Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review
Abstract
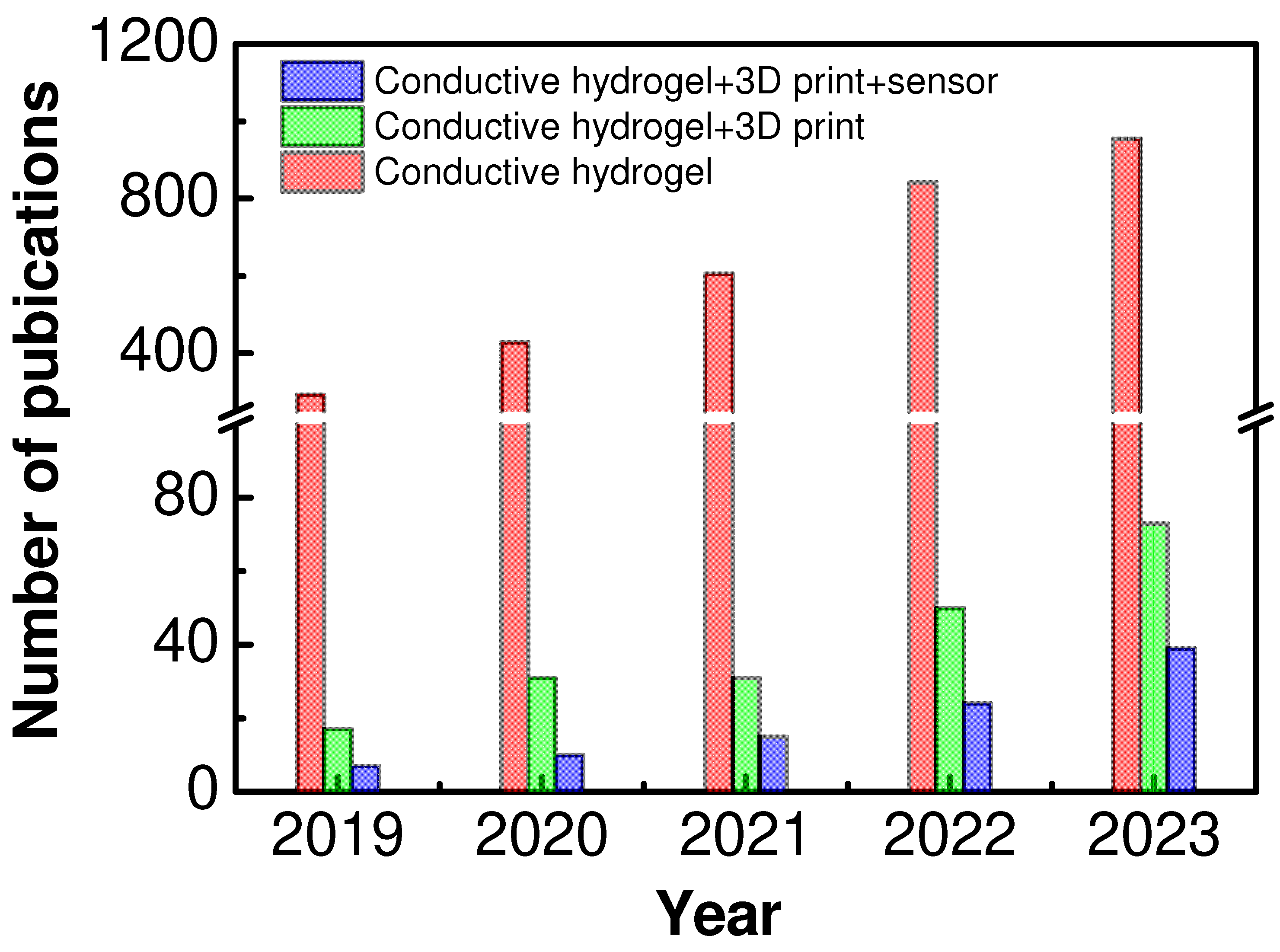
:1. Introduction
2. Conductive Hydrogels
2.1. Electronically Conductive Hydrogels (ECHs)
2.2. Ionic Conductive Hydrogels (ICHs)
2.3. Composite Conductive Hydrogels
3. 3D Printing Technology for Conductive Hydrogel Fabrication
3.1. Inkjet Printing
3.2. Direct Ink Writing (DIW)
3.3. Digital Light Processing (DLP) and Stereolithography (SLA)
3.4. Two-Photon Polymerization (TPP)
4. Application of 3D-Printed Conductive Hydrogel Sensor
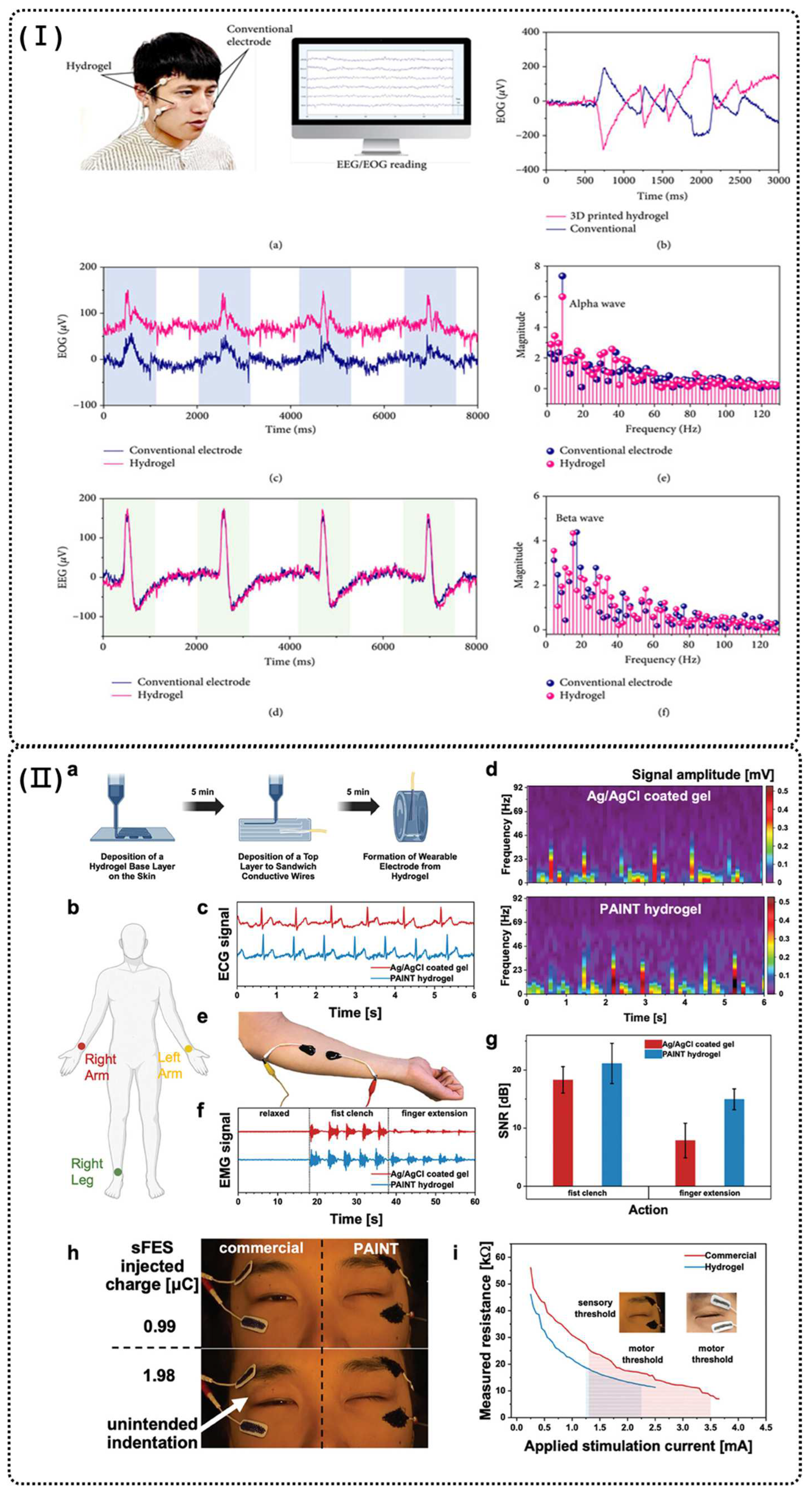
4.1. Human Motion Detection Sensors
4.2. Healthcare Detection Sensors
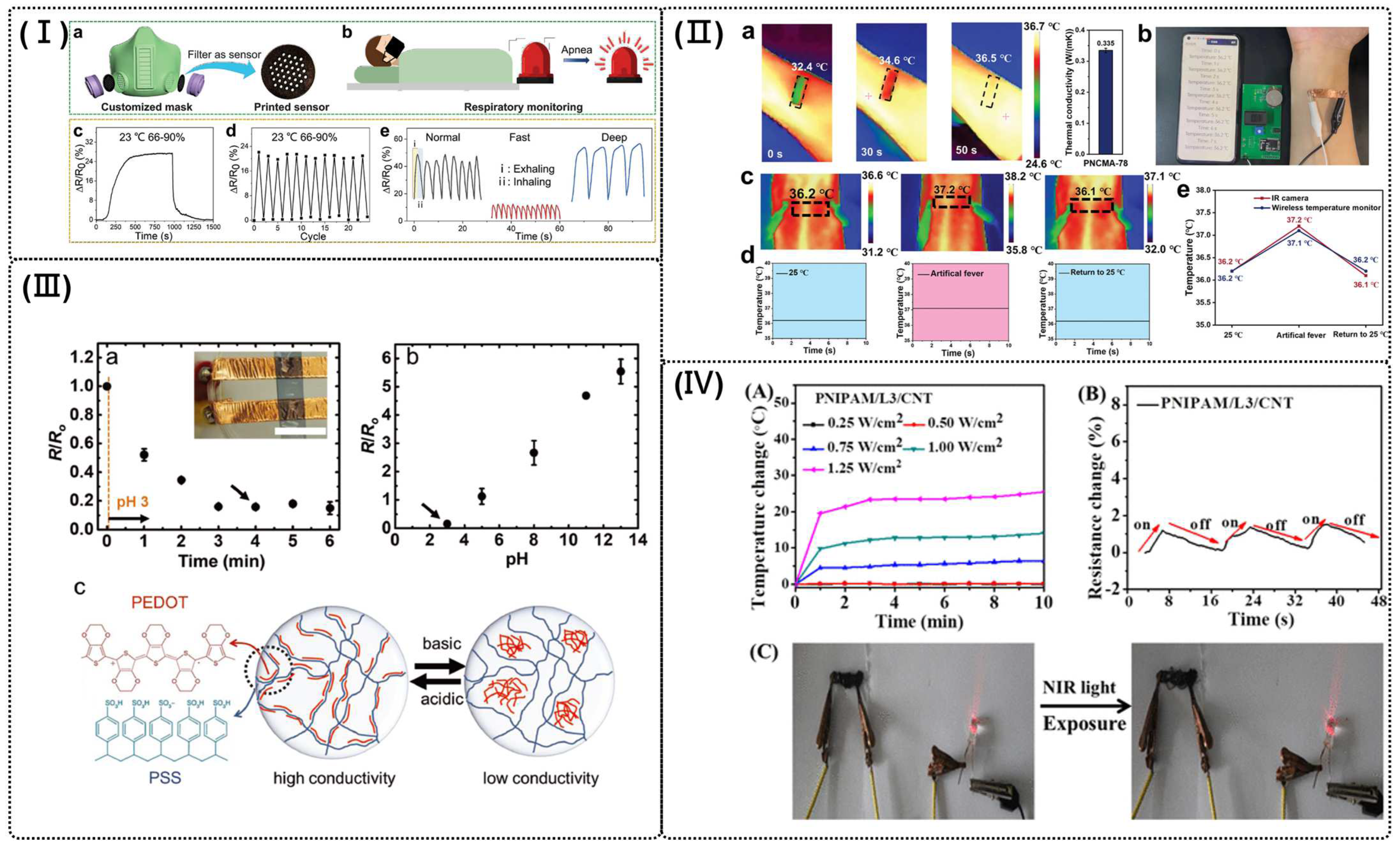
4.3. Environmental Detection Sensor
4.3.1. Humidity Sensors
4.3.2. Temperature Sensor
4.3.3. pH Sensor
4.3.4. Light Sensor
4.4. Biochemical Detection Sensors
| Hydrogels System and Component | Printing Techniques | Conductivity | Gauge Factor | Sensing Range | Application | Ref. |
|---|---|---|---|---|---|---|
| PPNGC hydrogel | N/A | 2.1 S/m | 0.78 (0–120% strain), 1.52 (120–600% strain) | 0–600% | human motions | [111] |
| PANI hybrid hydrogel | DIW | N/A | 2.2 (0–3% strain), 0.8 (40 ppm NH3), 7.3 (400 ppm NH3) | (1) stress sensing of 0–899.8 MPa, (2) strain sensing of 0–764.4% | human motions, NH3 detection, temperature detection | [108] |
| SMF/RSF/PAM composite hydrogel | DIW | 0.056 S/m | 0.9 | 0–200% | human motions | [112] |
| poly(ACMO)/pt hydrogel | DLP | 1.6 S/cm | 1.5 (0–10% strain), 7.2 (10–100% strain) | 0–100% | human motions | [74] |
| polyaniline hybrid (PHH) hydrogels | DIW | 0.04–0.21 S/m | ∼1.77 | 0–820% (the resistive strain sensors) | human motions, human–machine interfaces, physiological signal detections | [32] |
| dorsal root ganglion (DRG) cell-encapsulated gelatin methacryloyl (GelMA) hydrogels | SLA | 662.0~ 968.0 Ω/sq | N/A | N/A | healthcare applications, neural tissue regeneration | [85] |
| PPy/PEGDA hydrogel | DLP | 2 MΩcm | N/A | N/A | biosensors, drug delivery, tissue engineering | [19] |
| PEGDA/AAm LiCl/nHAp hydrogels | projection microstereolithography (PμSL) | ~0.1 S/cm | N/A | both large-scale and tiny human motions | human motions, conductor, neural interface | |
| PAINT hydrogels | DIW | 0.26~0.58 S/m | signal-to-noise ratio by 88% | N/A | healthcare applications | [2] |
| GNPs–CNTs (GC) hydrogels | multi-jet fusion (MJF) | 1.48 × 10−2 S/m | 20.1(0–5% strain), 2.3 (6–26% strain), 360.8 (26–70% strain) | 0–70% strain, 10~90% humidity | strain/humidity sensors | [115] |
| PEGDA/MWCNT-based composite hydrogel | multimaterial mask image projection-based stereolithography | 770 kΩ~17.25 MΩ | Rrel = 5.73 for 150 μm thickness to 2.17 for 450 μm thickness with 2 μL water | N/A | liquid sensing | [116] |
| PVA/agarose/ borax/liquid metal composite | three-dimensional printed molds | N/A | SNR = 4.05 | N/A | multimodular sensor system biosignal detection | [33] |
| poly (N-isopropyl acrylamide)/polyaniline hydrogels | DIW | 7.1 × 10−6 S/m | N/A | N/A | stimulus-response electronics, flexible electronics, artificial intelligence wearables | [124] |
| polyNCMA/LiTFSI gel | N/A | 3.6 × 10−5 S/m at 30 °C, 2 × 10−3 S/m at 90 °C | 8.4%/K | 30~40 °C | temperature monitor | [117] |
| PEDOT:PSS/HPU gel | extrusion printing | N/A | N/A | pH 3~11 | pH sensor | [123] |
| PEGDA–PANI electroconductive hydrogel | SLA | 2.5 × 10−2 S/m | pH 2~7 | smart biomedical sensors, pH sensor | [107] | |
| (poly(ethylene glycol)-b-poly(propylene glycol)-b-poly(ethylene glycol) (PF127))/CNTs | N/A | 0.16~0.20 S/m | N/A | N/A | wearable electronic devices, health monitoring | [38] |
| PANI-based biosensors | inkjet printing | N/A | (1) 7.4927 μA·mM−1·cm−2 of triglyceride, (2) 3.94 μA·mM−1·cm−2 of lactic acid, (3) 5.028 μA·mM−1·cm−2 of glucose | (1) 0.1–6 mM of triglyceride, (2) 0.5–1.7 mM of lactic acid, (3) 1–25 mM of glucose | biosensors for human health monitoring | [78] |
| GOx-loaded H/G4–PANI Hydrogel: | inkjet printing | N/A | N/A | H2O2 of (1–10) × 10−3 M | flexible bioelectronics | [130] |
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suneetha, M.; Sun Moo, O.; Mo Choi, S.; Zo, S.; Madhusudana Rao, K.; Soo Han, S. Tissue-adhesive, stretchable, and self-healable hydrogels based on carboxymethyl cellulose-dopamine/PEDOT:PSS via mussel-inspired chemistry for bioelectronic applications. Chem. Eng. J. 2021, 426, 130847. [Google Scholar] [CrossRef]
- Chen, J.X.M.; Chen, T.; Zhang, Y.; Fang, W.; Li, W.E.; Li, T.; Popovic, M.R.; Naguib, H.E. Conductive Bio-based Hydrogel for Wearable Electrodes via Direct Ink Writing on Skin. Adv. Funct. Mater. 2024, 2403721. [Google Scholar] [CrossRef]
- Hui, Z.; Zhang, L.; Ren, G.; Sun, G.; Yu, H.-D.; Huang, W. Green Flexible Electronics: Natural Materials, Fabrication, and Applications. Adv. Mater. 2023, 35, 2211202. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Han, W.; Lin, H.; Li, R.; Zhu, J.; Huang, W. 3D Printed Flexible Strain Sensors: From Printing to Devices and Signals. Adv. Mater. 2021, 33, 2004782. [Google Scholar] [CrossRef]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable Electrochemical Sensors for the Monitoring and Screening of Drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yao, F.; Wang, C.; Qin, Z.; Zhang, H.; Yu, Q.; Zhang, H.; Dong, X.; Wei, Y.; Li, J. Ionically Conductive Hydrogel with Fast Self-Recovery and Low Residual Strain as Strain and Pressure Sensors. Macromol. Rapid Commun. 2020, 41, 2000185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Ren, Y.; Jin, G.; Zhang, C.; Chen, W.; Yan, F. Poly(ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper. Mater. Horiz. 2020, 7, 919–927. [Google Scholar] [CrossRef]
- Sun, G.; Li, Z.; Liang, R.; Weng, L.; Zhang, L. Super stretchable hydrogel achieved by non-aggregated spherulites with diameters <5 nm. Nat. Commun. 2016, 7, 12095. [Google Scholar] [PubMed]
- Yu, J.; Wan, R.; Tian, F.; Cao, J.; Wang, W.; Liu, Q.; Yang, H.; Liu, J.; Liu, X.; Lin, T.; et al. 3D Printing of Robust High-Performance Conducting Polymer Hydrogel-Based Electrical Bioadhesive Interface for Soft Bioelectronics. Small 2024, 20, 2308778. [Google Scholar] [CrossRef]
- Zhao, S.; Tseng, P.; Grasman, J.; Wang, Y.; Li, W.; Napier, B.; Yavuz, B.; Chen, Y.; Howell, L.; Rincon, J.; et al. Programmable Hydrogel Ionic Circuits for Biologically Matched Electronic Interfaces. Adv. Mater. 2018, 30, 1800598. [Google Scholar] [CrossRef]
- He, X.-C.; Chen, X.-N.; Liu, Y.-H.; Zhong, X.; Qiang, L.; Wang, H.-Q.; Wang, F.-Z.; Wang, J.-S.; Li, C.-H.; Zheng, P.-F. A blue light 3D printable hydrogel with water absorption, antibacterial, and hemostatic properties for skin wound healing. Chem. Eng. J. 2024, 493, 152439. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, L.; Jiang, K.; Wei, Z.; Shen, G. Reviews of wearable healthcare systems: Materials, devices and system integration. Mater. Sci. Eng. R. Rep. 2020, 140, 100523. [Google Scholar] [CrossRef]
- Yang, H.; Leow, W.R.; Chen, X. 3D Printing of Flexible Electronic Devices. Small Methods 2018, 2, 1700259. [Google Scholar] [CrossRef]
- Bin, T.; Jing, L.; Wei, W. Functional Conductive Hydrogel: From Performance to Flexible Sensors Applications. Mater. Chem. Front. 2023, 7, 2925–2957. [Google Scholar]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Yin, X.-Y.; Zhang, Y.; Xiao, J.; Moorlag, C.; Yang, J. Monolithic Dual-Material 3D Printing of Ionic Skins with Long-Term Performance Stability. Adv. Funct. Mater. 2019, 29, 1904716. [Google Scholar] [CrossRef]
- Kuang, X.; Arıcan, M.O.; Zhou, T.; Zhao, X.; Zhang, Y.S. Functional Tough Hydrogels: Design, Processing, and Biomedical Applications. Acc. Mater. Res. 2023, 4, 101–114. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, S.Y.; Xiao, R.; Yin, J.; Wu, Z.L.; Zheng, Q.; Qian, J. Constitutive behaviors of tough physical hydrogels with dynamic metal-coordinated bonds. J. Mech. Phys. Solids 2020, 139, 103935. [Google Scholar] [CrossRef]
- Fantino, E.; Roppolo, I.; Zhang, D.; Xiao, J.; Chiappone, A.; Castellino, M.; Guo, Q.; Pirri, C.F.; Yang, J. 3D Printing/Interfacial Polymerization Coupling for the Fabrication of Conductive Hydrogel. Macromol. Mater. Eng. 2018, 303, 1700356. [Google Scholar] [CrossRef]
- Ding, H.; Liang, X.; Wang, Q.; Wang, M.; Li, Z.; Sun, G. A semi-interpenetrating network ionic composite hydrogel with low modulus, fast self-recoverability and high conductivity as flexible sensor. Carbohydr. Polym. 2020, 248, 116797. [Google Scholar] [CrossRef]
- Ding, H.; Liang, X.; Xu, J.; Tang, Z.; Li, Z.; Liang, R.; Sun, G. Hydrolyzed Hydrogels with Super Stretchability, High Strength, and Fast Self-Recovery for Flexible Sensors. ACS Appl. Mater. Interfaces 2021, 13, 22774–22784. [Google Scholar] [CrossRef]
- Ding, H.; Liu, J.; Shen, X.; Li, H. Advances in the Preparation of Tough Conductive Hydrogels for Flexible Sensors. Polymers 2023, 15, 4001. [Google Scholar] [CrossRef]
- Xie, X.; Xu, Z.; Yu, X.; Jiang, H.; Li, H.; Feng, W. Liquid-in-liquid printing of 3D and mechanically tunable conductive hydrogels. Nat. Commun. 2023, 14, 4289. [Google Scholar] [CrossRef]
- Yu, J.; Tian, F.; Wang, W.; Wan, R.; Cao, J.; Chen, C.; Zhao, D.; Liu, J.; Zhong, J.; Wang, F.; et al. Design of Highly Conductive, Intrinsically Stretchable, and 3D Printable PEDOT:PSS Hydrogels via PSS-Chain Engineering for Bioelectronics. Chem. Mater. 2023, 35, 5936–5944. [Google Scholar] [CrossRef]
- Arwani, R.T.; Tan, S.C.L.; Sundarapandi, A.; Goh, W.P.; Liu, Y.; Leong, F.Y.; Yang, W.; Zheng, X.T.; Yu, Y.; Jiang, C.; et al. Stretchable ionic–electronic bilayer hydrogel electronics enable in situ detection of solid-state epidermal biomarkers. Nat. Mater. 2024, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Xu, B.; Tao, X.; Hu, H. 3D Flexible, Conductive and Wearable Fabric Strain Sensors. Int. J. Mater. Mech. Eng. 2015, 4, 10–13. [Google Scholar] [CrossRef]
- Huang, H.; Cong, H.-T.; Lin, Z.; Liao, L.; Shuai, C.-X.; Qu, N.; Luo, Y.; Guo, S.; Xu, Q.-C.; Bai, H.; et al. Manipulation of Conducting Polymer Hydrogels with Different Shapes and Related Multifunctionality. Small 2024, 20, 2309575. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D Printing of Multifunctional Hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Tian, K.; Bae, J.; Bakarich, S.E.; Yang, C.; Gately, R.D.; Spinks, G.M.; in het Panhuis, M.; Suo, Z.; Vlassak, J.J. 3D Printing of Transparent and Conductive Heterogeneous Hydrogel-Elastomer Systems. Adv. Mater. 2017, 29, 1604827. [Google Scholar] [CrossRef]
- Tan, H.W.; Choong, Y.Y.C.; Kuo, C.N.; Low, H.Y.; Chua, C.K. 3D printed electronics: Processes, materials and future trends. Prog. Mater. Sci. 2022, 127, 100945. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.-Q.; Lin, B.; Liu, M.-C.; Zhang, Y.; Liu, S.-J.; Li, Y.; Zhao, Q. Two-photon polymerization-based 3D micro-scaffolds toward biomedical devices. Chem. Eng. J. 2024, 493, 152469. [Google Scholar] [CrossRef]
- Meichen, Y.; Yufeng, W.; Hele, G.; Chao, Z.; Tianxi, X.L. 3D reactive printing of polyaniline hybrid hydrogel microlattices with large stretchability and high fatigue resistance for wearable pressure sensors. Compos. Sci. Technol. 2022, 220, 109263. [Google Scholar]
- Choi, Y.Y.; Ho, D.H.; Cho, J.H. Self-Healable Hydrogel–Liquid Metal Composite Platform Enabled by a 3D Printed Stamp for a Multimodular Sensor System. ACS Appl. Mater. Interfaces 2020, 12, 9824–9832. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Hao, F.; Maimaitiyiming, X.; Sun, S. 3D Printed Multifunctional Self-Adhesive and Conductive Polyacrylamide/Chitosan/Sodium Carboxymethyl Cellulose/CNT Hydrogels as Flexible Sensors. Macromol. Chem. Phys. 2023, 224, 2200272. [Google Scholar] [CrossRef]
- Kong, D.; Li, Y.; Yang, B.; Pang, Y.; Yuan, H.; Du, C.; Tan, Y. 3D-Printed Hydrogels with High-Strength and Anisotropy Mediated by Chain Rigidity. Small 2024, 2403052. [Google Scholar] [CrossRef]
- Dutta, S.D.; Ganguly, K.; Randhawa, A.; Patil, T.V.; Kim, H.; Acharya, R.; Lim, K.-T. 3D Printable Hydrogel Bioelectronic Interfaces for Healthcare Monitoring and Disease Diagnosis: Materials, Design Strategies, and Applications. Adv. Mater. Technol. 2024, 9, 2301874. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-Responsive Conductive Nanocomposite Hydrogels with High Stretchability, Self-Healing, Adhesiveness, and 3D Printability for Human Motion Sensing. ACS Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Li, Y.; Wang, H.; Zhang, Y.; Guo, B.; Yu, J.; Wang, Y. 3D Printed Silk Fibroin-Based Hydrogels with Tunable Adhesion and Stretchability for Wearable Sensing. Adv. Funct. Mater. 2024, 2404451. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, Y.; Xu, J.; Qian, R.; Yu, Z.; Ye, C. Stretchable, adhesive and self-healing conductive hydrogels based on PEDOT:PSS-stabilized liquid metals for human motion detection. Chem. Eng. J. 2024, 494, 152971. [Google Scholar] [CrossRef]
- Cui, L.; Wang, W.; Zheng, J.; Hu, C.; Zhu, Z.; Liu, B. Wide-humidity, anti-freezing and stretchable multifunctional conductive carboxymethyl cellulose-based hydrogels for flexible wearable strain sensors and arrays. Carbohydr. Polym. 2024, 342, 122406. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.W.; Portillo Lara, R.; Mogadam, E.; Hsiang Yu, C.; Kimball, W.; Annabi, N. Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, H.; Wang, Y.; Fan, X.; Li, Z.; Zhang, X.; Liu, T. Highly Stretchable, Ultra-Soft, and Fast Self-Healable Conductive Hydrogels Based on Polyaniline Nanoparticles for Sensitive Flexible Sensors. Adv. Funct. Mater. 2022, 32, 2204366. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, H.; Zhao, Y.; Huyan, C.; Wang, Z.; Torun, H.; Guo, Z.; Dai, S.; Xu, B.B.; Chen, F. A Strand Entangled Supramolecular PANI/PAA Hydrogel Enabled Ultra-Stretchable Strain Sensor. Small 2022, 18, 2203258. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, N.; Yu, X.; Guo, Z.; Liu, Z.; Sun, X.; Li, M.-H.; Hu, J. Natural glycyrrhizic acid-tailored hydrogel with in-situ gradient reduction of AgNPs layer as high-performance, multi-functional, sustainable flexible sensors. Chem. Eng. J. 2022, 430, 132779. [Google Scholar] [CrossRef]
- Peng, Y.; Pi, M.; Zhang, X.; Yan, B.; Li, Y.; Shi, L.; Ran, R. High strength, antifreeze, and moisturizing conductive hydrogel for human-motion detection. Polymer 2020, 196, 122469. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Xu, S.; Song, H. Stretchable ionic conductive gels for wearable human-activity detection. Chem. Eng. J. 2024, 489, 151231. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Li, G.; Li, C.; Li, G.; Yu, D.; Song, Z.; Wang, H.; Liu, X.; Liu, H.; Liu, W. Development of Conductive Hydrogels for Fabricating Flexible Strain Sensors. Small 2022, 18, 2101518. [Google Scholar] [CrossRef]
- Han, D.; Wang, G.; Xu, X.; Chen, J.; Lu, M.; Liu, X.; Zhang, L.; Lai, L. Ultra-stretchable, self-healing, bonding, and skin-inspired conductive triple network hydrogel for wearable strain sensors and friction nanogenerators. Polymer 2024, 305, 127169. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, X.; Tian, J.; Xu, L.; Li, Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J. Colloid Interface Sci. 2022, 606, 192–203. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Zhang, Y.; Zheng, J.; Wu, H.; Chen, Y.; Xu, S.; Yang, J.; Liu, C.; Zhang, Y. Dual Cross-Linked Ion-Based Temperature-Responsive Conductive Hydrogels with Multiple Sensors and Steady Electrocardiogram Monitoring. Chem. Mater. 2020, 32, 7670–7678. [Google Scholar] [CrossRef]
- Zhou, L.; Pei, X.; Fang, K.; Zhang, R.; Fu, J. Super tough, ultra-stretchable, and fast recoverable double network hydrogels physically crosslinked by triple non-covalent interactions. Polymer 2020, 192, 122319. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Chen, H.; Ren, X.; Gao, G. Skin-Inspired Gels with Toughness, Antifreezing, Conductivity, and Remoldability. ACS Appl. Mater. Interfaces 2019, 11, 28336–28344. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, Y.; Yang, F.; Liu, Y.; Wang, X.; Yang, J.; Gong, X.; Zheng, J. Highly stretchable, self-adhesive, biocompatible, conductive hydrogels as fully polymeric strain sensors. J. Mater. Chem. A 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Han, X.; Fan, Z.; Zhang, H.; Li, Q. High-strength and highly electrically conductive hydrogels for wearable strain sensor. Chem. Phys. Lett. 2021, 769, 138437. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Cao, W.-T.; Ma, M.-G.; Wan, P. Ultrasensitive Wearable Soft Strain Sensors of Conductive, Self-healing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, Y.; Ma, Z.; Bai, Y.; Wang, J.; Zeng, P.; Gong, P.; Shi, F.; Ji, Z.; Qiao, Y.; et al. Highly Sensitive Strain Sensor Based on a Stretchable and Conductive Poly(vinyl alcohol)/Phytic Acid/NH2-POSS Hydrogel with a 3D Microporous Structure. ACS Appl. Mater. Interfaces 2020, 12, 26496–26508. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, J.; Xiang, K.; Hu, W.; Sun, J.; Zhang, H.; Wang, L. Highly Stretchable, Ultra-Sensitive, and Self-Healable Multifunctional Flexible Conductive Hydrogel Sensor for Motion Detection and Information Transmission. ACS Appl. Mater. Interfaces 2023, 15, 29499–29510. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, M.; Sun, Y.; Zuo, B. Self-Healing, Wet-Adhesion silk fibroin conductive hydrogel as a wearable strain sensor for underwater applications. Chem. Eng. J. 2022, 446, 136931. [Google Scholar] [CrossRef]
- Hossein, O. High-Performing Conductive Hydrogels for Wearable Applications. Gels 2023, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Li, H.; Huo, P.; Teng, P.; Ding, H.; Shen, X. Recent progress in fabrications, properties and applications of multifunctional conductive hydrogels. Eur. Polym. J. 2024, 208, 112895. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Cao, D.; Zhang, G.; Li, J.; Li, K.; Yang, Y.; Wang, W.; Jin, Y.; Sun, R.; et al. Recent Advancements in Flexible and Stretchable Electrodes for Electromechanical Sensors: Strategies, Materials, and Features. ACS Appl. Mater. Interfaces 2017, 9, 12147. [Google Scholar] [CrossRef]
- Yan, Y.; Han, M.; Jiang, Y.; Ng, E.L.L.; Zhang, Y.; Owh, C.; Song, Q.; Li, P.; Loh, X.J.; Chan, B.Q.Y.; et al. Electrically Conductive Polymers for Additive Manufacturing. ACS Appl. Mater. Interfaces 2024, 16, 5337–5354. [Google Scholar] [CrossRef]
- Yin, X.-Y.; Zhang, Y.; Cai, X.; Guo, Q.; Yang, J.; Wang, Z.L. 3D printing of ionic conductors for high-sensitivity wearable sensors. Mater. Horiz. 2019, 6, 767–780. [Google Scholar] [CrossRef]
- Lai, C.-W.; Yu, S.-S. 3D Printable Strain Sensors from Deep Eutectic Solvents and Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 34235–34244. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, X.; Zheng, Y.; Chen, K.; Zeng, W.; Wu, X. Recent advances in the 3D printing of electrically conductive hydrogels for flexible electronics. J. Mater. Chem. C 2022, 10, 5380–5399. [Google Scholar] [CrossRef]
- Guo, B.; Zhong, Y.; Chen, X.; Yu, S.; Bai, J. 3D printing of electrically conductive and degradable hydrogel for epidermal strain sensor. Compos. Commun. 2023, 37, 101454. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors—A review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.S.; Wang, Y. 3D printing of conjugated polymers. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1592–1605. [Google Scholar] [CrossRef]
- Kalkal, A.; Kumar, S.; Kumar, P.; Pradhan, R.; Willander, M.; Packirisamy, G.; Kumar, S.; Malhotra, B.D. Recent advances in 3D printing technologies for wearable (bio)sensors. Addit. Manuf. 2021, 46, 102088. [Google Scholar] [CrossRef]
- Li, L.; Pan, L.; Ma, Z.; Yan, K.; Cheng, W.; Shi, Y.; Yu, G. All Inkjet-Printed Amperometric Multiplexed Biosensors Based on Nanostructured Conductive Hydrogel Electrodes. Nano Lett. 2018, 18, 3322–3327. [Google Scholar] [CrossRef]
- Teo, M.Y.; RaviChandran, N.; Kim, N.; Kee, S.; Stuart, L.; Aw, K.C.; Stringer, J. Direct Patterning of Highly Conductive PEDOT:PSS/Ionic Liquid Hydrogel via Microreactive Inkjet Printing. ACS Appl. Mater. Interfaces 2019, 11, 37069–37076. [Google Scholar] [CrossRef]
- Pataky, K.; Braschler, T.; Negro, A.; Renaud, P.; Lutolf, M.P.; Brugger, J. Microdrop printing of hydrogel bioinks into 3D tissue-like geometries. Adv. Mater. 2012, 24, 391. [Google Scholar] [CrossRef]
- Aggas, J.R.; Abasi, S.; Phipps, J.F.; Podstawczyk, D.A.; Guiseppi-Elie, A. Microfabricated and 3-D printed electroconductive hydrogels of PEDOT:PSS and their application in bioelectronics. Biosens. Bioelectron. 2020, 168, 112568. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Qu, G.; Luo, G.; Huang, Y.; Zhang, H.; Zhou, X.; Wang, L.; Liu, Z.; Kong, T. Scalable and Automated Fabrication of Conductive Tough-Hydrogel Microfibers with Ultrastretchability, 3D Printability, and Stress Sensitivity. ACS Appl. Mater. Interfaces 2018, 10, 11204–11212. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jia, R.; Gan, Z.; Du, Y.; Wang, D.; Xu, X. Tough and conductive polymer hydrogel based on double network for photo-curing 3D printing. Mater. Res. Express 2020, 7, 055304. [Google Scholar] [CrossRef]
- He, Y.; Yu, R.; Li, X.; Zhang, M.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. Digital Light Processing 4D Printing of Transparent, Strong, Highly Conductive Hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 36286–36294. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Lee, S.-J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Lichade, K.M.; Shiravi, S.; Finan, J.D.; Pan, Y. Direct printing of conductive hydrogels using two-photon polymerization. Addit. Manuf. 2024, 84, 104123. [Google Scholar] [CrossRef]
- Sanjuan-Alberte, P.; Vaithilingam, J.; Moore, J.C.; Wildman, R.D.; Tuck, C.J.; Alexander, M.R.; Hague, R.J.M.; Rawson, F.J. Development of Conductive Gelatine-Methacrylate Inks for Two-Photon Polymerisation. Polymers 2021, 13, 1038. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Yan, M.; Hu, G.; Li, Z.; He, W.; Wang, X.; Abulimit, A.; Li, R. Research progress on the application of inkjet printing technology combined with hydrogels. Appl. Mater. Today 2024, 36, 102036. [Google Scholar] [CrossRef]
- Kong, X.; Dong, M.; Du, M.; Qian, J.; Yin, J.; Zheng, Q.; Wu, Z.L. Recent Progress in 3D Printing of Polymer Materials as Soft Actuators and Robots. Chem. Bio. Eng. 2024, 1, 312–329. [Google Scholar] [CrossRef]
- Cheng, Y.; Chan, K.H.; Wang, X.-Q.; Ding, T.; Li, T.; Lu, X.; Ho, G.W. Direct-Ink-Write 3D Printing of Hydrogels into Biomimetic Soft Robots. ACS Nano 2019, 13, 13176–13184. [Google Scholar] [CrossRef]
- Guo, S.-Z.; Qiu, K.; Meng, F.; Park, S.H.; McAlpine, M.C. 3D Printed Stretchable Tactile Sensors. Adv. Mater. 2017, 29, 1701218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Q.; Wu, P. A multifunctional skin-like sensor based on a 3D printed thermo-responsive hydrogel. Mater. Horiz. 2017, 4, 694–700. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Panhuis, M.I.H.; Beirne, S.; Wallace, G.G.; Spinks, G.M. Extrusion printing of ionic–covalent entanglement hydrogels with high toughness. J. Mater. Chem. B 2013, 1, 4939–4946. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Li, X.; Guan, S.; Chen, S.; Liu, S.; Cui, E.; Yu, Y.; Pan, W.; Tang, N.; et al. Design strategies for environmentally friendly polyvinyl alcohol hydrogel sensors: Research progress and Perspectives. Mater. Today Commun. 2024, 39, 109401. [Google Scholar] [CrossRef]
- Peng, S.; Guo, Q.; Thirunavukkarasu, N.; Zheng, Y.; Wang, Z.; Zheng, L.; Wu, L.; Weng, Z. Tailoring of photocurable ionogel toward high resilience and low hysteresis 3D printed versatile porous flexible sensor. Chem. Eng. J. 2022, 439, 135593. [Google Scholar] [CrossRef]
- Pang, Y.; Xu, X.; Chen, S.; Fang, Y.; Shi, X.; Deng, Y.; Wang, Z.-L.; Cao, C. Skin-inspired textile-based tactile sensors enable multifunctional sensing of wearables and soft robots. Nano Energy 2022, 96, 107137. [Google Scholar] [CrossRef]
- Robert, S.J.; Jacob, F.; Victor, H.; Isabel, P.; Adrian, G.; Nastaran, A.; Miguel, F.-M.; Kiana, S.; Bohao, X.; Ian, M.H.; et al. 3D printed architected conducting polymer hydrogels. J. Mater. Chem. B 2021, 9, 7258–7270. [Google Scholar]
- Mu, Q.; Wang, L.; Dunn, C.K.; Kuang, X.; Duan, F.; Zhang, Z.; Qi, H.J.; Wang, T. Digital light processing 3D printing of conductive complex structures. Addit. Manuf. 2017, 18, 74–83. [Google Scholar] [CrossRef]
- Ennis, A.; Nicdao, D.; Kolagatla, S.; Dowling, L.; Tskhe, Y.; Thompson, A.J.; Trimble, D.; Delaney, C.; Florea, L. Two-Photon Polymerization of Sugar Responsive 4D Microstructures. Adv. Funct. Mater. 2023, 33, 2213947. [Google Scholar] [CrossRef]
- Torgersen, J.; Qin, X.-H.; Li, Z.; Ovsianikov, A.; Liska, R.; Stampfl, J. Hydrogels for Two-Photon Polymerization: A Toolbox for Mimicking the Extracellular Matrix. Adv. Funct. Mater. 2013, 23, 4542–4554. [Google Scholar] [CrossRef]
- Jaiswal, A.; Rastogi, C.K.; Rani, S.; Singh, G.P.; Saxena, S.; Shukla, S. Two decades of two-photon lithography: Materials science perspective for additive manufacturing of 2D/3D nano-microstructures. Iscience 2023, 26, 106374. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D Printed Bionic Ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally sourced hydrogels: Emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, P.; Cheng, W.; Yan, K.; Pan, L.; Shi, Y.; Yu, G. Highly Sensitive, Printable Nanostructured Conductive Polymer Wireless Sensor for Food Spoilage Detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar] [CrossRef] [PubMed]
- Zhaolong, W.; Chen, L.; Yiqin, C.; Peng, L.; Huigao, D.; Ping, C. 3D Printed Ultrastretchable, Hyper-Antifreezing Conductive Hydrogel for Sensitive Motion and Electrophysiological Signal Monitoring. Research 2020, 2020, 1426078. [Google Scholar]
- Carcione, R.; Pescosolido, F.; Montaina, L.; Toschi, F.; Orlanducci, S.; Tamburri, E.; Battistoni, S. Self-Standing 3D-Printed PEGDA–PANIs Electroconductive Hydrogel Composites for pH Monitoring. Gels 2023, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hao, F.; Maimaitiyiming, X. 3D Print Polyaniline/Gelatin Hydrogels as Wearable Multifunctional Sensors. ChemistrySelect 2022, 7, e202203286. [Google Scholar] [CrossRef]
- Kang, B.; Yan, X.; Zhao, Z.; Song, S. Dual-Sensing, Stretchable, Fatigue-Resistant, Adhesive, and Conductive Hydrogels Used as Flexible Sensors for Human Motion Monitoring. Langmuir 2022, 38, 7013–7023. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, W.; Chen, D.; Yu, D.; Song, Z.; Wang, H.; Li, G.; Liu, X.; Ge, S. Hydrophobic association-improved multi-functional hydrogels with liquid metal droplets stabilized by xanthan gum and PEDOT:PSS for strain sensors. Int. J. Biol. Macromol. 2024, 271, 132494. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, Y.; Wang, Q.; Zhu, T.; Gao, Y.; Gao, G.; Yan, L.; Chen, K. Biocompatible and 3D-printable conductive hydrogels driven by sodium carboxymethyl cellulose for wearable strain sensors. Polymer 2024, 295, 126763. [Google Scholar] [CrossRef]
- Niu, Q.; Huang, L.; Fan, S.; Yao, X.; Zhang, Y. 3D Printing Silk Fibroin/Polyacrylamide Triple-Network Composite Hydrogels with Stretchability, Conductivity, and Strain-Sensing Ability as Bionic Electronic Skins. ACS Biomater. Sci. Eng. 2024, 10, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ding, Q.; Li, Z.; Zhou, Z.; Luo, L.; Tao, K.; Xie, X.; Wu, J. Ultrasensitive, stretchable, and transparent humidity sensor based on ion-conductive double-network hydrogel thin films. Sci. China Mater. 2022, 65, 2540–2552. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, S.S.; Tran, T.S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.K.; Roy Choudhury, N. 3D Printable Electrically Conductive Hydrogel Scaffolds for Biomedical Applications: A Review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Gao, M.; Gao, J.; Zhao, L.; Teo, E.H.T.; Wang, D.; Qi, H.J.; Zhou, K. 3D Printed Conformal Strain and Humidity Sensors for Human Motion Prediction and Health Monitoring via Machine Learning. Adv. Sci. 2023, 10, 2304132. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Xie, B.; Chu, M.; Sun, H.; Hao, S.; Chen, Y.; Chen, Y. 3D Printing of Flexible Liquid Sensor Based on Swelling Behavior of Hydrogel with Carbon Nanotubes. Adv. Mater. Technol. 2019, 4, 1800476. [Google Scholar] [CrossRef]
- Yao, P.; Bao, Q.; Yao, Y.; Xiao, M.; Xu, Z.; Yang, J.; Liu, W. Environmentally Stable, Robust, Adhesive, and Conductive Supramolecular Deep Eutectic Gels as Ultrasensitive Flexible Temperature Sensor. Adv. Mater. 2023, 35, 2300114. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Sun, H.; Xu, Y.; Wang, X.; Luo, H.; Liu, Z.; Zhang, T. Porous Ionic Membrane Based Flexible Humidity Sensor and its Multifunctional Applications. Adv. Sci. 2017, 4, 1600404. [Google Scholar] [CrossRef]
- Morais, R.M.; Klem, M.d.S.; Nogueira, G.L.; Gomes, T.C.; Alves, N. Low Cost Humidity Sensor Based on PANI/PEDOT:PSS Printed on Paper. IEEE Sens. J. 2018, 18, 2647–2651. [Google Scholar] [CrossRef]
- Gao, N.; Yu, J.; Tian, Q.; Shi, J.; Zhang, M.; Chen, S.; Zang, L. Application of PEDOT:PSS and Its Composites in Electrochemical and Electronic Chemosensors. Chemosensors 2021, 9, 79. [Google Scholar] [CrossRef]
- Bian, Z.; Li, Y.; Sun, H.; Shi, M.; Zheng, Y.; Liu, H.; Liu, C.; Shen, C. Transparent, intrinsically stretchable cellulose nanofiber-mediated conductive hydrogel for strain and humidity sensing. Carbohydr. Polym. 2023, 301, 120300. [Google Scholar] [CrossRef] [PubMed]
- Naficy, S.; Oveissi, F.; Patrick, B.; Schindeler, A.; Dehghani, F. Printed, Flexible pH Sensor Hydrogels for Wet Environments. Adv. Mater. Technol. 2018, 3, 1800137. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Zhang, J.; Xu, L.; Li, B.; Dong, J.; Gao, G.-L.; Jiang, Z. 3D printed thermo-responsive electroconductive hydrogel and its application for motion sensor. Front. Mater. 2023, 10, 1096475. [Google Scholar] [CrossRef]
- Lindner, E.; Cosofret, V.V.; Ufer, S.; Buck, R.P.; Kusy, R.P.; Ash, R.B.; Nagle, H.T. Flexible (Kapton-based) microsensor arrays of high stability for cardiovascular applications. J. Chem. Soc. Faraday Trans. 1993, 89, 361–367. [Google Scholar] [CrossRef]
- Huang, W.-D.; Cao, H.; Deb, S.; Chiao, M.; Chiao, J.C. A flexible pH sensor based on the iridium oxide sensing film. Sens. Actuators A Phys. 2011, 169, 1–11. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An Injectable Self-Assembling Collagen-Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy. Adv. Mater. (Deerfield Beach Fla.) 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Zhong, R.; Tang, Q.; Wang, S.; Zhang, H.; Zhang, F.; Xiao, M.; Man, T.; Qu, X.; Li, L.; Zhang, W.; et al. Self-Assembly of Enzyme-Like Nanofibrous G-Molecular Hydrogel for Printed Flexible Electrochemical Sensors. Adv. Mater. 2018, 30, 1706887. [Google Scholar] [CrossRef]




| 3D Printing Technology | Material Requirements | Resolution | Characteristics | Limitations | Ref. |
|---|---|---|---|---|---|
| Inkjet Printing | Conductive inks (e.g., silver nanoparticle ink, graphene ink) | ~20–50 μm | Precise material deposition, good for thin and complex layers | Lower mechanical strength, ink formulation critical | [78,79,80] |
| Direct Ink Writing (DIW) | Shear-thinning materials, conductive hydrogels | ~100–300 μm | Customized viscosity, highly adaptable to various materials | Complex multi-step process, a temporary sacrificial material | [34,81,82] |
| Stereolithography (SLA) | Photopolymers doped with conductive materials | ~50–200 μm | High resolution, complex structures achievable | Limited material choices, brittle structures | [85] |
| Digital Light Processing (DLP) | Photopolymers combined with conductive powders or fibers | ~25–100 μm | High resolution, fast printing speed | Material restrictions, post-processing needed | [16,19,83,84] |
| Two-Photon Polymerization (TPP) | Photosensitive conductive hydrogels | <1 μm | Ultra-high resolution, capable of nanoscale features | Expensive equipment, limited scalability of photo-initiators | [86,87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Zhang, M.; Chong, C.-M.; Lin, D.; Chen, S.; Zhen, Y.; Ding, H.; Zhong, H.-J. Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers 2024, 16, 2131. https://doi.org/10.3390/polym16152131
Liang X, Zhang M, Chong C-M, Lin D, Chen S, Zhen Y, Ding H, Zhong H-J. Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers. 2024; 16(15):2131. https://doi.org/10.3390/polym16152131
Chicago/Turabian StyleLiang, Xiaoxu, Minghui Zhang, Cheong-Meng Chong, Danlei Lin, Shiji Chen, Yumiao Zhen, Hongyao Ding, and Hai-Jing Zhong. 2024. "Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review" Polymers 16, no. 15: 2131. https://doi.org/10.3390/polym16152131
APA StyleLiang, X., Zhang, M., Chong, C.-M., Lin, D., Chen, S., Zhen, Y., Ding, H., & Zhong, H.-J. (2024). Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers, 16(15), 2131. https://doi.org/10.3390/polym16152131








