In Vitro and Ex Vivo Evaluation of Novel Methacrylated Chitosan-PNIPAAm-Hyaluronic Acid Hydrogels Loaded with Progesterone for Applications in Vaginal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
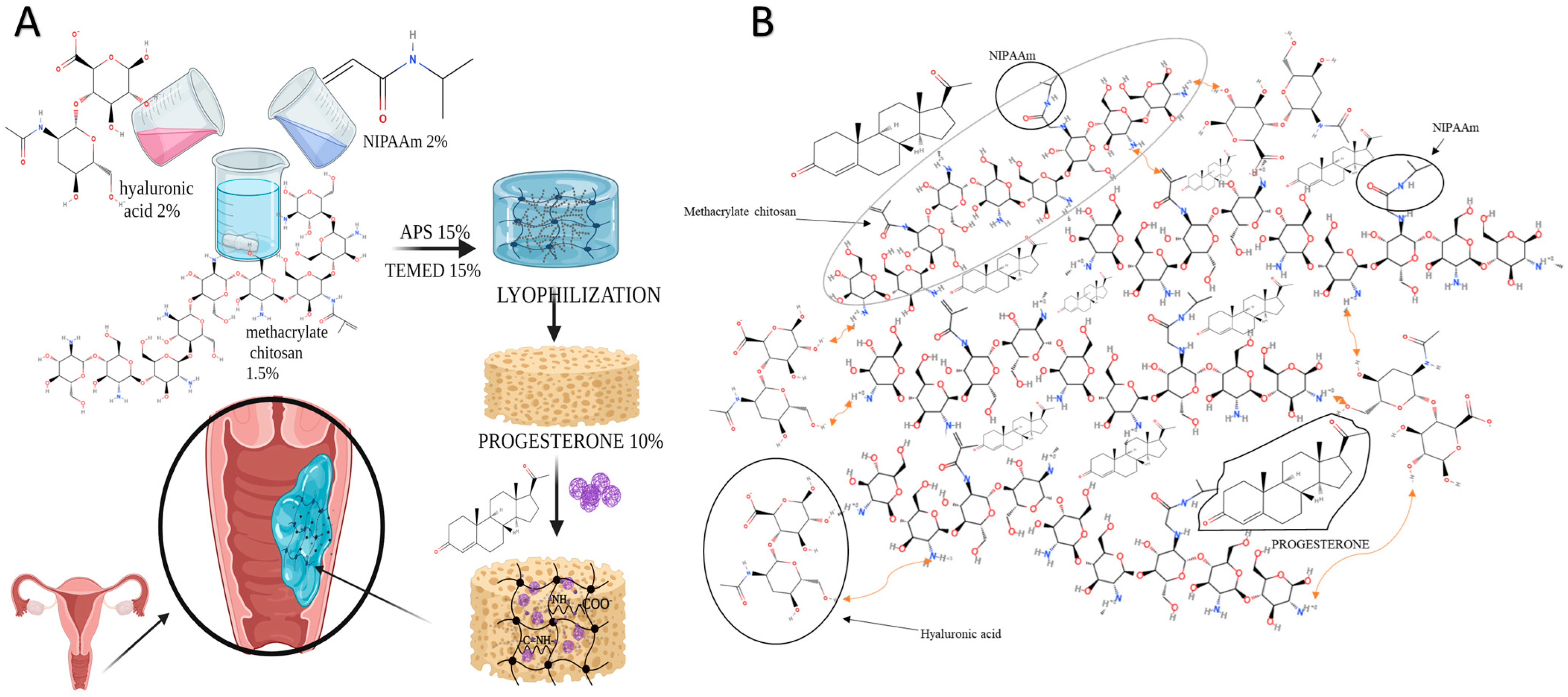
Preparation of CHT-HA/PNIPAAm Hydrogels
2.3. Characterization Methods
2.3.1. FTIR Spectroscopy
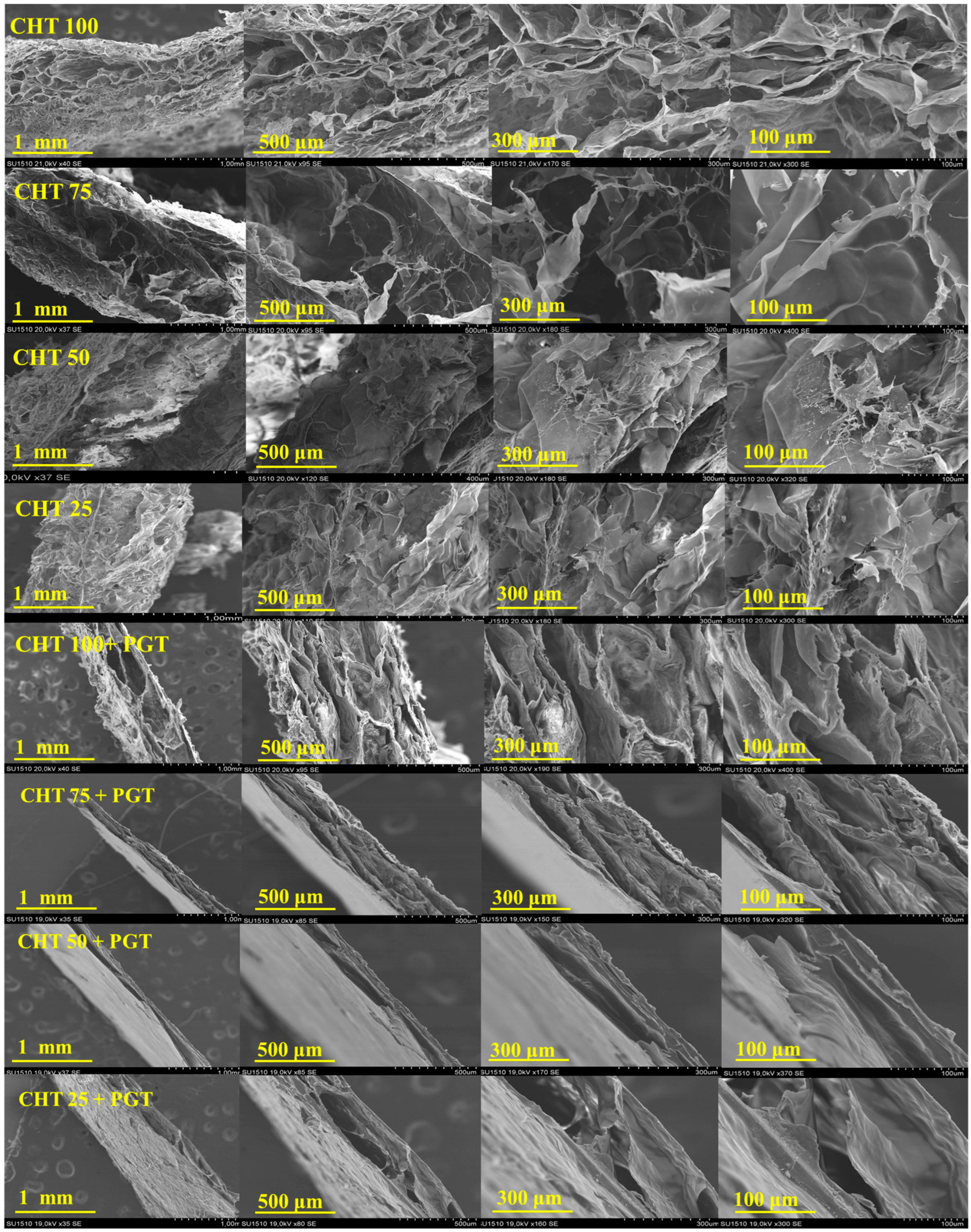
2.3.2. SEM Microscopy
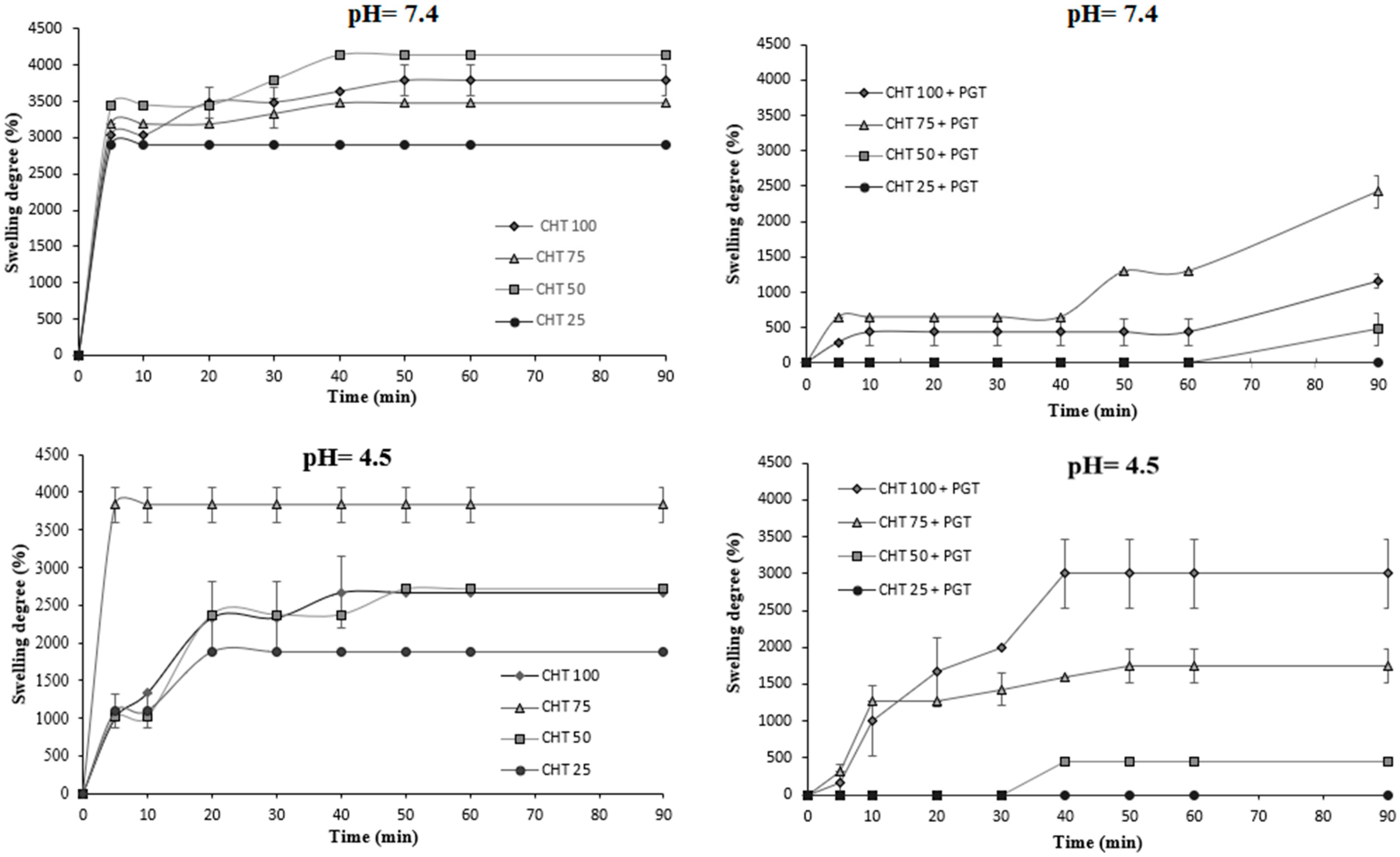
2.3.3. Swelling Properties
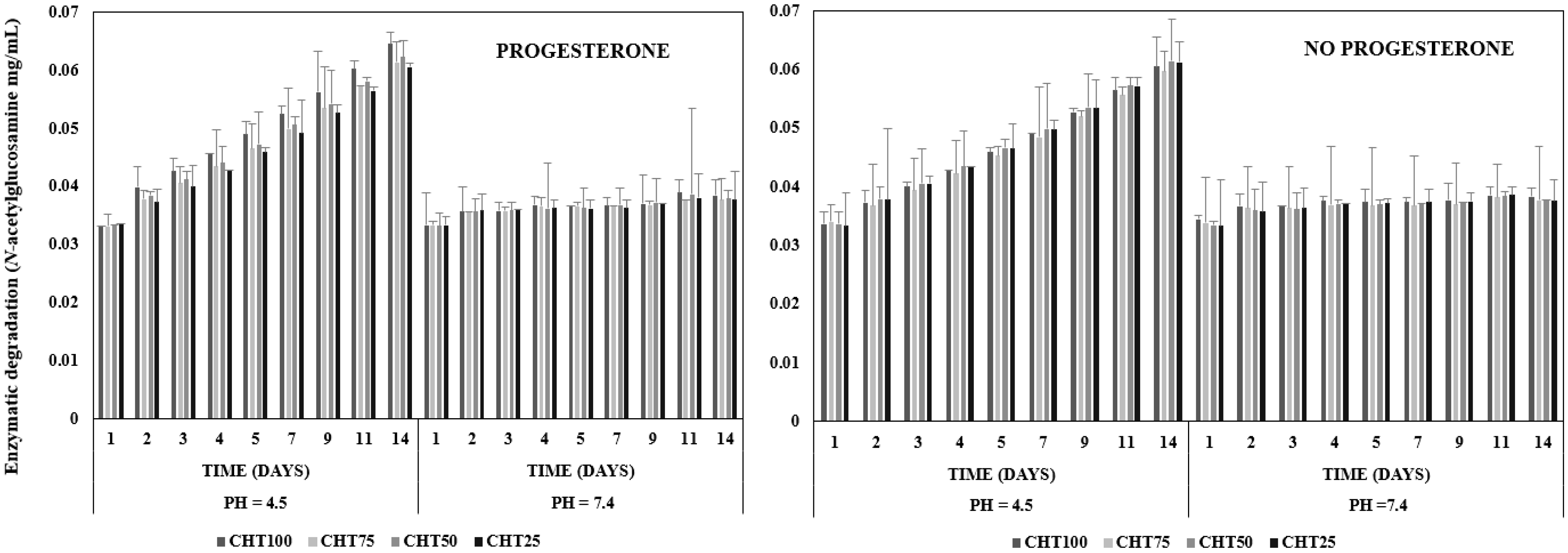
2.3.4. Enzymatic Degradation
2.3.5. In Vitro Release Profiles
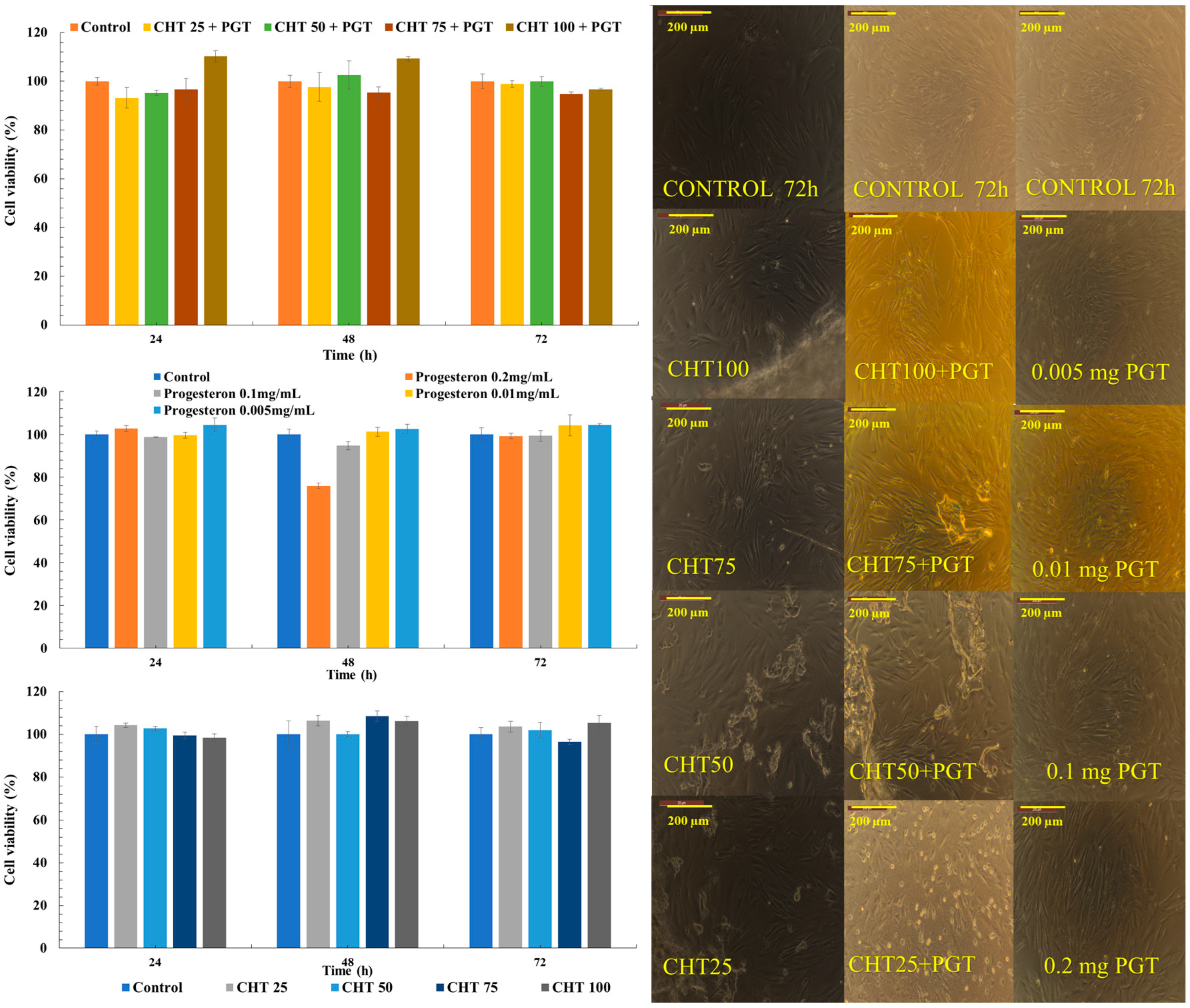
2.3.6. Cytocompatibility Tests
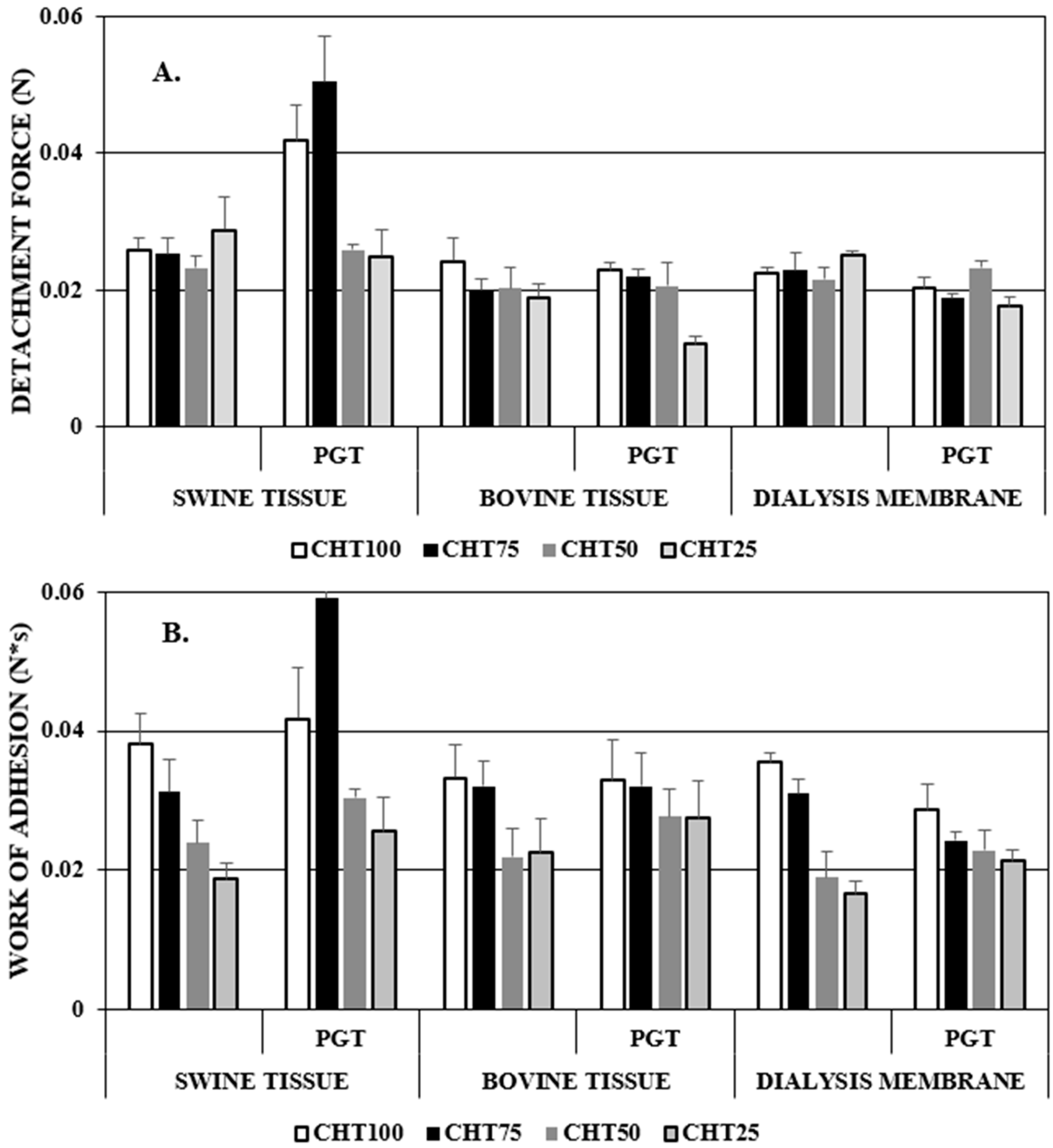
2.3.7. Bioadhesion Tests
Preparation of Simulated Vaginal Fluid (SVF)
3. Results and Discussion
3.1. Hydrogels Structure
3.2. Hydrogels Morphology
3.3. Swelling Properties
3.4. Enzymatic Degradation
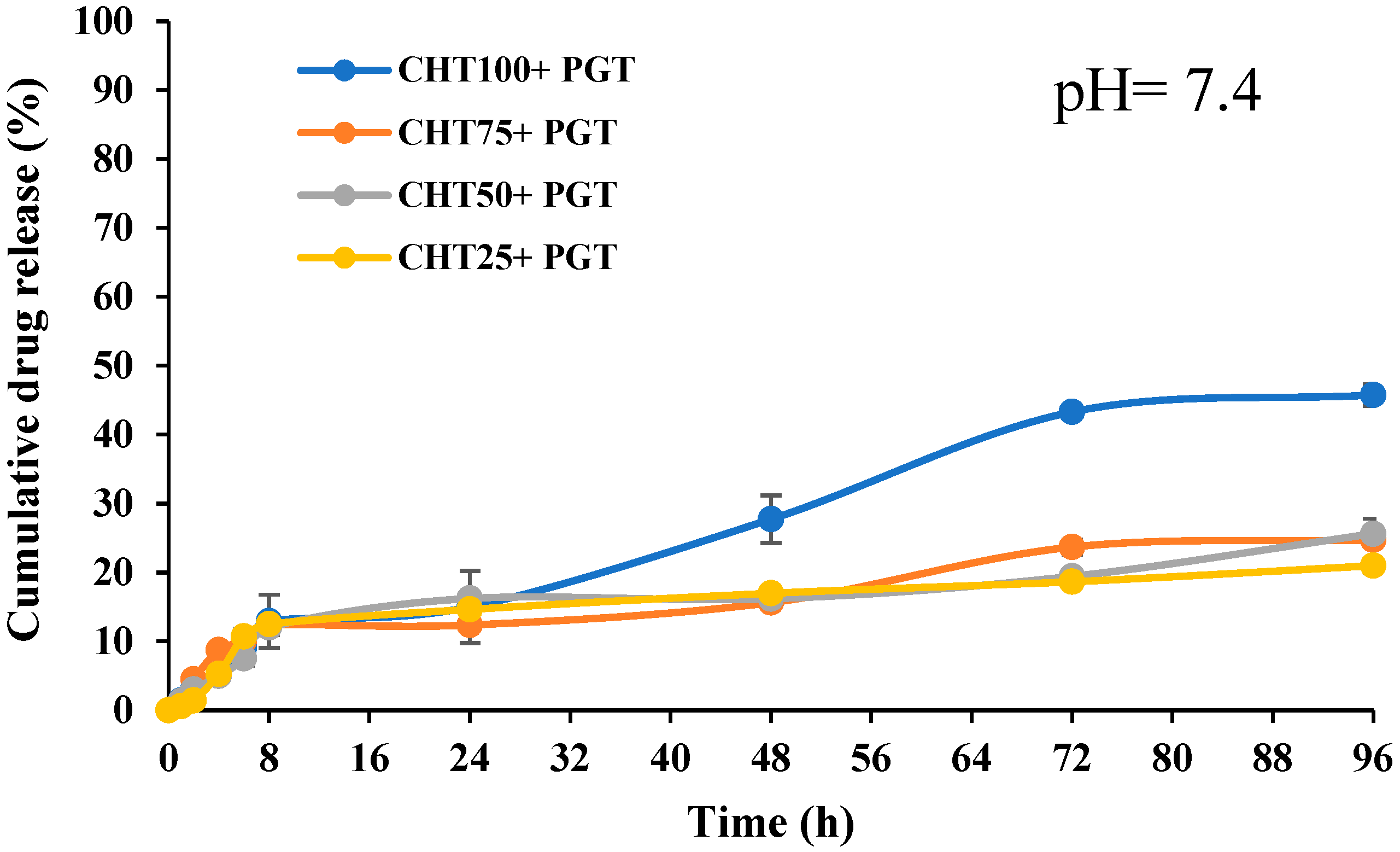
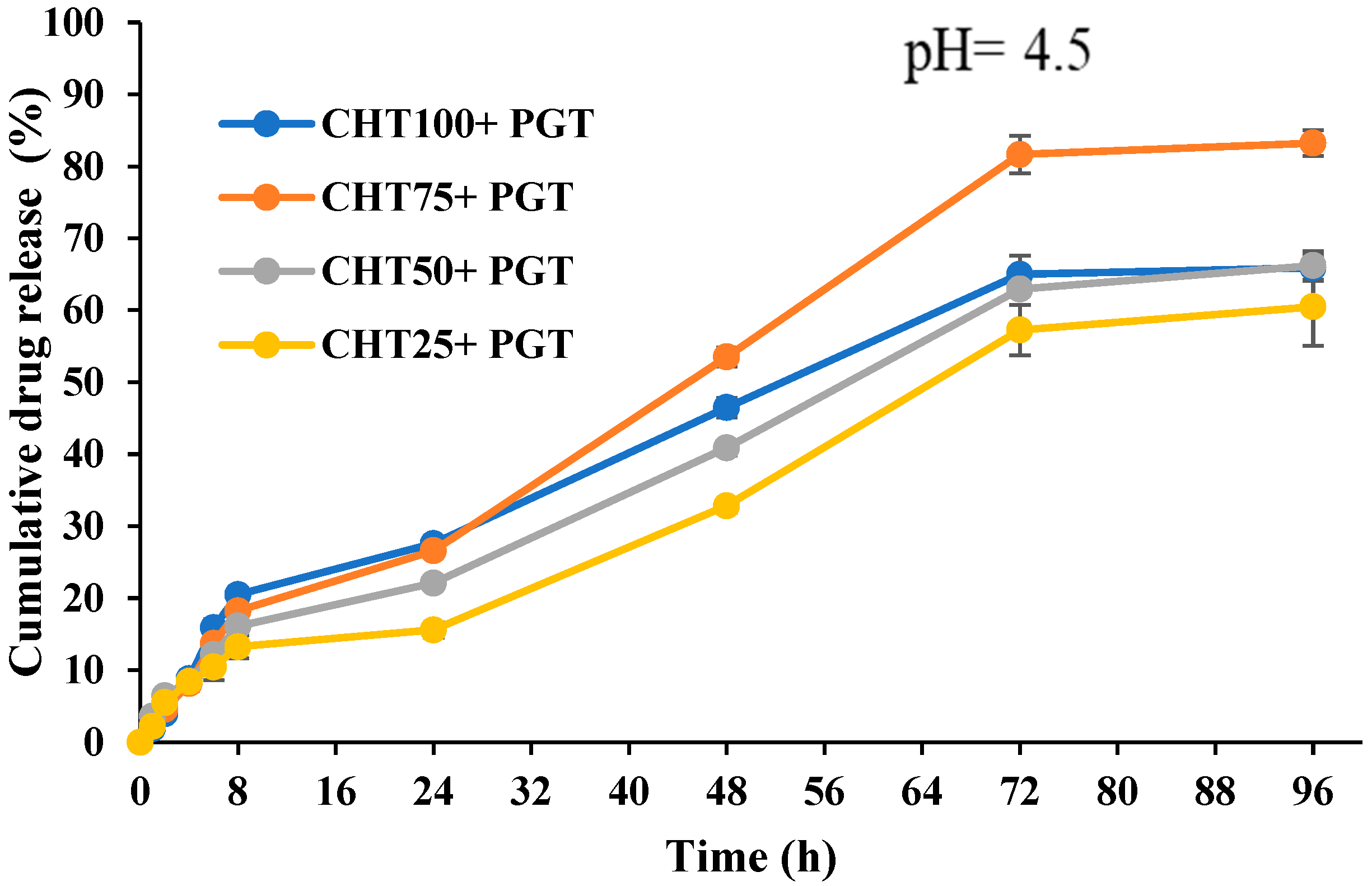
3.5. In Vitro Release Profiles
3.6. Cytocompatibility Tests
3.7. Bioadhesion Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero, R.; Conde-Agudelo, A.; Da Fonseca, E.; O’Brien, J.M.; Cetingoz, E.; Creasy, G.W.; Hassan, S.S.; Nicolaides, K.H. Vaginal Progesterone for Preventing Preterm Birth and Adverse Perinatal Outcomes in Singleton Gestations with a Short Cervix: A Meta-Analysis of Individual Patient Data. Am. J. Obstet. Gynecol. 2018, 218, 161–180. [Google Scholar] [CrossRef]
- Vaisbuch, E.; Leong, M.; Shoham, Z. Progesterone Support in IVF: Is Evidence-Based Medicine Translated to Clinical Practice? A Worldwide Web-Based Survey. Reprod. Biomed. Online 2012, 25, 139–145. [Google Scholar] [CrossRef]
- Sroussi, J.; Bourret, A.; Pourcelot, A.-G.; Thubert, T.; Lesavre, M.; Legendre, G.; Tuffet, S.; Rousseau, A.; Benifla, J.-L. Does Hyaluronic Acid Gel Reduce Intrauterine Adhesions after Dilation and Curettage in Women with Miscarriage? A Multicentric Randomized Controlled Trial (HYFACO Study). Am. J. Obstet. Gynecol. 2022, 227, 597.e1–597.e8. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Nallasamy, S.; Mahendroo, M. Progesterone and Its Receptor Signaling in Cervical Remodeling: Mechanisms of Physiological Actions and Therapeutic Implications. J. Steroid Biochem. Mol. Biol. 2022, 223, 106137. [Google Scholar] [CrossRef]
- Sugita, Y.; Kuwabara, Y.; Katayama, A.; Matsuda, S.; Manabe, I.; Suzuki, S.; Oishi, Y. Characteristic Impairment of Progesterone Response in Cultured Cervical Fibroblasts Obtained from Patients with Refractory Cervical Insufficiency. Sci. Rep. 2023, 13, 11709. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Dyson, M.T.; Coon, V.J.S.; Furukawa, Y.; Yilmaz, B.D.; Maruyama, T.; Bulun, S.E. Generation of Progesterone-Responsive Endometrial Stromal Fibroblasts from Human Induced Pluripotent Stem Cells: Role of the WNT/CTNNB1 Pathway. Stem Cell Rep. 2018, 11, 1136–1155. [Google Scholar] [CrossRef] [PubMed]
- Sundström-Poromaa, I.; Comasco, E.; Sumner, R.; Luders, E. Progesterone—Friend or Foe? Front. Neuroendocrinol. 2020, 59, 100856. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Beneficial Effects of Hyaluronic Acid. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 72, pp. 137–176. [Google Scholar] [CrossRef]
- Bai, Q.; Gao, Q.; Hu, F.; Zheng, C.; Chen, W.; Sun, N.; Liu, J.; Zhang, Y.; Wu, X.; Lu, T. Chitosan and Hyaluronic-Based Hydrogels Could Promote the Infected Wound Healing. Int. J. Biol. Macromol. 2023, 232, 123271. [Google Scholar] [CrossRef]
- Buckley, C.; Murphy, E.J.; Montgomery, T.R.; Major, I. Hyaluronic Acid: A Review of the Drug Delivery Capabilities of This Naturally Occurring Polysaccharide. Polymers 2022, 14, 3442. [Google Scholar] [CrossRef]
- Kikani, T.; Dave, S.; Thakore, S. Functionalization of Hyaluronic Acid for Development of Self-Healing Hydrogels for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2023, 242, 124950. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and Thermogelling Systems for Vaginal Drug Delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.H.C.; Prado, I.M. Hydrogels Based on Natural Polysaccharides and Their Applications. In Comprehensive Glycoscience; Elsevier: Amsterdam, The Netherlands, 2021; pp. 71–92. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Yang, F.; Wang, F.; Li, H.; Tian, H.; Wang, G. Chitosan Hydrogel Loaded with Recombinant Protein Containing Epitope C from HSP90 of Candida Albicans Induces Protective Immune Responses against Systemic Candidiasis. Int. J. Biol. Macromol. 2021, 173, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Guo, A.; Zhao, Y.; Sun, X. A High Strength, Low Friction, and Biocompatible Hydrogel from PVA, Chitosan and Sodium Alginate for Articular Cartilage. Carbohydr. Polym. 2022, 286, 119268. [Google Scholar] [CrossRef] [PubMed]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds. J. Tissue Repair Regen. 2017, 1, 12–22. [Google Scholar] [CrossRef]
- Talaei, F.; Azhdarzadeh, M.; Hashemi Nasel, H.; Moosavi, M.; Foroumadi, A.; Dinarvand, R.; Atyabi, F. Core Shell Methyl Methacrylate Chitosan Nanoparticles: In Vitro Mucoadhesion and Complement Activation. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 257–265. [Google Scholar]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated Chitosan as a Polymer with Enhanced Mucoadhesive Properties for Transmucosal Drug Delivery. Int. J. Pharm. 2018, 550, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Pandey, S. Methyl Methacrylate Modified Chitosan: Synthesis, Characterization and Application in Drug and Gene Delivery. Carbohydr. Polym. 2019, 211, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Lim, S.; Choi, J.S. On the Nature of Wetting Transition on High-Aspect-Ratio pNIPAAm Micropillar Structures. Surf. Interfaces 2022, 31, 102062. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, R.; Xu, Y.; Cao, Y.; Xiao, L. A One-Pot Synthesis of Thermosensitive PNIPAAM Interpenetration Polymer Networks(IPN) Hydrogels. JCIS Open 2021, 1, 100002. [Google Scholar] [CrossRef]
- Puleo, G.L.; Zulli, F.; Piovanelli, M.; Giordano, M.; Mazzolai, B.; Beccai, L.; Andreozzi, L. Mechanical and Rheological Behavior of pNIPAAM Crosslinked Macrohydrogel. React. Funct. Polym. 2013, 73, 1306–1318. [Google Scholar] [CrossRef]
- Fang, J. Temperature-Sensitive Hydrogels Composed of Chitosan and Hyaluronic Acid as Injectable Carriers for Drug Delivery. Eur. J. Pharm. Biopharm. 2008, 68, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, T.; Zhang, H.; Dai, H.; Gao, H.; Feng, W.; Xu, D.; Duan, J. The Application of pH-Responsive Hyaluronic Acid-Based Essential Oils Hydrogels with Enhanced Anti-Biofilm and Wound Healing. Int. J. Biol. Macromol. 2024, 275, 133559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Peng, X.; Zheng, Y.; Cheng, Z.; Sun, S.; Ding, Q.; Liu, W.; Ding, C. A Poloxamer/Hyaluronic Acid/Chitosan-Based Thermosensitive Hydrogel That Releases Dihydromyricetin to Promote Wound Healing. Int. J. Biol. Macromol. 2022, 216, 475–486. [Google Scholar] [CrossRef]
- Almomen, A.; Cho, S.; Yang, C.-H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janát-Amsbury, M.M. Thermosensitive Progesterone Hydrogel: A Safe and Effective New Formulation for Vaginal Application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef]
- Shapiro, R.L.; Bockley, K.M.; Hsueh, H.T.; Appell, M.B.; Carter, D.M.; Ortiz, J.; Brayton, C.; Ensign, L.M. Hypotonic, Gel-Forming Delivery System for Vaginal Drug Administration. J. Control. Release 2024, 371, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Zou, P.; Dong, J.; Sun, M.; Ding, P.; Han, Y.; Ji, C.; Zhou, Q.; Yuan, H.; Suo, J. Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception. Appl. Sci. 2019, 9, 1638. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and Characterization of Hybrid Hyaluronic Acid-Gelatin Hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; Di Cagno, M.P. Interpreting Non-Linear Drug Diffusion Data: Utilizing Korsmeyer-Peppas Model to Study Drug Release from Liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Knuth, K.; Amiji, M.; Robinson, J.R. Hydrogel Delivery Systems for Vaginal and Oral Applications. Adv. Drug Deliv. Rev. 1993, 11, 137–167. [Google Scholar] [CrossRef]
- Tester, R.; Al-Ghazzewi, F.H. Intrinsic and Extrinsic Carbohydrates in the Vagina: A Short Review on Vaginal Glycogen. Int. J. Biol. Macromol. 2018, 112, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Zhang, H. Solvent Effects on Infrared Spectra of Progesterone in CHCl3/Cyclo-C6H12 Binary Solvent Systems. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2007, 66, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, N.S.; Turino, L.N.; Luna, J.A.; Mengatto, L.N. Progesterone Loaded Thermosensitive Hydrogel for Vaginal Application: Formulation and in Vitro Comparison with Commercial Product. Saudi Pharm. J. 2019, 27, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Koohzad, F.; Asoodeh, A. Development of a Highly Porous Bioscaffold by the Combination of Bubble Entrapping and Freezing-Thawing Techniques to Fabricate Hyaluronic Acid/Gelatin Tri-Layer Wound Dressing. Int. J. Biol. Macromol. 2024, 260, 129206. [Google Scholar] [CrossRef] [PubMed]
- Ziminska, M.; Wilson, J.J.; McErlean, E.; Dunne, N.; McCarthy, H.O. Synthesis and Evaluation of a Thermoresponsive Degradable Chitosan-Grafted PNIPAAm Hydrogel as a “Smart” Gene Delivery System. Materials 2020, 13, 2530. [Google Scholar] [CrossRef] [PubMed]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze–Thaw Hydrogel Fabrication Method: Basic Principles, Synthesis Parameters, Properties, and Biomedical Applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. Controlled Freezing and Freeze Drying: A Versatile Route for Porous and Micro-/Nano-Structured Materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Das Purkayastha, S.; Bhattacharya, M.K.; Prasad, H.K.; De Mandal, S. Diversity and the Antimicrobial Activity of Vaginal Lactobacilli: Current Status and Future Prospective. In Recent Advancements in Microbial Diversity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 397–422. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Barroso, N.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. 3D Printable Self-Healing Hyaluronic Acid/Chitosan Polycomplex Hydrogels with Drug Release Capability. Int. J. Biol. Macromol. 2021, 188, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Afloarea, O.-T.; Cheaburu Yilmaz, C.N.; Verestiuc, L.; Bibire, N. Development of Vaginal Carriers Based on Chitosan-Grafted-PNIPAAm for Progesterone Administration. Gels 2022, 8, 596. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Photocrosslinkable and Self-Healable Hydrogels of Chitosan and Hyaluronic Acid. Int. J. Biol. Macromol. 2022, 216, 291–302. [Google Scholar] [CrossRef]
- Ritt, C.L.; Werber, J.R.; Wang, M.; Yang, Z.; Zhao, Y.; Kulik, H.J.; Elimelech, M. Ionization Behavior of Nanoporous Polyamide Membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 30191–30200. [Google Scholar] [CrossRef] [PubMed]
- Polexe, R.; Delair, T. Elaboration of Stable and Antibody Functionalized Positively Charged Colloids by Polyelectrolyte Complexation between Chitosan and Hyaluronic Acid. Molecules 2013, 18, 8563–8578. [Google Scholar] [CrossRef]
- Collado-González, M.; González Espinosa, Y.; Goycoolea, F.M. Interaction Between Chitosan and Mucin: Fundamentals and Applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The Enzymatic Degradation and Swelling Properties of Chitosan Matrices with Different Degrees of N-Acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, M.; Sun, H.; Yang, Y.; Sun, S.; Yu, H.; He, M.; Sun, Y.; Cheng, Y. Preparation and Characterization of Photo-Oxidative Dual-Crosslinked Chitosan/Hyaluronic Acid Hydrogels. React. Funct. Polym. 2022, 180, 105378. [Google Scholar] [CrossRef]
- Zhu, L.; Bratlie, K.M. pH Sensitive Methacrylated Chitosan Hydrogels with Tunable Physical and Chemical Properties. Biochem. Eng. J. 2018, 132, 38–46. [Google Scholar] [CrossRef]
- El Idrissi, A.; Channab, B.; Essamlali, Y.; Zahouily, M. Superabsorbent Hydrogels Based on Natural Polysaccharides: Classification, Synthesis, Physicochemical Properties, and Agronomic Efficacy under Abiotic Stress Conditions: A Review. Int. J. Biol. Macromol. 2024, 258, 128909. [Google Scholar] [CrossRef] [PubMed]
- Argin, S.; Kofinas, P.; Lo, Y.M. The Cell Release Kinetics and the Swelling Behavior of Physically Crosslinked Xanthan–Chitosan Hydrogels in Simulated Gastrointestinal Conditions. Food Hydrocoll. 2014, 40, 138–144. [Google Scholar] [CrossRef]
- Casey-Power, S.; Ryan, R.; Behl, G.; McLoughlin, P.; Byrne, M.E.; Fitzhenry, L. Hyaluronic Acid: Its Versatile Use in Ocular Drug Delivery with a Specific Focus on Hyaluronic Acid-Based Polyelectrolyte Complexes. Pharmaceutics 2022, 14, 1479. [Google Scholar] [CrossRef]
- Busatto, C.; Pesoa, J.; Helbling, I.; Luna, J.; Estenoz, D. Effect of Particle Size, Polydispersity and Polymer Degradation on Progesterone Release from PLGA Microparticles: Experimental and Mathematical Modeling. Int. J. Pharm. 2018, 536, 360–369. [Google Scholar] [CrossRef]
- Zhang, Y.; Shams, T.; Harker, A.H.; Parhizkar, M.; Edirisinghe, M. Effect of Copolymer Composition on Particle Morphology and Release Behavior in Vitro Using Progesterone. Mater. Des. 2018, 159, 57–67. [Google Scholar] [CrossRef]
- Chantilis, S.J.; Zeitoun, K.M.; Patel, S.I.; Johns, D.A.; Madziar, V.A.; McIntire, D.D. Use of Crinone∗ Vaginal Progesterone Gel for Luteal Support in in Vitro Fertilization Cycles. Fertil. Steril. 1999, 72, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, M.; Jeyakumar, S.; Veerappa, V.G.; Pushpadhas, H.A.; Ramesha, K.P.; Kumaresan, A.; Manimaran, A.; Eljeeva Emerald, F.M. Fabrication and Characterization of Progesterone Loaded Pullulan Nanofibers for Controlled Release. J. Drug Deliv. Sci. Technol. 2024, 91, 105193. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in Vitro Drug Release of pH- and Temperature-Sensitive Double Cross-Linked Interpenetrating Polymer Network Hydrogels Based on Hyaluronic Acid/Poly (N-Isopropylacrylamide) for Transdermal Delivery of Luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Kapuscinska, A.; Schroeder, G.; Nowak, I. Physico-Chemical Characterization of Formulations Containing Endomorphin-2 Derivatives. Amino Acids 2017, 49, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Shaibie, N.A.; Ramli, N.A.; Mohammad Faizal, N.D.F.; Srichana, T.; Mohd Amin, M.C.I. Poly( N -isopropylacrylamide)-Based Polymers: Recent Overview for the Development of Temperature-Responsive Drug Delivery and Biomedical Applications. Macromol. Chem. Phys. 2023, 224, 2300157. [Google Scholar] [CrossRef]
- Li, G.; Guo, L.; Chang, X.; Yang, M. Thermo-Sensitive Chitosan Based Semi-IPN Hydrogels for High Loading and Sustained Release of Anionic Drugs. Int. J. Biol. Macromol. 2012, 50, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova, L.; Vitku, J.; Suchopar, J.; Hill, M.; Parizek, A. Progesterone: A Steroid with Wide Range of Effects in Physiology as Well as Human Medicine. Int. J. Mol. Sci. 2022, 23, 7989. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Partheniadis, I.; Nikolakakis, I.; Al-Obaidi, H. Solubility Improvement of Progesterone from Solid Dispersions Prepared by Solvent Evaporation and Co-Milling. Polymers 2020, 12, 854. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, X.; Wang, H.; Zhou, J.; Lin, X.; Yang, P. Vagina, a Promising Route for Drug Delivery. J. Drug Deliv. Sci. Technol. 2024, 93, 105397. [Google Scholar] [CrossRef]
- Cicinelli, E.; Rubini, G.; De Ziegler, D.; Barba, B.; Pinto, V.; Di Stefano, M.G.; Mele, M. Absorption and Preferential Vagina-to-Uterus Distribution after Vaginal Administration of 99mTc-Pertechnetate in Postmenopausal Women. Fertil. Steril. 2001, 76, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Nacu, I.; Bercea, M.; Niță, L.E.; Peptu, C.A.; Butnaru, M.; Vereștiuc, L. 3D Bioprinted Scaffolds Based on Functionalized Gelatin for Soft Tissue Engineering. React. Funct. Polym. 2023, 190, 105636. [Google Scholar] [CrossRef]
- Shukla, V.; Barnhouse, V.; Ackerman, W.E.; Summerfield, T.L.; Powell, H.M.; Leight, J.L.; Kniss, D.A.; Ghadiali, S.N. Cellular Mechanics of Primary Human Cervical Fibroblasts: Influence of Progesterone and a Pro-Inflammatory Cytokine. Ann. Biomed. Eng. 2018, 46, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Capella, V.; Rivero, R.E.; Liaudat, A.C.; Ibarra, L.E.; Roma, D.A.; Alustiza, F.; Mañas, F.; Barbero, C.A.; Bosch, P.; Rivarola, C.R.; et al. Cytotoxicity and Bioadhesive Properties of Poly-N-Isopropylacrylamide Hydrogel. Heliyon 2019, 5, e01474. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Wojasiński, M.; Dąbrowska, J.; Krzyżowska, M.; Nowicka, M.; Ciach, T.; Winnicka, K. Chitosan-Poly(Ethylene Oxide) Nanofibrous Mat as a Vaginal Platform for Tenofovir Disoproxyl FumarateThe Effect of Vaginal pH on Drug Carrier Performance. Int. J. Biol. Macromol. 2022, 222, 856–867. [Google Scholar] [CrossRef]
- Deshkar, S.S.; Shirolkar, S.V.; Patil, A.T. Vaginal Bioadhesive Drug Delivery Systems and Their Applications. In Bioadhesives in Drug Delivery; Mittal, K.L., Bakshi, I.S., Narang, J.K., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 307–369. [Google Scholar] [CrossRef]
- Valamla, B.; Thakor, P.; Phuse, R.; Dalvi, M.; Kharat, P.; Kumar, A.; Panwar, D.; Singh, S.B.; Giorgia, P.; Mehra, N.K. Engineering Drug Delivery Systems to Overcome the Vaginal Mucosal Barrier: Current Understanding and Research Agenda of Mucoadhesive Formulations of Vaginal Delivery. J. Drug Deliv. Sci. Technol. 2022, 70, 103162. [Google Scholar] [CrossRef]
- Brockhausen, I. Biosynthesis of Complex Mucin-Type O-Glycans. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; pp. 315–350. [Google Scholar] [CrossRef]
- Tuğcu-DemiRöz, F. Development of in Situ Poloxamer-Chitosan Hydrogels for Vaginal Drug Delivery of Benzydamine Hydrochloride: Textural, Mucoadhesive and in Vitro Release Properties. Marmara Pharm. J. 2017, 21, 762–770. [Google Scholar] [CrossRef]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of Mucoadhesive Vaginal Hydrogels Insensitive to Dilution with Vaginal Fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef]
- Babelyte, M.; Peciulyte, L.; Navikaite-Snipaitiene, V.; Bendoraitiene, J.; Samaryk, V.; Rutkaite, R. Synthesis and Characterization of Thermoresponsive Chitosan-Graft-Poly(N-Isopropylacrylamide) Copolymers. Polymers 2023, 15, 3154. [Google Scholar] [CrossRef]
- Peppas, N.A.; Huang, Y. Nanoscale Technology of Mucoadhesive Interactions. Adv. Drug Deliv. Rev. 2004, 56, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Khutoryanskiy, V.V. Mucoadhesion and Mucosa-Mimetic Materials—A Mini-Review. Int. J. Pharm. 2015, 495, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Haugstad, K.; Håti, A.; Nordgård, C.; Adl, P.; Maurstad, G.; Sletmoen, M.; Draget, K.; Dias, R.; Stokke, B. Direct Determination of Chitosan–Mucin Interactions Using a Single-Molecule Strategy: Comparison to Alginate–Mucin Interactions. Polymers 2015, 7, 161–185. [Google Scholar] [CrossRef]
- Campaña-Seoane, M.; Peleteiro, A.; Laguna, R.; Otero-Espinar, F.J. Bioadhesive Emulsions for Control Release of Progesterone Resistant to Vaginal Fluids Clearance. Int. J. Pharm. 2014, 477, 495–505. [Google Scholar] [CrossRef] [PubMed]


| COD | CHITOSAN wt/wt % | NIPAAm wt/wt % | Formulation |
|---|---|---|---|
| CHT 100 | 100 | 0 | The polymer solution (CHT-HA) was mixed with NIPAAm and the initiator solutions, for 4 h at 60 °C, purification and freeze-thaw cycling (3 cycles), hydrogels lyophilization. |
| CHT 75 | 75 | 25 | |
| CHT 50 | 50 | 50 | |
| CHT 25 | 25 | 75 | |
| CHT 100 PGT | 100 | 0 | The polymer solution (CHT-HA) was mixed with NIPAAm and the initiator solutions, 4 h 60 °C, purification and freeze-thaw cycling (3 cycles). Finally, the hydrogels were lyophilized and loaded with 10% progesterone. |
| CHT 75 PGT | 75 | 25 | |
| CHT 50 PGT | 50 | 50 | |
| CHT 25 PGT | 25 | 75 |
| pH | CHT100 | CHT75 | CHT50 | CHT25 | CHT100+PGT | CHT75+PGT | CHT50+PGT | CHT25+PGT |
|---|---|---|---|---|---|---|---|---|
| 7.4 | 3788% | 3478% | 4138% | 2899% | 1159% | 2903% | 1270% | 615% |
| 4.5 | 2667% | 3833% | 2712% | 1875% | 3000% | 1746% | 455% | 0% |
| pH 4.5 | CHT25+PGT | CHT50+PGT | CHT75+PGT | CHT100+PGT | pH 7.4 | CHT25+PGT | CHT50+PGT | CHT75+PGT | CHT100+PGT |
|---|---|---|---|---|---|---|---|---|---|
| k | 0.194 | 0.351 | 0.351 | 0.434 | k | 0.22 | 0.381 | 0.32 | 0.368 |
| n | 0.744 | 0.628 | 0.71 | 0.589 | n | 0.655 | 0.386 | 0.423 | 0.369 |
| R2 | 0.984 | 0.988 | 0.99 | 0.99 | R2 | 0.988 | 0.966 | 0.97 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afloarea, O.-T.; Nacu, I.; Vereștiuc, L.; Yilmaz, C.N.; Panainte, A.D.; Peptu, C.A.; Ostafe, I.-G.; Bibire, N. In Vitro and Ex Vivo Evaluation of Novel Methacrylated Chitosan-PNIPAAm-Hyaluronic Acid Hydrogels Loaded with Progesterone for Applications in Vaginal Delivery. Polymers 2024, 16, 2160. https://doi.org/10.3390/polym16152160
Afloarea O-T, Nacu I, Vereștiuc L, Yilmaz CN, Panainte AD, Peptu CA, Ostafe I-G, Bibire N. In Vitro and Ex Vivo Evaluation of Novel Methacrylated Chitosan-PNIPAAm-Hyaluronic Acid Hydrogels Loaded with Progesterone for Applications in Vaginal Delivery. Polymers. 2024; 16(15):2160. https://doi.org/10.3390/polym16152160
Chicago/Turabian StyleAfloarea, Oana-Teodora, Isabella Nacu, Liliana Vereștiuc, Cătălina Natalia Yilmaz, Alina Diana Panainte, Cătălina Anișoara Peptu, Iulia-Giorgiana Ostafe, and Nela Bibire. 2024. "In Vitro and Ex Vivo Evaluation of Novel Methacrylated Chitosan-PNIPAAm-Hyaluronic Acid Hydrogels Loaded with Progesterone for Applications in Vaginal Delivery" Polymers 16, no. 15: 2160. https://doi.org/10.3390/polym16152160





