Maximizing Degumming Efficiency for Firmiana simplex Bark Using Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Deep Eutectic Solvents
2.2.2. Firmiana Simplex Bark Fiber Preparation
2.2.3. DES Treatment with Firmiana Simplex Bark Fibers
2.3. Characterization
3. Results and Discussion
3.1. Chemical Composition Analysis
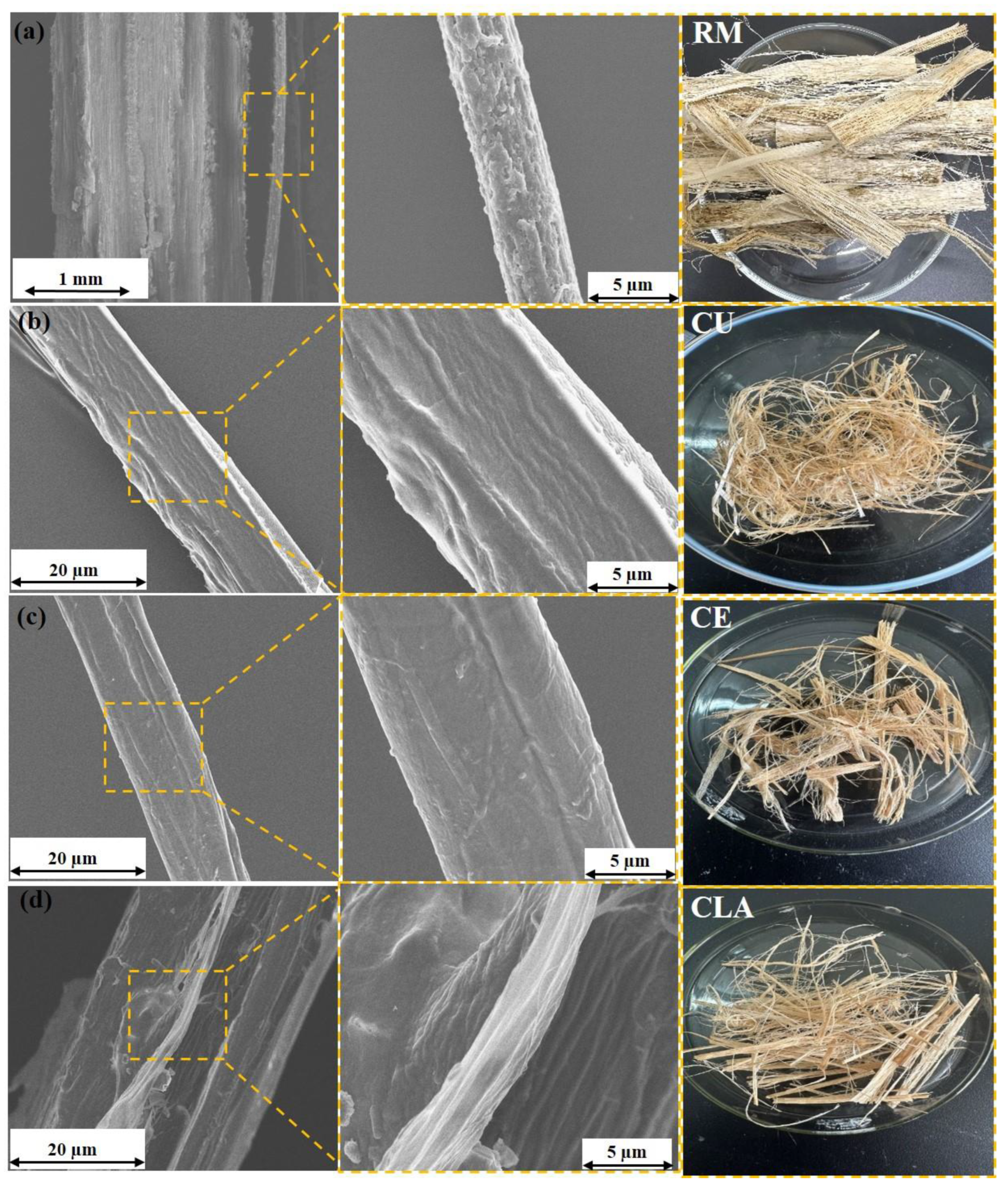
3.2. Morphological Analysis
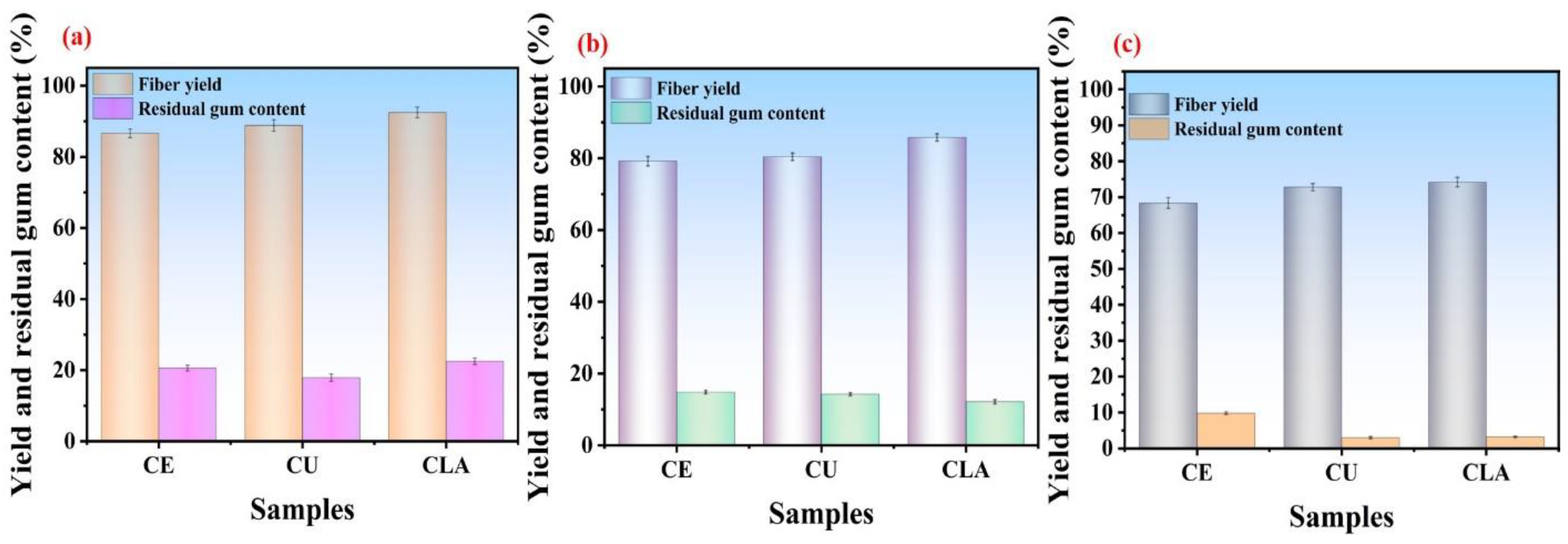
3.3. Residual Gum Content
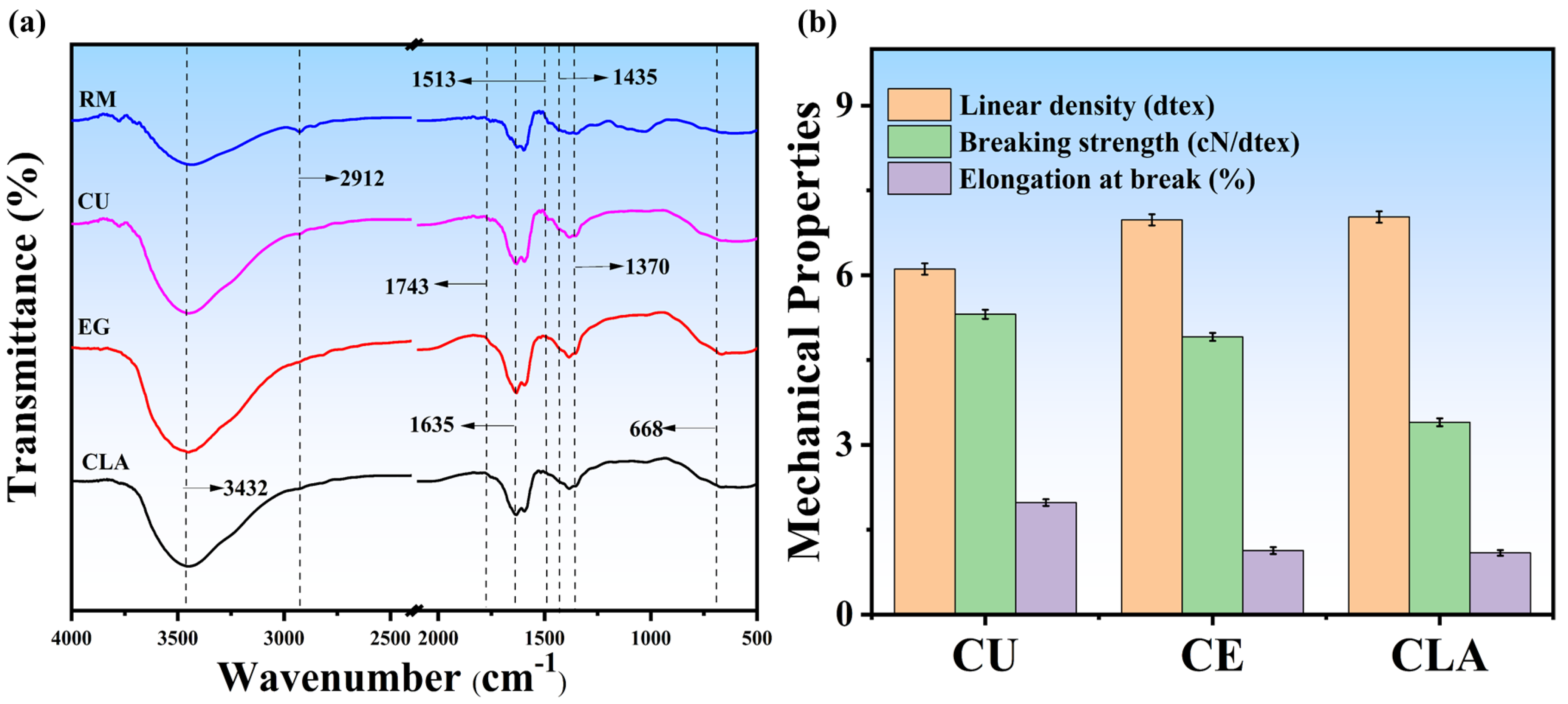
3.4. Chemical Structural Changes and Mechanical Properties Analysis
3.5. Optimization Analysis of CU-Based DES Treatment
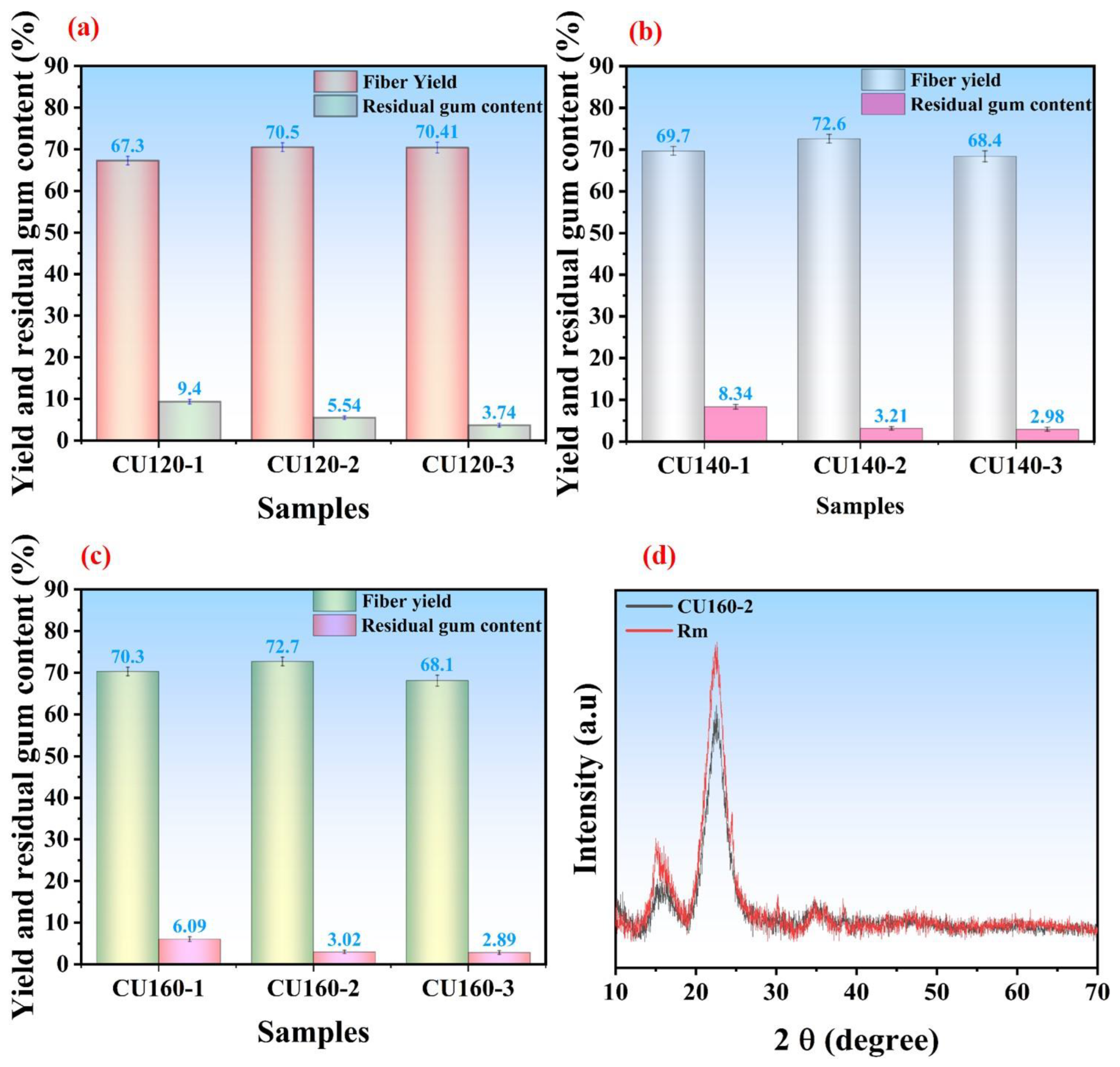
3.5.1. Influence of CU-Based DES Treatment on Fiber Yield and Residual Gum Content
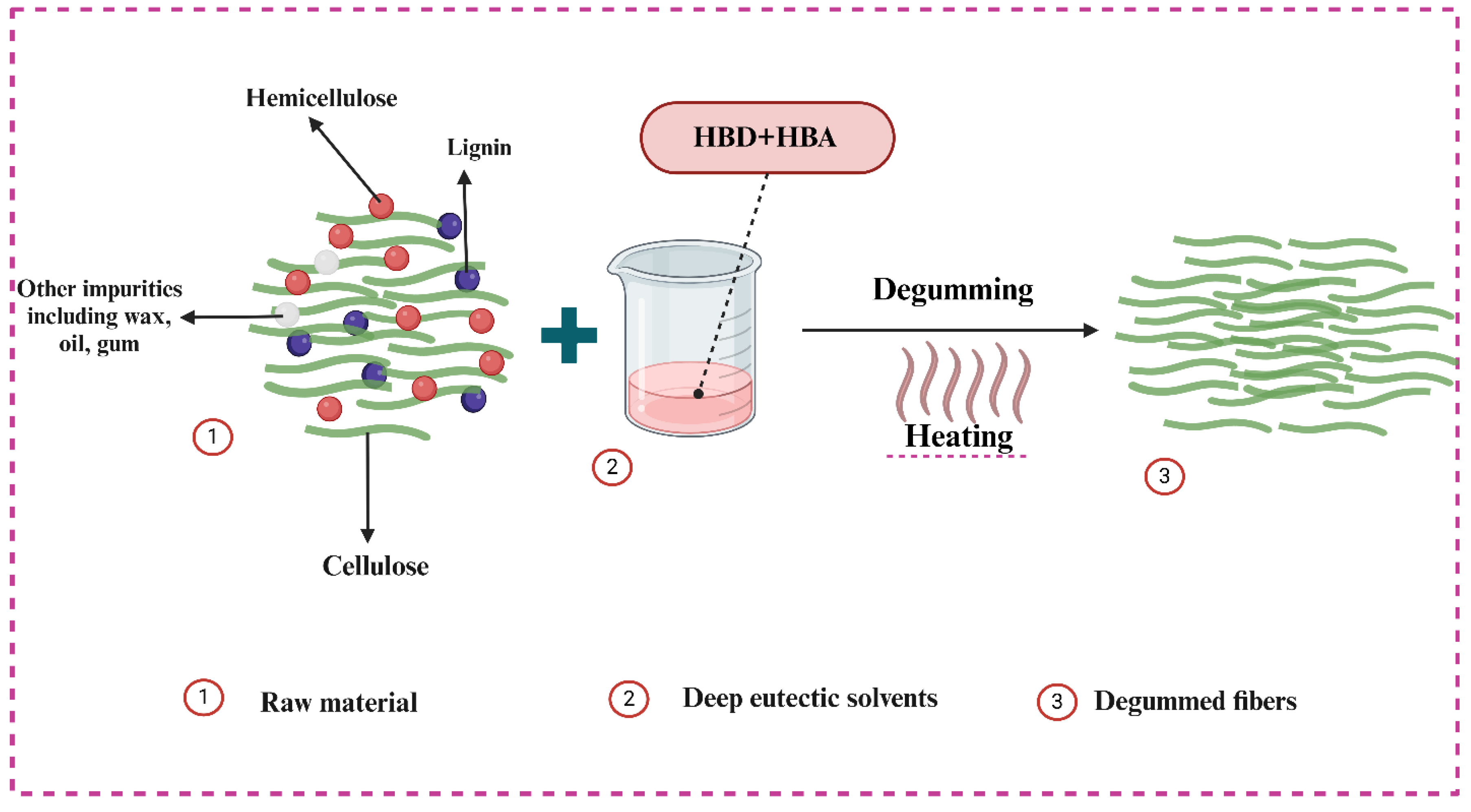
3.5.2. Morphological Analysis and Degumming Mechanism of DES
3.5.3. Chemical Composition after CU-Based DES Pretreatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farooq, A.; Ying, L.; Yang, H.; Sarkodie, B.; Ding, Y.; Zhu, M.; Susu, B.; Hu, C.; Tian, M.; Wang, Z. Continuous oil–water separation by utilizing novel natural hollow fibers: Evaluation and potential applications. Cellulose 2024, 31, 3029–3051. [Google Scholar] [CrossRef]
- Shadhin, M.; Rahman, M.; Jayaraman, R.; Chen, Y.; Mann, D.; Zhong, W. Natural biomass & waste biomass fibers–structures, environmental footprints, sustainability, degumming methods, & surface modifications. Ind. Crops Prod. 2023, 204, 117252. [Google Scholar]
- Wulandari, A.P.; Kusmoro, J.; Ernawati, E.E. Standardization of Chemical Degumming and Biodegumming of Ramie Fiber Processing for Natural Fiber Supply Chain Strategy as a Source of Textile Raw Materials in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2023, 1211, 012008. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Huang, X.; Huang, R.; Liang, Z.; Xiao, W.; Xu, Y.; Liu, Z. Evaluation of Aqueous Ammonia Degumming of Ramie Fiber and Recyclability of Black Liquor. ACS Sustain. Chem. Eng. 2023, 11, 17885–17895. [Google Scholar] [CrossRef]
- Farooq, A.; Yang, H.; Ding, Z.; Bu, F.; Guo, M.; Sun, W.; Wang, Z.; Tian, M. Exploring the versatility of biodegradable biomass aerogels: In-depth evaluation of Firmiana simplex bark microfibers depolymerized by deep eutectic solvent. Int. J. Biol. Macromol. 2024, 275, 133629. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Ohtsu, M.; Kokudo, N.; Shibata, H.; Aragaki, N.; Higuchi, T.; Hashimoto, T. Phenolic constituents from the branch of Firmiana simplex. Shoyakugaku Zasshi Jpn. J. Pharmacogn. 2010, 64, 102–103. [Google Scholar]
- Ajaib, M.; Qayyum Wahla, S.; Mohammed Khan, K.; Perveen, S.; Shah, S. Firmiana Simplex: A Potential Source of Antimicrobials. J. Chem. Soc. Pak. 2014, 36, 744. [Google Scholar]
- Son, Y.K.; Lee, M.H.; Han, Y.N. A new antipsychotic effective neolignan from Firmiana simplex. Arch. Pharmacal Res. 2005, 28, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.W.; Park, J.E.; Cha, J.M.; Subedi, L.; Kim, S.Y.; Lee, K.R. Three new lignan glycosides from the Firmiana simplex. Chem. Pharm. Bull. 2019, 67, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Chiliveri, S.R.; Koti, S.; Linga, V.R. Retting and degumming of natural fibers by pectinolytic enzymes produced from Bacillus tequilensis SV11-UV37 using solid state fermentation. Springerplus 2016, 5, 559. [Google Scholar] [CrossRef]
- Magalhães, S.; Fernandes, C.; Pedrosa, J.F.; Alves, L.; Medronho, B.; Ferreira, P.J.; Rasteiro, M.d.G. Eco-friendly methods for extraction and modification of cellulose: An overview. Polymers 2023, 15, 3138. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Li, M.; Alasood, A.; Farooq, A.; Ashraf, M.; Patoary, M.K.; Liu, L. Novel pretreatment performance evaluation for cellulose nanofibrils extraction from Ficus natalensis barkcloth. J. Polym. Environ. 2022, 30, 1547–1559. [Google Scholar] [CrossRef]
- Farooq, A.; Jiang, S.; Farooq, A.; Naeem, M.A.; Ahmad, A.; Liu, L. Structure and properties of high quality natural cellulose nano fibrils from a novel material Ficus natalensis barkcloth. J. Ind. Text. 2021, 51, 664–680. [Google Scholar] [CrossRef]
- Shen, M.; Wang, L.; Chen, F.; Long, J.-J.; Rui, Y.-N. Effect of low-temperature oxygen plasma on the degumming of ramie fabric. J. Clean. Prod. 2015, 92, 318–326. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Liu, G.; Zhang, Y.; Han, G.; Vomiero, A.; Zhao, H. Colloidal carbon quantum dots as light absorber for efficient and stable ecofriendly photoelectrochemical hydrogen generation. Nano Energy 2021, 86, 106122. [Google Scholar] [CrossRef]
- Yiin, C.L.; Yap, K.L.; Ku, A.Z.E.; Chin, B.L.F.; Lock, S.S.M.; Cheah, K.W.; Loy, A.C.M.; Chan, Y.H. Recent advances in green solvents for lignocellulosic biomass pretreatment: Potential of choline chloride (ChCl) based solvents. Bioresour. Technol. 2021, 333, 125195. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Liu, Y.; Su, Z.; Li, B.; Gao, C.; Tian, W. Comprehensive analysis of important parameters of choline chloride-based deep eutectic solvent pretreatment of lignocellulosic biomass. Bioresour. Technol. 2021, 319, 124209. [Google Scholar] [CrossRef] [PubMed]
- Omar, K.A.; Sadeghi, R. New chloroacetic acid-based deep eutectic solvents for solubilizing metal oxides. J. Mol. Liq. 2022, 347, 118393. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents as catalysts for upgrading biomass. Catalysts 2021, 11, 178. [Google Scholar] [CrossRef]
- Vigier, K.D.O.; Chatel, G.; Jérôme, F. Contribution of deep eutectic solvents for biomass processing: Opportunities, challenges, and limitations. ChemCatChem 2015, 7, 1250–1260. [Google Scholar] [CrossRef]
- Xu, G.-C.; Ding, J.-C.; Han, R.-Z.; Dong, J.-J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef]
- Song, Y.; Kai, N.; Jiang, W.; Zhang, Y.; Ben, H.; Han, G.; Ragauskas, A.J. Utilization of deep eutectic solvent as a degumming protocol for Apocynum venetum bast. Cellulose 2019, 26, 8047–8057. [Google Scholar] [CrossRef]
- Nie, K.; Liu, B.; Zhao, T.; Wang, H.; Song, Y.; Ben, H.; Ragauskas, A.J.; Han, G.; Jiang, W. A facile degumming method of kenaf fibers using deep eutectic solution. J. Nat. Fibers 2022, 19, 1115–1125. [Google Scholar] [CrossRef]
- Yu, W.; Wang, C.; Yi, Y.; Wang, H.; Zeng, L.; Li, M.; Yang, Y.; Tan, Z. Comparison of deep eutectic solvents on pretreatment of raw ramie fibers for cellulose nanofibril production. ACS Omega 2020, 5, 5580–5588. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Loow, Y.-L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 2017, 24, 3591–3618. [Google Scholar] [CrossRef]
- Pandey, A.; Bhawna; Dhingra, D.; Pandey, S. Hydrogen bond donor/acceptor cosolvent-modified choline chloride-based deep eutectic solvents. J. Phys. Chem. B 2017, 121, 4202–4212. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.-J. Effect of choline-based deep eutectic solvent pretreatment on the structure of cellulose and lignin in bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.; Saravanakumar, S. Isolation and characterization of cellulose fibers from Thespesia populnea barks: A study on physicochemical and structural properties. Int. J. Biol. Macromol. 2019, 129, 396–406. [Google Scholar] [CrossRef]
- Hossain, S.; Jalil, M.A.; Islam, T.; Rahman, M.M. A low-density cellulose rich new natural fiber extracted from the bark of jack tree branches and its characterizations. Heliyon 2022, 8, e11667. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, K.; Kumaravel, A.; Saravanakumar, S.; Arthanarieswaran, V. Characterization of new natural cellulosic fiber from the Ipomoea staphylina plant. Int. J. Polym. Anal. Charact. 2016, 21, 267–274. [Google Scholar] [CrossRef]
- Sarikanat, M.; Seki, Y.; Sever, K.; Durmuşkahya, C. Determination of properties of Althaea officinalis L. (Marshmallow) fibres as a potential plant fibre in polymeric composite materials. Compos. Part B Eng. 2014, 57, 180–186. [Google Scholar] [CrossRef]
- Saravanakumar, S.; Kumaravel, A.; Nagarajan, T.; Sudhakar, P.; Baskaran, R. Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark. Carbohydr. Polym. 2013, 92, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, V. Process Optimization of Cellulose Fibril Production—The Effect of Process Medium Composition on Energy Efficiency and Product Quality. Master’s Thesis, Aalto University, Espoo, Finland, 2016. [Google Scholar]
- Ayeni, A.O.; Adeeyo, O.A.; Oresegun, O.M.; Oladimeji, T.E. Compositional analysis of lignocellulosic materials: Evaluation of an economically viable method suitable for woody and non-woody biomass. Am. J. Eng. Res. 2015, 4, 14–19. [Google Scholar]
- Beakou, A.; Ntenga, R.; Lepetit, J.; Ateba, J.A.; Ayina, L.O. Physico-chemical and microstructural characterization of “Rhectophyllum camerunense” plant fiber. Compos. Part A Appl. Sci. Manuf. 2008, 39, 67–74. [Google Scholar] [CrossRef]
- ASTM D 2654:1967; Tentative Methods of Test for Amount of Moisture in Textile Materials. ASTM: West Conshohocken, PA, USA, 1967.
- ASTM E1755-01; Standard Test Method for Ash in Biomass. ASTM: West Conshohocken, PA, USA, 2010.
- Kürschner, K.; Hoffer, A.; Jenkins, S.; Vieweg, W.; Schwarzkopf, O.; Schramek, W.; Schubert, C.; Velten, H.; Hess, K.; Trogus, C.J.Z.f.a.C. Cellulose und cellulosederivate. Z. Für Anal. Chem. 1933, 92, 145–154. [Google Scholar] [CrossRef]
- Nimz, H.J.A.C. The Chemistry of Lignin. Von IA Pearl. Edward Arnold (Publishers) Ltd., London 1967. Marcel Dekker, Inc., New York 1. Aufl., XI, 339 S., 24 Abb., geb. $15.75. Angew. Chem. 1968, 80, 328. [Google Scholar] [CrossRef]
- Lin, G.; Tang, Q.; Huang, H.; Yu, J.; Li, Z.; Ding, B. Process optimization and comprehensive utilization of recyclable deep eutectic solvent for the production of ramie cellulose fibers. Cellulose 2022, 29, 3689–3701. [Google Scholar] [CrossRef]
- Qu, Y.; Zhao, S.; Shi, Z.; Zhang, R.; Liu, L.; Ji, F.; Yu, J. High-efficiency organosolv degumming of ramie fiber by autocatalysis of high-boiling alcohols: An evaluation study of solvents. Cellulose 2020, 27, 4271–4285. [Google Scholar] [CrossRef]
- GB/T 5889-1986; Method of Quantitative Analysis of Ramie Chemical Components. Standardization Administration of the People’s Republic of China: Beijing, China, 1986.
- Huang, H.; Tang, Q.; Lin, G.; Liu, Y.; Yu, J.; Ding, B.; Li, Z. Anthraquinone-assisted deep eutectic solvent degumming of ramie fibers: Evaluation of fiber properties and degumming performance. Ind. Crops Prod. 2022, 185, 115115. [Google Scholar] [CrossRef]
- GB/T 18147.5-2015; Test Method for Hemp Fiber—Part 5: Test Method for Breaking Strength of Hemp Fiber. Standardization Administration of the People’s Republic of China: Beijing, China, 2015.
- Kabir, M.; Wang, H.; Lau, K.; Cardona, F. Effects of chemical treatments on hemp fibre structure. Appl. Surf. Sci. 2013, 276, 13–23. [Google Scholar] [CrossRef]
- Thiamngoen, P.; Pinpatthanapong, K.; Juntadech, N.C.; Rangseesuriyachai, T. Preparation of Cellulose Fiber from Cattail Using Two-Stage Pretreatment and Choline Chloride-Oxalic Acid Deep Eutectic Solvent Extraction for Oil Adsorption. Environ. Process. 2024, 11, 35. [Google Scholar] [CrossRef]
- Hossain, A.; Rahaman, M.S.; Yelle, D.; Shang, H.; Sun, Z.; Renneckar, S.; Dong, J.; Tulaphol, S.; Sathitsuksanoh, N. Effects of polyol-based deep eutectic solvents on the efficiency of rice straw enzymatic hydrolysis. Ind. Crops Prod. 2021, 167, 113480. [Google Scholar] [CrossRef]
- Tang, W.; Fan, B.; Wang, X.; Huang, C.; Tang, Z.; He, Y. Facilitating enzymatic hydrolysis efficiency of rape straw by pretreatment with a novel glycerol-based deep eutectic solvent. Ind. Crops Prod. 2023, 206, 117587. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Ren, Q.; Jiang, C.; Feng, J.; Hou, Q. Upgrading lignin macromolecular by green and recyclable ternary deep eutectic solvents. Bioresour. Technol. 2024, 394, 130230. [Google Scholar] [CrossRef] [PubMed]
- Jablonský, M.; Jančíková, V. Comparison of the acidity of systems based on choline chloride and lactic acid. J. Mol. Liq. 2023, 380, 121731. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H.; et al. Efficient cleavage of strong hydrogen bonds in cotton by deep eutectic solvents and facile fabrication of cellulose nanocrystals in high yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Yu, W.; Wang, C.; Yi, Y.; Zhou, W.; Wang, H.; Yang, Y.; Tan, Z. Choline chloride-based deep eutectic solvent systems as a pretreatment for nanofibrillation of ramie fibers. Cellulose 2019, 26, 3069–3082. [Google Scholar] [CrossRef]
- Qu, Y.; Yin, W.; Zhang, R.; Zhao, S.; Liu, L.; Yu, J. Isolation and characterization of cellulosic fibers from ramie using organosolv degumming process. Cellulose 2020, 27, 1225–1237. [Google Scholar] [CrossRef]
- Sun, S.-F.; Yang, H.-Y.; Yang, J.; Shi, Z.-J. The effect of alkaline extraction of hemicellulose on cocksfoot grass enzymatic hydrolysis recalcitrance. Ind. Crops Prod. 2022, 178, 114654. [Google Scholar] [CrossRef]
- Meng, C.; Li, Z.; Wang, C.; Yu, C. Sustained-release alkali source used in the oxidation degumming of ramie. Text. Res. J. 2017, 87, 1155–1164. [Google Scholar] [CrossRef]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- GB/T 20793-2015; Degummed Ramie. Standardization Administration of the People’s Republic of China: Beijing, China, 2015.
- Poletto, M.; Ornaghi Junior, H.L.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Gonçalves, J.V.; Suela, J.; Silva, M.V.D.; Bianchi, R.F.; Costa, C.C.D. Conductive Amazon açaí/polyaniline composite fiber: Fabrication and properties. Polímeros 2023, 33, e20230037. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.-L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.-C. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Patoary, M.K.; Zhao, Y.; Jiang, S.; Zhang, M.; Liu, L. An Eco-friendly Route to Prepare Cellulose Based Multifunctional Lyocell Fabrics Using Zinc Oxide and Cellulose Nanofibrils Network. Fibers Polym. 2022, 23, 1275–1283. [Google Scholar] [CrossRef]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Lynam, J.G.; Kumar, N.; Wong, M.J. Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour. Technol. 2017, 238, 684–689. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Efficient cleavage of lignin–carbohydrate complexes and ultrafast extraction of lignin oligomers from wood biomass by microwave-assisted treatment with deep eutectic solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef]
- Hou, X.-D.; Li, A.-L.; Lin, K.-P.; Wang, Y.-Y.; Kuang, Z.-Y.; Cao, S.-L. Insight into the structure-function relationships of deep eutectic solvents during rice straw pretreatment. Bioresour. Technol. 2018, 249, 261–267. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, A.T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Shen, X.-J.; Xue, Z.; Sun, Z.; Yuan, T.-Q. Structure–function relationships of deep eutectic solvents for lignin extraction and chemical transformation. Green Chem. 2020, 22, 7219–7232. [Google Scholar] [CrossRef]
- Credou, J.; Berthelot, T. Cellulose: From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Manimaran, P.; Solai Senthil Kumar, K.; Prithiviraj, M. Investigation of physico chemical, mechanical and thermal properties of the albizia lebbeck bark fibers. J. Nat. Fibers 2021, 18, 1151–1162. [Google Scholar] [CrossRef]
- Almeshaal, M.; Palanisamy, S.; Murugesan, T.M.; Palaniappan, M.; Santulli, C. Physico-chemical characterization of Grewia Monticola Sond (GMS) fibers for prospective application in biocomposites. J. Nat. Fibers 2022, 19, 15276–15290. [Google Scholar] [CrossRef]
- Wirawan, W.A.; Choiron, M.A.; Siswanto, E.; Widodo, T.D. Morphology, structure, and mechanical properties of new natural cellulose fiber reinforcement from waru (Hibiscus tiliaceus) bark. J. Nat. Fibers 2022, 19, 12385–12397. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M.; Rajulu, A.V. Chemical composition and structural characterization of Napier grass fibers. Mater. Lett. 2012, 67, 35–38. [Google Scholar] [CrossRef]
- Manimaran, P.; Sanjay, M.R.; Senthamaraikannan, P.; Yogesha, B.; Barile, C.; Siengchin, S. A new study on characterization of Pithecellobium dulce fiber as composite reinforcement for light-weight applications. J. Nat. Fibers 2020, 17, 359–370. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, A.; Khawar, M.T.; Wang, Z.; Tian, M.; Mushtaq, M. Maximizing Degumming Efficiency for Firmiana simplex Bark Using Deep Eutectic Solvents. Polymers 2024, 16, 2112. https://doi.org/10.3390/polym16152112
Farooq A, Khawar MT, Wang Z, Tian M, Mushtaq M. Maximizing Degumming Efficiency for Firmiana simplex Bark Using Deep Eutectic Solvents. Polymers. 2024; 16(15):2112. https://doi.org/10.3390/polym16152112
Chicago/Turabian StyleFarooq, Amjad, Muhammad Tauseef Khawar, Zongqian Wang, Mingwei Tian, and Muhammad Mushtaq. 2024. "Maximizing Degumming Efficiency for Firmiana simplex Bark Using Deep Eutectic Solvents" Polymers 16, no. 15: 2112. https://doi.org/10.3390/polym16152112





