Novel Thermosensitive and Mucoadhesive Nasal Hydrogel Containing 5-MeO-DMT Optimized Using Box-Behnken Experimental Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Thermosensitive Gels
2.3. Experimental Design
2.4. Gelation Temperature Determination
2.5. Rheological Studies
2.6. Mucoadhesion Analysis
2.7. Confocal Raman Spectroscopy
2.8. In Vitro Drug Release Study
3. Results and Discussion
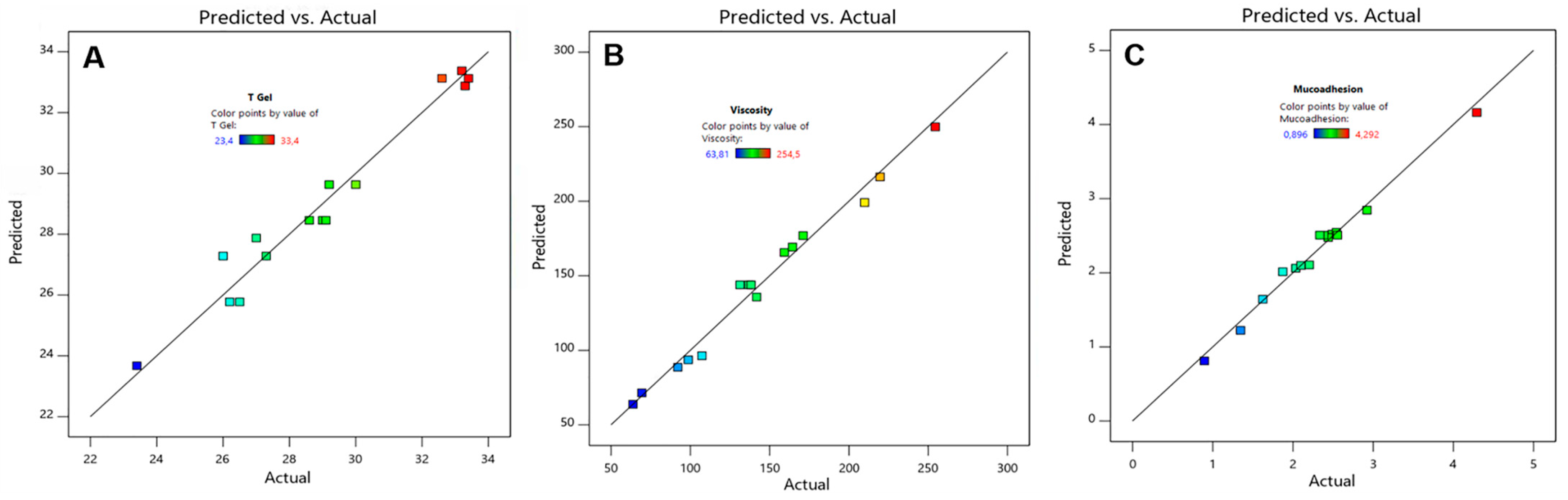
3.1. Experimental Design
3.1.1. Model Fitting
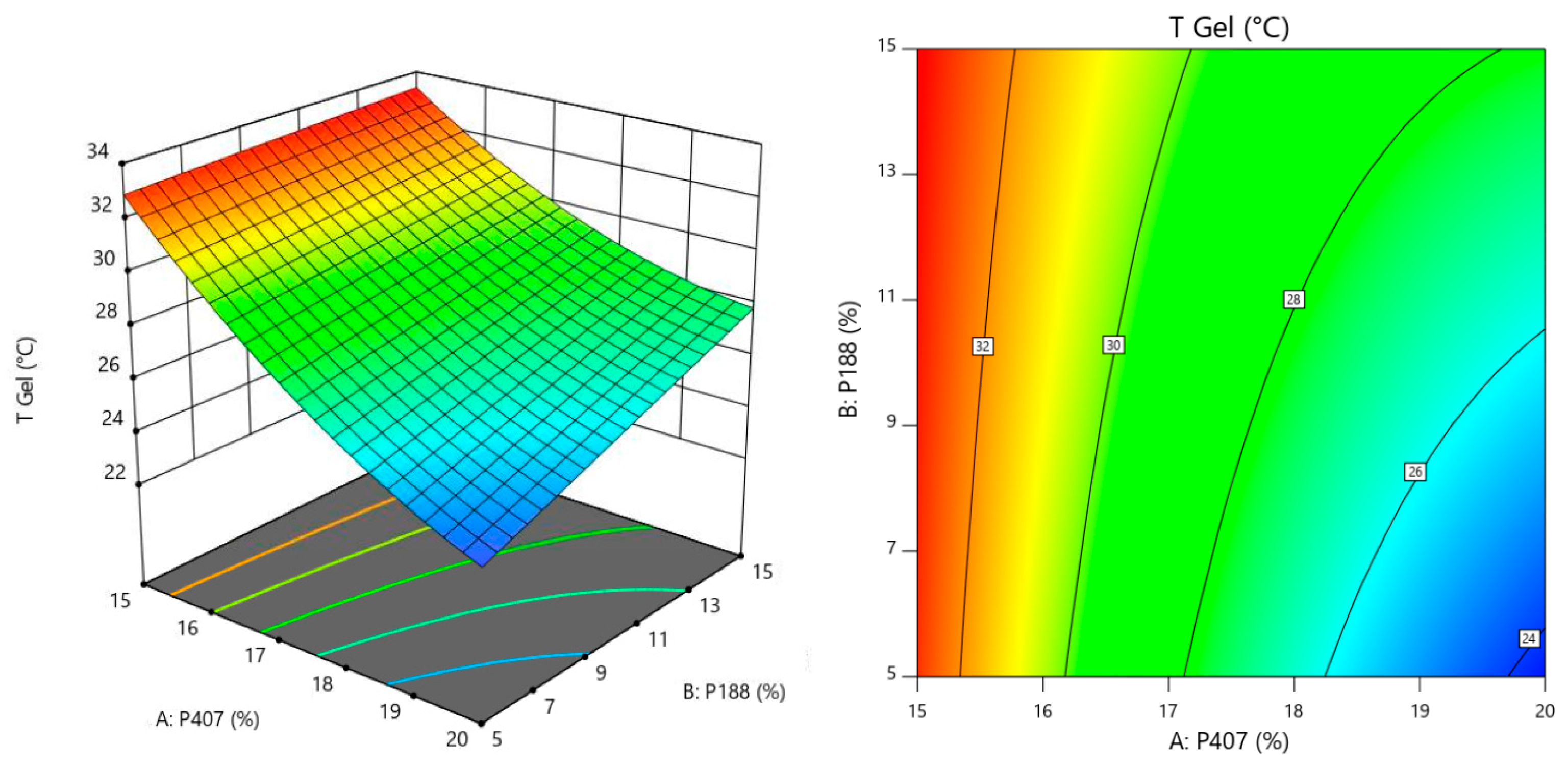
3.1.2. Response Surface for Tgel
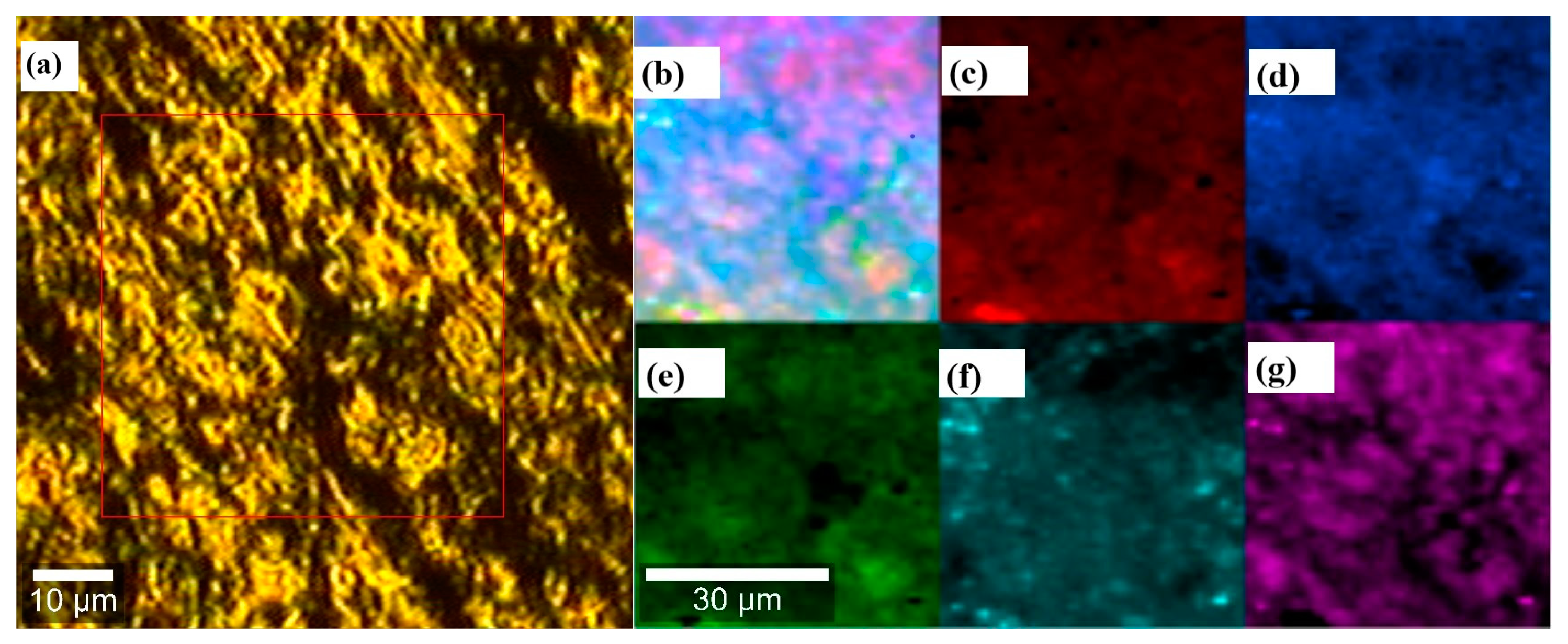
3.2. Raman Spectroscopy
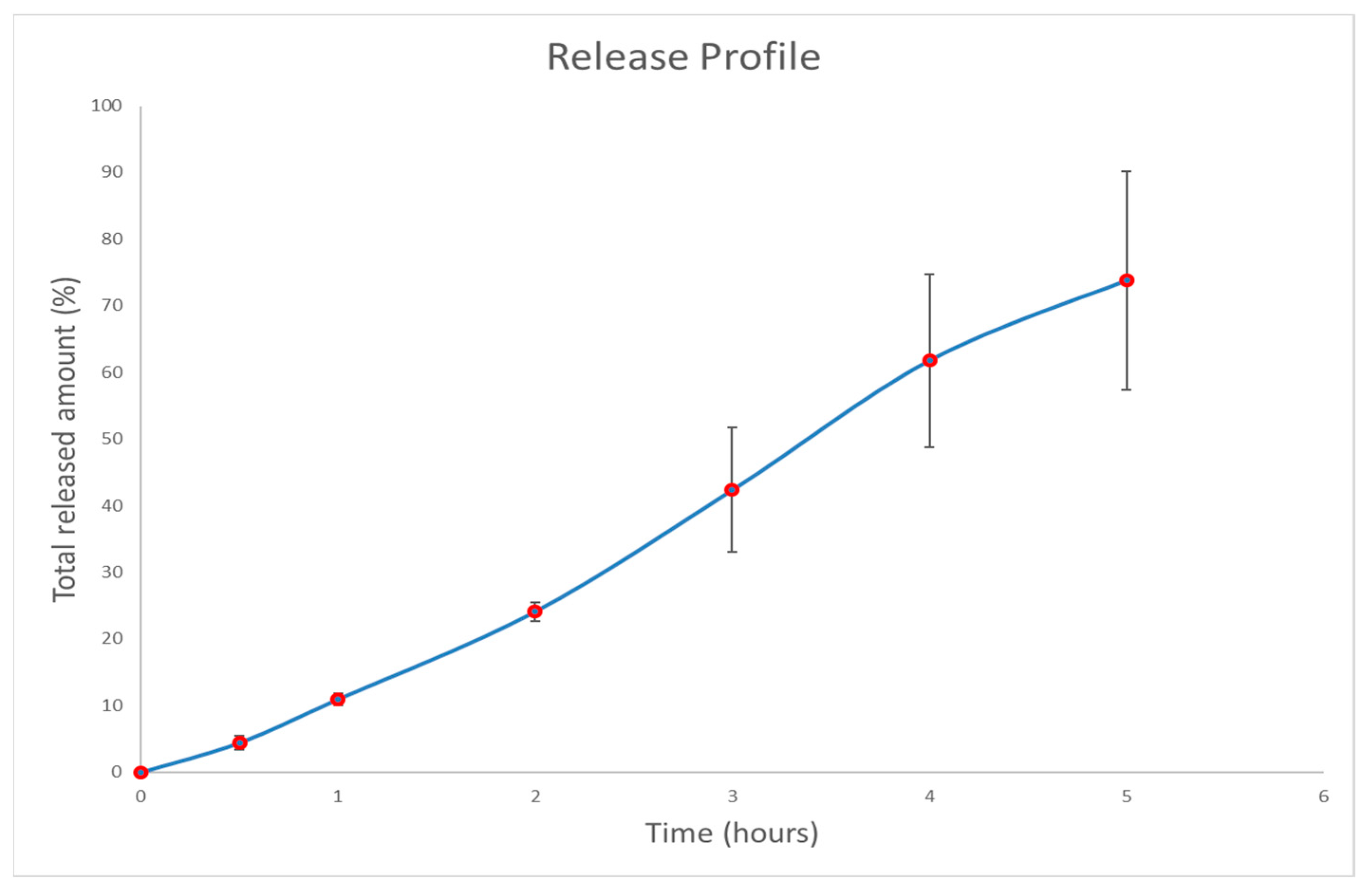
3.3. In Vitro Drug Release Study
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, K.A.A.; Carhart-Harris, R.; Nutt, D.J.; Erritzoe, D. Therapeutic effects of classic serotonergic psychedelics: A systematic review of modern-era clinical studies. Acta Psychiatr. Scand. 2021, 143, 101–118. [Google Scholar] [CrossRef]
- Reckweg, J.T.; Uthaug, M.V.; Szabo, A.; Davis, A.K.; Lancelotta, R.; Mason, N.L. The clinical pharmacology and potential therapeutic applications of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT). J. Neurochem. 2022, 162, 128–146. [Google Scholar] [CrossRef]
- Weil, A.T.; Davis, W. Bufo alvarius: A potent hallucinogen of animal origin. J. Ethnopharmacol. 1994, 41, 1–8. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Frecska, E.; Rajnavolgyi, E. Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS ONE 2014, 9, e106533. [Google Scholar] [CrossRef]
- Barker, S.A. N, N-dimethyltryptamine (DMT), an endogenous hallucinogen: Past, present, and future research to determine its role and function. Front. Neurosci. 2018, 12, 536. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Feduccia, A.A.; Jerome, L.; Mithoefer, A.; Wagner, M.; Walsh, Z.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Doblin, R. MDMA-assisted psychotherapy for treatment of PTSD: Study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology 2019, 236, 2735–2745. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Wang, J.B.; Lin, J.; Bedrosian, L.; Coker, A.; Jerome, I.; Feduccia, A.; Lilienstein, A.; Harrison, C.; Heimler, E.; Mithoefer, M.; et al. Scaling Up: Multisite Open-Label Clinical Trials of MDMA-Assisted Therapy for Severe Posttraumatic Stress Disorder. J. Humanist Psychol. 2021, 00221678211023663. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.; Strassman, R.J. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef]
- Winkelman, M.J.; Szabo, A.; Frecska, E. The potential of psychedelics for the treatment of Alzheimer’s disease and related dementias. Eur. Neuropsychopharmacol. 2023, 76, 3–16. [Google Scholar] [CrossRef]
- Buot, A.; Pallares, C.; Oganesyan, A.; Dauré, C.; Bonnelle, V.; Burguière, E.; Dos Santos, J.F.A.; N’diaye, K.; Ljuslin, M.; Smith, P.; et al. Improvement in OCD symptoms associated with serotoninergic psychedelics: A retrospective online survey. Sci. Rep. 2023, 13, 13378. [Google Scholar] [CrossRef]
- Davis, A.K.; Barsuglia, J.P.; Lancelotta, R.; Grant, R.M.; Renn, E. The epidemiology of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J. Psychopharmacol. 2018, 32, 779–792. [Google Scholar] [CrossRef]
- Shen, H.W.; Jiang, X.L.; Winter, J.; Yu, A.M. Psychedelic 5-Methoxy-N,N-Dimethyltryptamine: Metabolism, Pharmacokinetics, Drug Interactions, and Pharmacological Actions. Curr. Drug Metab. 2011, 11, 659–666. [Google Scholar] [CrossRef]
- Halberstadt, A.L. Behavioral and pharmacokinetic interactions between monoamine oxidase inhibitors and the hallucinogen 5-methoxy-N,N-dimethyltryptamine. Pharmacol. Biochem. Behav. 2016, 143, 1–10. [Google Scholar] [CrossRef]
- Jogani, V.; Jinturkar, K.; Vyas, T.; Misra, A. Recent patents review on intranasal administration for CNS drug delivery. Recent Pat. Drug Deliv. Formul. 2008, 2, 25–40. [Google Scholar]
- Illum, L. Nasal drug delivery—Recent developments and future prospects. J. Control. Release 2012, 161, 254–263. [Google Scholar] [CrossRef]
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef]
- Good, M.; Joel, Z.; Benway, T.; Routledge, C.; Timmermann, C.; Erritzoe, D.; Weaver, R.; Allen, G.; Hughes, C.; Topping, H.; et al. Pharmacokinetics of N,N-dimethyltryptamine in Humans. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.W.; Jiang, X.L.; Yu, A.M. Nonlinear pharmacokinetics of 5-methoxy-N,N-dimethyltryptamine in mice. Drug Metab. Dispos. 2011, 39, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; da Silva, A.B.; da Costa, L.M.; Zatta, K.C.; Onzi, G.R.; da Fonseca, F.N.; Guterres, S.S.; Paese, K. Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma. Drug Deliv. Transl. Res. 2023, 13, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Thouvenin, A.; Toussaint, B.; Marinovic, J.; Gilles, A.L.; Dufaӱ Wojcicki, A.; Boudy, V. Development of Thermosensitive and Mucoadhesive Hydrogel for Buccal Delivery of (S)-Ketamine. Pharmaceutics 2022, 14, 2039. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Asri, R.M.; Amir, M.N.; Himawan, A.; Arjuna, A.; Juniarti, N.; Utami, R.N.; Mardikasari, S.A. Development of Thermoresponsive Hydrogels with Mucoadhesion Properties Loaded with Metronidazole Gel-Flakes for Improved Bacterial Vaginosis Treatment. Pharmaceutics 2023, 15, 1529. [Google Scholar] [CrossRef]
- Chen, I.C.; Su, C.Y.; Chen, P.Y.; Hoang, T.C.; Tsou, Y.S.; Fang, H.W. Investigation and Characterization of Factors Affecting Rheological Properties of Poloxamer-Based Thermo-Sensitive Hydrogel. Polymers 2022, 14, 5353. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Nirmayanti, N.; Alhidayah, A.; Usman, J.T.; Nur, J.F.; Amir, M.N.; Permana, A.D. Combinatorial Approach of Thermosensitive Hydrogels and Solid Microneedles to Improve Transdermal Delivery of Valsartan: An In Vivo Proof of Concept Study. AAPS Pharm. Sci. Tech. 2023, 24, 5. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 1–72. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L. Long-Acting Gel Formulations: Advancing Drug Delivery across Diverse Therapeutic Areas. Pharmaceuticals 2024, 17, 493. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Ongun, M.; Tunçel, E.; Kodan, E.; Tuğcu Demiröz, F.N.; Tirnaksiz, F.F. Development and Characterization of Mucoadhesive-Thermosensitive Buccal Gel Containing Metronidazole for the Treatment of Oral Mucositis. J. Fac. Pharm. Ank. Ank. Ecz. Fak. Derg. 2020, 44, 517–539. [Google Scholar] [CrossRef]
- Pagano, C.; Giovagnoli, S.; Perioli, L.; Tiralti, M.C.; Ricci, M. Development and characterization of mucoadhesive-thermoresponsive gels for the treatment of oral mucosa diseases. Eur. J. Pharm. Sci. 2020, 142, 105125. [Google Scholar] [CrossRef]
- Nair, A.B.; Sreeharsha, N.; Al-Dhubiab, B.E.; Hiremath, J.G.; Shinu, P.; Attimarad, M.; Venugopala, K.N.; Mutahar, M. HPMC- and PLGA- Based Nanoparticles for the Mucoadhesive Delivery of Sitagliptin: Optimization and In Vivo Evaluation in Rats. Materials 2019, 12, 4239. [Google Scholar] [CrossRef]
- Li, L.; He, N.; Jiang, B.; Yu, K.; Zhang, Q.; Zhang, H.; Tang, D.; Song, Y. Highly Salt-Resistant 3D Hydrogel Evaporator for Continuous Solar Desalination via Localized Crystallization. Adv. Funct. Mater. 2021, 31, 2104380. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P.; Tantishaiyakul, V. Thermosensitive Polymer Blend Composed of Poloxamer 407, Poloxamer 188 and Polycarbophil for the Use as Mucoadhesive In Situ Gel. Polymers 2022, 14, 1836. [Google Scholar] [CrossRef]
- Wong, Y.L.; Pandey, M.; Choudhury, H.; Lim, W.M.; Bhattamisra, S.K.; Gorain, B. Development of In-Situ Spray for Local Delivery of Antibacterial Drug for Hidradenitis Suppurativa: Investigation of Alternative Formulation. Polymers 2021, 13, 2770. [Google Scholar] [CrossRef]
- Arpa, M.D.; Kesmen, E.E.; Biltekin, S.N. Novel Sprayable Thermosensitive Benzydamine Hydrogels for Topical Application: Development, Characterization, and In Vitro Biological Activities. AAPS Pharm. Sci. Tech. 2023, 24, 214. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Wang, J.; Chen, J. Conformational structure of triblock copolymers by FT-Raman and FTIR spectroscopy. J. Colloid Interface Sci. 1999, 209, 368–373. [Google Scholar] [CrossRef]
- Cook, M.T.; Abou-Shamat, M.A.; Stair, J.L.; Calvo-Castro, J. Raman spectroscopy coupled to computational approaches towards understanding self-assembly in thermoreversible poloxamer gels. J. Mol. Liq. 2022, 351, 118660. [Google Scholar] [CrossRef]
- Shi, S.C.; Wu, J.Y.; Huang, T.F. Raman, FTIR, and XRD study of MoS2 enhanced hydroxypropyl methylcellulose green lubricant. Opt. Quantum Electron. 2016, 48, 474. [Google Scholar] [CrossRef]
- Wu, X.; Cañamares, M.V.; Kakoulli, I. Sanchez-Cortes, S. Chemical Characterization and Molecular Dynamics Simulations of Bufotenine by Surface-Enhanced Raman Scattering (SERS) and Density Functional Theory (DFT). J. Phys. Chem. Lett. 2022, 13, 5831–5837. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, J.; Guirguis, A.; Samaras, E.G.; Zloh, M.; Kirton, S.B.; Stair, J.L. Detection of newly emerging psychoactive substances using Raman spectroscopy and chemometrics. RSC Adv. 2018, 8, 31924–31933. [Google Scholar] [CrossRef] [PubMed]
- Šapić, I.M.; Bistričić, L.; Volovšek, V.; Dananić, V.; Furić, K. DFT study of molecular structure and vibrations of 3-glycidoxypropyltrimethoxysilane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Pastorczak, M.; Kozanecki, M.; Ulanski, J. Water–Polymer interactions in PVME hydrogels—Raman spectroscopy studies. Polymer 2009, 50, 4535–4542. [Google Scholar] [CrossRef]
- Gibaldi, M.; Feldman, S. Establishment of Sink Conditions in Dissolution Rate Determinations. J. Pharm. Sci. 1967, 56, 1238–1242. [Google Scholar] [CrossRef]


| Levels | |||
| Independent variables | Low | Medium | High |
| X1 = P407 (% w/v) | 15 | 17.50 | 20 |
| X2 = P188 (% w/v) | 5 | 5 | 15 |
| X3 = HPMC (% w/v) | 0.10 | 0.55 | 1 |
| Dependent variables | Constraints | ||
| Y1 = Tgel (°C) | Y1 ≤ 35 | ||
| Y2 = Viscosity (mPas) | Y2 ≤ 200 | ||
| Y3 = Mucoahesion (N·m) | Maximize | ||
| Experiment | P407 (%) | P188 (%) | HPMC (%) | Tgel (°C) | Viscosity (mPas) | Mucoadhesion (N·m) |
|---|---|---|---|---|---|---|
| 1 | 20 | 10 | 1 | 26.2 | 254.5 | 1.347 |
| 2 | 20 | 5 | 0.55 | 23.4 | 209.9 | 0.896 |
| 3 | 17.5 | 10 | 0.55 | 28.6 | 131.3 | 2.556 |
| 4 | 17.5 | 5 | 1 | 26 | 171.1 | 1.873 |
| 5 | 17.5 | 5 | 0.10 | 27.3 | 98.7 | 1.624 |
| 6 | 15 | 15 | 0.55 | 33.2 | 92.1 | 2.489 |
| 7 | 17.5 | 15 | 1 | 29.2 | 164.4 | 2.439 |
| 8 | 20 | 10 | 0.10 | 26.5 | 159.3 | 2.541 |
| 9 | 15 | 10 | 0.10 | 33.4 | 63.81 | 2.10 |
| 10 | 15 | 5 | 0.55 | 33.3 | 69.42 | 2.923 |
| 11 | 15 | 10 | 1 | 32.6 | 107.3 | 4.292 |
| 12 | 20 | 15 | 0.55 | 27 | 219.6 | 2.035 |
| 13 | 17.5 | 15 | 0.10 | 30 | 141.8 | 2.204 |
| 14 | 17.5 | 10 | 0.55 | 29 | 136.9 | 2.384 |
| 15 | 17.5 | 10 | 0.55 | 29.1 | 138.3 | 2.335 |
| Tgel | p Value |
| Model | <0.0001 |
| A (P407) | <0.0001 |
| B (P188) | 0.0007 |
| AB | 0.0235 |
| A2 | 0.0199 |
| Lack of Fit Test | 0.1116 |
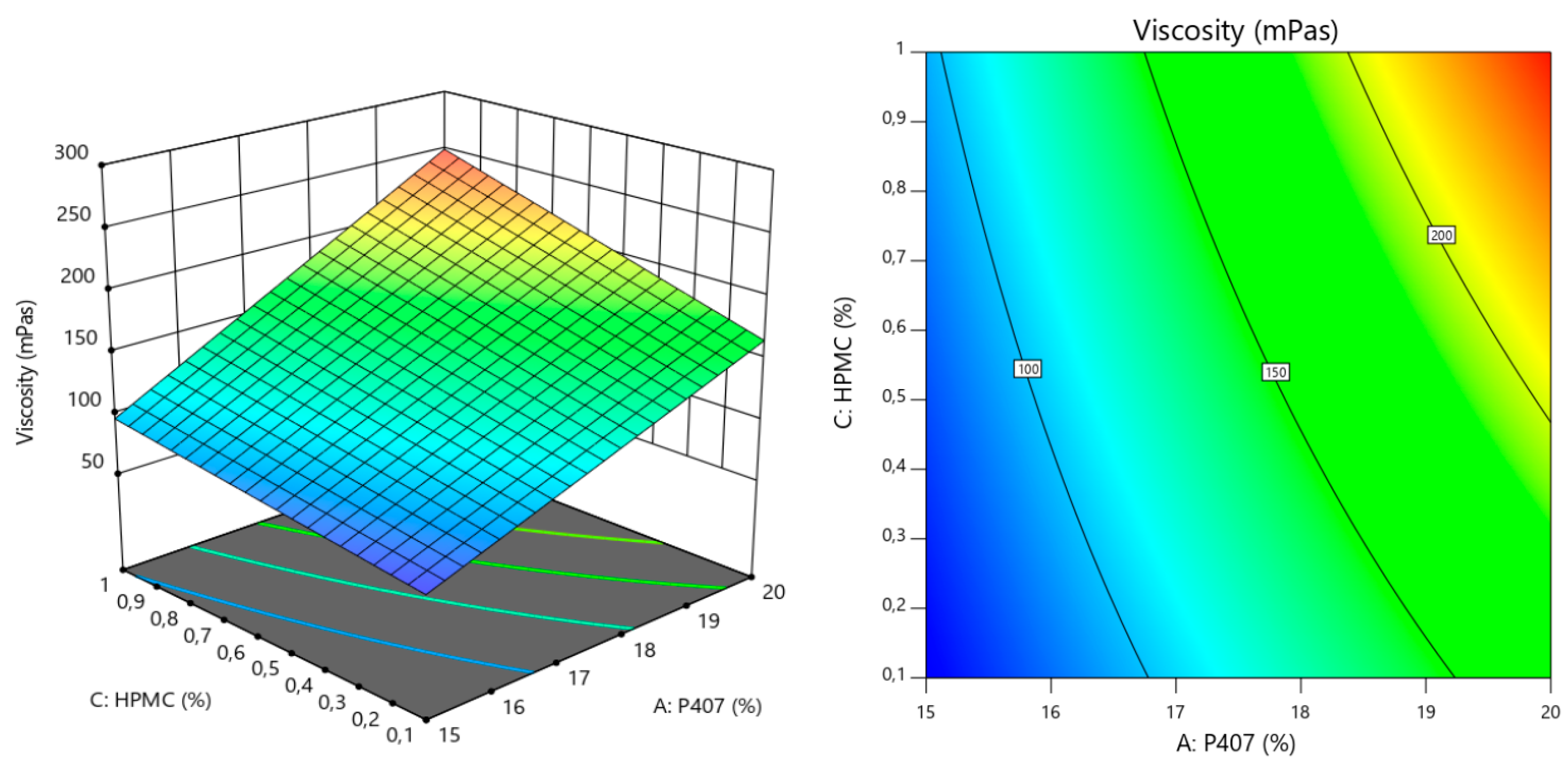
| Viscosity | p value |
| Model | <0.0001 |
| A (P407) | <0.0001 |
| B (P188) | 0.021 |
| C (HPMC) | <0.0001 |
| AC | 0.0158 |
| BC | 0.0188 |
| Lack of Fit Test | 0.1337 |
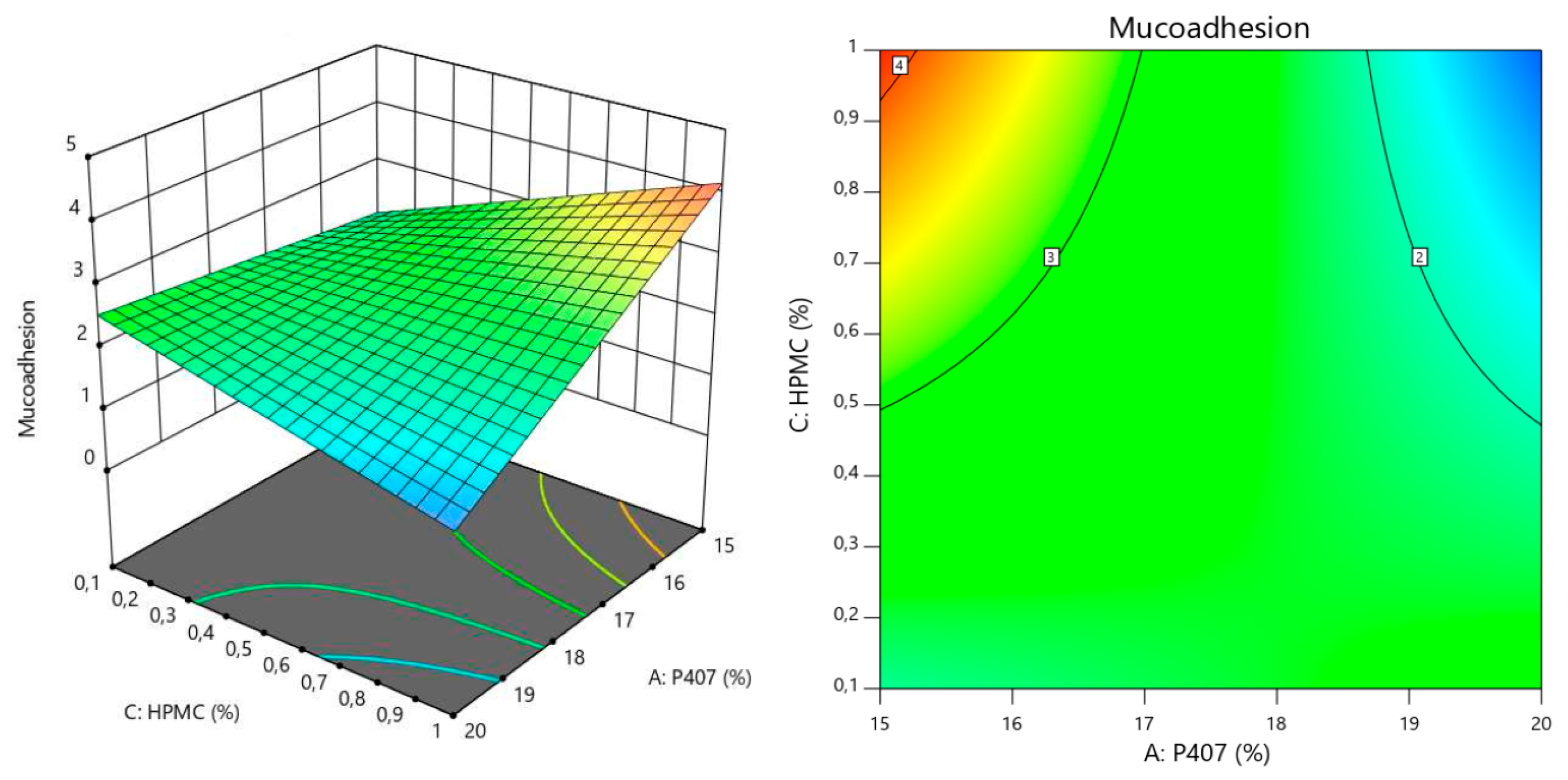
| Mucoadhesion | p value |
| Model | <0.0001 |
| A (P407) | <0.0001 |
| B (P188) | 0.0008 |
| C (HPMC) | 0.0031 |
| AB | 0.0002 |
| AC | <0.0001 |
| B2 | 0.0001 |
| Lack of Fit Test | 0.5154 |
| Fit Equation | |
|---|---|
| Tgel (°C) | Y1 = 113.43 − 7.77X1 − 1.06X2 + 0.07X1X2 + 0.16X12 Adjusted r2 = 0.9487 Predicted r2 = 0.8929 |
| Viscosity (mPas) | Y2 = −275.67 + 19.21X1 + 4.76X2 − 80.85X3 + 11.49X1X3 − 5.53X2X3 Adjusted r2 = 0.9747 Predicted r2 = 0.9480 |
| Mucoadhesion (N·m) | Y3 = −2.51 + 0.23X2 + 0.18X3 + 0.39X1X2 − 0.85X1X3 − 0.45X22 Adjusted r2 = 0.9731 Predicted r2 = 0.9387 |
| Formulation | Tgel (°C) | Viscosity (mPas) | Mucoadhesion (N·m) |
|---|---|---|---|
| Best solution (0.966 desirability) | 32.6798 | 97.0418 | 4.17737 |
| Solution number 19 (0.962 desirability) | 32.7233 | 96.3711 | 4.16372 |
| Point 11 (0.962 desirability) | 32.7249 | 96.3465 | 4.16271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, P.; Castro, A.; Díaz, P.; Minini, L.; Ferraro, F.; Paulsen, E.; Faccio, R.; Pardo, H. Novel Thermosensitive and Mucoadhesive Nasal Hydrogel Containing 5-MeO-DMT Optimized Using Box-Behnken Experimental Design. Polymers 2024, 16, 2148. https://doi.org/10.3390/polym16152148
Miranda P, Castro A, Díaz P, Minini L, Ferraro F, Paulsen E, Faccio R, Pardo H. Novel Thermosensitive and Mucoadhesive Nasal Hydrogel Containing 5-MeO-DMT Optimized Using Box-Behnken Experimental Design. Polymers. 2024; 16(15):2148. https://doi.org/10.3390/polym16152148
Chicago/Turabian StyleMiranda, Pablo, Analía Castro, Paola Díaz, Lucía Minini, Florencia Ferraro, Erika Paulsen, Ricardo Faccio, and Helena Pardo. 2024. "Novel Thermosensitive and Mucoadhesive Nasal Hydrogel Containing 5-MeO-DMT Optimized Using Box-Behnken Experimental Design" Polymers 16, no. 15: 2148. https://doi.org/10.3390/polym16152148







