Controllable Assemblies of Au NPs/P5A for Enhanced Catalytic Reduction of 4-Nitrophenol
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of P5A
2.3. Preparation of Au NPs/P5A
2.4. Catalytic Reduction Experiment
2.5. Techniques of Characterizations
3. Results and Discussion
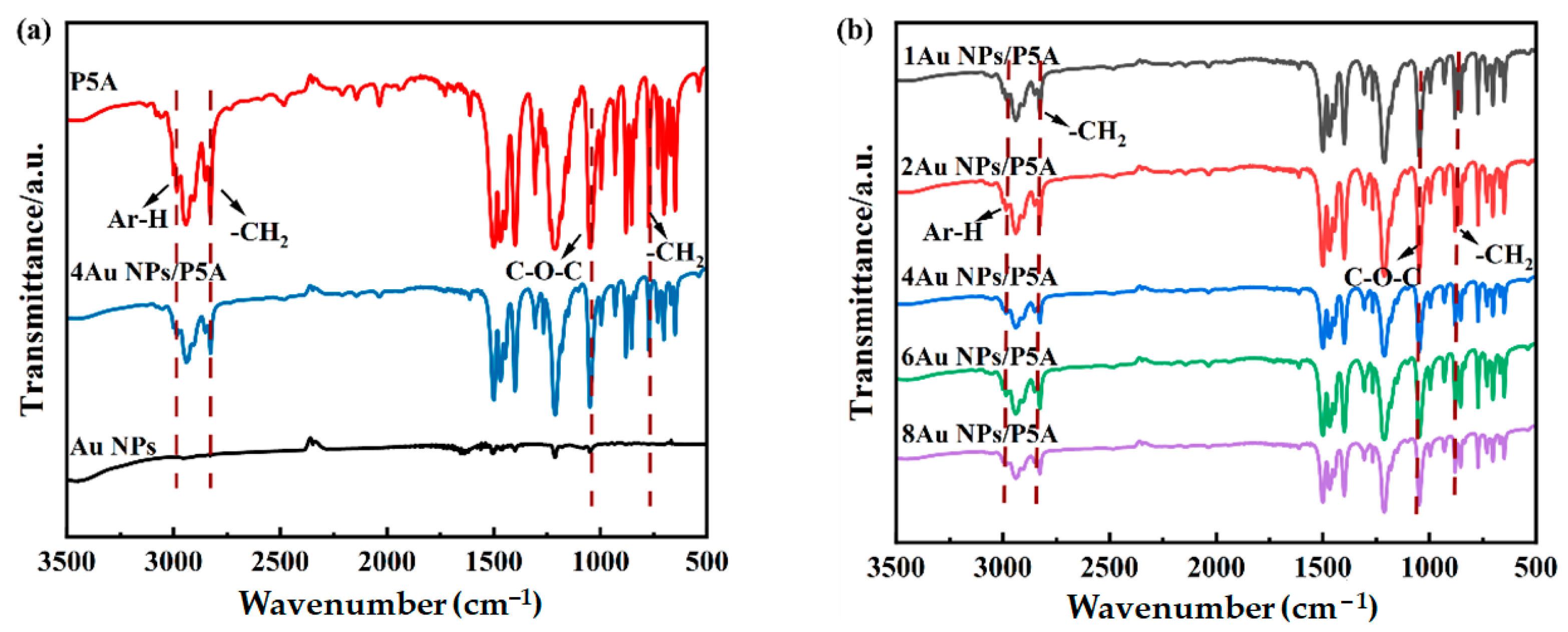
3.1. Characterization of the Au NPs/P5A Composite
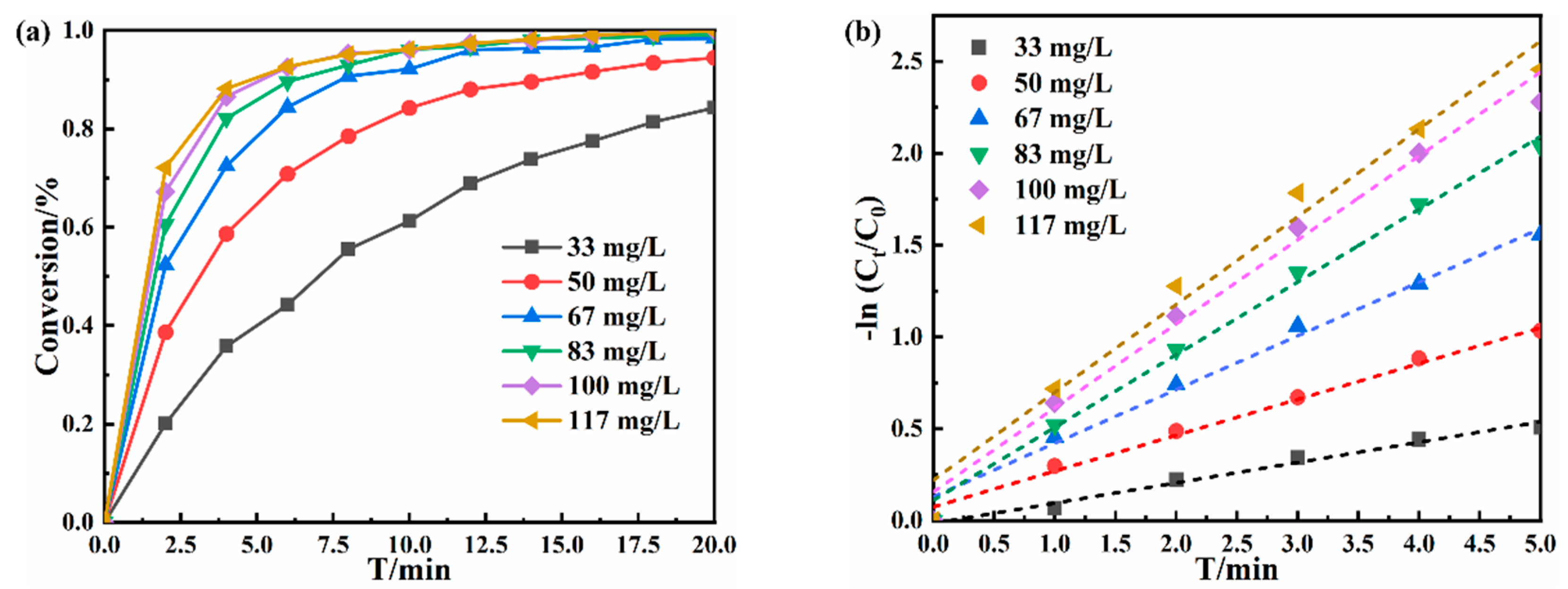
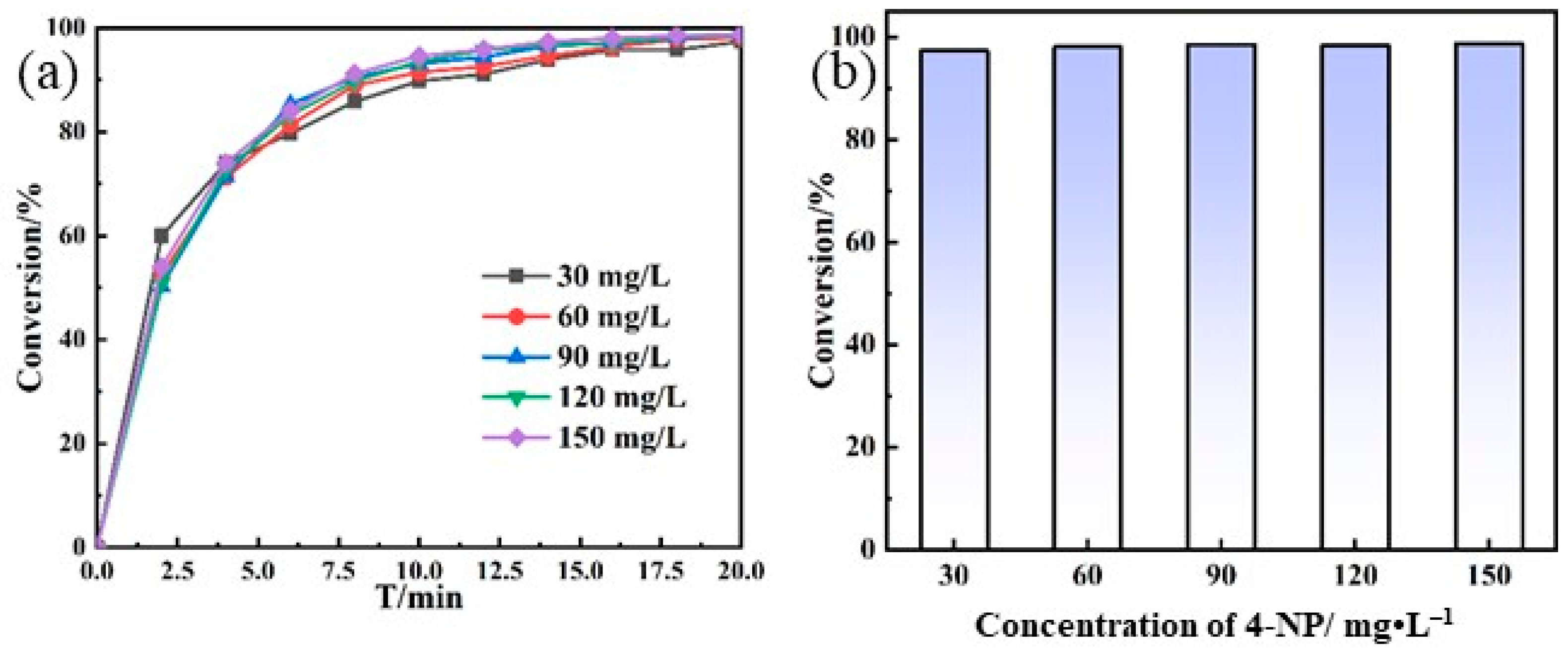
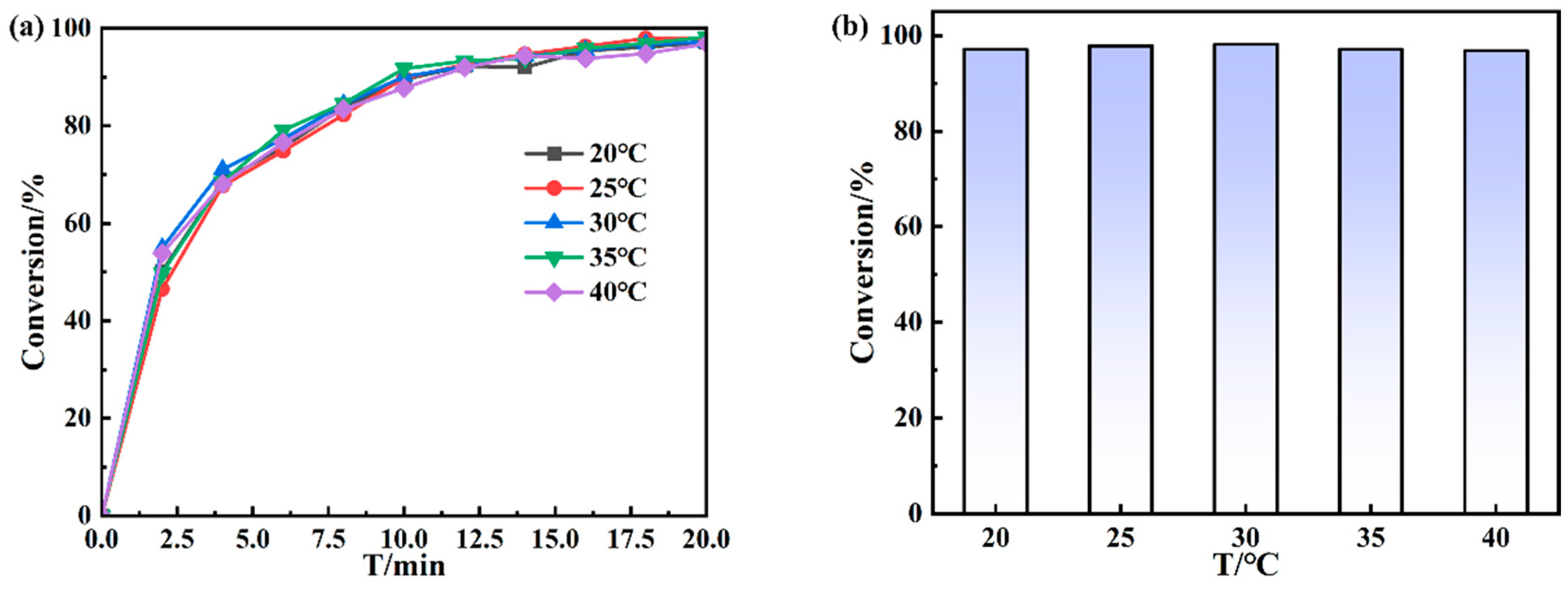
3.2. Catalytic Performance of Au NPs/P5A on Reducing 4-NP
4. Catalytic Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.Q.; Murugananthan, M.; Zhang, Y.R. Degradation of p-Nitrophenol by thermally activated persulfate in soil system. Chem. Eng. J. 2016, 283, 1357–1365. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.Q.; Zhou, H.; Murugananthan, M.; Zhang, Y.R. Degradation of p-nitrophenol by heat and metal ions co-activated persulfate. Chem. Eng. J. 2015, 264, 39–47. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic Assemblies for Catalytic Reduction of Nitrophenols: A Critical Review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, A.Y.; He, R.B.; Wu, Y.; Hou, L.; Yang, G.P.; Zhang, W.Y.; Wang, Y.Y. Enhancement of catalytic activity for hydrogenation of nitroaromatic by anionic metal-organic framework. Chin. Chem. Lett. 2024, 35, 108639. [Google Scholar] [CrossRef]
- Wang, D.; Li, P.; Xi, J.B. Active metals decorated NiCo2O4 yolk-shell nanospheres as nanoreactors for catalytic reduction of nitroarenes and azo dyes. Chemosphere 2024, 350, 141102. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zeng, Z.T.; Zeng, G.M.; Lai, C.; Duan, A.; Xiao, R.; Huang, D.L.; Fu, Y.K.; Yi, H.; Li, B.S.; et al. Cooperative catalytic performance of bimetallic Ni-Au nanocatalyst for highly efficient hydrogenation of nitroaromatics and corresponding mechanism insight. Appl. Catal. B Environ. 2019, 259, 118035. [Google Scholar] [CrossRef]
- Li, H.; Chen, D.X.; Sun, Y.L.; Zheng, Y.B.; Tan, L.L.; Weiss, P.S.; Yang, Y.W. Viologen-Mediated Assembly of and Sensing with Carboxylatopillar[5]arene-Modified Gold Nanoparticles. J. Am. Chem. Soc. 2013, 135, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, X.; Liu, D.B.; Chen, X.Y. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhou, Y.J.; Zhao, R.; Jie, K.C.; Huang, F.H. Dihalobenzene Shape Sorting by Nonporous Adaptive Crystals of Perbromoethylated Pillararenes. Angew. Chem. Int. Ed. 2019, 58, 3981–3985. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.a.; Nakamoto, Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Song, L.Q.; Zhang, H.C.; Han, J.; Zhao, Y.L. Self-assembly of partially oxidized pillar[5]arenes induced by intermolecular charge transfer interactions. Chin. Chem. Lett. 2023, 34, 108479. [Google Scholar] [CrossRef]
- Guo, S.W.; Song, Y.S.; He, Y.L.; Hu, X.Y.; Wang, L.Y. Highly Efficient Artificial Light-Harvesting Systems Constructed in Aqueous Solution Based on Supramolecular Self-Assembly. Angew. Chem. Int. Ed. 2018, 57, 3163–3167. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, S.; Kato, K.; Fa, S.X.; Ogoshi, T. Host-Guest chemistry based on solid-state pillar[n]arenes. Coord. Chem. Rev. 2022, 462, 214503. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kakuta, T.; Yamagishi, T.a. Applications of Pillar[n]arene-Based Supramolecular Assemblies. Angew. Chem. Int. Ed. 2019, 58, 2197–2206. [Google Scholar] [CrossRef]
- Ma, J.K.; Yan, H.W.; Quan, J.X.; Bi, J.H.; Tian, D.M.; Li, H.B. Enantioselective Dynamic Self-Assembly of Histidine Droplets on Pillar[5]arene-Modified Interfaces. ACS Appl. Mater. 2019, 11, 1665–1671. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, B.; Zhang, H. Controllable Assemblies of Au NPs/P5A for Enhanced Catalytic Reduction of 4-Nitrophenol. Polymers 2024, 16, 2104. https://doi.org/10.3390/polym16152104
Liu Z, Li B, Zhang H. Controllable Assemblies of Au NPs/P5A for Enhanced Catalytic Reduction of 4-Nitrophenol. Polymers. 2024; 16(15):2104. https://doi.org/10.3390/polym16152104
Chicago/Turabian StyleLiu, Zhaona, Bing Li, and Huacheng Zhang. 2024. "Controllable Assemblies of Au NPs/P5A for Enhanced Catalytic Reduction of 4-Nitrophenol" Polymers 16, no. 15: 2104. https://doi.org/10.3390/polym16152104
APA StyleLiu, Z., Li, B., & Zhang, H. (2024). Controllable Assemblies of Au NPs/P5A for Enhanced Catalytic Reduction of 4-Nitrophenol. Polymers, 16(15), 2104. https://doi.org/10.3390/polym16152104






