Bio-Based Epoxy Vitrimers with Excellent Properties of Self-Healing, Recyclability, and Welding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
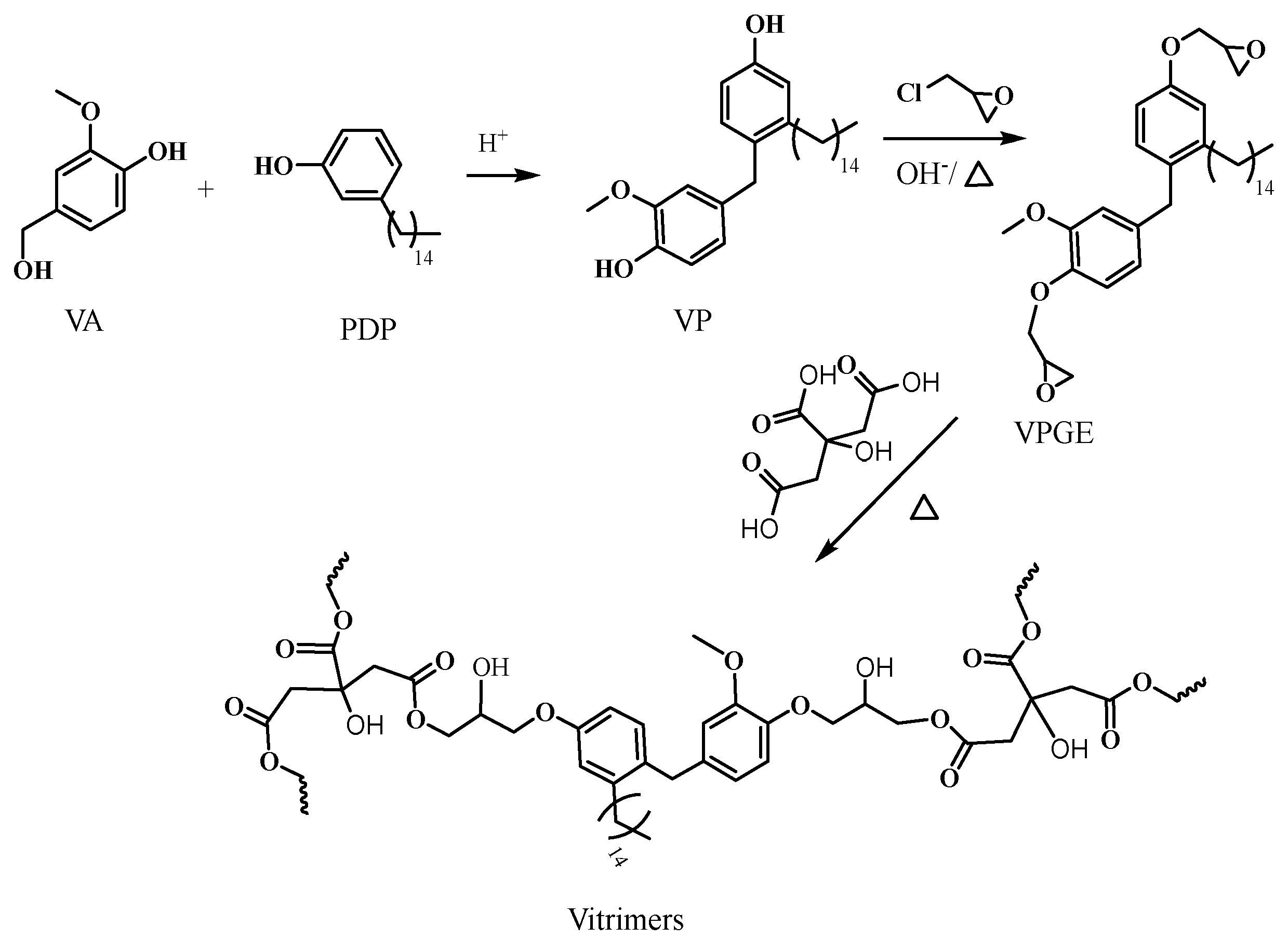
2.2. Preparation of VP
2.3. Preparation of VPGE
2.4. Preparation of VPGE−CA Vitrimers
2.5. Characterizations
3. Results and Discussion
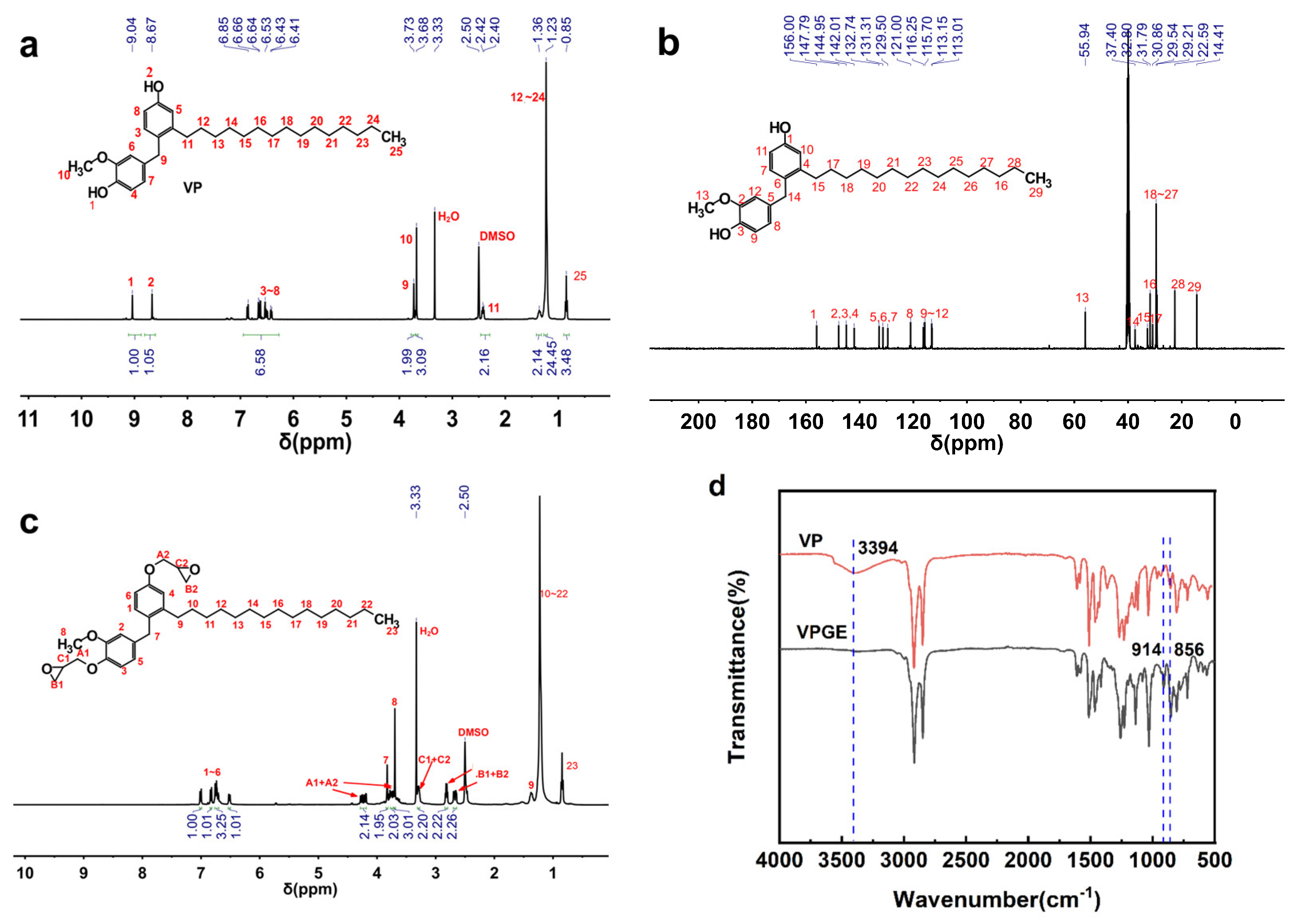
3.1. Structure Characterization of VP and VPGE
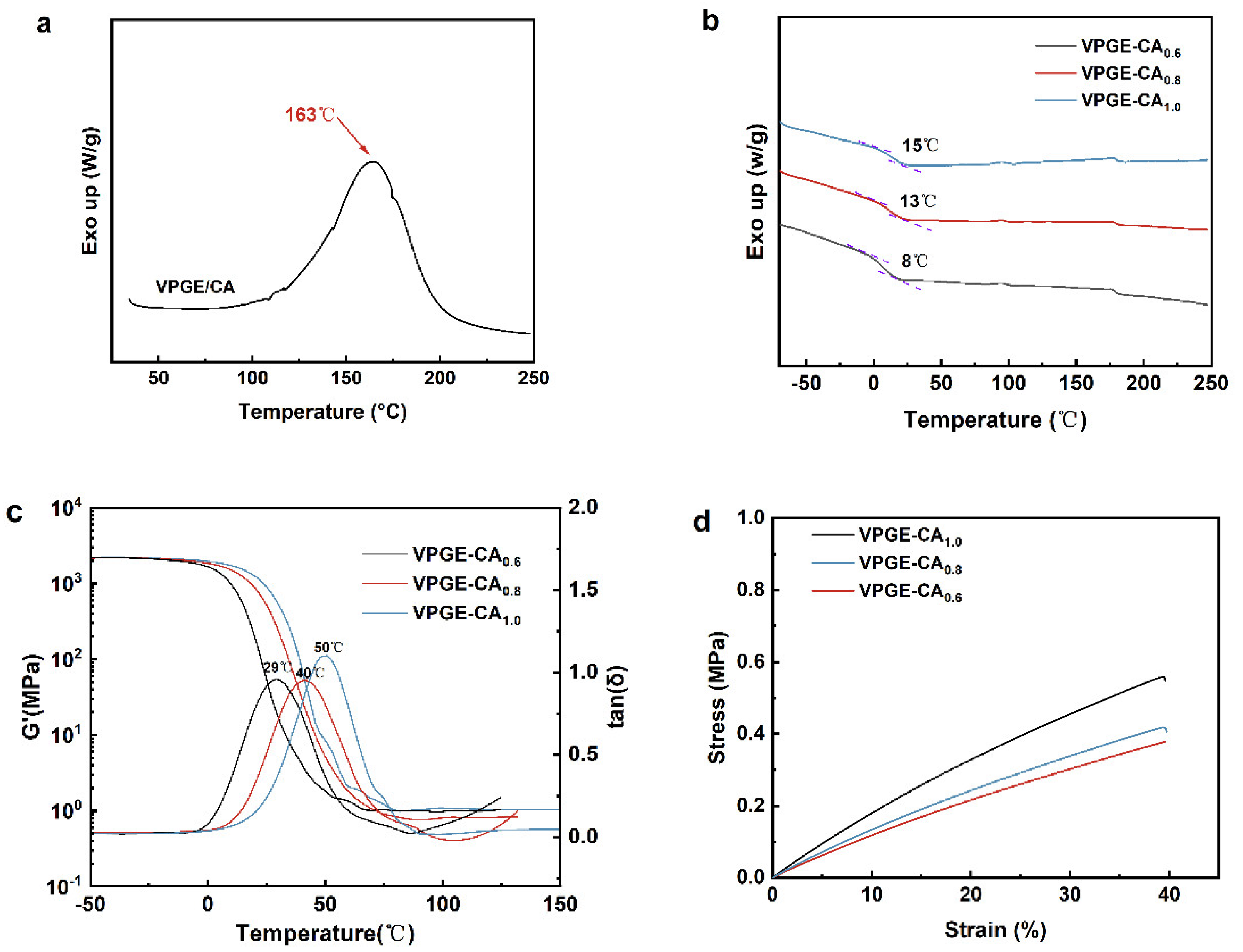
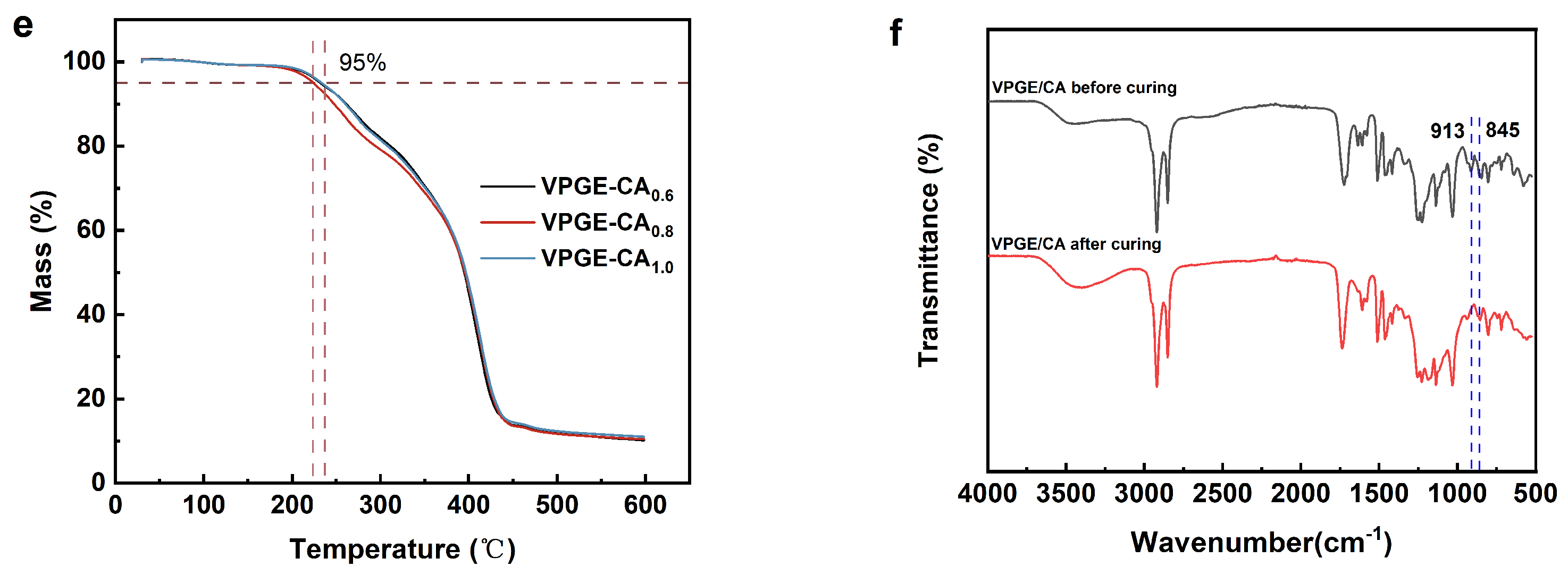
3.2. Curing and Structure Characterization of VPGE−CA Networks
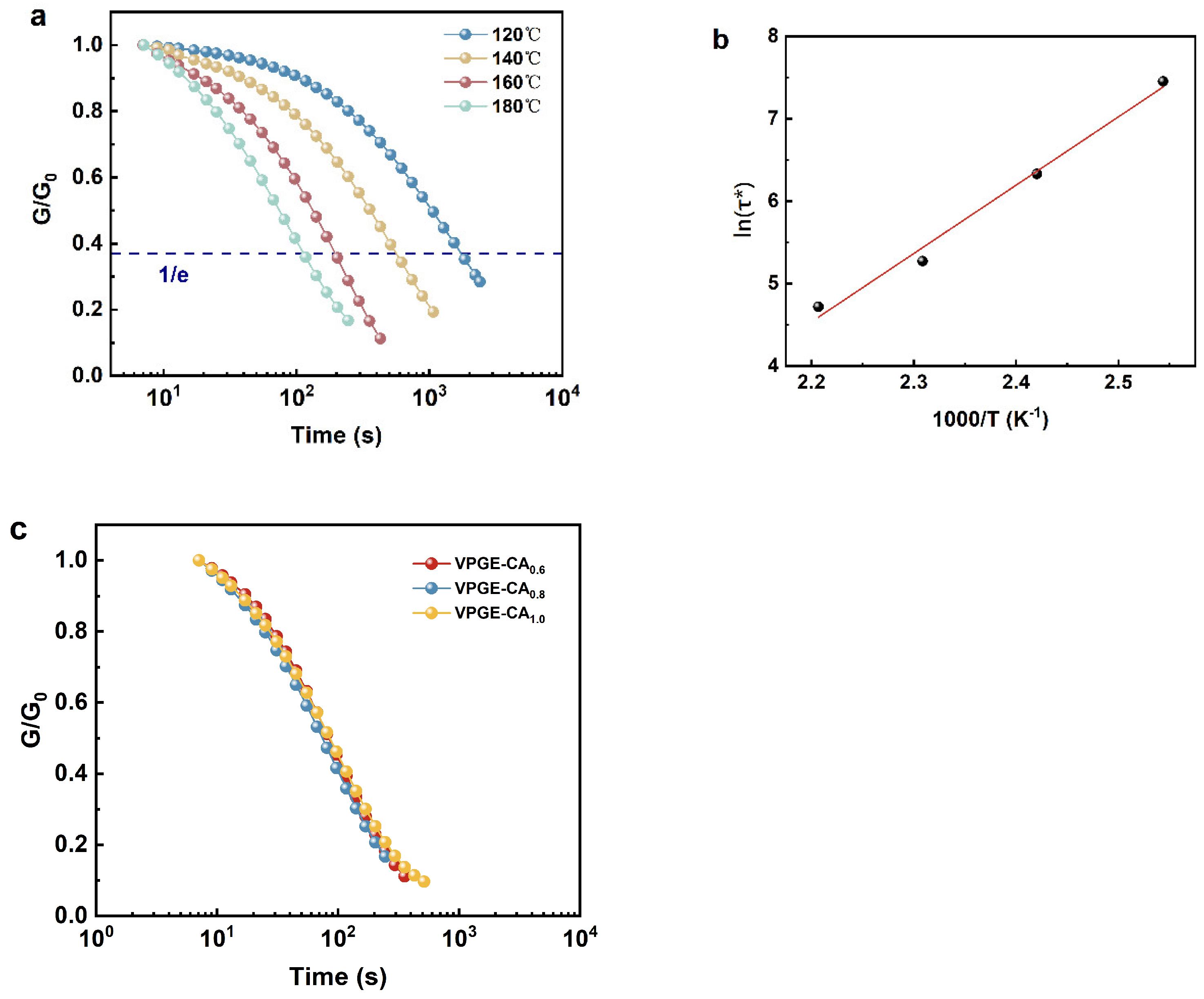
3.3. Network Rearrangement Properties
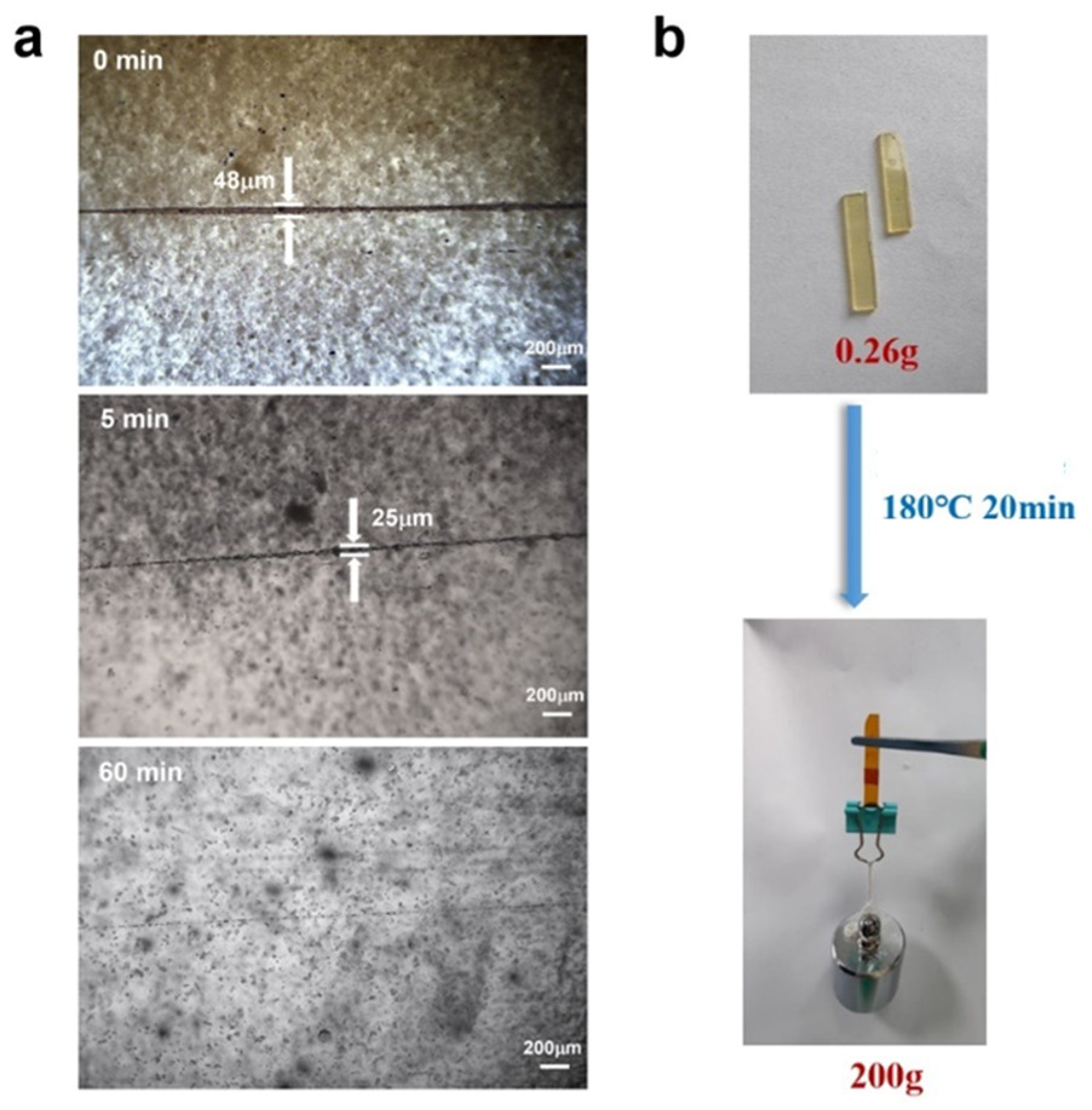
3.4. Repairing, Welding, and Shape−Changing Properties
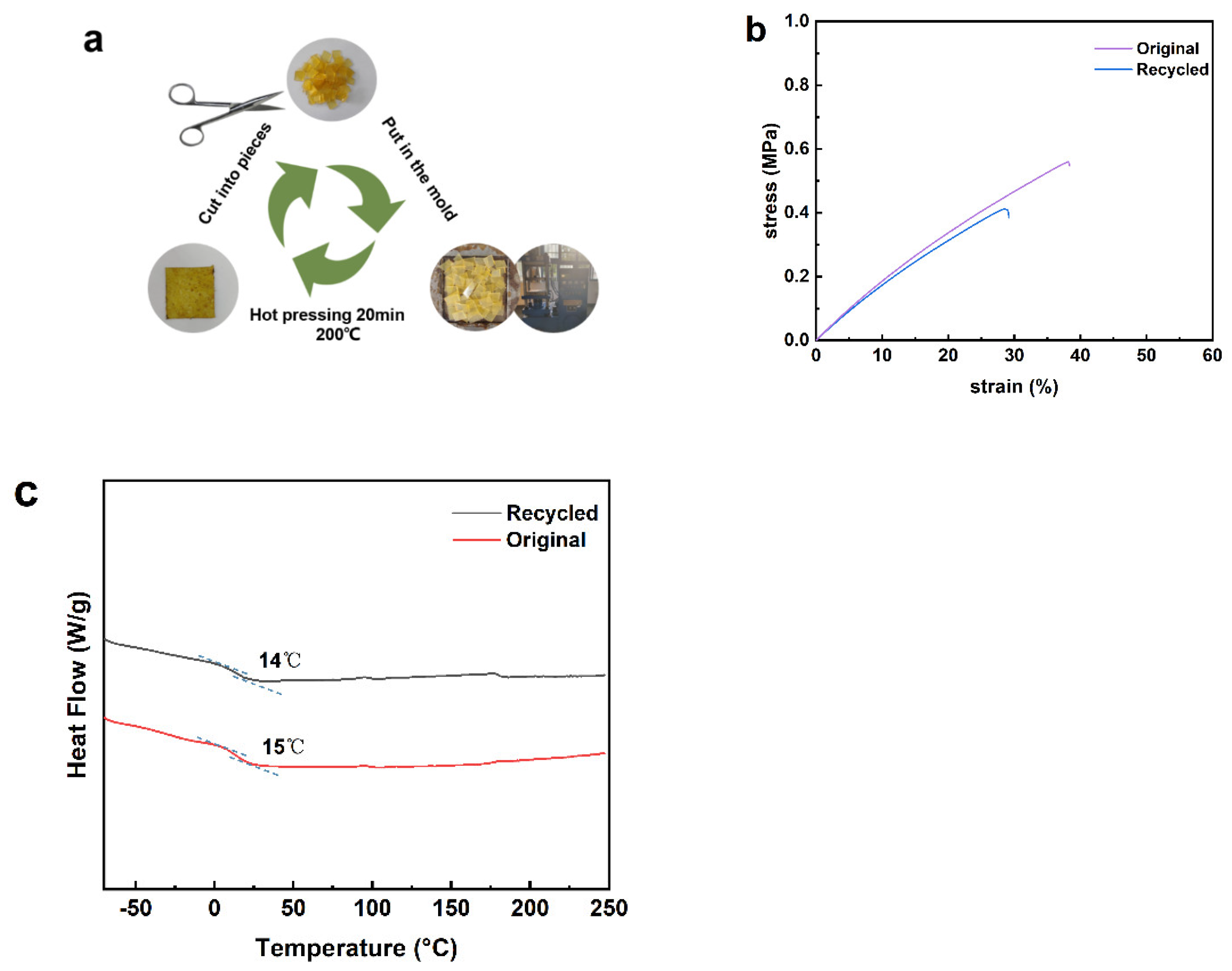
3.5. Recycling Properties
3.6. Chemical Degradability and Solvent Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Raquez, J.M.; Deléglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio) materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Yousefi, A.; Lafleur, P.G.; Gauvin, R. Kinetic studies of thermoset cure reactions: A review. Polym. Compos. 1997, 18, 157–168. [Google Scholar] [CrossRef]
- Williams, C.K.; Hillmyer, M.A. Polymers from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling technologies for thermoset composite materials-current status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, X.; Fang, Y.; Gao, K.; Yue, T.; Zhang, L.; Ganesan, V.; Meng, F.; Liu, J. Molecular dynamics simulation of the structural, mechanical, and reprocessing properties of vitrimers based on a dynamic covalent polymer network. Macromolecules 2022, 55, 1091–1103. [Google Scholar] [CrossRef]
- Rohewal, S.S.; Kanbargi, N.; Young, R.; Kearney, L.T.; Damron, J.T.; Hinton, H.; Tetard, L.; Naskar, A.K. Fast relaxing sustainable soft vitrimer with enhanced recyclability. Polym. Chem. 2024, 15, 714–724. [Google Scholar] [CrossRef]
- Guerre, M.; Taplan, C.; Winne, J.M.; Du Prez, F.E. Vitrimers: Directing chemical reactivity to control material properties. Chem. Sci. 2020, 11, 4855–4870. [Google Scholar] [CrossRef] [PubMed]
- Qiang, H.; Wang, J.; Liu, H.; Zhu, Y. From vanillin to biobased aromatic polymers. Polym. Chem. 2023, 14, 4255–4274. [Google Scholar] [CrossRef]
- Elling, B.R.; Dichtel, W.R. Reprocessable cross-linked polymer networks: Are associative exchange mechanisms desirable? ACS Cent. Sci. 2020, 6, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward stimuli-responsive dynamic thermosets through continuous development and improvements in covalent adaptable networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Zheng, L.J.; Zeng, J.B. Dynamic crosslinking: An efficient approach to fabricate epoxy vitrimer. Materials 2021, 14, 919. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wang, F.; Kui, X.; Zhang, R.; Wang, X.; Li, X.; Chen, T.; Sun, P.; Shi, A.-C. Dual cross-linked vinyl vitrimer with efficient self-catalysis achieving triple-shape-memory properties. Macromol. Rapid Commun. 2019, 40, 1900313. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, T.; Liu, X.; Zhu, J. Research progress on bio-based thermosetting resins. Polym. Int. 2016, 65, 164–173. [Google Scholar] [CrossRef]
- Fei, M.; Chang, Y.; Hao, C.; Shao, L.; Liu, W.; Zhao, B.; Zhang, J. Highly engineerable Schiff base polymer matrix with facile fiber composite manufacturability and hydrothermal recyclability. Compos. B Eng. 2023, 248, 110366. [Google Scholar] [CrossRef]
- Li, J.; Ju, B.; Zhang, S. Catalyst-free, sustainable epoxy vitrimers from epoxidized soybean oil and natural sugar alcohols. Ind. Crops Products 2023, 205, 117466. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, P.; Lamm, M.E.; Sha, Y.; Kurnaz, L.B.; Ma, Y. Plant oil-derived vitrimers-graphene composites with self-healing ability triggered by multiple stimuli. Compos. B Eng. 2023, 259, 110704. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Ma, J.; Xu, C.-A.; Liang, X.; Yuan, T.; Hu, Y.; Yang, Z. Fully bio-based thermosetting polyimine vitrimers with excellent adhesion, rapid self-healing, multi-recyclability and antibacterial ability. Ind. Crops Products 2023, 204, 117288. [Google Scholar] [CrossRef]
- Olejnik, O.; Masek, A. Recent progress in bio-based elastomers with intrinsic self-healing mechanisms-part I: Natural rubber modifications. J. Saudi Chem. Soc. 2023, 27, 101676. [Google Scholar] [CrossRef]
- Trinh, B.; Owen, P.; Vanderheide, A.; Gupta, A.; Mekonnen, T.H. Recyclable and Self-Healing Natural Rubber Vitrimers from Anhydride-Epoxy Exchangeable Covalent Bonds. ACS Appl. Polym. Mater. 2023, 5, 8890–8906. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, M.-L.; Li, Y.-D.; Li, L.-Y.; Zeng, J.-B. Fully Biobased and Mechanically Robust Elastomeric Vitrimer based on Epoxidized Natural Rubber and Dynamic Imine Bonds. ACS Sustain. Chem. Eng. 2023, 11, 17190–17198. [Google Scholar] [CrossRef]
- Salaeh, S.; Das, A.; Wiessner, S.; Stapor, M. Vitrimer-like material based on a biorenewable elastomer crosslinked with a dimeric fatty acid. Eur. Polym. J. 2021, 151, 110452. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, Y.; Wang, X.; Zhang, J.; Cheng, J.; Gao, F. A fully bio-based Schiff base vitrimer with self-healing ability at room temperature. Polym. Chem. 2023, 14, 862–871. [Google Scholar] [CrossRef]
- Yang, X.; Ke, Y.; Chen, Q.; Shen, L.; Xue, J.; Quirino, R.; Yan, Z.; Luo, Y.; Zhang, C. Efficient transformation of renewable vanillin into reprocessable, acid-degradable and flame retardant polyimide vitrimers. J. Clean. Prod. 2022, 333, 130043. [Google Scholar] [CrossRef]
- Guggari, S.; Magliozzi, F.; Malburet, S.; Graillot, A.; Destarac, M.; Guerre, M. Vanillin-based dual dynamic epoxy building block: A promising accelerator for disulfide vitrimers. Polym. Chem. 2024, 15, 1347–1357. [Google Scholar] [CrossRef]
- Altuna, F.I.; Pettarin, V.; Williams, R.J.J. Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem. 2013, 15, 3360–3366. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Design 2020, 186, 108248. [Google Scholar] [CrossRef]
- Wu, J.; Yu, X.; Zhang, H.; Guo, J.; Hu, J.; Li, M.-H. Fully biobased vitrimers from glycyrrhizic acid and soybean oil for self-healing, shape memory, weldable, and recyclable materials. ACS Sustain. Chem. Eng. 2020, 8, 6479–6487. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Catechol-mediated glycidylation toward epoxy vitrimers/polymers with tunable properties. Macromolecules 2019, 52, 3646–3654. [Google Scholar] [CrossRef]
- Bloise, E.; Carbone, L.; Colafemmina, G.; D’Accolti, L.; Mazzetto, S.E.; Vasapollo, G.; Mele, G. First example of a lipophilic porphyrin-cardanol hybrid embedded in a cardanol-based micellar nanodispersion. Molecules 2012, 17, 12252–12261. [Google Scholar] [CrossRef]
- Darroman, E.; Bonnot, L.; Auvergne, R.; Boutevin, B.; Caillol, S. New aromatic amine based on cardanol giving new biobased epoxy networks with cardanol. Eur. J. Lipid Sci. Technol. 2015, 117, 178–189. [Google Scholar] [CrossRef]
- Jaillet, F.; Darroman, E.; Ratsimihety, A.; Auvergne, R.; Boutevin, B.; Caillol, S. New biobased epoxy materials from cardanol. Eur. J. Lip. Sci. Tech. 2014, 116, 63–73. [Google Scholar] [CrossRef]
- Samanta, S.; Kim, S.; Saito, T.; Sokolov, A.P. Polymers with dynamic bonds: Adaptive functional materials for a sustainable future. J. Phys. Chem. B 2021, 125, 9389–9401. [Google Scholar] [CrossRef] [PubMed]
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104, 101233. [Google Scholar] [CrossRef]

| Sample | VPGE (g) | CA (g) | TBD (g) | R Epoxy/–COOH |
|---|---|---|---|---|
| VPGE−CA1.0 | 1.0000 | 0.2317 | 0.0252 | 1.0 |
| VPGE−CA0.8 | 1.0000 | 0.1854 | 0.0201 | 0.8 |
| VPGE−CA0.6 | 1.0000 | 0.1390 | 0.0151 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Li, S.; Gao, R.; Zhang, Y.; Wang, L.; Ye, Y.; Cao, C.; Xue, H. Bio-Based Epoxy Vitrimers with Excellent Properties of Self-Healing, Recyclability, and Welding. Polymers 2024, 16, 2113. https://doi.org/10.3390/polym16152113
Xia J, Li S, Gao R, Zhang Y, Wang L, Ye Y, Cao C, Xue H. Bio-Based Epoxy Vitrimers with Excellent Properties of Self-Healing, Recyclability, and Welding. Polymers. 2024; 16(15):2113. https://doi.org/10.3390/polym16152113
Chicago/Turabian StyleXia, Jianrong, Shuyun Li, Renjin Gao, Yuchi Zhang, Liwei Wang, Yuansong Ye, Changlin Cao, and Hanyu Xue. 2024. "Bio-Based Epoxy Vitrimers with Excellent Properties of Self-Healing, Recyclability, and Welding" Polymers 16, no. 15: 2113. https://doi.org/10.3390/polym16152113
APA StyleXia, J., Li, S., Gao, R., Zhang, Y., Wang, L., Ye, Y., Cao, C., & Xue, H. (2024). Bio-Based Epoxy Vitrimers with Excellent Properties of Self-Healing, Recyclability, and Welding. Polymers, 16(15), 2113. https://doi.org/10.3390/polym16152113





