From Synthesis to Functionality: Tailored Ionic Liquid-Based Electrospun Fibers with Superior Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
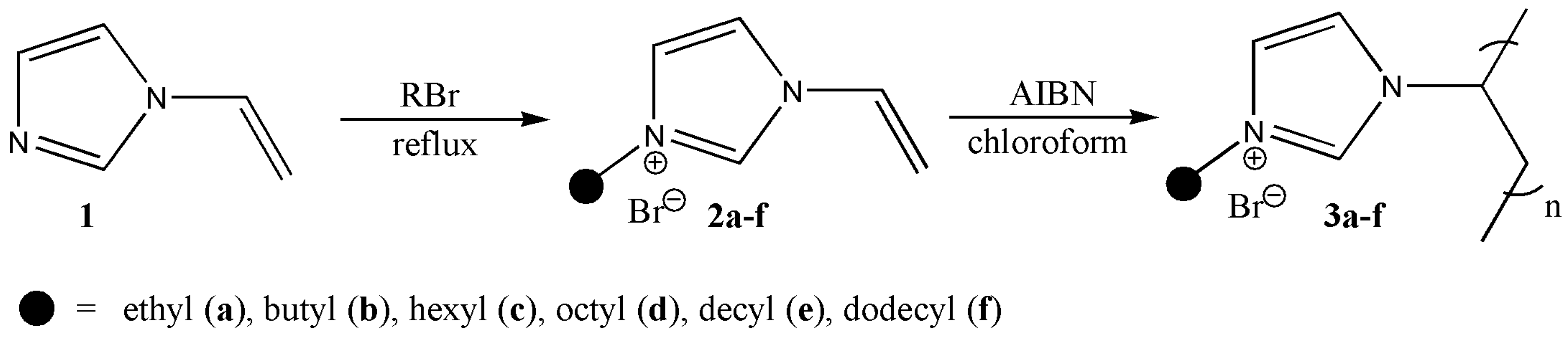
2.2. General Synthetic Procedure for the 1-Alkyl-3-vinylimidazolium Bromide, Poly(1-alkyl-3-vinylimidazolium bromide), and Preparation of Solutions for Electrospinning
2.3. Microbial Strains and Culture Conditions
2.4. Minimal Inhibitory Concentration (MIC) Determination
2.5. Apparatus and Procedures
3. Results and Discussion
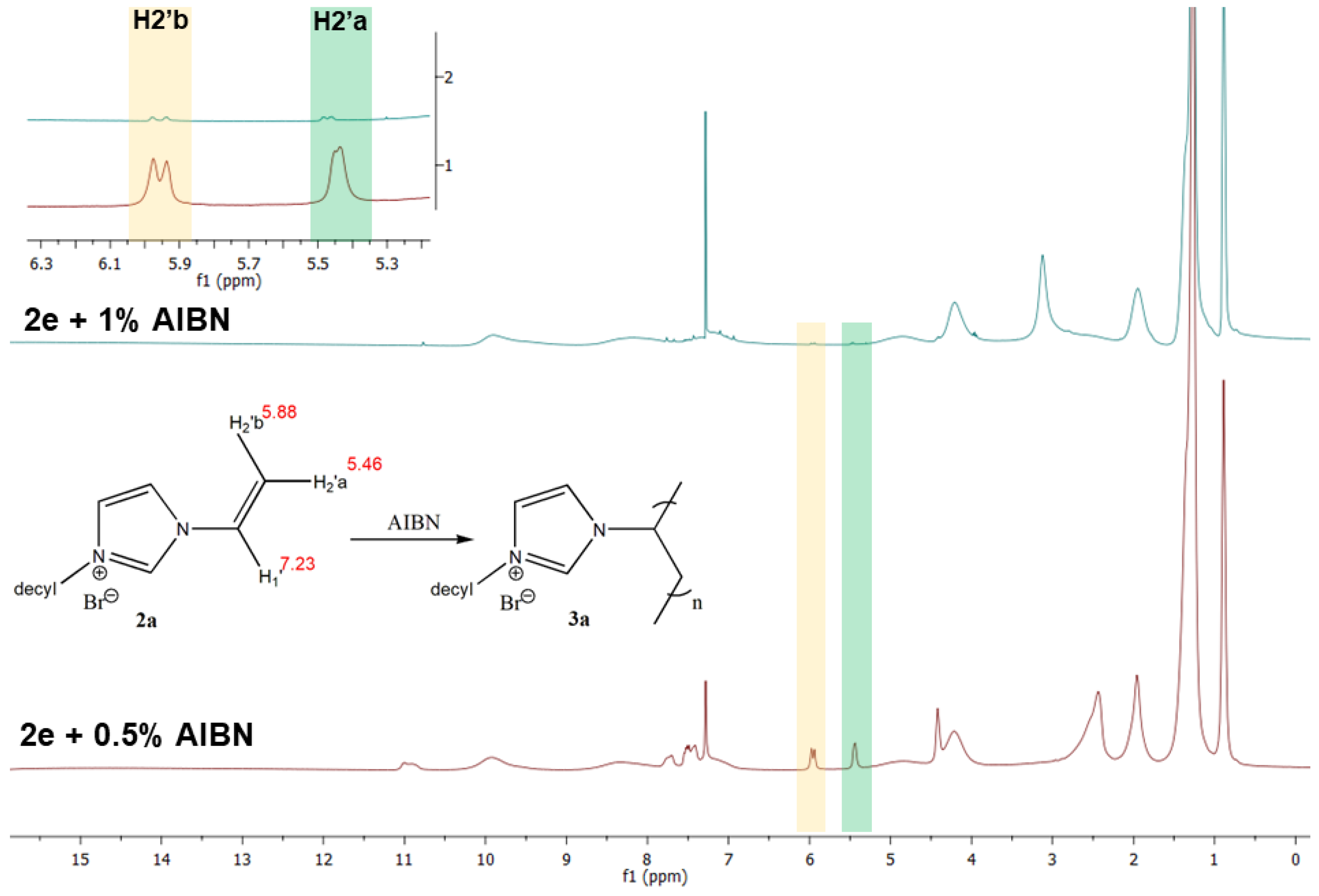
3.1. Synthetic Procedure and Structural Characterization of Poly(1-alkyl-3-vinylimidazolium bromide)
3.2. Thermal Properties of Poly(1-alkyl-3-vinylimidazolium bromide)
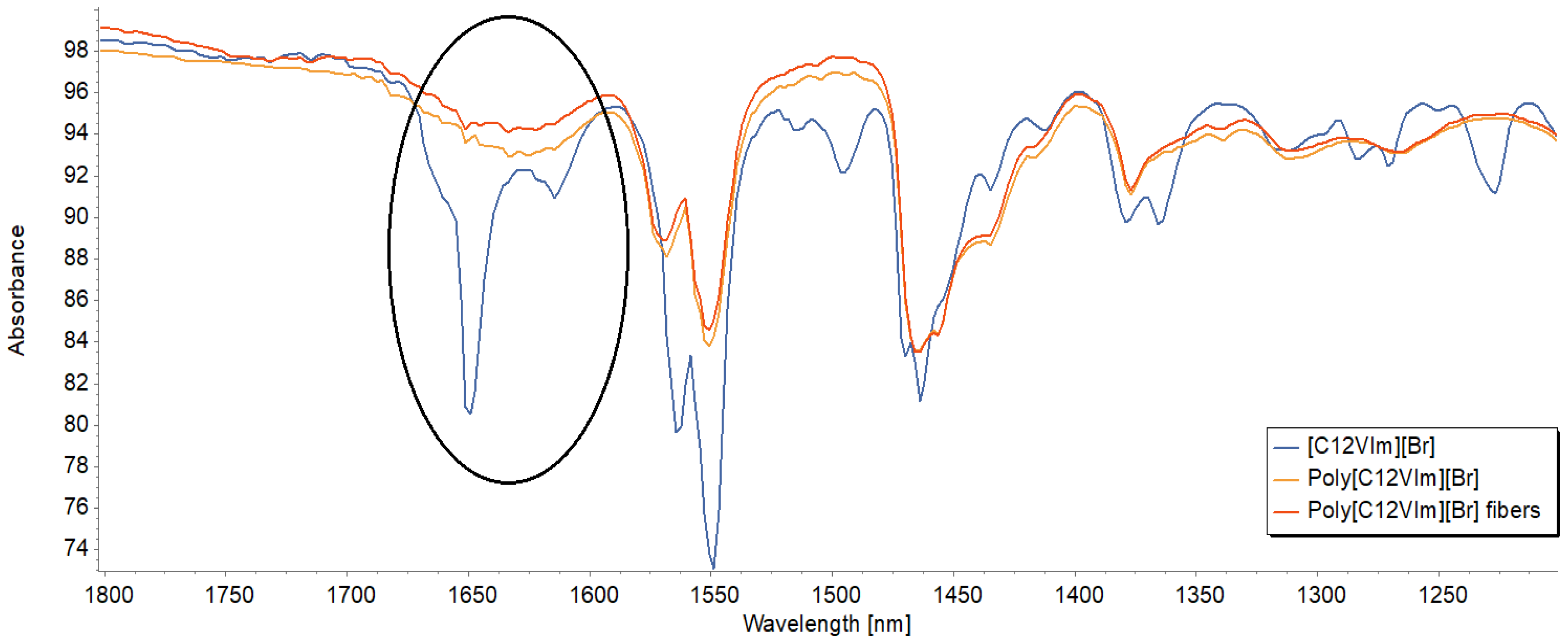
3.3. Chemical Structure Evaluation of IL, PIL, and Electrospun Fibers
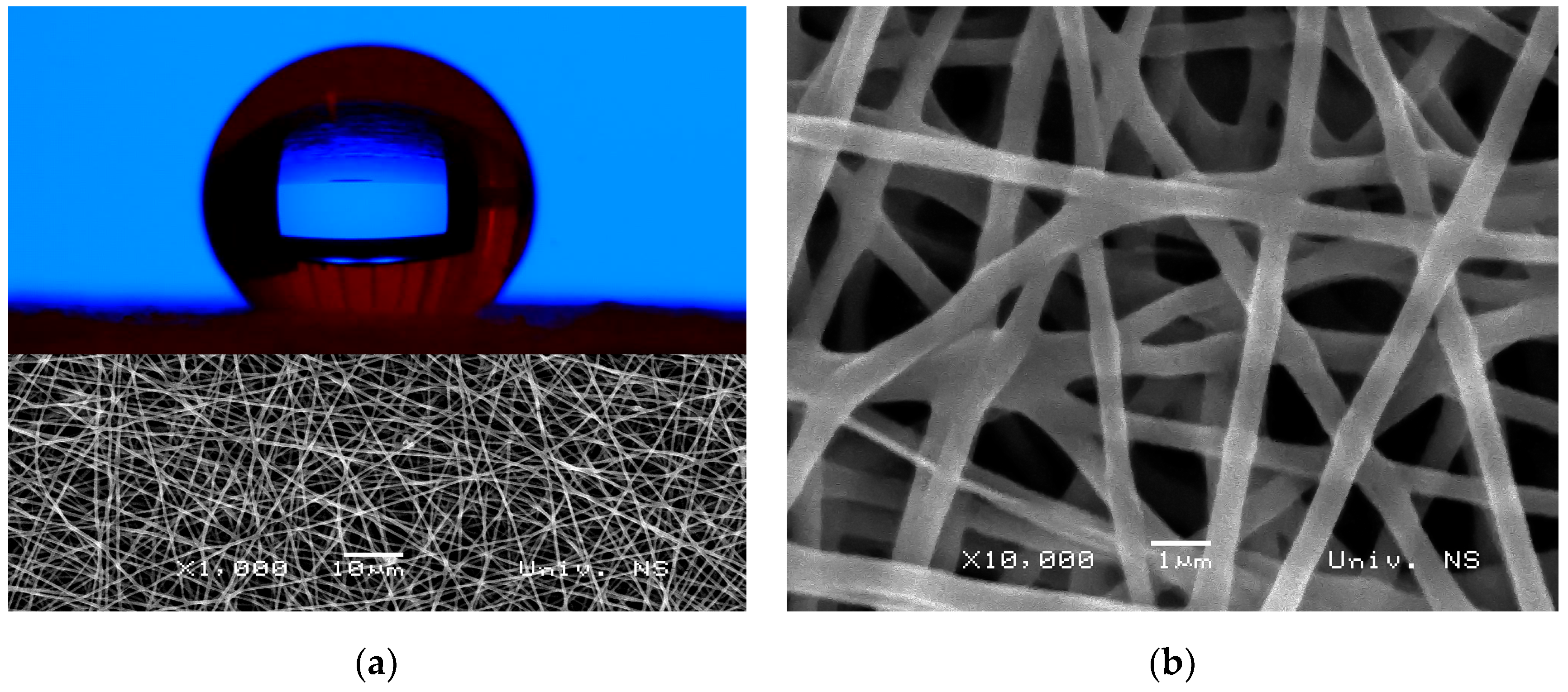
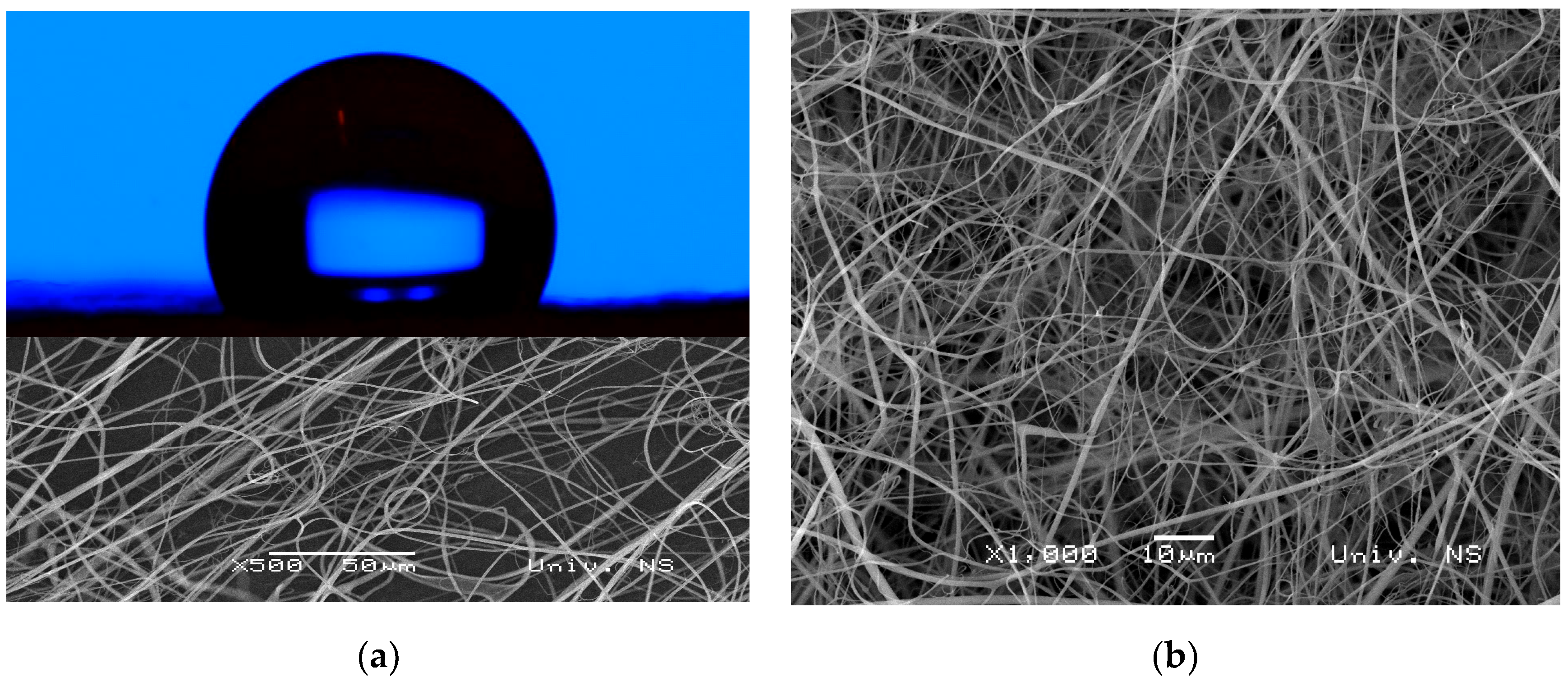
3.4. Morphology and Wettability Evaluation of the Electrospun Fibers
3.5. Antimicrobial Properties of ILs, PILs, and Electrospun Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 7 February 2024).
- Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 7 February 2024).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Microbial pollution and food safety. AIMS Microbiol. 2018, 4, 377–396. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Finland, M.; Haight, T.H. Antibiotic resistance of pathogenic staphylococci. Study of five hundred strains isolated at Boston City Hospital from October, 1951. to February, 1952. Arch. Intern. Med. 1953, 1, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 19 November 2023).
- Piddock, L.V.J. The crisis of no new antibiotics-what is the way forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2019, 5, 726–749. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/europe/publications/i/item/9789289058537 (accessed on 20 November 2023).
- Leon-Buitimea, A.; Garza-Cardenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramirez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Salatin, S.; Kherzi, K.; Lee, J.-Y.; Lotfipour, F. Antimicrobial Peptide-Decorated Nanoparticles and Polymers. Front. Microbiol. 2022, 13, 831655. [Google Scholar]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, A.; Tong, W.; Xu, G.-J. Biodegradable Antibacterial Polymeric Nanosystems: A New Hope to Cope with Multidrug-Resistant Bacteria. Small 2019, 151, 1900999. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.F.; Maginn, E.J. Ionic Liquids: Innovative Fluids for Chemical Processing. AIChE J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Carvalho, E.O.; Correia, D.M.; Esperança, J.M.S.S.; Padrão, J.; Ivanova, K.; Hoyo, J.; Tzanov, T.; Lanceros-Mendez, S. Ionic Liquids as Biocompatible Antibacterial Agents: A Case Study on Structure-Related Bioactivity on Escherichia coli. ACS Appl. Bio Mater. 2022, 11, 5181–5189. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Pendleton, J.K.; Gilmore, B.F. The antimicrobial potential of ionic liquids: A source of chemical diversity for infection and biofilm control. Int. J. Antimicrob. Agents 2015, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.M.S.; Saraiva, M.L.M.F.S.; Passos, M.L.C. Ionic liquids and organic salts with antimicrobial activity as a strategy against resistant microorganisms. J. Mol. Liq. 2022, 368, 120750. [Google Scholar] [CrossRef]
- Fang, Z.; Zheng, X.; Li, Y.; Qi, J.; Wu, W.; Lu, Y. Ionic Liquids: Emerging Antimicrobial Agents. Pharm. Res. 2022, 39, 2391–2404. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Galinski, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta. 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids-Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Fallah, Z.; Zare, E.N.; Khan, M.A.; Iftekhar, S.; Ghomi, M.; Sharifi, E.; Tajbakhsh, M.; Nikfarjam, N.; Makvandi, P.; Lichtfouse, E.; et al. Ionic liquid-based antimicrobial materials for water treatment, air filtration, food packaging and anticorrosion coatings. Adv. Colloid Interface Sci. 2021, 294, 102454. [Google Scholar] [CrossRef]
- Busetti, A.; Crawford, D.E.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P. Antimicrobial and antibiofilm activities of 1-alkylquinolinium bromide ionic liquids. Green Chem. 2010, 12, 420–425. [Google Scholar] [CrossRef]
- Bergamo, V.Z.; Donato, R.K.; Dalla Lana, D.F.; Donato, K.J.Z.; Ortega, G.G.; Schrekker, H.S. Imidazolium salts as antifungal agents: Strong antibiofilm activity against multidrug-resistant Candida tropicalis isolates. Lett. Appl. Microbiol. 2014, 60, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Cornellas, A.; Perez, L.; Comelles, F.; Ribosa, I.; Manresa, A.; Garcia, M.T. Self-aggregation and antimicrobial activity of imidazolium and pyridinium based ionic liquids in aqueous solution. J. Colloid Interface Sci. 2011, 355, 164–171. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Gonzales, J.J.; Comelles, F. Surface activity, self-aggregation and antimicrobial activity of catanionic mixtures of surface active imidazolium- or pyridinium-based ionic liquids and sodium bis(2-ethylhexyl) sulfosuccionate. J. Mol. Liq. 2020, 303, 112637. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, L. Aggregation Behavior of Surface Active Imidazolium Ionic Liquids in Ethylammonium Nitrate: Effect of Alkyl Chain Length, Cations, and Counterions. J. Phys. Chem. 2011, 116, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Nakayama, K.; Kitazume, T. Antibacterial activities of imidazolium, pyrrolidinium and piperidinium salts. Bioorg. Med. Chem. Lett. 2011, 21, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Antimicrobial activities of ionic liquids. Green Chem. 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Florio, W.; Becherini, S.; D’Andrea, F.; Lupetti, A.; Chiappe, C.; Guazzelli, L. Comparative Evaluation of Antimicrobial Activity of Different Types of Ionic Liquids. Mater. Sci. Eng. C 2019, 104, 109907. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Di Pippo, F.; Di Gregorio, L.; Congestri, R.; Tandoi, V.; Rossetti, S. Biofilm growth and control in cooling water industrial systems. FEMS Microbiol. Ecol. 2018, 94, fiy044. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Poly(ionic liquid)s as antimicrobial materials. Eur. Polym. J. 2018, 105, 135–149. [Google Scholar] [CrossRef]
- Zhu, X.; Du, M.; Feng, J.; Wang, H.; Xu, Z.; Wang, L.; Zuo, S.; Wang, C.; Wang, Z.; Zhang, C.; et al. High-Efficiency Perovskite Solar Cells with Imidazolium-Based Ionic Liquid for Surface Passivation and Charge Transport. Angew. Chem. 2020, 60, 4238–4244. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kong, M.; Niu, Y.; Li, G. Entanglement and Relaxation of Poly(methyl methacrylate) Chains in Imidazolium-Based Ionic Liquids with Different Cationic Structures. Macromocelules 2020, 53, 7865–7875. [Google Scholar] [CrossRef]
- Tchalala, M.r.; El-Demellawi, J.K.; Kalakonda, P.; Chaieb, S. High thermally stable hybrid materials based on amorphous porous silicon nanoparticles and imidazolium-based ionic liquids: Structural and chemical analysis. Mater. Today Proc. 2021, 39, 1132–1140. [Google Scholar] [CrossRef]
- Macerreyes, D. Polymeric ionic liquids: Broadening the properties and application of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Smith, C.A.; Cataldo, V.A.; Dimke, T.; Stephan, I.; Guterman, R. Antibacterial and Degradable Thioimidazolium Poly(ionic liquid). ACS Sustain. Chem. Eng. 2020, 8, 8419–8424. [Google Scholar] [CrossRef]
- Zhou, C.; Sheng, C.; Gao, L.; Guo, J.; Li, P.; Liu, B. Engineering poly(ionic liquid) semi-IPN hydrogels with fast antibacterial and anti-inflammatory properties for wound healing. Chem. Eng. J. 2021, 413, 127429. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, Q.; Guo, J.; Qin, J.; Mao, H.; Wang, B.; Yan, F. Structure-Antibacterial Activity Relationships of Imidazolium-Type Ionic Liquid Monomers, Poly(ionic liquids) and Poly(ionic liquid) Membranes: Effect of Alkyl Chain Length and Cations. ACS Appl. Mater. Interfaces 2016, 20, 12684–12692. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Yuan, J.; Marquez, A.G.; Recnacher, J.; Giordano, C.; Janek, J.; Antonetti, M. Nitrogen-doped carbon fibers and membranes by carbonization of electrospun poly(ionic liquid)s. Polym. Chem. 2011, 2, 1654–1657. [Google Scholar] [CrossRef]
- Chen, H.; Elabd, Y.A. Polymerized Ionic Liquids: Solution Properties and Electrospinning. Macromolecules 2009, 42, 3368–3373. [Google Scholar] [CrossRef]
- Josef, E.; Guterman, R. Designing Solutions for Electrospinning of Poly(ionic liquid)s. Macromolecules 2019, 52, 5223–5230. [Google Scholar] [CrossRef]
- Vraneš, M.; Rackov, S.; Papović, S.; Tomaš, R.; Pilić, B. Effect of Alkyl Chain Elongation on Thermophysical Properties of 1-Alkyl-3-Vinylimidazolium Bromide-Based Ionic Liquids and Salts. J. Chem. Eng. Data. 2022, 67, 3329–3339. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Montolio, S.; Abarca, G.; Porcar, R.; Dupont, J.; Burguete, M.I.; Garcia-Verdugo, E.; Luis, S.V. Hierarchically structured polymeric ionic liquids and polyvinylpyrrolidone mat-fibers fabricated by electrospinning. J. Mater. Chem. A. 2017, 5, 9733–9744. [Google Scholar] [CrossRef]
- Thomas, M.; Jose, S. Electrospun membrane of PVA and functionalized agarose with polymeric ionic liquid and conductive carbon for efficient dye sensitized solar cell. J. Photochem. Photobiol. 2022, 425, 113666. [Google Scholar] [CrossRef]
- Smith, T.W.; Zhao, M.; Yang, F.; Smith, D.; Cebe, P. Imidazole Polymers Derived from Ionic Liquid 4-Vinylimidazolium Monomers: Their Synthesis and Thermal and Dielectric Properties. Macromolecules 2013, 46, 1133–1143. [Google Scholar] [CrossRef]
- Agapov, A. Decoupling Phenomena in Dynamics of Soft Matter. Ph.D. Dissertation, University of Akron, Akron, OH, USA, 2011. [Google Scholar]
- Xie, Z.; Zhang, P.; Zhang, Z.; Chen, C.; Wang, X. The choice of antimicrobial polymers: Hydrophilic or hydrophobic? Chin. Chem. Lett. 2024, 35, 109768. [Google Scholar] [CrossRef]
- Ciuzas, D.; Krugly, E.; Sriubaite, S.; Pauliukaityte, I.; Baniukaitiene, O.; Bulota, M.; Martuzevicius, D. Electrospun cellulose fibers from ionic liquid: Practical implications toward robust morphology. J. Appl. Polym. Sci. 2021, 139, 51525. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of Gelatin with Tunable Fiber Morphology from Round to Flat/Ribbon. Mater. Sci. Eng. C 2017, 80, 371–378. [Google Scholar] [CrossRef]
- Liu, Z.; Ju, K.; Wang, Z.; Li, W.; Ke, H.; He, J. Electrospun Jets Number and nanofiber Morphology Effected by Voltage Value: Numerical Simulation and Experimental Verification. Nanoscale Res. Lett. 2019, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.K.; Nancharaiah, Y.V. Alkylimidazolium Ionic Liquids as Antifungal Alternatives: Antibiofilm Activity against Candida albicans and Underlying Mechanism of Action. Front Microbiol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Schrekker, H.S.; Donato, R.K.; Fuentefria, A.M.; Bergamo, V.; Oliveira, L.F.; Machado, M.M. Imidazolium salts as antifungal agents: Activity against emerging yeast pathogens, without human leukocyte toxicity. MedChemComm 2013, 4, 1457–1460. [Google Scholar] [CrossRef]
- Ribas, A.D.; Ponte, E.M.; Dalbem, A.M.; Dalla-Lana, D.; Bundchen, C.; Donato, R.K.; Schrekker, H.S.; Fuentefria, A.M. Imidazolium salts with antifungal potential for the control of head blight of wheat caused by Fusarium graminearum. J. Appl. Microbiol. 2016, 124, 445–452. [Google Scholar] [CrossRef]
- Farkaš, V. Structure and biosynthesis of fungal cell walls: Methodological approaches. Folia Microbiol. 2003, 48, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Joksimović, N.; Petronijević, J.; Milović, E.; Janković, N.; Kosanić, M.; Petrović, N. Antioxidant and Antimicrobial Potential, BSA and DNA Binding Properties of Some 3-Hydroxy-3-Pyrrolin-2-Ones Bearing Thenoyl Fragment. Med. Chem. 2022, 18, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.; Feder-Kubis, J.; Krasowska, A. Antifungal activity of ionic liquids based on (−)-menthol: A mechanism study. Microbiol. Res. 2017, 197, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Vranes, M.; Tot, A.; Papovic, S.; Miljakovic, D.; Gadzuric, S.; Ignjatov, M. Ionic liquids as potentially new antifungal agents against Alternaria species. RSC Adv. 2020, 10, 22318–22323. [Google Scholar] [CrossRef] [PubMed]
- Forero Doria, O.; Castro, R.; Gutierrez, M.; Gonzalez Valenzuela, D.; Santos, L.; Ramirez, D.; Guzman, L. Novel Alkylimidazolium Ionic Liquids as an Antibacterial Alternative to Pathogens of the Skin and Soft Tissue Infections. Molecules 2018, 23, 2354. [Google Scholar] [CrossRef]
- Quin, J.; Guo, J.; Xu, Q.; Zheng, Z.; Mao, H.; Yan, F. Synthesis of Pyrrolidinium-Type Poly(ionic liquid) Membranes for Antimicrobial Applications. ACS Appl. Mater. Interfaces 2017, 9, 10504–10511. [Google Scholar] [CrossRef]

| ILs/PILs | Chemical Structure | Abbreviation |
|---|---|---|
| 1-ethyl-3-vinylimidazolium bromide |  | [C2VIm][Br] 2a |
| 1-butyl-3-vinylimidazolium bromide | 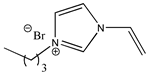 | [C4VIm][Br] 2b |
| 1-hexyl-3-vinylimidazolium bromide | 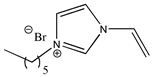 | [C6VIm][Br] 2c |
| 1-octyl-3-vinylimidazolium bromide | 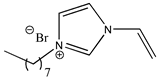 | [C8VIm][Br] 2d |
| 1-decyl-3-vinylimidazolium bromide | 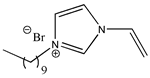 | [C10VIm][Br] 2e |
| 1-dodecyl-3-vinylimidazolium bromide | 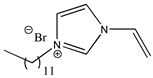 | [C12VIm][Br] 2f |
| Poly(1-ethyl-3-vinylimidazolium bromide) | 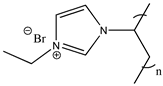 | Poly([C2VIm][Br]) 3a |
| Poly(1-butyl-3-vinylimidazolium bromide) | 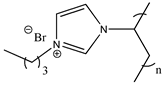 | Poly([C4VIm][Br]) 3b |
| Poly(1-hexyl-3-vinylimidazolium bromide) | 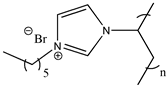 | Poly([C6VIm][Br]) 3c |
| Poly(1-octyl-3-vinylimidazolium bromide) | 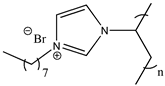 | Poly([C8VIm][Br]) 3d |
| Poly(1-decyl-3-vinylimidazolium bromide) | 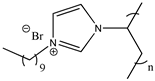 | Poly([C10VIm][Br]) 3e |
| Poly(1-dodecyl-3-vinylimidazolium bromide) |  | Poly([C12VIm][Br]) 3f |
| ILs/PILs | Tg/°C | Tm/°C | Tc/°C |
|---|---|---|---|
| [C2VIm][Br] | - | 52.26 [50] | 34.50 [50] |
| [C4VIm][Br] | −41.87 [50] | - | - |
| [C6VIm][Br] | −61 [50] | - | - |
| [C8VIm][Br] | −54.76 [50] | - | - |
| [C10VIm][Br] | −62.70 [50] | - | - |
| [C12VIm][Br] | - | 39.51 [50] | 1.83 [50] |
| Poly([C2VIm][Br]) | - | 75.75 | 52.66 |
| Poly([C4VIm][Br]) | 100.80 | - | - |
| Poly([C6VIm][Br]) | 175.04 | 242.98 | - |
| Poly([C8VIm][Br]) | - | - | - |
| Poly([C10VIm][Br]) | 104.56 | 180.65 | - |
| Poly([C12VIm][Br]) | 176.08 | - | - |
| Bacteria | Staphylococcus aureus | Bacillus subtilis | Klebsiella pneumoniae | Escherichia coli | Proteus mirabilis |
|---|---|---|---|---|---|
| Tested Compounds and Fibers | MIC Values (mg/mL) | ||||
| [C2VIm][Br] | 0.34 | 0.17 | 0.34 | 0.68 | 0.34 |
| [C4VIm][Br] | 3.31 | 0.825 | 1.65 | 3.31 | 1.65 |
| [C6VIm][Br] | 1.05 | 0.525 | 1.05 | 1.05 | 1.055 |
| [C8VIm][Br] | 0.366 | 0.09 | 0.366 | 0.732 | 0.366 |
| [C10VIm][Br] | 0.52 | 0.026 | 0.052 | 0.105 | 0.105 |
| [C12VIm][Br] | 0.005 | 0.001 | 0.005 | 0.01 | 0.01 |
| Poly([C2VIm][Br]) | 0.79 | 0.39 | 0.79 | 0.79 | 0.79 |
| Poly([C4VIm][Br]) | 0.747 | 0.186 | 0.373 | 0.747 | 0.33 |
| Poly([C6VIm][Br]) | 0.165 | 0.041 | 0.082 | 0.165 | 0.082 |
| Poly([C8VIm][Br]) | 0.74 | 0.186 | 0.372 | 0.745 | 0.372 |
| Poly([C10VIm][Br]) | 0.64 | 0.32 | 0.64 | 0.64 | 0.64 |
| Poly([C12VIm][Br]) | 0.09 | 0.022 | 0.045 | 0.09 | 0.09 |
| Poly([C10VIm][Br]) fibers | 0.83 | 0.42 | 0.83 | 0.83 | 1.66 |
| Poly([C12VIm][Br]) fibers | 0.015 | 0.06 | 0.06 | 0.03 | 0.11 |
| Streptomycin | 0.031 | 0.016 | 0.031 | 0.062 | 0.062 |
| Doxycycline | 0.0004 | 0.0019 | 0.004 | 0.0156 | 0.0156 |
| Fungi | Aspergillus niger | Aspergillus flavus | Penicillium italicum | Mucor mucedo | Trichophyton mentagrophytes |
|---|---|---|---|---|---|
| Tested Compounds and Fibers | MIC Values (mg/mL) | ||||
| [C2VIm][Br] | 5.47 | 10.95 | 2.74 | 10.95 | 0.68 |
| [C4VIm][Br] | 13.23 | 26.46 | 26.45 | 26.46 | 0.08 |
| [C6VIm][Br] | 8.39 | 8.39 | 8.39 | 8.39 | 0.52 |
| [C8VIm][Br] | 1.465 | 1.465 | 1.465 | 0.732 | 0.183 |
| [C10VIm][Br] | 0.85 | 0.425 | 0.21 | 1.71 | 0.105 |
| [C12VIm][Br] | 0.01 | 0.02 | 0.02 | 0.041 | 0.01 |
| Poly([C2VIm][Br]) | 12.76 | 12.76 | 12.76 | 1.276 | 0.79 |
| Poly([C4VIm][Br]) | 5.98 | 5.98 | 1.495 | 5.98 | 0.186 |
| Poly([C6VIm][Br]) | 0.66 | 0.66 | 0.66 | 2.63 | 0.33 |
| Poly([C8VIm][Br]) | 2.99 | 1.49 | 1.49 | 2.99 | 0.74 |
| Poly([C10VIm][Br]) | 5.15 | 2.06 | 2.06 | 1.03 | 0.64 |
| Poly([C12VIm][Br]) | 0.72 | 0.36 | 0.72 | 2.91 | 0.09 |
| Poly([C10VIm][Br]) fibers | 1.66 | 1.66 | 1.66 | 0.83 | 0.83 |
| Poly([C12VIm][Br]) fibers | 0.46 | 0.46 | 0.06 | 0.12 | 0.06 |
| Ketoconazole | 0.07 | 0.31 | 0.15 | 0.25 | 0.15 |
| Fluconazole | 0.5 | 1 | 1 | 1 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rackov, S.; Pilić, B.; Janković, N.; Kosanić, M.; Petković, M.; Vraneš, M. From Synthesis to Functionality: Tailored Ionic Liquid-Based Electrospun Fibers with Superior Antimicrobial Properties. Polymers 2024, 16, 2094. https://doi.org/10.3390/polym16152094
Rackov S, Pilić B, Janković N, Kosanić M, Petković M, Vraneš M. From Synthesis to Functionality: Tailored Ionic Liquid-Based Electrospun Fibers with Superior Antimicrobial Properties. Polymers. 2024; 16(15):2094. https://doi.org/10.3390/polym16152094
Chicago/Turabian StyleRackov, Sanja, Branka Pilić, Nenad Janković, Marijana Kosanić, Marijana Petković, and Milan Vraneš. 2024. "From Synthesis to Functionality: Tailored Ionic Liquid-Based Electrospun Fibers with Superior Antimicrobial Properties" Polymers 16, no. 15: 2094. https://doi.org/10.3390/polym16152094








