Thermal, Morphological, Mechanical, and Biodegradation Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/High-Density Polyethylene Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLLA/HDPE and PLLA-PEG-PLLA/HDPE Blends
2.3. Characterization of PLLA/HDPE and PLLA-PEG-PLLA/HDPE Blends
3. Results and Discussion
3.1. Thermal Transition Properties
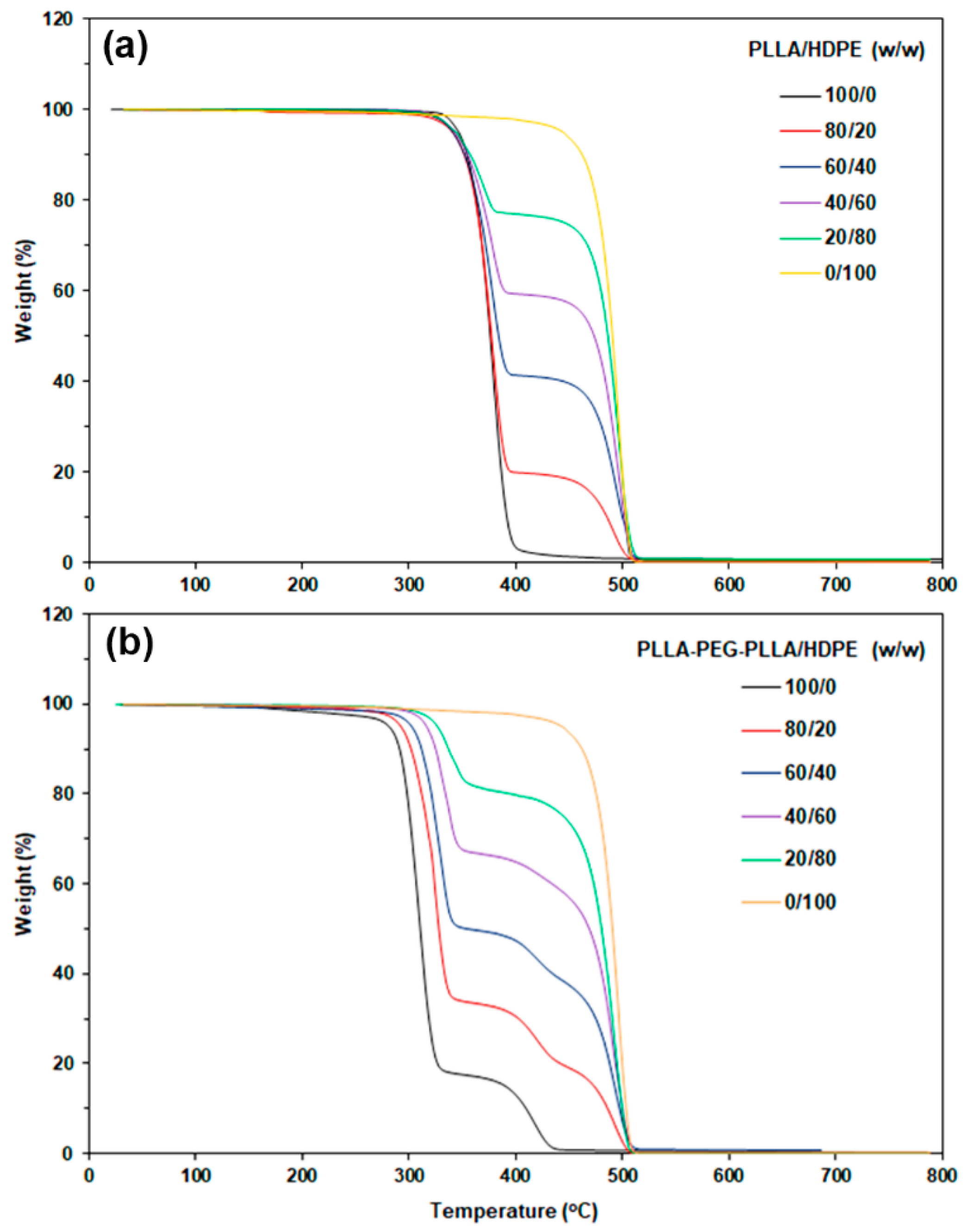
3.2. Thermal Decomposition Properties
3.3. Crystalline Structures
3.4. Phase Morphology
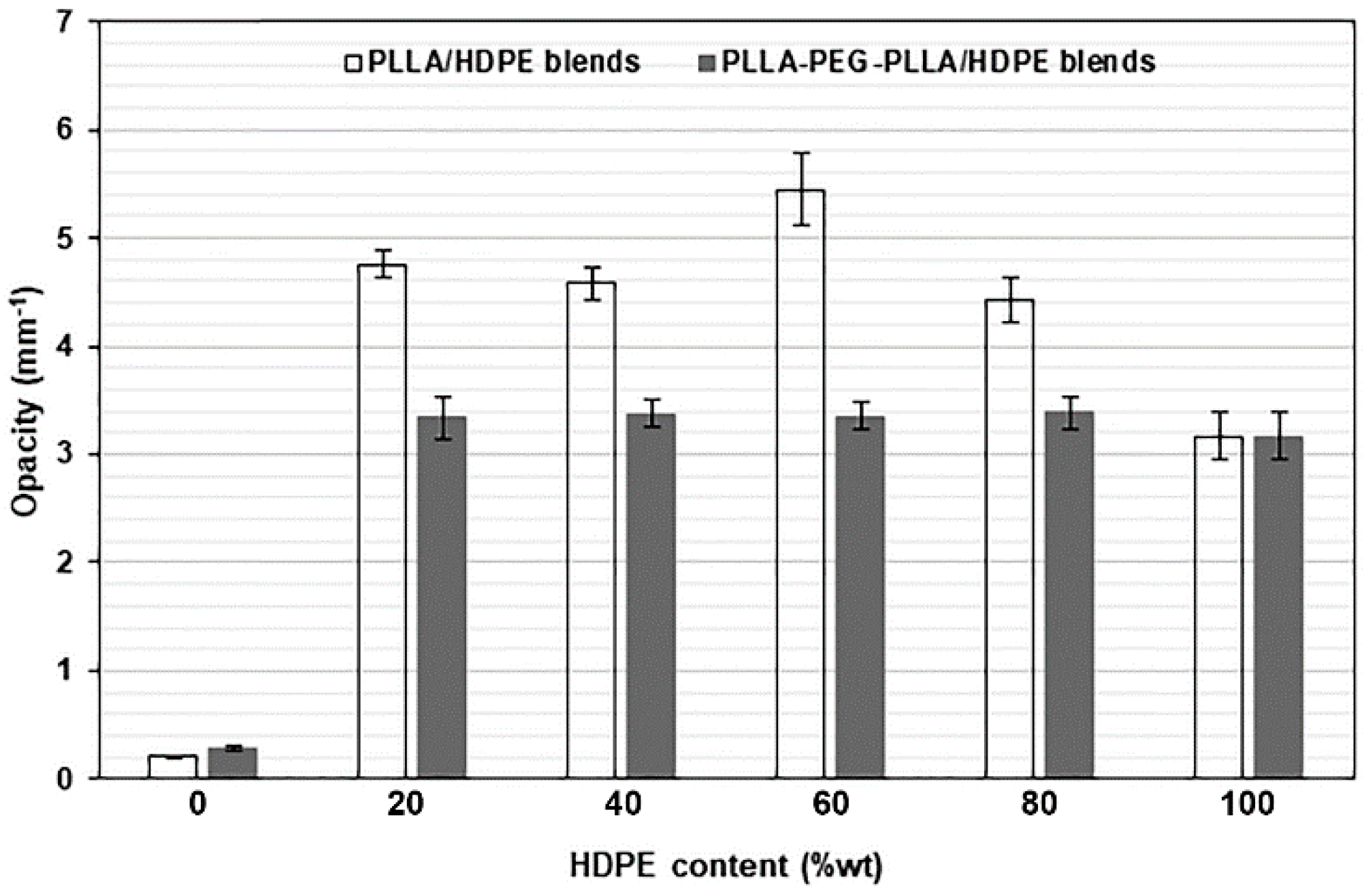
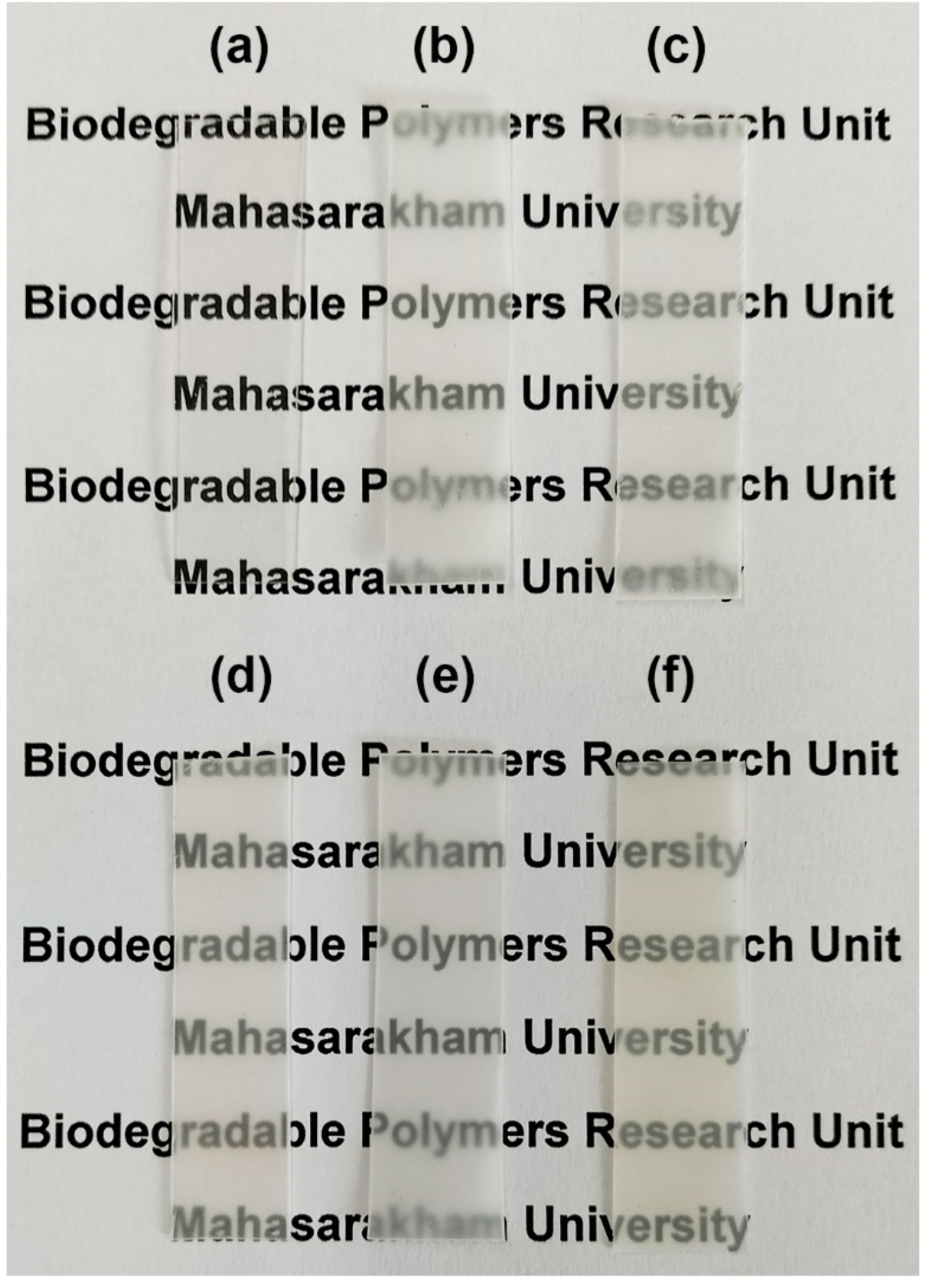
3.5. Film Opacity
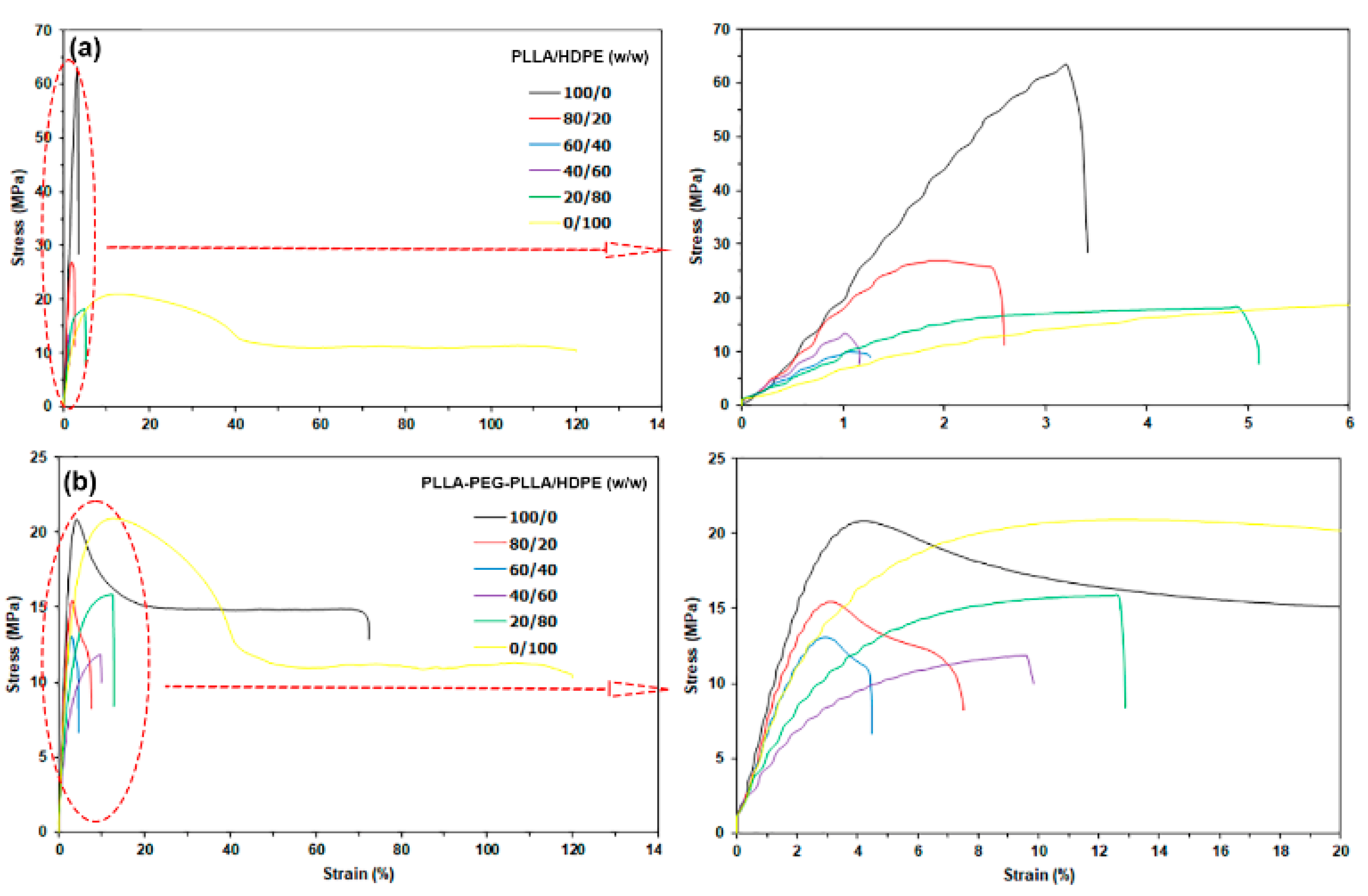
3.6. Tensile Properties
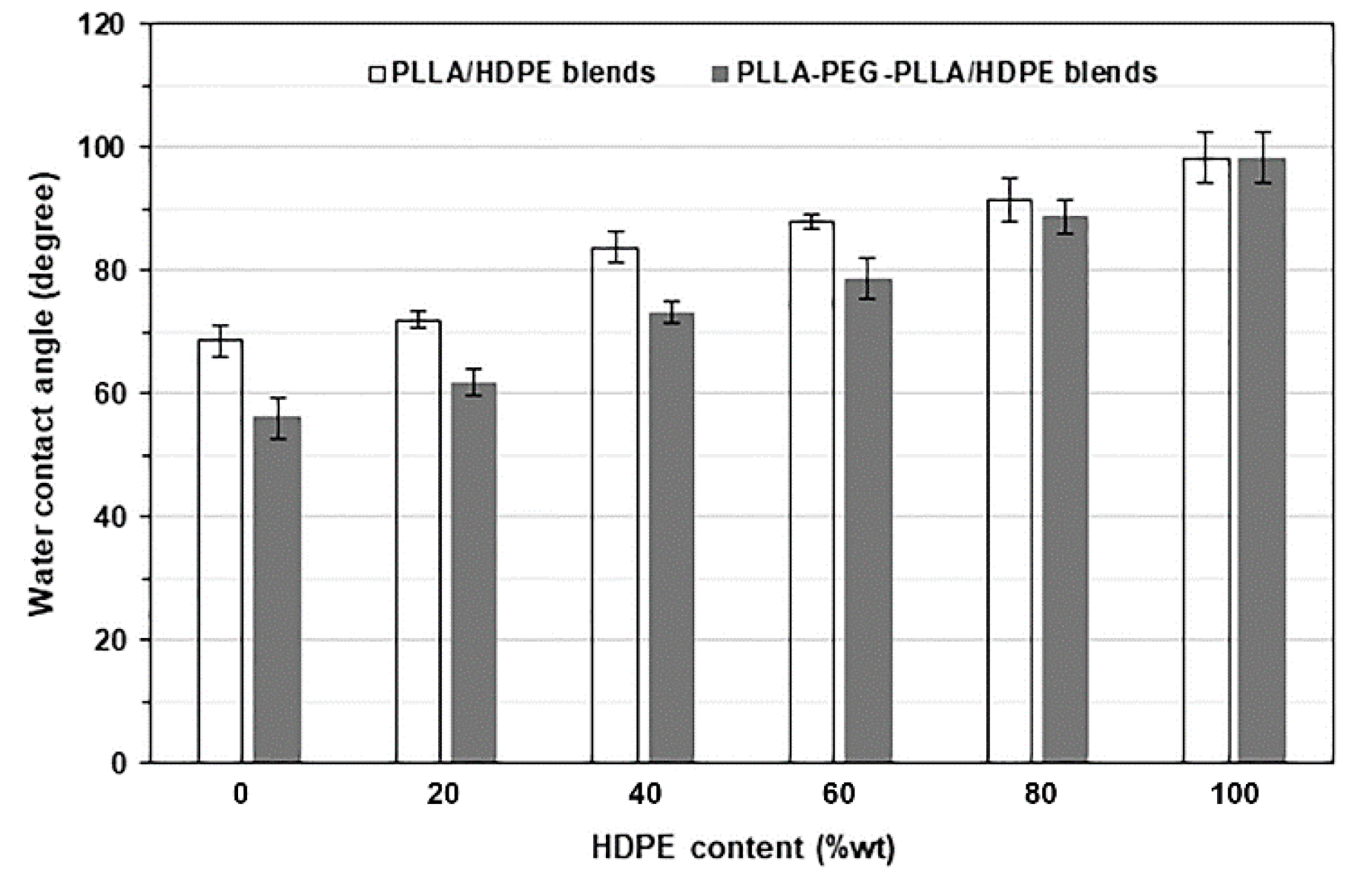
3.7. Wettability
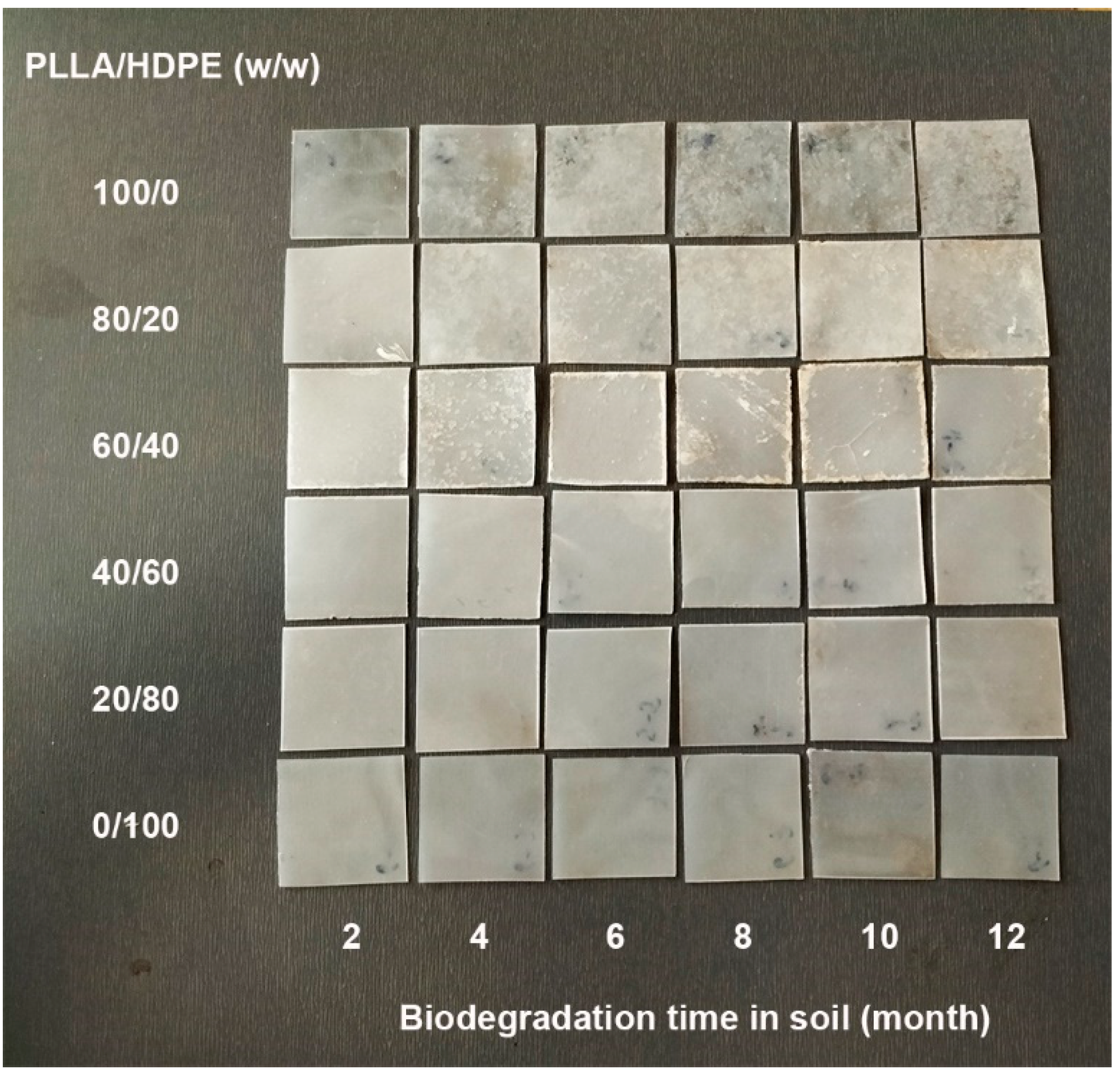
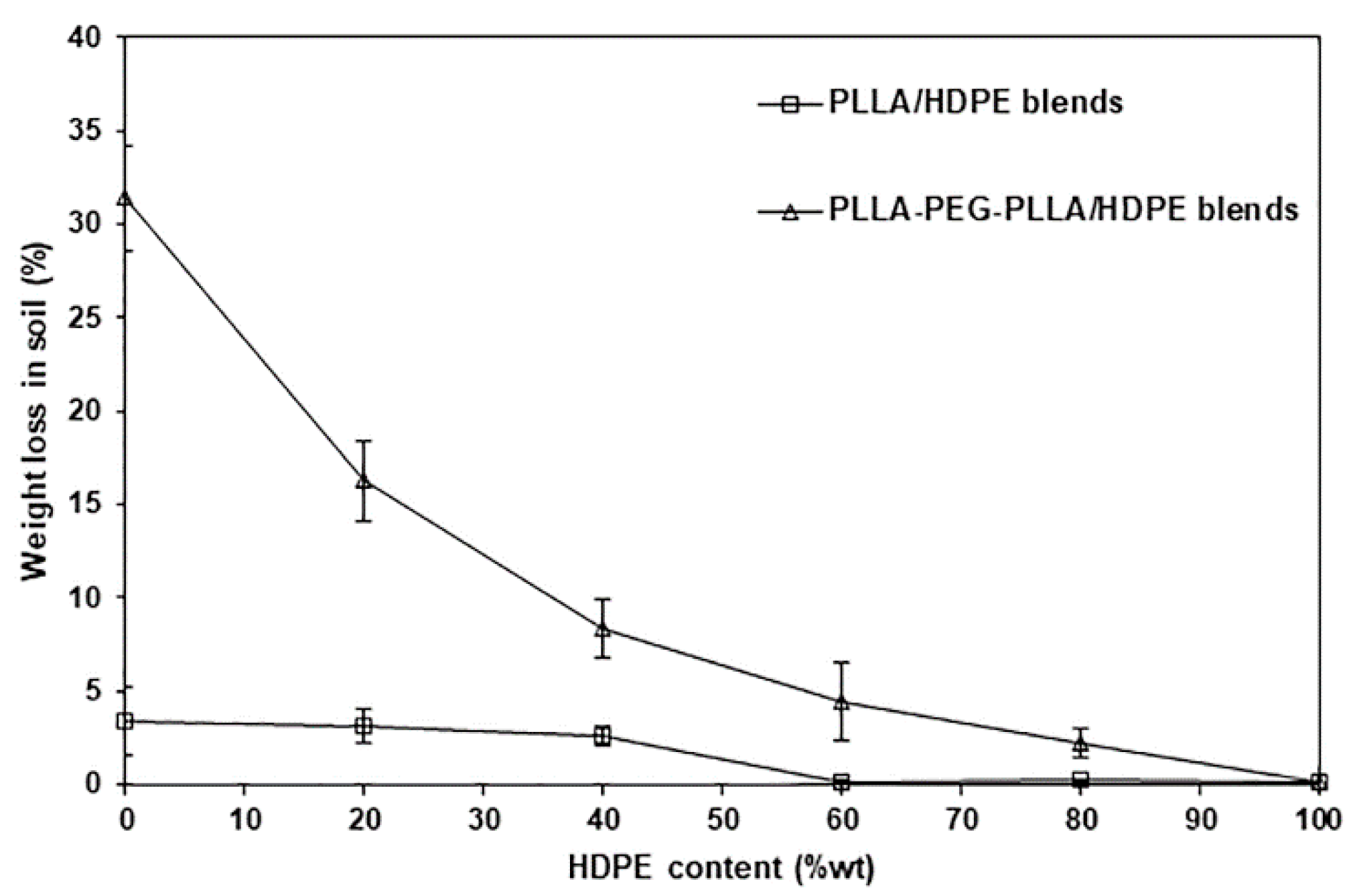
3.8. Biodegradation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable polylactic acid (PLA)-based sustainable engineered blends and biocomposites: Recent developments, challenges, and opportunities. ACS Eng. Au 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Andreeßen, C.; Steinbüchel, A. Recent developments in non-biodegradable biopolymers: Precursors, production processes, and future perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the gap for bioplastic use in food packaging: An update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef] [PubMed]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in research and development of bioplastic for food packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Kakadellis, S.; Harris, Z.M. Don’t scrap the waste: The need for broader system boundaries in bioplastic food packaging life cycle assessment—A critical review. J. Clean. Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Rodrigues, P.V.; Cunha, A.B.; Andrade, M.A.; Vilarinho, F.; Machado, A.V.; Castro, M.C.R. Blown film of PLA for packaging with green tea and fish industrial residues: An insight on their properties. Food Packag. Shelf. 2024, 43, 101283. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Yanat, M.; Muthurajan, M.; Strubel, M.; Grolle, K.; Schroen, K. Polylactic acid films reinforced with chitin nanocrystals: Biodegradation and migration behavior. Food Packag. Shelf. 2023, 40, 101217. [Google Scholar] [CrossRef]
- Petrovics, N.; Kirchkeszner, C.; Patkó, A.; Tábi, T.; Magyar, N.; Székely, I.K.; Szabó, B.S.; Nyiri, Z.; Eke, Z. Effect of crystallinity on the migration of plastic additives from polylactic acid-based food contact plastics. Food Packag. Shelf. 2023, 36, 101054. [Google Scholar] [CrossRef]
- Vidal, C.P.; Luzi, F.; Puglia, D.; López-Carballo, G.; Rojas, A.; Galotto, M.J.; de Dicastillo, C.L. Development of a sustainable and antibacterial food packaging material based in a biopolymeric multilayer system composed by polylactic acid, chitosan, cellulose nanocrystals and ethyl lauroyl alginate. Food Packag. Shelf. 2023, 36, 10150. [Google Scholar]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Simunec, D.P.; Kyratzis, I.; Sola, A.; Wen, C. Biodegradable PLA-ZnO nanocomposite biomaterials with antibacterial properties, tissue engineering viability, and enhanced biocompatibility. Smart Mater. Manufac. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Rayung, M.; Abu, F.; Ahmad, S.; Fadil, F.; Karim, A.A.; Norizan, M.N.; Sarifuddin, N.; Mat Desa, M.S.Z.; Mohd Basri, M.S.; et al. A review on antimicrobial packaging from biodegradable polymer composites. Polymers 2022, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Mastalygina, E.E.; Aleksanyan, K.V. Recent approaches to the plasticization of poly(lactic acid) (PLA) (A review). Polymers 2024, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, M.; Yeganehzad, S.; Hesarinejad, M.A.; Kiumarsi, M.; Abdollahi Moghaddam, M.R. Polylactic acid/saqqez gum blends for chewing gum applications: Impact of plasticizers on thermo-mechanical and morphological Properties. Polymers 2024, 16, 1469. [Google Scholar] [CrossRef] [PubMed]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M. A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohyd. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Chotiprayon, P.; Chaisawad, B.; Yoksan, R. Thermoplastic cassava starch/poly(lactic acid) blend reinforced with coir fibres. Int. J. Biol. Macromol. 2020, 156, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, N.; Wesslen, B. Tributyl citrate oligomers as plasticizers for poly(lactic acid): Thermo-mechanical film properties and aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, Y.S.; Topolkaraev, V.; Hiltner, A.; Baer, E. Crystallization and phase separation in blends of high stereoregular poly(lactide) with poly(ethylene glycol). Polymer 2003, 44, 5681–5689. [Google Scholar] [CrossRef]
- Hu, Y.; Topolkaraev, V.; Hiltner, A.; Baer, E. Aging of poly(lactide)/poly(ethylene glycol) blends. Part 1. Poly(lactide) with low stereoregularity. Polymer 2003, 44, 5701–5710. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly(l-lactide) with poly(propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Li, X.; Jin, Y.; Sun, W.; Dong, T. Fast crystallization and toughening of poly(l-lactic acid) by incorporating with poly(ethylene glycol) as a middle block chain. Polym. Sci. Ser. A 2018, 60, 141–155. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymoramas, N. Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender. e-Polymers 2020, 20, 423–429. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef] [PubMed]
- Thongsomboon, W.; Srihanam, P.; Baimark, Y. Preparation of flexible poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/talcum/thermoplastic starch ternary composites for use as heat-resistant and single-use bioplastics. Int. J. Biol. Macromol. 2023, 230, 123172. [Google Scholar] [CrossRef]
- Gallego, R.; López-Quintana, S.; Basurto, F.; Núñez, K.; Villarreal, N.; Merino, J.C. Synthesis of new compatibilizers to poly(lactic acid) blends. Polym. Eng. Sci. 2014, 54, 522–530. [Google Scholar] [CrossRef]
- Madhu, G.; Bhunia, H.; Bajpai, P.K. Blends of high density polyethylene and poly(l-lactic acid): Mechanical and thermal properties. Polym. Eng. Sci. 2014, 54, 2155–2160. [Google Scholar] [CrossRef]
- Quitadamo, A.; Massardier, V.; Santulli, C.; Valente, M. Optimization of thermoplastic blend matrix HDPE/PLA with different types and levels of coupling agents. Materials 2018, 11, 2527. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Loi, J.; Delgado, P.; Topolkaraev, V.; McEneany, R.J.; Macosko, C.W.; Hillmyer, M.A. Reactive compatibilization of polylactide/polypropylene blends. Ind. Eng. Chem. Res. 2015, 54, 6108–6114. [Google Scholar] [CrossRef]
- Li, S.C.; Zhou, W.J.; Wu, W.J.; Shao, J.; Chen, S.L.; Hou, H.Q.; Xiang, S. The crystallization behavior of l-poly(lactic acid)/polypropylene blends: The acceleration for both l-poly(lactic acid) and polypropylene. Chin. J. Polym. Sci. 2024, 42, 775–786. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F.; Ko, Y.G. Mechanical properties and compatibility of polylactic acid/polystyrene polymer blend. Mater. Lett. 2016, 164, 409–412. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Lu, X.; Tang, L.; Wang, L.; Zhao, J.; Li, D.; Wu, Z.; Xiao, P. Morphology and properties of bio-based poly(lactic acid)/high-density polyethylene blends and their glass fiber reinforced composites. Polym. Test. 2016, 54, 90–97. [Google Scholar] [CrossRef]
- Srihanam, P.; Thongsomboon, W.; Baimark, Y. Phase Morphology, mechanical, and thermal properties of calcium carbonate-reinforced poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide) bioplastics. Polymers 2023, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Madhua, G.; Bhuniaa, H.; Bajpaia, P.K.; Nando, G.B. Physico-mechanical properties and biodegradation of oxo-degradable HDPE/PLA blends. Polym. Sci. Ser. A 2016, 58, 57–75. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Pakkethati, K.; Srihanam, P.; Manphae, A.; Rungseesantivanon, W.; Prakymoramas, N.; Lan, P.N.; Baimark, Y. Improvement in Crystallization, Thermal, and Mechanical Properties of Flexible Poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide) Bioplastic with Zinc Phenylphosphate. Polymers 2024, 16, 975. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Gordon, S.H.; Biresaw, G. Poly(lactic acid)/polystyrene bioblends characterized by thermogravimetric analysis, differential scanning calorimetry, and photoacoustic infrared spectroscopy. J. Appl. Polym. Sci. 2007, 106, 1689–1696. [Google Scholar] [CrossRef]
- Muhammad Harris, Johan Potgieter, Sudip Ray, Richard Archer, Khalid Mahmood Arif Polylactic acid and high-density polyethylene blend: Characterization and application in additive manufacturing. J. Appl. Polym. Sci. 2020, 137, 49602. [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Rayón, E.; Samper, M.D.; Balart, R. Compatibilization and characterization of polylactide and biopolyethylene binary blends by non-reactive and reactive compatibilization approaches. Polymers 2020, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent semi-crystalline polymeric materials and their nanocomposites: A review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
- Srisuwan, Y.; Baimark, Y. Thermal, morphological and mechanical properties of flexible poly (l-lactide)-b-polyethylene glycol-b-poly(l-lactide)/thermoplastic starch blends. Carbohyd. Polym. 2022, 283, 119155. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Ahmadi, S.; Faramarzi, M.; Faghihi, J. Reactive blending of polylactic acid/polyethylene glycol toward biodegradable film. Macromol. Res. 2023, 31, 873–881. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Abdelhamid, D. Biodegradable and Bioerodible Polymers for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 63–83. [Google Scholar]









| Blend Ratio (w/w) | Tcc (°C) | Tm,HDPE (°C) | Tm,PLLA (°C) | Xc,HDPE (%) | Xc,PLLA (%) |
|---|---|---|---|---|---|
| PLLA/HDPE | |||||
| 100/0 | 100 | - | 167 | - | 10.5 |
| 80/20 | 99 | 128 | 167 | 47.0 | 12.0 |
| 60/40 | 100 | 128 | 167 | 50.2 | 10.0 |
| 40/60 | 101 | 128 | 167 | 50.4 | 11.8 |
| 20/80 | - | 129 | - | 55.0 | - |
| 0/100 | - | 128 | - | 60.5 | - |
| PLLA-PEG-PLLA/HDPE | |||||
| 100/0 | 81 | - | 160 | - | 11.6 |
| 80/20 | 70 | 128 | 160 | 51.0 | 20.4 |
| 60/40 | 70 | 128 | 160 | 52.2 | 21.7 |
| 40/60 | 69 | 128 | 160 | 56.4 | 32.5 |
| 20/80 | - | 128 | 160 | 56.6 | 38.6 |
| 0/100 | - | 128 | - | 60.5 | - |
| Blend Ratio (w/w) | HDPE-Td.max (°C) | PEG-Td.max (°C) | HDPE-Td.max (°C) |
|---|---|---|---|
| PLLA/HDPE | |||
| 100/0 | 379 | - | - |
| 80/20 | 376 | - | 493 |
| 60/40 | 378 | - | 493 |
| 40/60 | 377 | - | 495 |
| 20/80 | 375 | - | 494 |
| 0/100 | - | - | 495 |
| PLLA-PEG-PLLA/HDPE | |||
| 100/0 | 310 | 417 | - |
| 80/20 | 325 | 417 | 493 |
| 60/40 | 328 | 418 | 494 |
| 40/60 | 334 | - | 492 |
| 20/80 | 339 | - | 493 |
| 0/100 | - | - | 495 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baimark, Y.; Srihanam, P.; Srisuwan, Y. Thermal, Morphological, Mechanical, and Biodegradation Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/High-Density Polyethylene Blends. Polymers 2024, 16, 2078. https://doi.org/10.3390/polym16142078
Baimark Y, Srihanam P, Srisuwan Y. Thermal, Morphological, Mechanical, and Biodegradation Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/High-Density Polyethylene Blends. Polymers. 2024; 16(14):2078. https://doi.org/10.3390/polym16142078
Chicago/Turabian StyleBaimark, Yodthong, Prasong Srihanam, and Yaowalak Srisuwan. 2024. "Thermal, Morphological, Mechanical, and Biodegradation Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/High-Density Polyethylene Blends" Polymers 16, no. 14: 2078. https://doi.org/10.3390/polym16142078
APA StyleBaimark, Y., Srihanam, P., & Srisuwan, Y. (2024). Thermal, Morphological, Mechanical, and Biodegradation Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/High-Density Polyethylene Blends. Polymers, 16(14), 2078. https://doi.org/10.3390/polym16142078









