Multilayer Film Comprising Polybutylene Adipate Terephthalate and Cellulose Nanocrystals with High Barrier and Compostable Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Application of CNC Coating
2.3. Electrospun Hot-Tack Layer from Food Waste PHBV
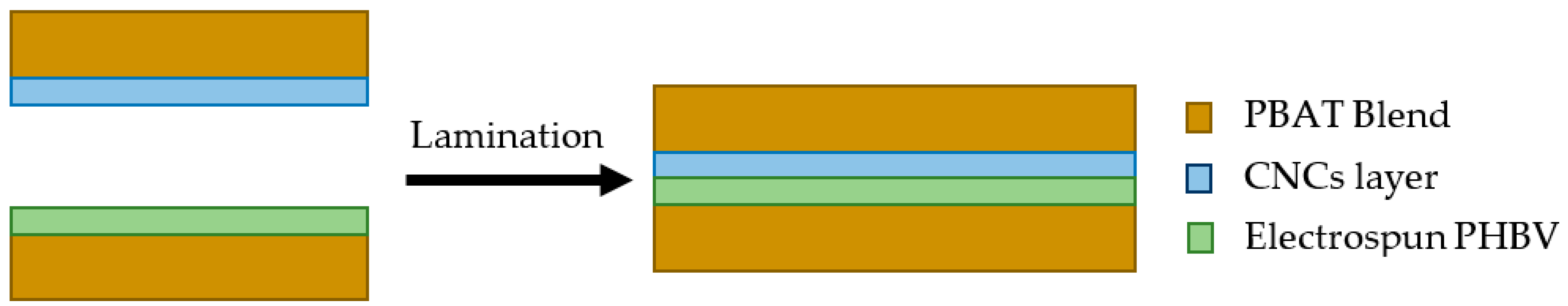
2.4. Lamination
2.5. Characterization
2.5.1. Confocal Laser Scanning Microscopy (CLSM) and Atomic Force Microscopy (AFM)
2.5.2. Surface Tension
2.5.3. Migration Test
Overall Migration
Specific Migration
2.5.4. Mechanical Tests
2.5.5. Adhesion Properties
2.5.6. Permeance Tests
2.5.7. Industrial Composting Disintegration
2.6. Statistical Analysis
3. Results and Discussion
3.1. Surface Roughness
3.2. Surface Tension
3.3. Migration Assessment of the Film
3.4. Mechanical Properties
3.5. Peeling Strength
3.6. Barrier Properties
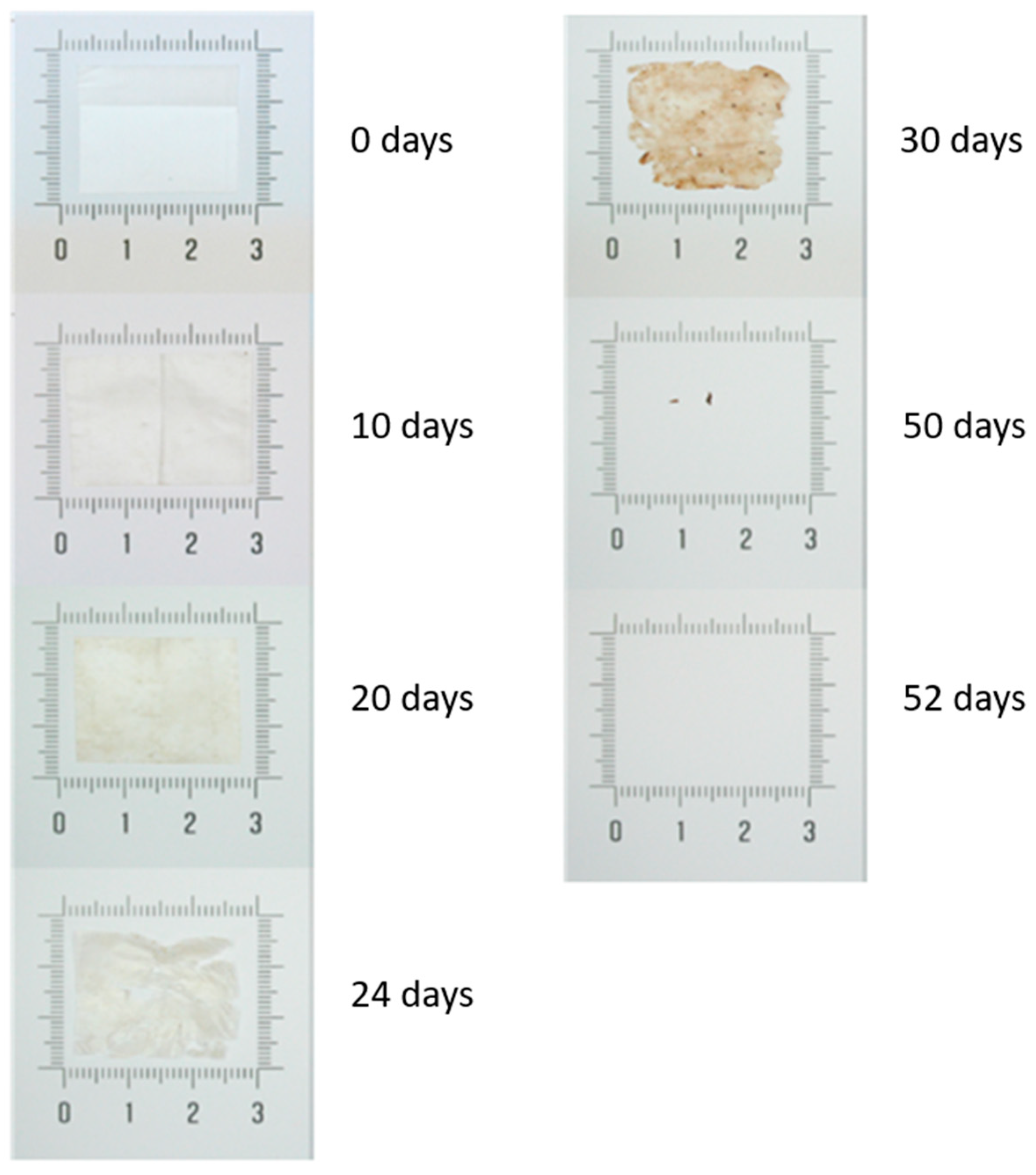
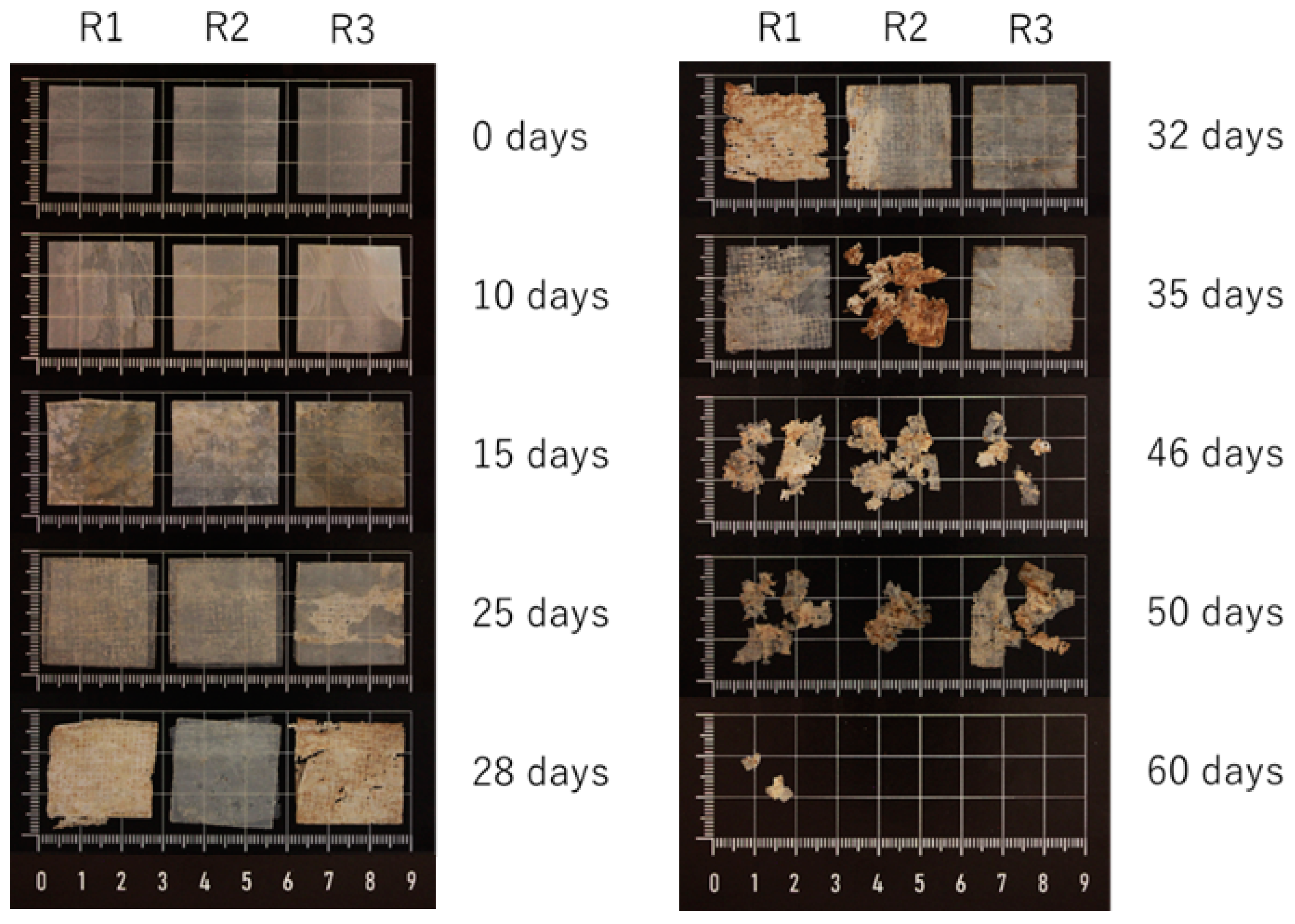
3.7. Disintegration Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhodes, C.J. Solving the Plastic Problem: From Cradle to Grave, to Reincarnation. Sci. Prog. 2019, 102, 218–248. [Google Scholar] [CrossRef] [PubMed]
- Millet, H.; Vangheluwe, P.; Block, C.; Sevenster, A.; Garcia, L.; Antonopoulos, R. The Nature of Plastics and Their Societal Usage; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Hernández-López, M.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Zavaleta-Avejar, L.; Benítez-Jiménez, J.J.; Sabino-Gutiérrez, M.A.; Ortega-Gudiño, P. Bio-Based Composite Fibers from Pine Essential Oil and PLA/PBAT Polymer Blend. Morphological, Physicochemical, Thermal and Mechanical Characterization. Mater. Chem. Phys. 2019, 234, 345–353. [Google Scholar] [CrossRef]
- Bhagwat, G.; Gray, K.; Wilson, S.P.; Muniyasamy, S.; Vincent, S.G.T.; Bush, R.; Palanisami, T. Benchmarking Bioplastics: A Natural Step Towards a Sustainable Future. J. Polym. Environ. 2020, 28, 3055–3075. [Google Scholar] [CrossRef]
- Ashter, S.A. New Developments. In Introduction to Bioplastics Engineering; Ashter, S.A., Ed.; Elsevier: Oxford, UK, 2016; pp. 251–274. ISBN 9780323393966. [Google Scholar]
- Wang, J.H.; Tian, Y.; Zhou, B. Degradation and Stabilization of Poly(Butylene Adipate-Co-Terephthalate)/Polyhydroxyalkanoate Biodegradable Mulch Films Under Different Aging Tests. J. Polym. Environ. 2022, 30, 1366–1379. [Google Scholar] [CrossRef]
- Keskin, G.; Klzll, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of Polyhydroxyalkanoate (PHA) Polymers Family as Substitutes of Petroleum Based Polymers for Packaging Applications and Solutions Brought by Their Composites to Form Barrier Materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun Poly(Hydroxybutyrate)/Chitosan Blend Fibrous Scaffolds for Cartilage Tissue Engineering. J. Appl. Polym. Sci. 2016, 133, 44171. [Google Scholar] [CrossRef]
- Javadi, A.; Kramschuster, A.J.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.S. Processing and Characterization of Microcellular PHBV/PBAT Blends. Polym. Eng. Sci. 2010, 50, 1440–1448. [Google Scholar] [CrossRef]
- Javadi, A.; Srithep, Y.; Lee, J.; Pilla, S.; Clemons, C.; Gong, S.; Turng, L.S. Processing and Characterization of Solid and Microcellular PHBV/PBAT Blend and Its RWF/Nanoclay Composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 982–990. [Google Scholar] [CrossRef]
- Svagan, A.J.; Åkesson, A.; Cárdenas, M.; Bulut, S.; Knudsen, J.C.; Risbo, J.; Plackett, D. Transparent Films Based on PLA and Montmorillonite with Tunable Oxygen Barrier Properties. Biomacromolecules 2012, 13, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, Y.; Mei, C.; Cai, L.; Nadda, A.; Le, Q.V.; Peng, Y.; Lam, S.S.; Sonne, C.; Xia, C. Advanced Nanocellulose-Based Gas Barrier Materials: Present Status and Prospects. Chemosphere 2022, 286, 131891. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive Review on Nanocellulose: Recent Developments, Challenges and Future Prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef] [PubMed]
- Seoane, I.T.; Fortunati, E.; Puglia, D.; Cyras, V.P.; Manfredi, L.B. Development and Characterization of Bionanocomposites Based on Poly(3-Hydroxybutyrate) and Cellulose Nanocrystals for Packaging Applications. Polym. Int. 2016, 65, 1046–1053. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Eects of Cellulose Nanocrystals and Cellulose Nanofibers on the Structure and Properties of Polyhydroxybutyrate Nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Trinh, B.M.; Mekonnen, T. Hydrophobic Esterification of Cellulose Nanocrystals for Epoxy Reinforcement. Polymers 2018, 155, 64–74. [Google Scholar] [CrossRef]
- de Dicastillo, C.L.; Garrido, L.; Velásquez, E.; Rojas, A.; Gavara, R. Designing Biodegradable and Active Multilayer System by Assembling an Electrospun Polycaprolactone Mat Containing Quercetin and Nanocellulose between Polylactic Acid Films. Polymers 2021, 13, 1288. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer Packaging: Advances in Preparation Techniques and Emerging Food Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Torres-Giner, S.; Lagaron, J.M. Nanostructured Multilayer Films. In Nanomaterials for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2018; pp. 147–171. [Google Scholar]
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High Throughput Electro-Hydrodynamic Processing in Food Encapsulation and Food Packaging Applications: Viewpoint. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- Vlachou, M.; Siamidi, A.; Kyriakou, S. Electrospinning and Drug Delivery. In Electrospinning and Electrospraying—Techniques and Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of Bioactive Compounds through Electrospinning/Electrospraying and Spray Drying: A Comparative Assessment of Food-Related Applications. Dry. Technol. 2017, 35, 139–162. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Release Kinetics and Antimicrobial Properties of Carvacrol Encapsulated in Electrospun Poly-(ε-Caprolactone) Nanofibres. Application in Starch Multilayer Films. Food Hydrocoll. 2018, 79, 158–169. [Google Scholar] [CrossRef]
- de Dicastillo, C.L.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.L.; Galotto, M.J. Improvement of Polylactide Properties through Cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer Structures Based on Annealed Electrospun Biopolymer Coatings of Interest in Water and Aroma Barrier Fiber-Based Food Packaging Applications. J. Appl. Polym. Sci. 2018, 135, 45501. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Escuin, J.M.; Bourbon, A.I.; Cabedo, L.; Nevo, Y.; Cerqueira, M.A.; Lagaron, J.M. Development of Active Barrier Multilayer Films Based on Electrospun Antimicrobial Hot-Tack Food Waste Derived Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Cellulose Nanocrystal Interlayers. Nanomaterials 2020, 10, 2356. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Hilliou, L.; Escuin, J.M.; Cabedo, L.; Nevo, Y.; Prieto, C.; Lagaron, J.M. High-Oxygen-Barrier Multilayer Films Based on Polyhydroxyalkanoates and Cellulose Nanocrystals. Nanomaterials 2021, 11, 1443. [Google Scholar] [CrossRef]
- Cruz, M.V.; Freitas, F.; Paiva, A.; Mano, F.; Dionísio, M.; Ramos, A.M.; Reis, M.A.M. Valorization of Fatty Acids-Containing Wastes and Byproducts into Short- and Medium-Chain Length Polyhydroxyalkanoates. N. Biotechnol. 2016, 33, 206–215. [Google Scholar] [CrossRef]
- ISO 25178-6:2010; Geometrical Product Specifications (GPS)—Surface Texture: Areal. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 4287:1997; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 1997.
- EN1186:2002; Materials and Articles in Contact with Foodstuffs-Plastics. European Committee for Standarization: Brussels, Belgium, 2002.
- EN13130-1:2004; Materials and Articles in Contact with Foodstuffs- Plastic Substances Subject to Limitation, Concerned with the Determination of Specific Migration from Plastic Materials into Food Stuffs and Food Simulants and the Determination of Specific Monomers and Additives in Plastics. European Committee for Standarization: Brussels, Belgium, 2004.
- ISO 527-3:2018; Plastics—Determination of Tensile Properties. International Organization for Standardization: Geneva, Switzerland, 2018.
- ASTM-D1876-08; Standard Test Method for Peel Resistance of Adhesives (T-Peel Test). American Society for Testing Materials: West Conshohocken, PA, USA, 2015.
- ASTM E398-03; Standard Test Method for Water Vapor Transmission Rate of Sheet Materials Using Dynamic Relative Humidity Measurement. American Society for Testing Materials: West Conshohocken, PA, USA, 2010.
- ASTM 3985-2010; ASTM Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. American Society for Testing Materials: West Conshohocken, PA, USA, 2010.
- ISO 20200:2015; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test. International Organization for Standardization: Geneva, Switzerland, 2015.
- Baby, M.; Periya, V.K.; Soundiraraju, B.; Balachandran, N.; Cheriyan, S.; Sankaranarayanan, S.K.; Maniyeri, S.C. Bio-Mimicking Hybrid Polymer Architectures as Adhesion Promoters for Low and High Surface Energy Substrates. J. Ind. Eng. Chem. 2021, 100, 351–363. [Google Scholar] [CrossRef]
- Shi, R.; Wang, B.; Yan, Z.; Wang, Z.; Dong, L. Effect of Surface Topography Parameters on Friction and Wear of Random Rough Surface. Materials 2019, 12, 2762. [Google Scholar] [CrossRef]
- Marinello, F.; La Storia, A.; Mauriello, G.; Passeri, D. Atomic Force Microscopy Techniques to Investigate Activated Food Packaging Materials. Trends Food Sci. Technol. 2019, 87, 84–93. [Google Scholar] [CrossRef]
- Singh, M.; Ohji, T.; Dong, S.; Koch, D.; Shimamura, K.; Clauss, B.; Heidenreich, B.; Akedo, J. Advances in High Temperature Ceramic Matrix Composites and Materials for Sustainable Development; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 263. [Google Scholar]
- Vatanpour, V.; Safarpour, M.; Khataee, A.; Zarrabi, H.; Yekavalangi, M.E.; Kavian, M. A Thin Film Nanocomposite Reverse Osmosis Membrane Containing Amine-Functionalized Carbon Nanotubes. Sep. Purif. Technol. 2017, 184, 135–143. [Google Scholar] [CrossRef]
- Louzi, V.C.; Campos, J.S. de C. Corona Treatment Applied to Synthetic Polymeric Monofilaments (PP, PET, and PA-6). Surf. Interfaces 2019, 14, 98–107. [Google Scholar] [CrossRef]
- Sellin, N.; Sinézio, J.; Campos, C. Surface Composition Analysis of PP Films Treated by Corona Discharge. Mater. Res. 2003, 6, 163–166. [Google Scholar] [CrossRef]
- Podgorski, L.; Chevet, B.; Onic, L.; Merlin, A. Modification of Wood Wettability by Plasma and Corona Treatments. Int. J. Adhes. Adhes. 2000, 20, 103–111. [Google Scholar] [CrossRef]
- Huang, Z.; Qiu, R.; Liu, T.; Huang, Y.; Zhu, Z.; Wang, L. Determination of Methacrylic Acid in Food Simulants by Pyrolytic Butylation-Gas Chromatography. J. Chromatogr. A 2016, 1454, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hao, Y.; Zhao, Y.; Lang, X.; Zhang, Y.; Wang, Z.; Zhang, H.; Dong, L. Improved Mechanical Properties, Barrier Properties and Degradation Behavior of Poly(Butylenes Adipate-Co-Terephthalate)/Poly(Propylene Carbonate) Films. Korean J. Chem. Eng. 2017, 34, 1294–1304. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent Advances in the Development of Biodegradable PHB-Based Toughening Materials: Approaches, Advantages and Applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Spagnol, C.; Fragal, E.H.; Witt, M.A.; Follmann, H.D.M.; Silva, R.; Rubira, A.F. Mechanically Improved Polyvinyl Alcohol-Composite Films Using Modified Cellulose Nanowhiskers as Nano-Reinforcement. Carbohydr. Polym. 2018, 191, 25–34. [Google Scholar] [CrossRef]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and Barrier Properties of Nanocrystalline Cellulose Reinforced Chitosan Based Nanocomposite Films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Šumiga, B.; Karlovits, I.; Šumiga, B. Adhesion Strength of Temperature Varied Nanocellulose Enhanced Water Based Paper and Cardboard Adhesives. In Proceedings of the International Symposium on Graphic Engineering and Design, Novi Sad, Serbia, 12–14 November 2020; pp. 191–195. [Google Scholar]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Vähä-Nissi, M.; Koivula, H.M.; Räisänen, H.M.; Vartiainen, J.; Ragni, P.; Kenttä, E.; Kaljunen, T.; Malm, T.; Minkkinen, H.; Harlin, A. Cellulose Nanofibrils in Biobased Multilayer Films for Food Packaging. J. Appl. Polym. Sci. 2017, 134, 44830. [Google Scholar] [CrossRef]
- Nuruddin, M.; Korani, D.M.; Jo, H.; Chowdhury, R.A.; Montes, F.J.; Howarter, J.A.; Youngblood, J.P. Gas and Water Vapor Barrier Performance of Cellulose Nanocrystal-Citric Acid-Coated Polypropylene for Flexible Packaging. ACS Appl. Polym. Mater. 2020, 2, 4405–4414. [Google Scholar] [CrossRef]

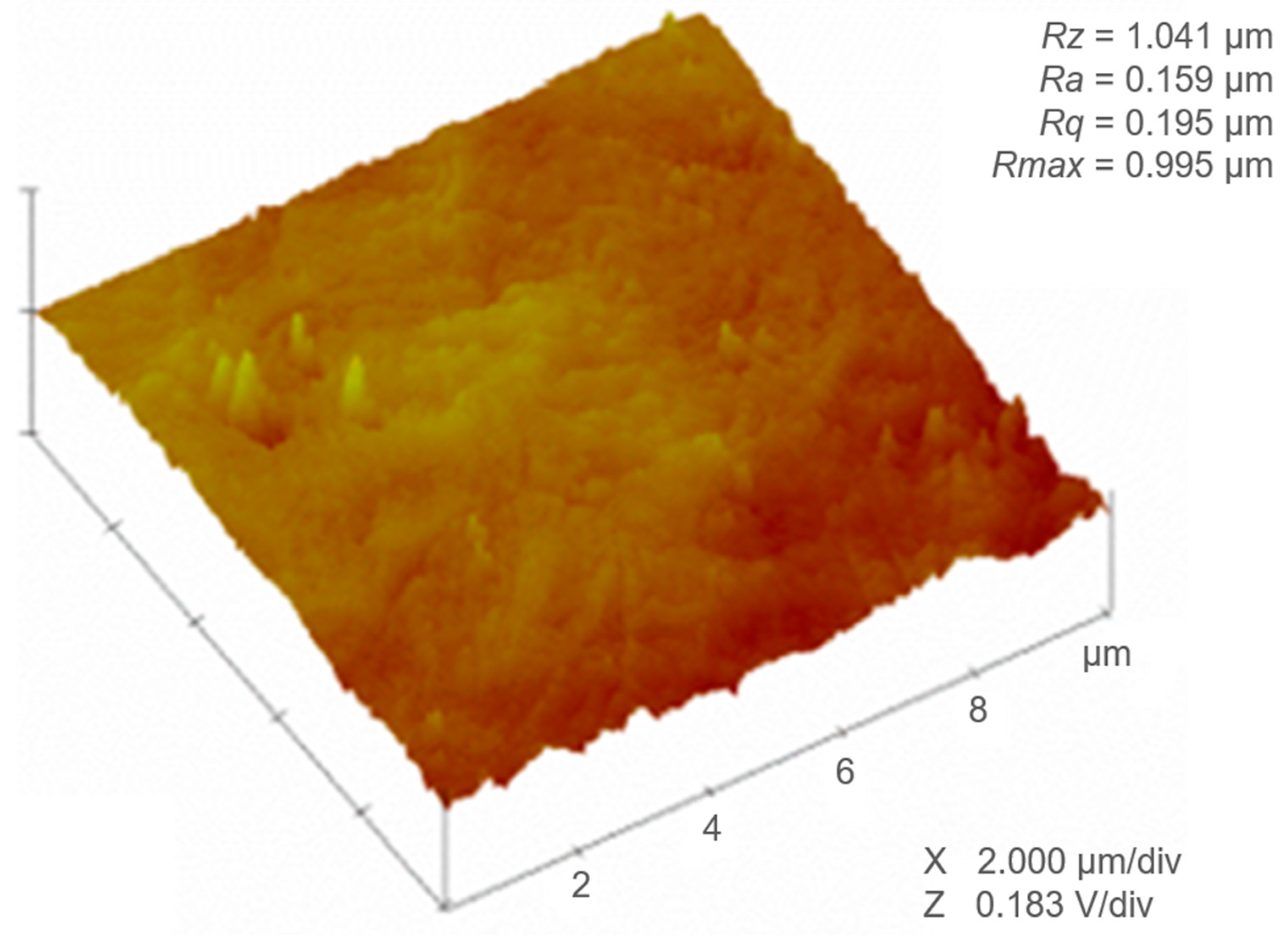
| Sample | L-Filter (µm) | Sq (µm) | Sa (µm) | Sz (µm) | Ssk | Sku | Sdr (%) |
|---|---|---|---|---|---|---|---|
| Film | 10 | 0.14 ± 0.01 | 0.079 ± 0.002 | 12.4 ± 2.3 | −4.9 ± 3.6 | 193.9 ± 96.5 | 4.1 ± 0.6 |
| Samples | |||
|---|---|---|---|
| Film without Surface Treatment | Film with Surface Treatment | ||
| Contact angle | Θ (o) Water | 73.9 ± 0.7 a | 53.2 ± 1.0 b |
| Θ (o) Ethylene glycol | 53.2 ± 0.8 a | 24.5 ± 1.1 b | |
| Θ (o) Diiodomethane | 31.6 ± 1.6 a | 46.0 ± 1.2 b | |
| Surface tension | Surface energy (mN/m) | 52.8 ± 0.2 a | 55.5 ± 0.3 b |
| Dispersive (mN/m) | 31.7 ± 0.6 a | 27.8 ± 0.5 b | |
| Polar (mN/m) | 18.2 ± 0.4 a | 12.0 ± 0.3 b | |
| H-H (mN/m) | 3.0 ± 0.1 a | 15.6 ± 0.3 b | |
| Sample | Test (40 °C, 10 Days) | Result (mg/dm2) |
|---|---|---|
| Multilayer film | Migration in ethanol 10% (A) | 1.7 ± 0.6 |
| Migration in acetic acid 3% (B) | 1.7 ± 0.6 | |
| Migration in olive oil (D2) | 2.5 ± 0.7 |
| Sample | Food Simulants | |||||
|---|---|---|---|---|---|---|
| 10% (v/v) Ethanol/Water | 3% (v/v) Acetic Acid/Water | 95% (v/v) Ethanol/Water * | ||||
| (mg/kg) | (mg/g Film) | (mg/kg) | (mg/g Film) | (mg/kg) | (mg/g Film) | |
| PBAT film | 10.70 ± 2.17 | 3.74 ± 1.12 | 7.49 ± 6.34 | 3.24 ± 1.53 | 0.63 ± 1.00 | 0.49 ± 0.25 |
| Multilayer film | ND | ND | ND | ND | ND | ND |
| Sample | MD | TD | |||||
|---|---|---|---|---|---|---|---|
| Thickness (mm) | E (MPa) | σy (MPa) | εb (%) | E (MPa) | σy (MPa) | εb (%) | |
| Monolayer | 0.05 | 1270 ± 64 a | 20.3 ± 1.3 a | 330 ± 22 a | 1030 ± 87 a | 17.8 ± 0.3 a | 243 ± 19 a |
| Multilayer | 0.105 | 950 ± 64 b | 20.6 ± 1.7 a | 27 ± 20 b | 1030 ± 64 a | 14.3 ± 0.5 b | 7.6 ± 2.3 b |
| Machine Direction | Peeling Load (N) | T-Peel Strength (N/mm) |
|---|---|---|
| MD | 0.083 ± 0.024 a | 0.006 ± 0.002 a |
| TD | 0.081 ± 0.009 a | 0.005 ± 0.001 a |
| Permeance | |||
|---|---|---|---|
| Sample | Thickness (mm) | WVP × 1012 (kg·m−2·s−1·Pa−1) | OP × 1015 (m3·m−2·s−1·Pa−1) |
| Monolayer PBAT Blend | 0.045 | 3.6 ± 0.3 a | 9.3 ± 0.1 a |
| Multilayer PBAT Blend with hot-tack | 0.107 | 2.0 ± 0.6 b | 5.9 ± 0.4 b |
| Multilayer PBAT Blend with hot-tack and CNCs | 0.105 | 2.4 ± 0.1 b | 0.5 ± 0.3 c |
| Time (Days) | Disintegration (%) |
|---|---|
| 0 | 0.0 ± 0.0 |
| 10 | −1.8 ± 0.2 |
| 15 | 2.8 ± 3.7 |
| 21 | 18.3 ± 7.5 |
| 23 | 24.6 ± 7.5 |
| 25 | 30.5 ± 0.7 |
| 28 | 47.9 ± 7.4 |
| 30 | 49.8 ± 5.7 |
| 32 | 53.4 ± 14.1 |
| 35 | 56.8 ± 10.6 |
| 37 | 58.7 ± 11.2 |
| 46 | 84.5 ± 5.2 |
| 50 | 82.7 ± 6.8 |
| 60 | 99.9 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melendez-Rodriguez, B.; Prieto, C.; Pardo-Figuerez, M.; Angulo, I.; Bourbon, A.I.; Amado, I.R.; Cerqueira, M.A.; Pastrana, L.M.; Hilliou, L.H.G.; Vicente, A.A.; et al. Multilayer Film Comprising Polybutylene Adipate Terephthalate and Cellulose Nanocrystals with High Barrier and Compostable Properties. Polymers 2024, 16, 2095. https://doi.org/10.3390/polym16152095
Melendez-Rodriguez B, Prieto C, Pardo-Figuerez M, Angulo I, Bourbon AI, Amado IR, Cerqueira MA, Pastrana LM, Hilliou LHG, Vicente AA, et al. Multilayer Film Comprising Polybutylene Adipate Terephthalate and Cellulose Nanocrystals with High Barrier and Compostable Properties. Polymers. 2024; 16(15):2095. https://doi.org/10.3390/polym16152095
Chicago/Turabian StyleMelendez-Rodriguez, Beatriz, Cristina Prieto, Maria Pardo-Figuerez, Inmaculada Angulo, Ana I. Bourbon, Isabel R. Amado, Miguel A. Cerqueira, Lorenzo M. Pastrana, Loic Hugues Gilles Hilliou, António A. Vicente, and et al. 2024. "Multilayer Film Comprising Polybutylene Adipate Terephthalate and Cellulose Nanocrystals with High Barrier and Compostable Properties" Polymers 16, no. 15: 2095. https://doi.org/10.3390/polym16152095







