Recent Progress in Circularly Polarized Luminescent Materials Based on Cyclodextrins
Abstract
1. Introduction
2. CPL Materials Based on Cyclodextrins
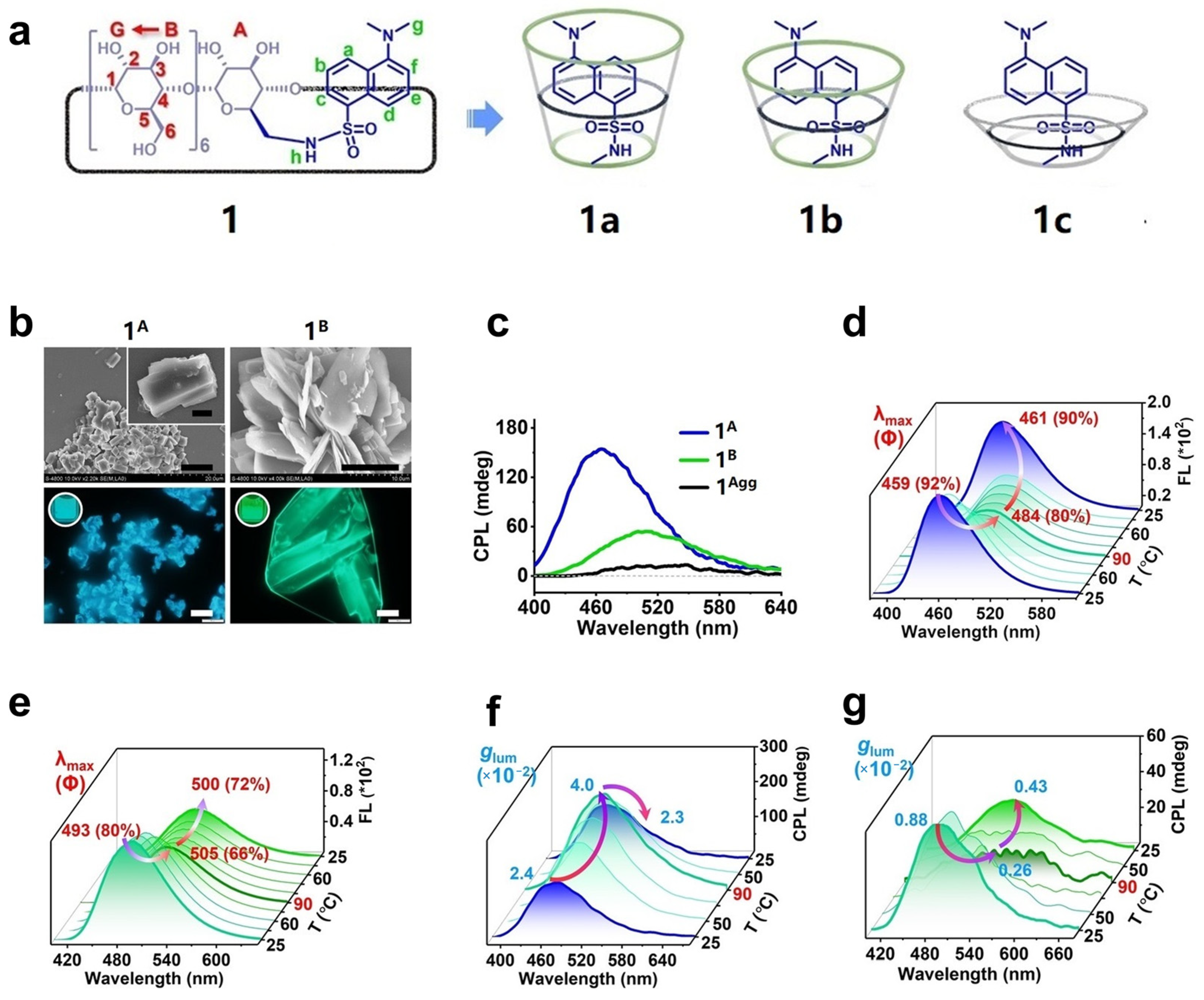
2.1. Covalent Grafting of Achiral Fluorescent Dyes with Cyclodextrins
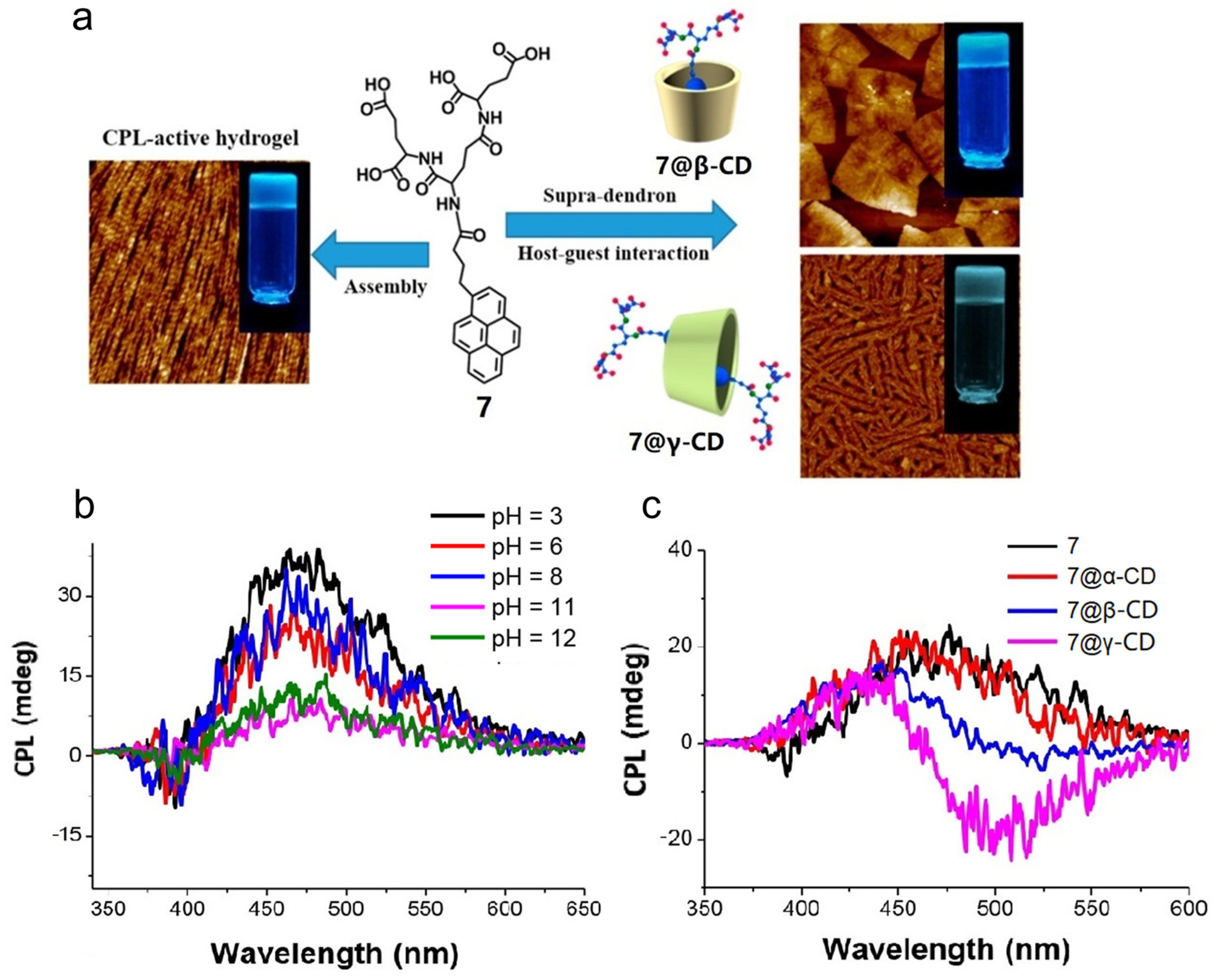
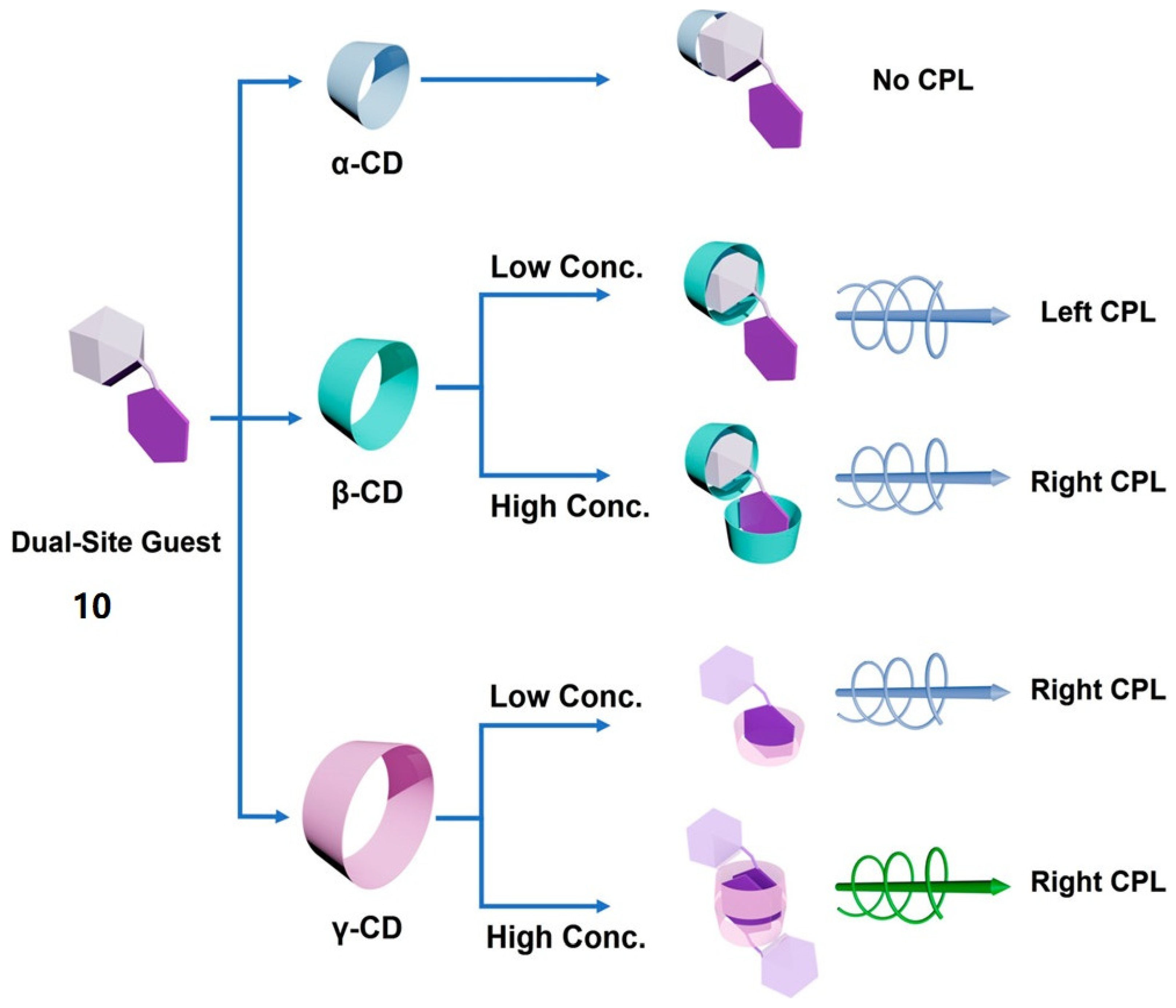
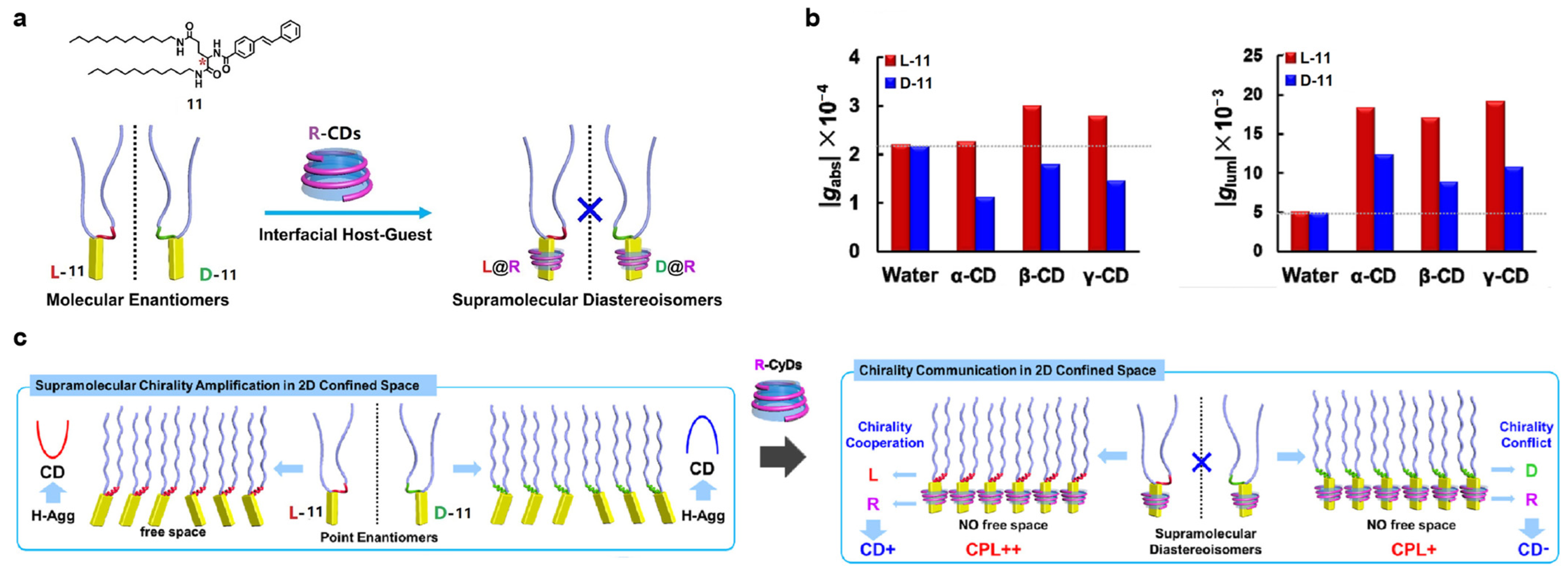
2.2. Host–Guest Interaction between Cyclodextrins and Achiral Fluorescent Dyes
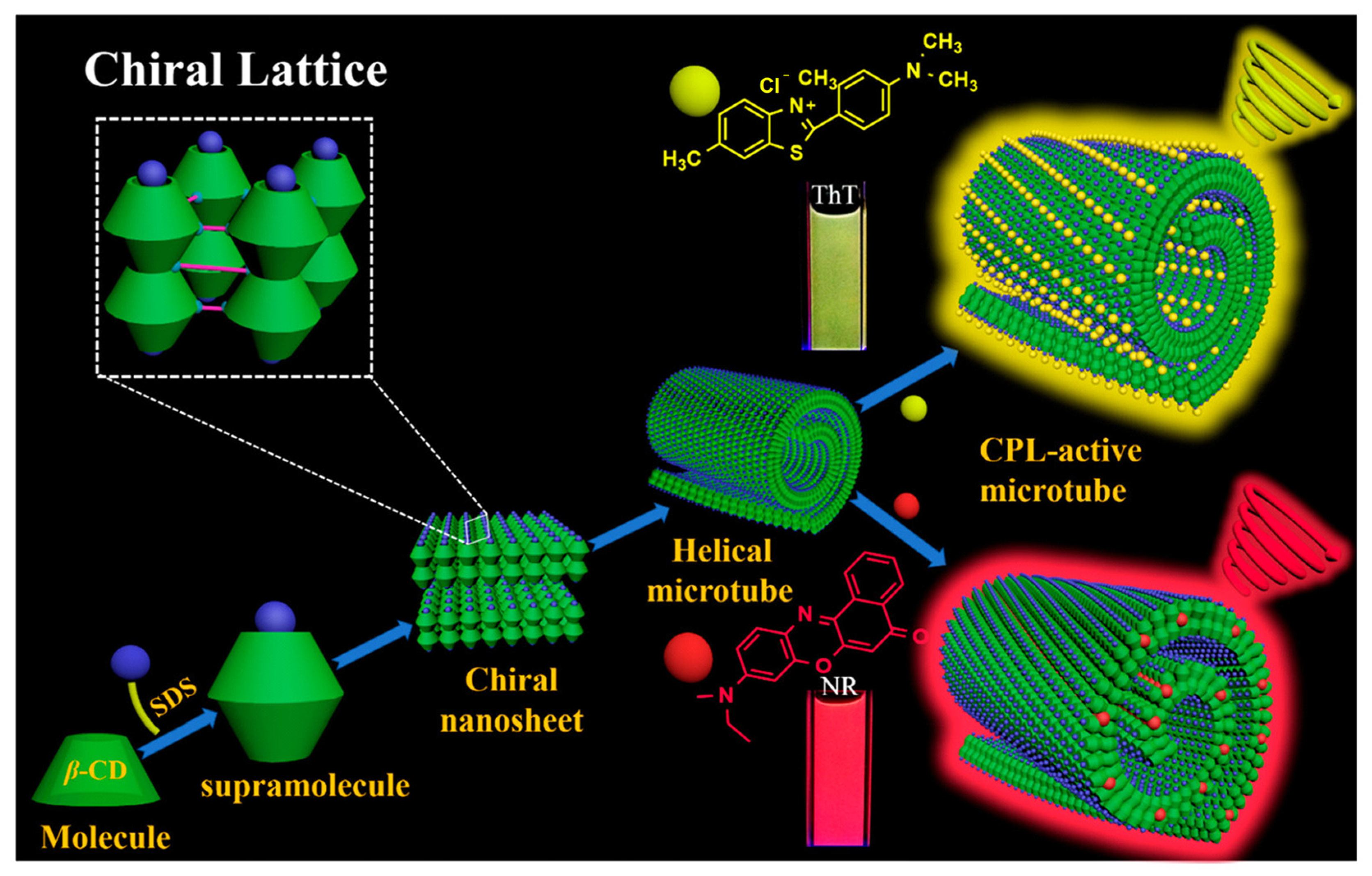
2.3. CPL Emission System Based on γ-CD-MOF
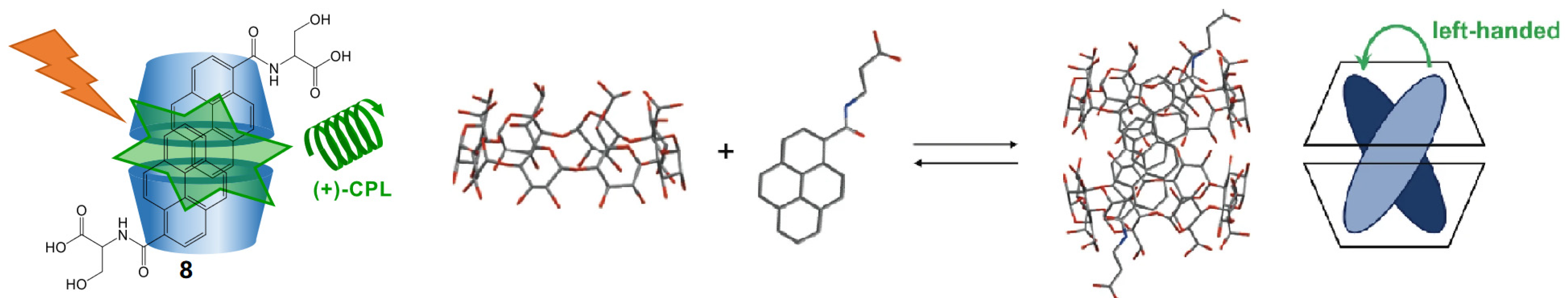
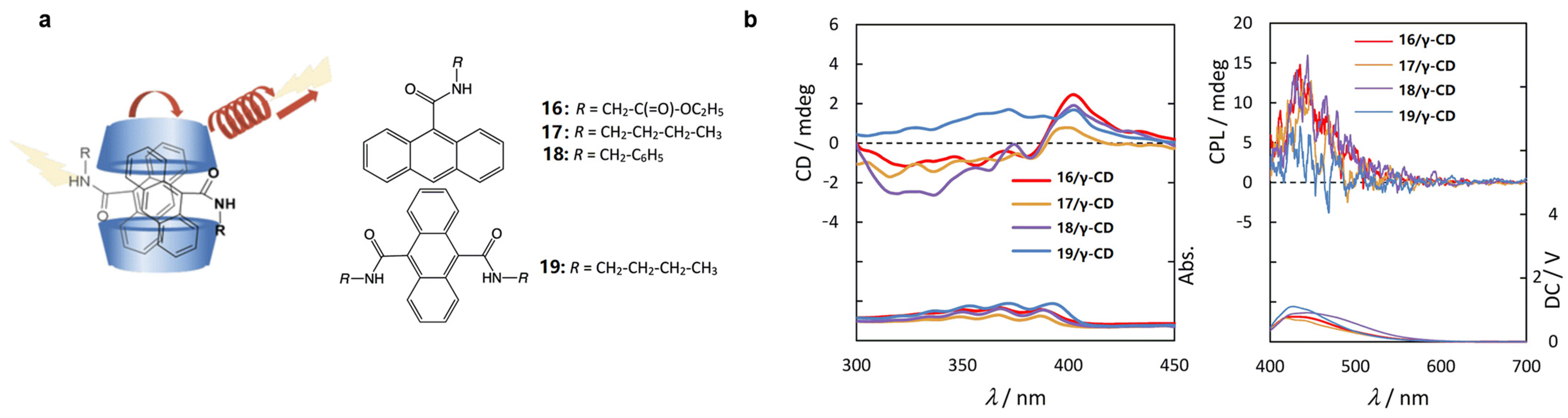
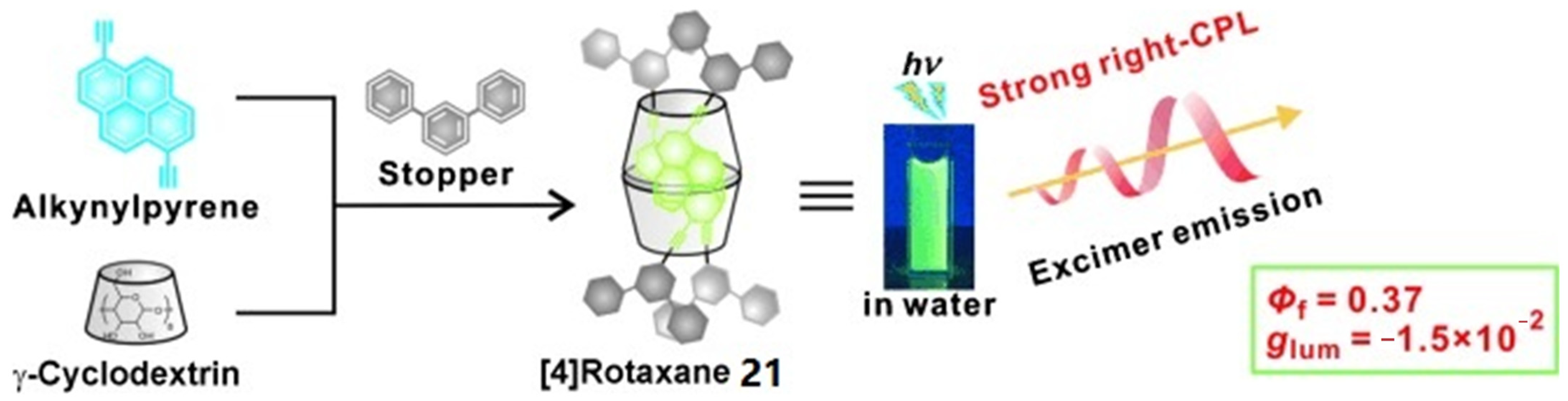
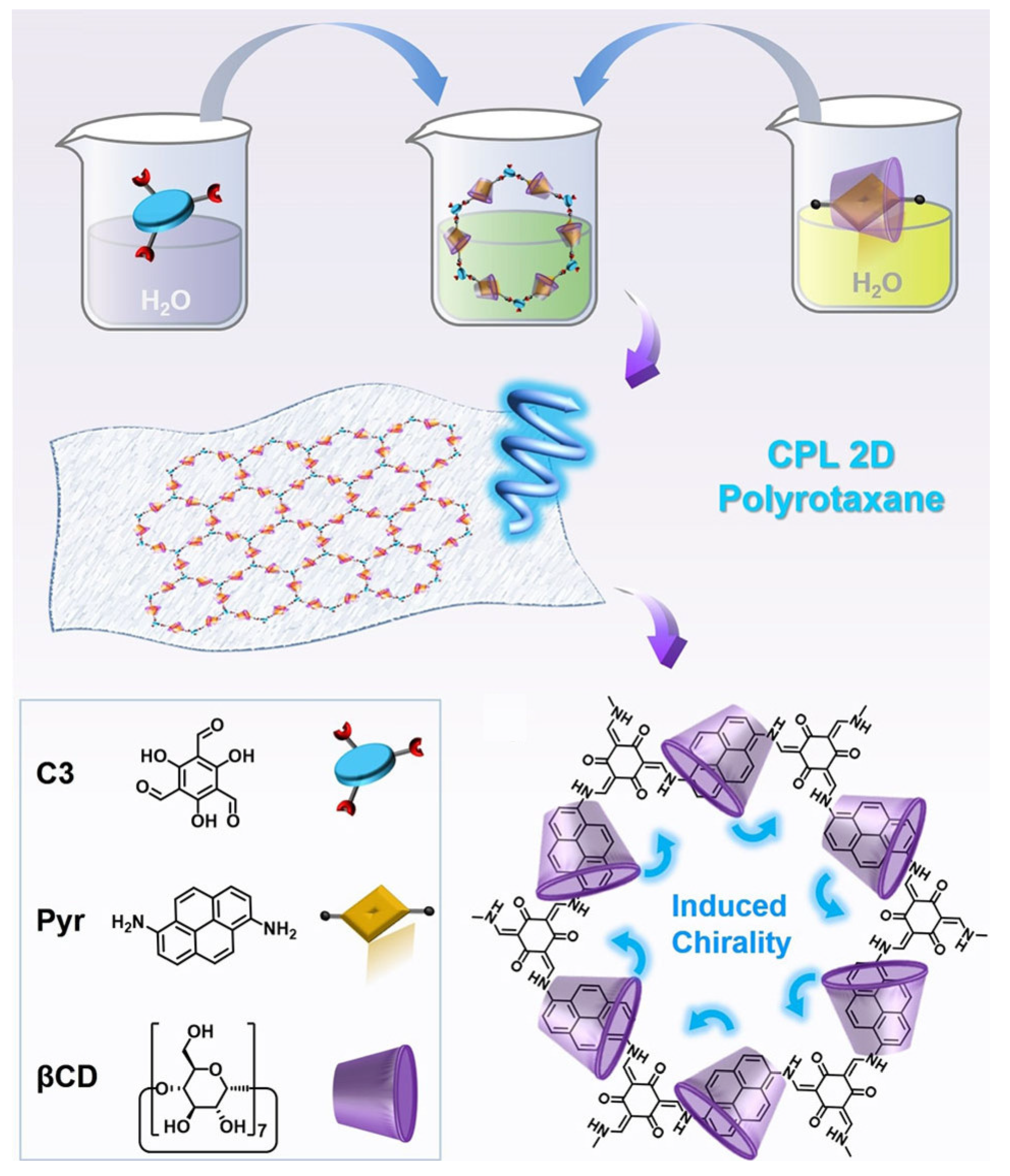
2.4. Cyclodextrin-Based Rotaxane Systems
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; da Costa, R.C.; Smilgies, D.-M.; Campbell, A.J.; Fuchter, M.J. Induction of Circularly Polarized Electroluminescence from an Achiral Light-Emitting Polymer via a Chiral Small-Molecule Dopant. Adv. Mater. 2013, 25, 2624–2628. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, J.; Zhao, C.; Feng, L.; Qu, X. Biomolecule-Based Circularly Polarized Luminescent Materials: Construction, Progress, and Applications. Angew. Chem. Int. Ed. 2022, 61, e202211822. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Li, K.; Tu, T.; Zhu, X.; Zhang, L.; Liu, M. ATP-Induced Emergent Circularly Polarized Luminescence and Encryption. Angew. Chem. Int. Ed. 2022, 61, e202200727. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Guo, S.; Lu, H.; Liu, S.; Zhao, Q.; Huang, W. Recent Progress on Circularly Polarized Luminescent Materials for Organic Optoelectronic Devices. Adv. Opt. Mater. 2018, 6, 1800538. [Google Scholar] [CrossRef]
- Gong, Z.-L.; Zhu, X.; Zhou, Z.; Zhang, S.-W.; Yang, D.; Zhao, B.; Zhang, Y.-P.; Deng, J.; Cheng, Y.; Zheng, Y.-X.; et al. Frontiers in circularly polarized luminescence: Molecular design, self-assembly, nanomaterials, and applications. Sci. China Chem. 2021, 64, 2060–2104. [Google Scholar] [CrossRef]
- Sang, Y.; Han, J.; Zhao, T.; Duan, P.; Liu, M. Circularly Polarized Luminescence in Nanoassemblies: Generation, Amplification, and Application. Adv. Mater. 2020, 32, 1900110. [Google Scholar] [CrossRef]
- Yu, N.; Aieta, F.; Genevet, P.; Kats, M.A.; Gaburro, Z.; Capasso, F. A Broadband, Background-Free Quarter-Wave Plate Based on Plasmonic Metasurfaces. Nano Lett. 2012, 12, 6328–6333. [Google Scholar] [CrossRef]
- Lin, W.-B.; He, D.-Q.; Lu, H.-Y.; Hu, Z.-Q.; Chen, C.-F. Sign inversions of circularly polarized luminescence for helical compounds by chemically fine-tuning operations. Chem. Commun. 2020, 56, 1863–1866. [Google Scholar] [CrossRef]
- Deng, M.; Mukthar, N.F.M.; Schley, N.D.; Ung, G. Yellow Circularly Polarized Luminescence from C1-Symmetrical Copper(I) Complexes. Angew. Chem. Int. Ed. 2020, 59, 1228–1231. [Google Scholar] [CrossRef]
- He, Y.; Lin, S.; Guo, J.; Li, Q. Circularly polarized luminescent self-organized helical superstructures: From materials and stimulus-responsiveness to applications. Aggregate 2021, 2, e141. [Google Scholar] [CrossRef]
- Xia, Q.; Meng, L.; He, T.; Huang, G.; Li, B.S.; Tang, B.Z. Direct Visualization of Chiral Amplification of Chiral Aggregation Induced Emission Molecules in Nematic Liquid Crystals. ACS Nano 2021, 15, 4956–4966. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.-H.; Zhang, D.; Cai, M.; Duan, L.; Fung, M.-K.; Chen, C.-F. Stable Enantiomers Displaying Thermally Activated Delayed Fluorescence: Efficient OLEDs with Circularly Polarized Electroluminescence. Angew. Chem. Int. Ed. 2018, 57, 2889–2893. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gao, B.-R.; Xu, X.-H.; Zhou, L.; Liu, N.; Wu, Z.-Q. Controlled Synthesis of Cyclic-Helical Polymers with Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2022, 61, e202204966. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Han, J.; Liu, M.; Duan, P. Photon Upconverted Circularly Polarized Luminescence via Triplet–Triplet Annihilation. Adv. Mater. 2019, 31, 1805683. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at All Scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Han, X.-N.; Han, Y.; Chen, C.-F. Advances in circularly polarized luminescence materials based on chiral macrocycles. Chem. Commun. 2023, 59, 13089–13106. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Kornowicz, A.; Lewiński, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. [Google Scholar] [CrossRef]
- Song, X.; Zhu, X.; Qiu, S.; Tian, W.; Liu, M. Self-Assembly of Adaptive Chiral [1]Rotaxane for Thermo-Rulable Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2022, 61, e202208574. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Kawakami, K.; Nagata, Y.; Kajiwara, R.; Yamada, S.; Mori, T.; Kida, T. Cyclodextrins with Multiple Pyrenyl Groups: An Approach to Organic Molecules Exhibiting Bright Excimer Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2022, 61, e202114700. [Google Scholar] [CrossRef]
- Tu, C.; Wu, W.; Liang, W.; Zhang, D.; Xu, W.; Wan, S.; Lu, W.; Yang, C. Host–Guest Complexation-Induced Aggregation Based on Pyrene-Modified Cyclodextrins for Improved Electronic Circular Dichroism and Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2022, 61, e202203541. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Han, J.; Zhou, J.; Jin, Q.; Liu, M.; Duan, P. Circularly Polarized Luminescence from a Pyrene-Cyclodextrin Supra-Dendron. Langmuir 2018, 34, 5821–5830. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Guo, P.; Qin, X.; Gao, X.; Ma, K.; Zhu, X.; Jin, X.; Xu, W.; Jiang, L.; Duan, P. Hierarchically Chiral Lattice Self-Assembly Induced Circularly Polarized Luminescence. ACS Nano 2020, 14, 3190–3198. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, W.; Ma, C.; Zhu, Z.; Qi, W.; Huang, J.; Yan, Y. Not by Serendipity: Rationally Designed Reversible Temperature-Responsive Circularly Polarized Luminescence Inversion by Coupling Two Scenarios of Harata–Kodaka’s Rule. JACS Au 2021, 1, 156–163. [Google Scholar] [CrossRef]
- Sawai, M.; Matsumoto, S.; Mimura, Y.; Imai, Y.; Yamazaki, S.; Kanehisa, N.; Tohnai, N.; Nakata, E.; Takashima, H. Circularly polarized luminescence (CPL) characteristics of hydrophobic pyrene derivatives/γ-cyclodextrin (γ-CD) complexes in aqueous solution dissolved by grinding. J. Incl. Phenom. Macrocycl. 2022, 102, 133–142. [Google Scholar] [CrossRef]
- Ji, L.; He, Q.; Niu, D.; Tan, J.; Ouyang, G.; Liu, M. Host–guest interaction enabled chiroptical photo-switching and enhanced circularly polarized luminescence. Chem. Commun. 2019, 55, 11747–11750. [Google Scholar] [CrossRef]
- Yang, C.; Chen, W.; Zhu, X.; Song, X.; Liu, M. Self-Assembly and Circularly Polarized Luminescence from Achiral Pyrene-Adamantane Conjugates by Selective Inclusion with Cyclodextrins. J. Phys. Chem. Lett. 2021, 12, 7491–7496. [Google Scholar] [CrossRef]
- Yang, C.; Yang, D.; Zhu, X.; Meng, Y.; Liu, M. Circularly Polarized Luminescence of Langmuir–Schaefer Films of Amphiphilic Stilbene Enhanced via Interfacial Reaction with Cyclodextrins. Langmuir 2020, 36, 12366–12374. [Google Scholar] [CrossRef]
- Imai, Y.; Mimura, Y.; Motomura, Y.; Ikemura, R.; Shizuma, M.; Kitamatsu, M. Controlling Excimer-Origin Circularly Polarized Luminescence of Bipyrenyl-Arginine Peptides by Cyclodextrin in Water. Bull. Chem. Soc. Jpn. 2023, 96, 268–273. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Ikemura, R.; Imai, Y.; Tohnai, N.; Yamazaki, S.; Nakata, E.; Takashima, H. Circularly polarised luminescence from excimer emission of anthracene derivatives complexed with γ-cyclodextrin in the solid state. RSC Adv. 2023, 13, 1914–1922. [Google Scholar] [CrossRef]
- Hu, L.; Li, K.; Shang, W.; Zhu, X.; Liu, M. Emerging Cubic Chirality in γCD-MOF for Fabricating Circularly Polarized Luminescent Crystalline Materials and the Size Effect. Angew. Chem. Int. Ed. 2020, 59, 4953–4958. [Google Scholar] [CrossRef] [PubMed]
- Kazem-Rostami, M.; Orte, A.; Ortuño, A.M.; David, A.H.G.; Roy, I.; Miguel, D.; Garci, A.; Cruz, C.M.; Stern, C.L.; Cuerva, J.M.; et al. Helically Chiral Hybrid Cyclodextrin Metal–Organic Framework Exhibiting Circularly Polarized Luminescence. J. Am. Chem. Soc. 2022, 144, 9380–9389. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Hayashi, K.; Yonenaga, Y.; Itou, T.; Fujimoto, K.; Uchida, T.-A.; Iwamura, M.; Nozaki, K. A Doubly Alkynylpyrene-Threaded [4]Rotaxane That Exhibits Strong Circularly Polarized Luminescence from the Spatially Restricted Excimer. Angew. Chem. Int. Ed. 2014, 53, 14392–14396. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhu, X.; Yang, C.; Liu, M. Two-Dimensional Chiral Polyrotaxane Monolayer with Emergent and Steerable Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2022, 61, e202114759. [Google Scholar] [CrossRef] [PubMed]





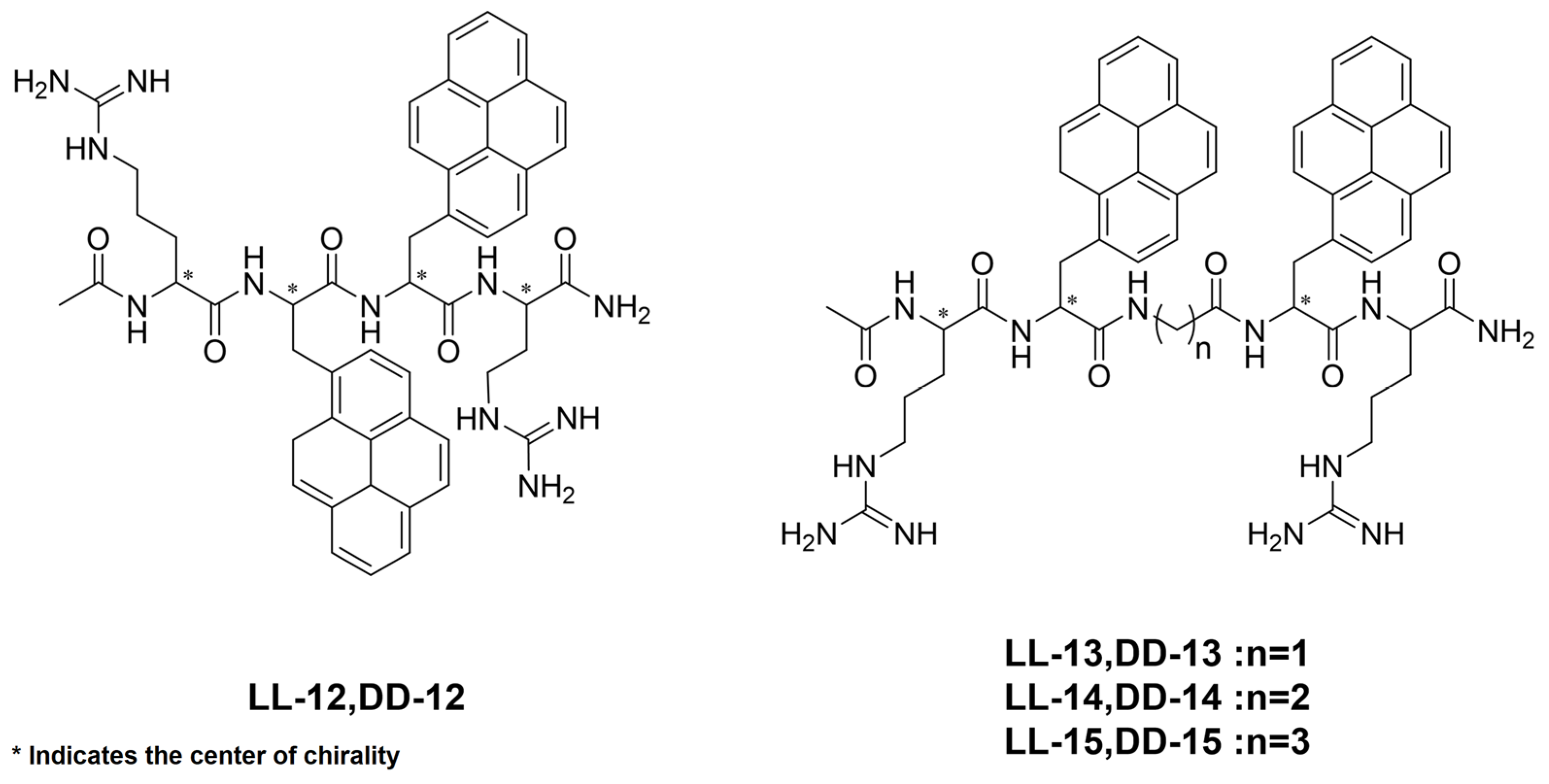


| Compounds | λCPL (nm) | glum (10−3) | Compounds | λCPL (nm) | glum (10−3) |
|---|---|---|---|---|---|
| LL-12 | 495 | +1.8 | LL-12/γ-CD | 468 | +3.0 |
| LL-13 | 486 | −5.3 | LL-13/γ-CD | 478 | −4.7 |
| LL-14 | 496 | +1.5 | LL-14/γ-CD | 485 | +8.5 |
| LL-15 | 476 | −3.3 | LL-15/γ-CD | 444 | +6.0 |
| DD-12 | 470 | −1.7 | DD-12/γ-CD | 470 | −3.8 |
| DD-13 | 483 | +5.2 | DD-13/γ-CD | 487 | +3.0 |
| DD-14 | 502 | −1.6 | DD-14/γ-CD | 483 | −14.0 |
| DD-15 | 487 | +3.3 | DD-15/γ-CD | 434 497 | −1.2 −0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Chang, W.; Liu, L.; Li, J. Recent Progress in Circularly Polarized Luminescent Materials Based on Cyclodextrins. Polymers 2024, 16, 2140. https://doi.org/10.3390/polym16152140
Zhou C, Chang W, Liu L, Li J. Recent Progress in Circularly Polarized Luminescent Materials Based on Cyclodextrins. Polymers. 2024; 16(15):2140. https://doi.org/10.3390/polym16152140
Chicago/Turabian StyleZhou, Chengkai, Weixing Chang, Lingyan Liu, and Jing Li. 2024. "Recent Progress in Circularly Polarized Luminescent Materials Based on Cyclodextrins" Polymers 16, no. 15: 2140. https://doi.org/10.3390/polym16152140
APA StyleZhou, C., Chang, W., Liu, L., & Li, J. (2024). Recent Progress in Circularly Polarized Luminescent Materials Based on Cyclodextrins. Polymers, 16(15), 2140. https://doi.org/10.3390/polym16152140







