polymers 2021, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Zhang, W.; Chen, X.; Yang, T.; Ma, G. Microspheres and Microcapsules for Protein Delivery: Strategies of Drug Activity Retention. Curr. Pharm. Des. 2013, 19, 6340–6352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, W.; Lv, P.; Wang, L.; Ma, G. Preparation and Evaluation of Alginate-Chitosan Microspheres for Oral Delivery of Insulin. Eur. J. Pharm. Biopharm. 2011, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Chen, W.; Zheng, X.; Ma, L. Preparation of PH-Sensitive Alginate-Based Hydrogel by Microfluidic Technology for Intestinal Targeting Drug Delivery. Int. J. Biol. Macromol. 2024, 254, 127649. [Google Scholar] [CrossRef] [PubMed]
- Thaarup, I.C.; Gummesson, C.; Bjarnsholt, T. Measuring Enzymatic Degradation of Degradable Starch Microspheres Using Confocal Laser Scanning Microscopy. Acta Biomater. 2021, 131, 464–471. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Cantarella, C.D.; Ragusa, D.; Giammanco, M.; Tosi, S. Folate Deficiency as Predisposing Factor for Childhood Leukaemia: A Review of the Literature. Genes Nutr. 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Zarou, M.M.; Vazquez, A.; Vignir Helgason, G. Folate Metabolism: A Re-Emerging Therapeutic Target in Haematological Cancers. Leukemia 2021, 35, 1539. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- Nickels, E.M.; Li, S.; Morimoto, L.; Kang, A.Y.; de Smith, A.J.; Metayer, C.; Wiemels, J.L. Periconceptional Folate Intake Influences DNA Methylation at Birth Based on Dietary Source in an Analysis of Pediatric Acute Lymphoblastic Leukemia Cases and Controls. Am. J. Clin. Nutr. 2022, 116, 1553. [Google Scholar] [CrossRef]
- Ortiz-Quezada, A.G.; Lombardini, L.; Cisneros-Zevallos, L. Antioxidants in Pecan Nut Cultivars [Carya Illinoinensis (Wangenh.) K. Koch]. Nuts Seeds Health Dis. Prev. 2011, 881–889. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Draszanowska, A.; Starowicz, M. Effect of Different Cooking Methods on the Folate Content, Organoleptic and Functional Properties of Broccoli and Spinach. LWT 2022, 167, 113825. [Google Scholar] [CrossRef]
- Gazzali, A.M.; Lobry, M.; Colombeau, L.; Acherar, S.; Azaïs, H.; Mordon, S.; Arnoux, P.; Baros, F.; Vanderesse, R.; Frochot, C. Stability of Folic Acid under Several Parameters. Eur. J. Pharm. Sci. 2016, 93, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Yücel, O.; Şengelen, A.; Emik, S.; Önay-Uçar, E.; Arda, N.; Gürdaǧ, G. Folic Acid-Modified Methotrexate-Conjugated Gold Nanoparticles as Nano-Sized Trojans for Drug Delivery to Folate Receptor-Positive Cancer Cells. Nanotechnology 2020, 31, 355101. [Google Scholar] [CrossRef]
- Muhammad, Z.; Raza, A.; Ghafoor, S.; Naeem, A.; Naz, S.S.; Riaz, S.; Ahmed, W.; Rana, N.F. PEG Capped Methotrexate Silver Nanoparticles for Efficient Anticancer Activity and Biocompatibility. Eur. J. Pharm. Sci. 2016, 91, 251–255. [Google Scholar] [CrossRef]
- Ganesh, A.N.; Heusser, C.; Garad, S.; Sánchez-Félix, M.V. Patient-Centric Design for Peptide Delivery: Trends in Routes of Administration and Advancement in Drug Delivery Technologies. Med. Drug Discov. 2021, 9, 100079. [Google Scholar] [CrossRef]
- Shahbaz, A.; Hussain, N.; Basra, M.A.R.; Bilal, M. Polysaccharides-Based Nano-Hybrid Biomaterial Platforms for Tissue Engineering, Drug Delivery, and Food Packaging Applications. Starch/Staerke 2022, 74, 2200023. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Xue, R.; Fan, J.; Yu, H.; Guan, J.; Wang, H.; Li, M.; Yu, W.; Xie, Z.; et al. All-in-One Theranostic Platform Based on Hollow Microcapsules for Intragastric-Targeting Antiulcer Drug Delivery, CT Imaging, and Synergistically Healing Gastric Ulcer. Small 2022, 18, 2104660. [Google Scholar] [CrossRef] [PubMed]
- López-Ramírez, E.; Chapa-González, C.; Martínez-Peréz, C.A.; Escobedo-González, R.; Nicolás Vázquez, M.I.; Medellín-Rodríguez, F.; García-Casillas, P.E. Citrulline Malate Transdermal Delivery through Integrating into Polyvinyl Alcohol (PVA) Nanofibers. J. Drug Deliv. Sci. Technol. 2021, 64, 102630. [Google Scholar] [CrossRef]
- Zanbak Çotaoğlu, E.M.; Köse Özkan, C.; Özkan, Y. Nanobiomaterials: Applications in Nanomedicine and Drug Delivery. In Handbook of Nanobioelectrochemistry; Springer: Singapore, 2023; pp. 519–539. [Google Scholar] [CrossRef]
- Roacho-Perez, J.A.; Gallardo-Blanco, H.L.; Sanchez-Dominguez, M.; Garcia-Casillas, P.; Chapa-Gonzalez, C.; Sanchez-Dominguez, C.N. Nanoparticles for Death-Induced Gene Therapy in Cancer (Review). Mol. Med. Rep. 2018, 17, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Chapa González, C.; Martínez Saráoz, J.V.; Roacho Pérez, J.A.; Olivas Armendáriz, I. Lipid Nanoparticles for Gene Therapy in Ocular Diseases. DARU J. Pharm. Sci. 2023, 31, 75–82. [Google Scholar] [CrossRef]
- Yadav, K.S.; Soni, G.; Choudhary, D.; Khanduri, A.; Bhandari, A.; Joshi, G. Microemulsions for Enhancing Drug Delivery of Hydrophilic Drugs: Exploring Various Routes of Administration. Med. Drug Discov. 2023, 20, 100162. [Google Scholar] [CrossRef]
- Barrón, S.; González, C.; Parrilla, Á.; la Rosa, D.; Nanoparticle-Mediated, L.; Bohara, R.A.; Cristina Stevens Barrón, J.; Chapa González, C.; Álvarez Parrilla, E.; Alejandra De la Rosa, L. Nanoparticle-Mediated Delivery of Flavonoids: Impact on Proinflammatory Cytokine Production: A Systematic Review. Biomolecules 2023, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, Q.; Chen, X.; Liu, J. Delivery Luteolin with Folacin-Modified Nanoparticle for Glioma Therapy. Int. J. Nanomed. 2019, 14, 7515. [Google Scholar] [CrossRef] [PubMed]
- Toscanini, M.A.; Limeres, M.J.; Garrido, A.V.; Cagel, M.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A.; Cuestas, M.L. Polymeric Micelles and Nanomedicines: Shaping the Future of next Generation Therapeutic Strategies for Infectious Diseases. J. Drug Deliv. Sci. Technol. 2021, 66, 102927. [Google Scholar] [CrossRef]
- Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872–930. [Google Scholar] [CrossRef]
- Rajan, R.; Pal, K.; Jayadev, D.; Jayan, J.S.; Aathira, U.; Appukuttan, S.; de Souza, F.G.; Joseph, K.; Kumar, S.S. Polymeric Nanoparticles in Hybrid Catalytic Processing and Drug Delivery System. Top. Catal. 2022, 65, 1860–1884. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M.; Bejenaru, C.; Bejenaru, L.E. Polymeric Protective Agents for Nanoparticles in Drug Delivery and Targeting. Int. J. Pharm. 2016, 510, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef]
- Alvarez-Barreto, J.F.; Viteri-Narvaez, D.; Proano, J.S.; Caicedo, A.; Grunauer, M.; Eguiguren, L.; Vargas, M. Development of Films, Based on Oxidized Ipomea Batatas Starch, with Protein Encapsulation. Rev. Mex. Ing. Biomed. 2021, 42, 119–131. [Google Scholar] [CrossRef]
- Gaona, C.G.C.; Alonso, M.C.I.; Céspedes, R.I.N.; Rosas, M.M.T.; Martínez, R.R.; Escareño, M.P.L. Novel Studies in the Designs of Natural, Synthetic, and Compound Hydrogels with Biomedical Applications. Rev. Mex. Ing. Biomed. 2023, 44, 74–96. [Google Scholar] [CrossRef]
- Morales, A.J.; López, I.D.; Sosa Savedra, J.C.; García García, A.L. A Review on the Advances of Biocompatible Materials and Their Processing Via Additive Manufacturing for Tissue Engineering Applications. Rev. Mex. Ing. Biomed. 2023, 44, 24–36. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A Review of Starch, a Unique Biopolymer—Structure, Metabolism and in Planta Modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Ali, K.A.; Roy, P.K.; Hossain, C.M.; Dutta, D.; Vichare, R.; Biswal, M.R. Starch-Based Nanomaterials in Drug Delivery Applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 31–56. [Google Scholar] [CrossRef]
- Marto, J.; Ribeiro, H.M.; Almeida, A.J. Starch-Based Nanocapsules as Drug Carriers for Topical Drug Delivery. In Smart Nanocontainers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 287–294. [Google Scholar] [CrossRef]
- Li, B.Z.; Wang, L.J.; Li, D.; Bhandari, B.; Li, S.J.; Lan, Y.; Chen, X.D.; Mao, Z.H. Fabrication of Starch-Based Microparticles by an Emulsification-Crosslinking Method. J. Food Eng. 2009, 92, 250–254. [Google Scholar] [CrossRef]
- Minakawa, A.F.K.; Faria-Tischer, P.C.S.; Mali, S. Simple Ultrasound Method to Obtain Starch Micro- and Nanoparticles from Cassava, Corn and Yam Starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, C.; Gong, C.; McClements, D.J.; Jin, Z.; Wang, J. Advances in Research on Preparation, Characterization, Interaction with Proteins, Digestion and Delivery Systems of Starch-Based Nanoparticles. Int. J. Biol. Macromol. 2020, 152, 117–125. [Google Scholar] [CrossRef]
- Ren, N.; Ma, Z.; Li, X.; Hu, X. Preparation of Rutin-Loaded Microparticles by Debranched Lentil Starch-Based Wall Materials: Structure, Morphology and in Vitro Release Behavior. Int. J. Biol. Macromol. 2021, 173, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Roacho-Pérez, J.A.; Ruiz-Hernandez, F.G.; Chapa-Gonzalez, C.; Martínez-Rodríguez, H.G.; Flores-Urquizo, I.A.; Pedroza-Montoya, F.E.; Garza-Treviño, E.N.; Bautista-Villarea, M.; García-Casillas, P.E.; Sánchez-Domínguez, C.N. Magnetite Nanoparticles Coated with PEG 3350-Tween 80: In Vitro Characterization Using Primary Cell Cultures. Polymers 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, S.H.A.; Garciá Casillas, P.; González, C.C. Seed-Mediated Synthesis and PEG Coating of Gold Nanoparticles for Controlling Morphology and Sizes. MRS Adv. 2020, 5, 3353–3360. [Google Scholar] [CrossRef]
- Dethe, M.R.; Prabakaran, A.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG Copolymer Based Injectable Thermosensitive Hydrogels. J. Control Release 2022, 343, 217. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Youngblood, R.; Cassinotti, L.; Skoumal, M.; Corfas, G.; Shea, L. An Injectable PEG Hydrogel Controlling Neurotrophin-3 Release by Affinity Peptides. J. Control Release 2021, 330, 575. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, D.W.; Cho, K.Y. The Influence of PEG Molecular Weight on the Structural Changes of Corn Starch in a Starch/PEG Blend. Polym. Bull. 2009, 63, 91–99. [Google Scholar] [CrossRef]
- Saputri, D.G.; Khairuddin; Nurhayati, N.D.; Pham, T. Optimization Properties of Environmentally Friendly Paper Coating Based Starch-Polyethylene Glycol (PEG) Mixture. J. Phys. Conf. Ser. 2017, 909, 012029. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Shi, Y.; Xi, G.; Wang, M.; Liang, B.; Feng, Y.; Ren, X.; Shi, C. Peptide-Immobilized Starch/PEG Sponge with Rapid Shape Recovery and Dual-Function for Both Uncontrolled and Noncompressible Hemorrhage. Acta Biomater. 2019, 99, 220–235. [Google Scholar] [CrossRef]
- Valenzuela Villela, K.S.; Betancourt Galindo, R.; Espinosa Neira, R.; Espinosa Solís, V.; García-Casillas, P.E. Stability of Starch-Folic Acid/ Polyethylene Glycol Particles for Gastrointestinal Drug Delivery. Food Hydrocoll. 2024, 22, 110318. [Google Scholar] [CrossRef]
- Sensoy, I. A Review on the Food Digestion in the Digestive Tract and the Used in Vitro Models. Curr. Res. Food Sci. 2021, 4, 308. [Google Scholar] [CrossRef] [PubMed]
- Momeni, S.; Ghomi, E.R.; Shakiba, M.; Shafiei-Navid, S.; Abdouss, M.; Bigham, A.; Khosravi, F.; Ahmadi, Z.; Faraji, M.; Abdouss, H.; et al. The Effect of Poly (Ethylene Glycol) Emulation on the Degradation of PLA/Starch Composites. Polymers 2021, 13, 1019. [Google Scholar] [CrossRef] [PubMed]
- Akakuru, O.U.; Louis, H.; Uwaoma, R.; Elemike, E.E.; Akakuru, O.C. Novel Highly-Swellable and PH-Responsive Slow Release Formulations of Clotrimazole with Chitosan-g-PEG/Starch Microparticles. React. Funct. Polym. 2019, 135, 32–43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela Villela, K.S.; Alvarado Araujo, K.V.; Garcia Casillas, P.E.; Chapa González, C. Protective Encapsulation of a Bioactive Compound in Starch–Polyethylene Glycol-Modified Microparticles: Degradation Analysis with Enzymes. Polymers 2024, 16, 2075. https://doi.org/10.3390/polym16142075
Valenzuela Villela KS, Alvarado Araujo KV, Garcia Casillas PE, Chapa González C. Protective Encapsulation of a Bioactive Compound in Starch–Polyethylene Glycol-Modified Microparticles: Degradation Analysis with Enzymes. Polymers. 2024; 16(14):2075. https://doi.org/10.3390/polym16142075
Chicago/Turabian StyleValenzuela Villela, Karen Sofia, Karen Valeria Alvarado Araujo, Perla Elvia Garcia Casillas, and Christian Chapa González. 2024. "Protective Encapsulation of a Bioactive Compound in Starch–Polyethylene Glycol-Modified Microparticles: Degradation Analysis with Enzymes" Polymers 16, no. 14: 2075. https://doi.org/10.3390/polym16142075





