Development of a Zr-Based Metal-Organic Framework (UiO-66) for a Cooperative Flame Retardant in the PC/ABS
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of UiO-66
2.3. Synthesis of UiO-66@HPCTP@PC/ABS
2.4. Characterizations
3. Results and Discussion
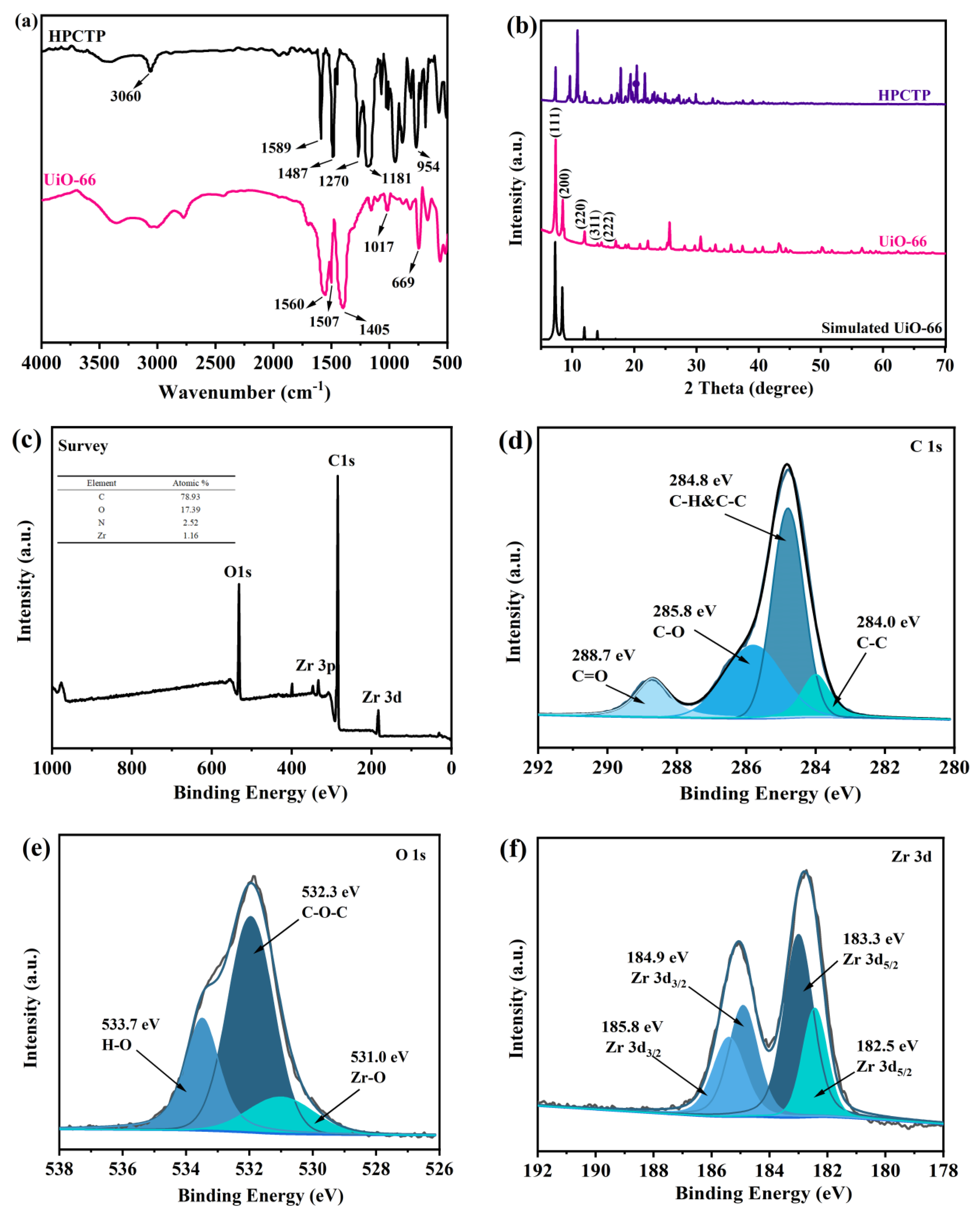
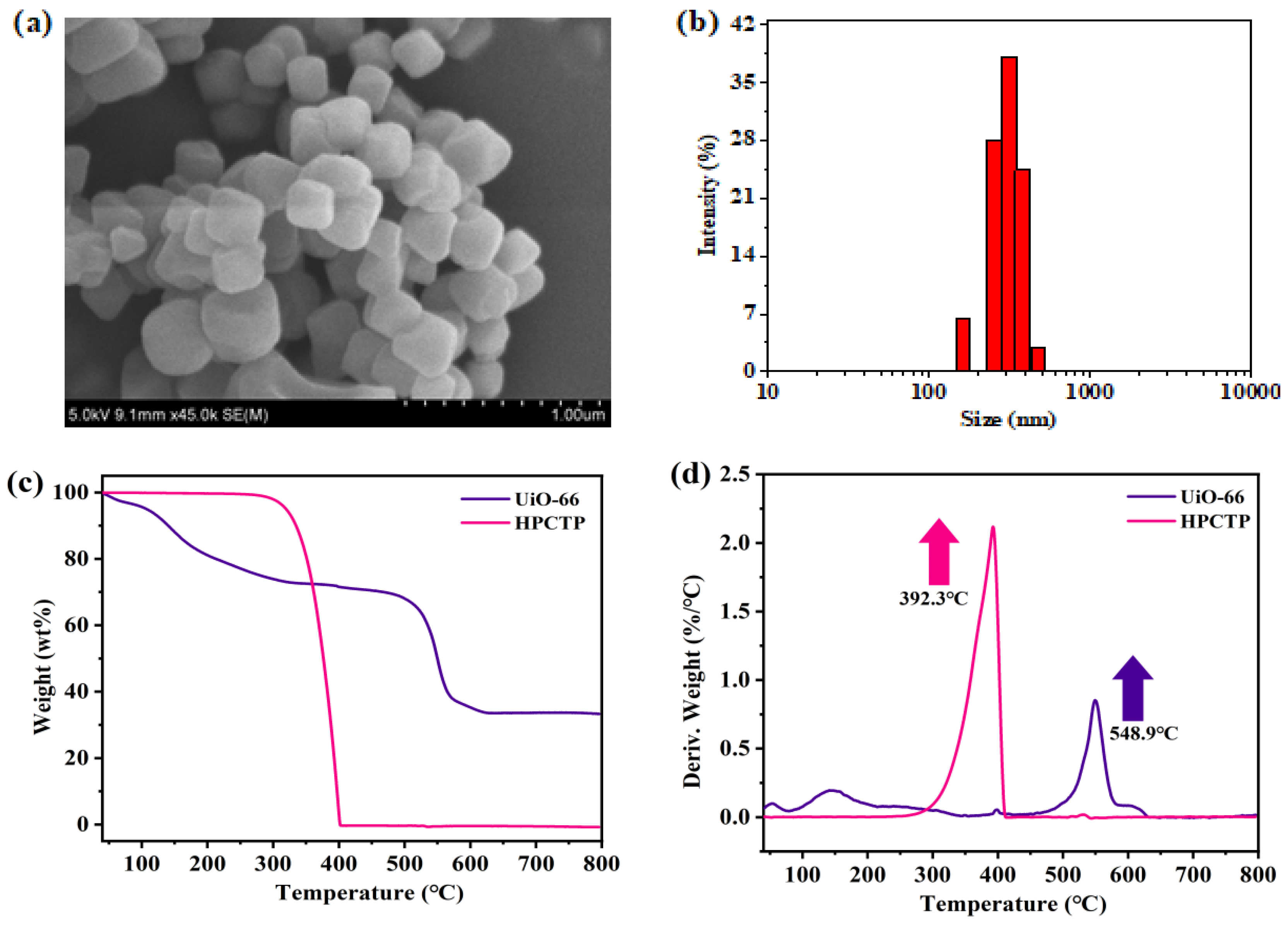
3.1. Preparation of UiO-66
3.2. Preparation of UiO-66@HPCTP@PC/ABS
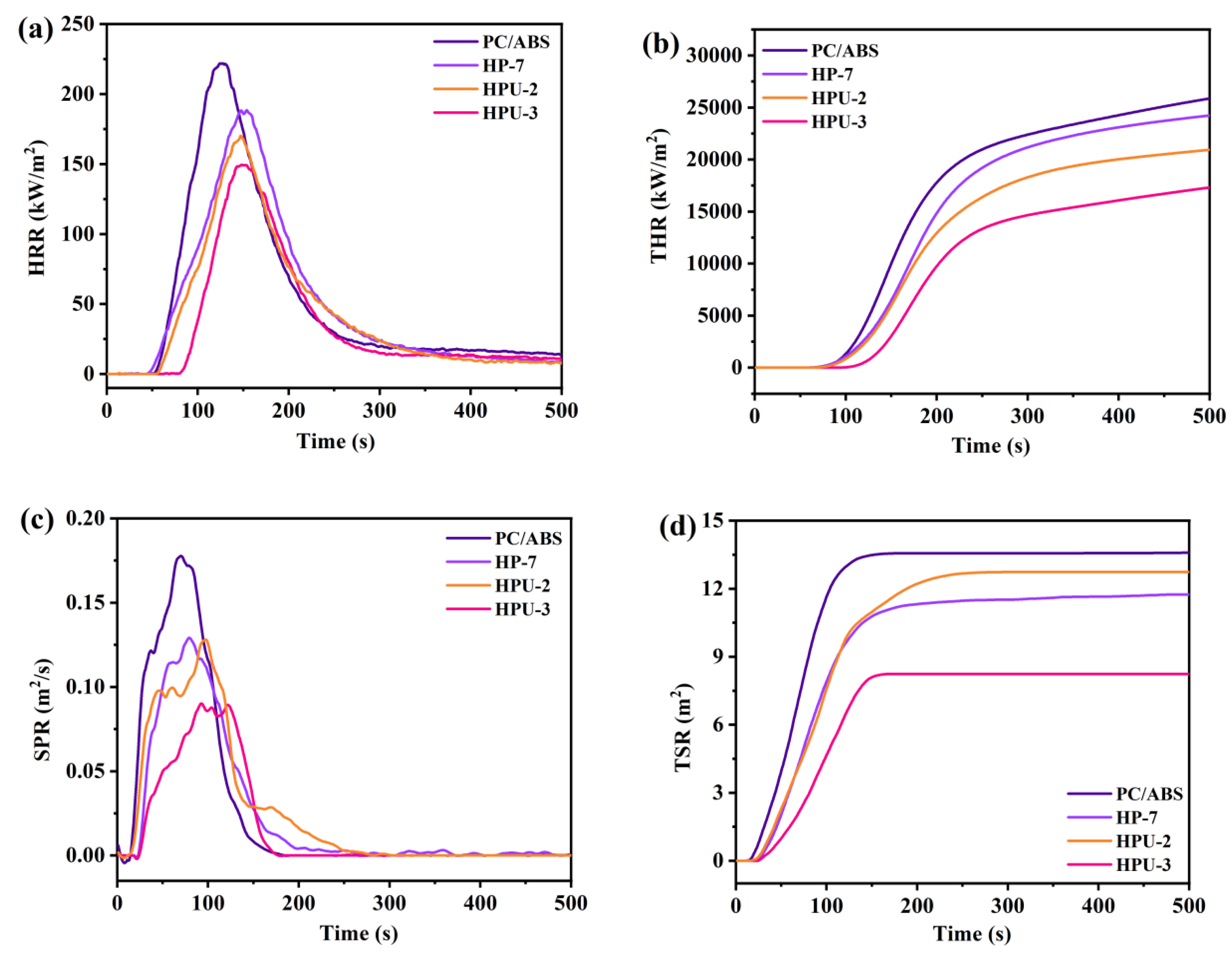
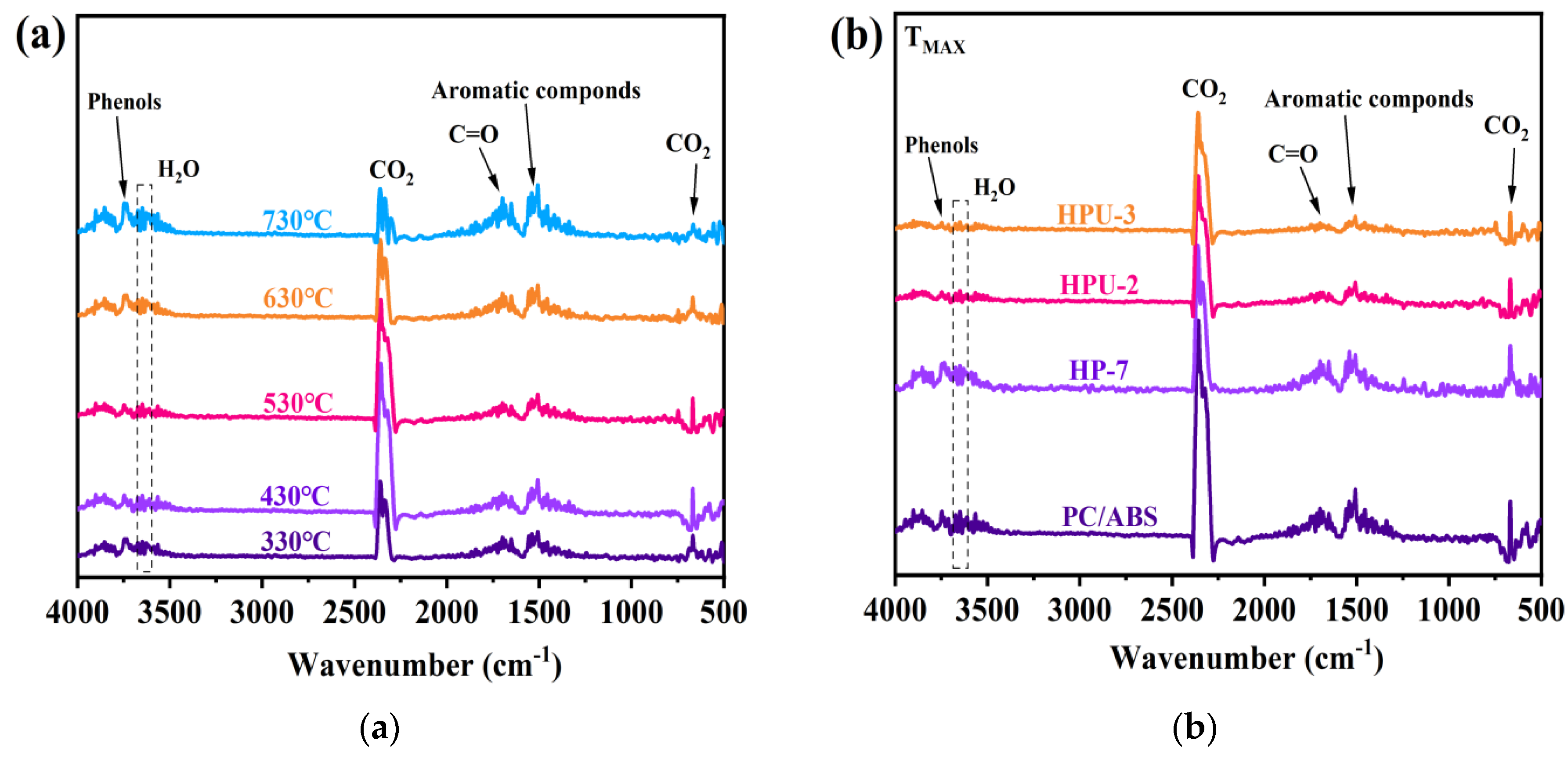
3.3. Performances of Flame Retardant
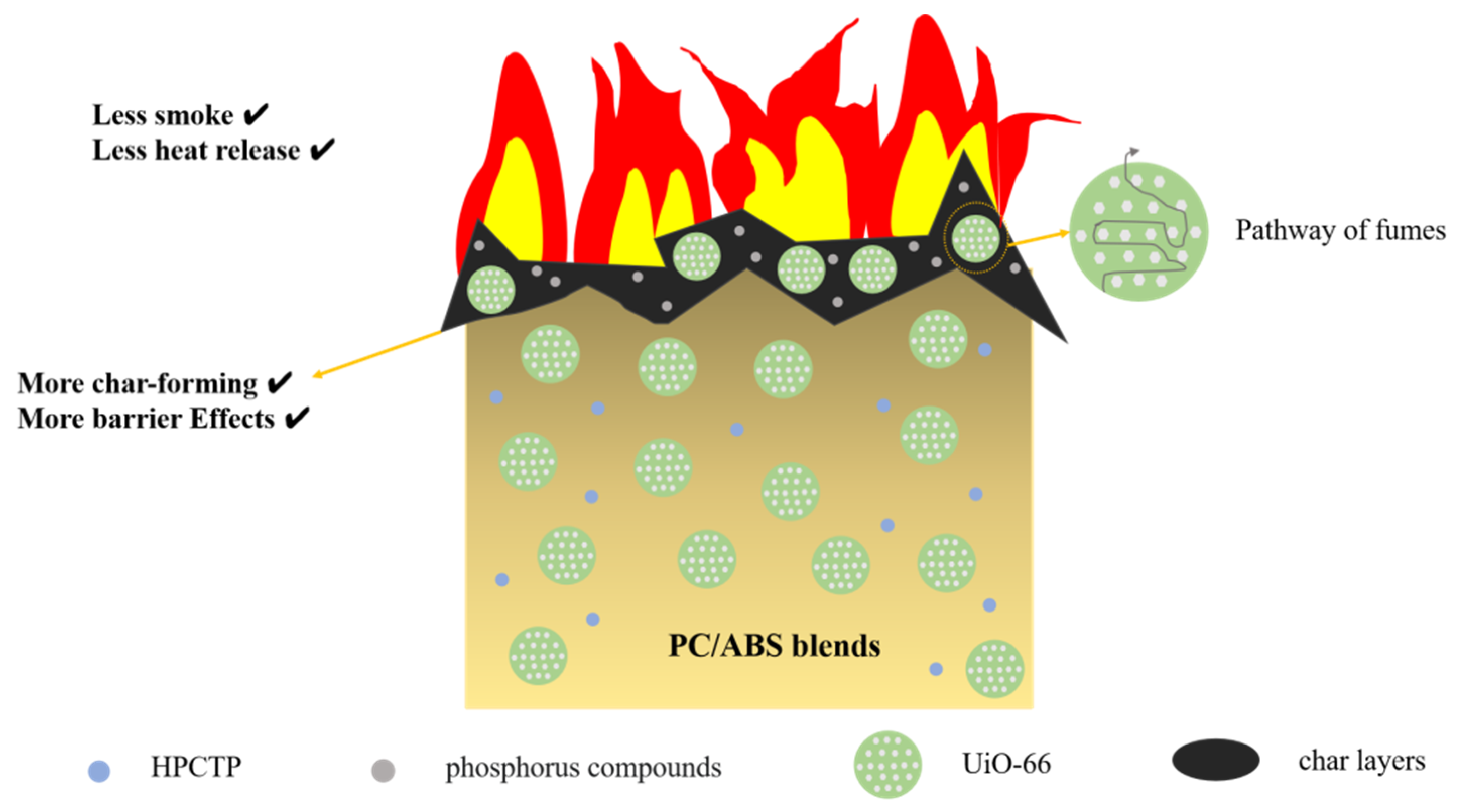
3.4. Flame-Retardant Mode of Action
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Truc, N.T.T.; Lee, B.-K. Selective separation of ABS/PC containing BFRs from ABSs mixture of WEEE by developing hydrophilicity with ZnO coating under microwave treatment. J. Hazard. Mater. 2017, 329, 84–91. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Mohanty, A.K.; Misra, M. Development of hybrid composites reinforced with biocarbon/carbon fiber system. The comparative study for PC, ABS and PC/ABS based materials. Compos. Part B Eng. 2020, 200, 108319. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Yue, D. Optimization of surface treatment using sodium hypochlorite facilitates coseparation of ABS and PC from WEEE plastics by flotation. Environ. Sci. Technol. 2019, 53, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yan, P.; Xu, H.; Liu, D.; Chen, G.; Cai, G.; Zhu, Y. Preparation of continuous glass fiber/polyamide 6 composites containing hexaphenoxycyclotriphosphazene: Mechanical properties, thermal stability, and flame retardancy. Polym. Compos. 2022, 43, 1022–1037. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Qian, L. Flame-retardant behavior and protective layer effect of phosphazene-triazine bi-group flame retardant on polycarbonate. J. Appl. Polym. Sci. 2020, 137, 49523. [Google Scholar] [CrossRef]
- Feng, H.; Qian, L.; Lu, L. Synergistic effect of polyimide charring agent and hexaphenoxycyclotriphosphazene on improving fire safety of polycarbonate: High graphitization to strengthen the char layer. Polym. Adv. Technol. 2021, 32, 1135–1149. [Google Scholar] [CrossRef]
- Seraji, S.M.; Gan, H.; Swan, S.R.; Varley, R.J. Phosphazene as an effective flame retardant for rapid curing epoxy resins. React. Funct. Polym. 2021, 164, 104910. [Google Scholar] [CrossRef]
- Hoang, D.; Kim, J. Synthesis and applications of biscyclic phosphorus flame retardants. Polym. Degrad. Stab. 2008, 93, 36–42. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Sadiq, M.M.; Batten, M.; Konstas, K.; Chen, D.; Doherty, C.M.; Caruso, R.A. Advancing metal-organic frameworks toward smart sensing: Enhanced fluorescence by a photonic metal-organic framework for organic vapor sensing. Adv. Opt. Mater. 2020, 8, 2000961. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Ongari, D.; Mohamed, M.H.; Xu, W.; Motkuri, R.K.; Haranczyk, M.; Thallapally, P.K. Metal organic frameworks for xenon storage applications. ACS Mater. Lett. 2020, 2, 233–238. [Google Scholar] [CrossRef]
- Hao, Y.-C.; Chen, L.-W.; Li, J.; Guo, Y.; Su, X.; Shu, M.; Zhang, Q.; Gao, W.-Y.; Li, S.; Yu, Z.-L. Metal-organic framework membranes with single-atomic centers for photocatalytic CO2 and O2 reduction. Nat. Commun. 2021, 12, 2682. [Google Scholar] [CrossRef]
- Birin, K.; Abdulaeva, I.; Polivanovskaya, D.; Sinel’shchikova, A.; Demina, L.; Baranchikov, A.; Gorbunova, Y.G.; Tsivadze, A.Y. Immobilization of Heterocycle-Appended Porphyrins on UiO-66 and UiO-67 MOFs. Russ. J. Inorg. Chem. 2021, 66, 193–201. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.M.; Ribeiro, S.O.; Castro, B.d.; Balula, S.S.; Cunha-Silva, L. Influence of UiO-66 (Zr) preparation strategies in its catalytic efficiency for desulfurization process. Materials 2019, 12, 3009. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, M.R.; Islamoglu, T.; Garibay, S.J.; Hupp, J.T.; Farha, O.K. Room-temperature synthesis of UiO-66 and thermal modulation of densities of defect sites. Chem. Mater. 2017, 29, 1357–1361. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal–organic frameworks: From nano to single crystals. Chem. A Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Sai, T.; Ran, S.; Guo, Z.; Fang, Z. A Zr-based metal organic frameworks towards improving fire safety and thermal stability of polycarbonate. Compos. Part B Eng. 2019, 176, 107198. [Google Scholar] [CrossRef]
- GB/T 17037.1-1997; Preparation of Injection Molding Samples for Thermoplastic Materials Part 1. China Standard Press: Beijing, China, 1997.
- GB/T 9341-2008; Determination of Plastic Bending Performance. China Standard Press: Beijing, China, 2008.
- GB/T 2406.2-2009; Determination of Combustion Behavior of Plastics by Oxygen Index Method. China Standard Press: Beijing, China, 2009.
- ISO5660-1; Reaction to Fire Tests-Heat Release, Smoke Production and Mass Loss Rate-Part 1: Heat Release Rate (Cone Calorimeter Method. ISO: Geneva, Switzerland, 2015.
- ASTM D7309; Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry. ASTM International: West Conshohocken, PA, USA, 2007.
- Stawowy, M.; Ciesielski, R.; Maniecki, T.; Matus, K.; Łużny, R.; Trawczynski, J.; Silvestre-Albero, J.; Łamacz, A. CO2 hydrogenation to methanol over Ce and Zr containing UiO-66 and Cu/UiO-66. Catalysts 2019, 10, 39. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Wang, D.; Xin, Y.; Li, X.; Wang, F.; Wang, Y.; Zhang, W.; Zheng, Y.; Yao, D.; Yang, Z.; Lei, X. A universal approach to turn UiO-66 into type 1 porous liquids via post-synthetic modification with corona-canopy species for CO2 capture. Chem. Eng. J. 2021, 416, 127625. [Google Scholar] [CrossRef]
- Rungswang, W.; Kotaki, M.; Shimojima, T.; Kimura, G.; Sakurai, S.; Chirachanchai, S. Directing thermoplastic elastomer microdomain parallel to fiber axis: A model case of SEBS with benzoxazine through π–π stacking. Macromolecules 2011, 44, 9276–9285. [Google Scholar] [CrossRef]
- Fang, F.; Song, P.; Ran, S.; Guo, Z.; Wang, H.; Fang, Z. A facile way to prepare phosphorus-nitrogen-functionalized graphene oxide for enhancing the flame retardancy of epoxy resin. Compos. Commun. 2018, 10, 97–102. [Google Scholar] [CrossRef]
- Fang, F.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Improved flame resistance and thermo-mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self-assembly in water. Compos. Part B Eng. 2019, 165, 406–416. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, W.; Zhou, X.; Gui, Z.; Hu, Y. Vertically aligned nickel 2-methylimidazole metal–organic framework fabricated from graphene oxides for enhancing fire safety of polystyrene. Ind. Eng. Chem. Res. 2017, 56, 8778–8786. [Google Scholar] [CrossRef]






| Sample | PC/ABS (wt%) | HPCTP (wt%) | UiO-66 (wt%) |
|---|---|---|---|
| PC/ABS | 70:30 | 0 | 0 |
| HP-3.5 | 70:30 | 3.5 | 0 |
| HP-7.0 | 70:30 | 7.0 | 0 |
| HP-10.5 | 70:30 | 10.5 | 0 |
| U-1.5 | 70:30 | 0 | 1.5 |
| U-3.0 | 70:30 | 0 | 3 |
| U-4.5 | 70:30 | 0 | 4.5 |
| HPU-1 | 70:30 | 7.0 | 1 |
| HPU-2 | 70:30 | 7.0 | 2 |
| HPU-3 | 70:30 | 7.0 | 3 |
| Sample Name | HPCTP (wt%) | UiO-66 (wt%) | LOI (%) | UL-94 | t1/s | t2/s | Dripping |
|---|---|---|---|---|---|---|---|
| PC/ABS | 0 | 0 | 21.2 | NR | >60 | >60 | YES |
| HP-3.5 | 3.5 | 0 | 22.7 | NR | >60 | >60 | YES |
| HP-7.0 | 7.0 | 0 | 24.0 | NR | 50.2 | >60 | YES |
| HP-10.5 | 10.5 | 0 | 25.3 | V-2 | 18.4 | 26.7 | YES |
| U-1.5 | 0 | 1.5 | 23.2 | NR | >60 | >60 | YES |
| U-3.0 | 0 | 3 | 23.7 | NR | 50.8 | >60 | YES |
| U-4.5 | 0 | 4.5 | 24.1 | NR | 39.6 | 45.1 | NO |
| HPU-1 | 7.0 | 1 | 23.8 | NR | 39.2 | >60 | YES |
| HPU-2 | 7.0 | 2 | 25.2 | V-2 | 17.2 | 28.8 | YES |
| HPU-3 | 7.0 | 3 | 27.0 | V-0 | 9.4 | 17.2 | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Chen, Z.; Bi, W.; Du, W.; Lin, L.; Hu, D.; Zhuo, H. Development of a Zr-Based Metal-Organic Framework (UiO-66) for a Cooperative Flame Retardant in the PC/ABS. Polymers 2024, 16, 2083. https://doi.org/10.3390/polym16142083
Chen S, Chen Z, Bi W, Du W, Lin L, Hu D, Zhuo H. Development of a Zr-Based Metal-Organic Framework (UiO-66) for a Cooperative Flame Retardant in the PC/ABS. Polymers. 2024; 16(14):2083. https://doi.org/10.3390/polym16142083
Chicago/Turabian StyleChen, Shaojun, Zerui Chen, Weifeng Bi, Wei Du, Ling Lin, Dasong Hu, and Haitao Zhuo. 2024. "Development of a Zr-Based Metal-Organic Framework (UiO-66) for a Cooperative Flame Retardant in the PC/ABS" Polymers 16, no. 14: 2083. https://doi.org/10.3390/polym16142083





