Addition of Coffee Waste-Derived Plasticizer Improves Processability and Barrier Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-Natural Rubber Bioplastic
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Plasticized Blends
3. Characterization of Plasticized Sheets
3.1. Thermal Characterization
3.1.1. Differential Scanning Calorimetry-DSC
3.1.2. Thermal Degradation Analysis-TGA
3.2. X-ray Diffraction (XRD)
3.3. Permeability Testing
3.3.1. Oxygen Transmission Rate (OTR)
3.3.2. Water Vapor Transmission Rate (WVTR)
3.4. Water Uptake Capacity
3.5. Water Contact Angle
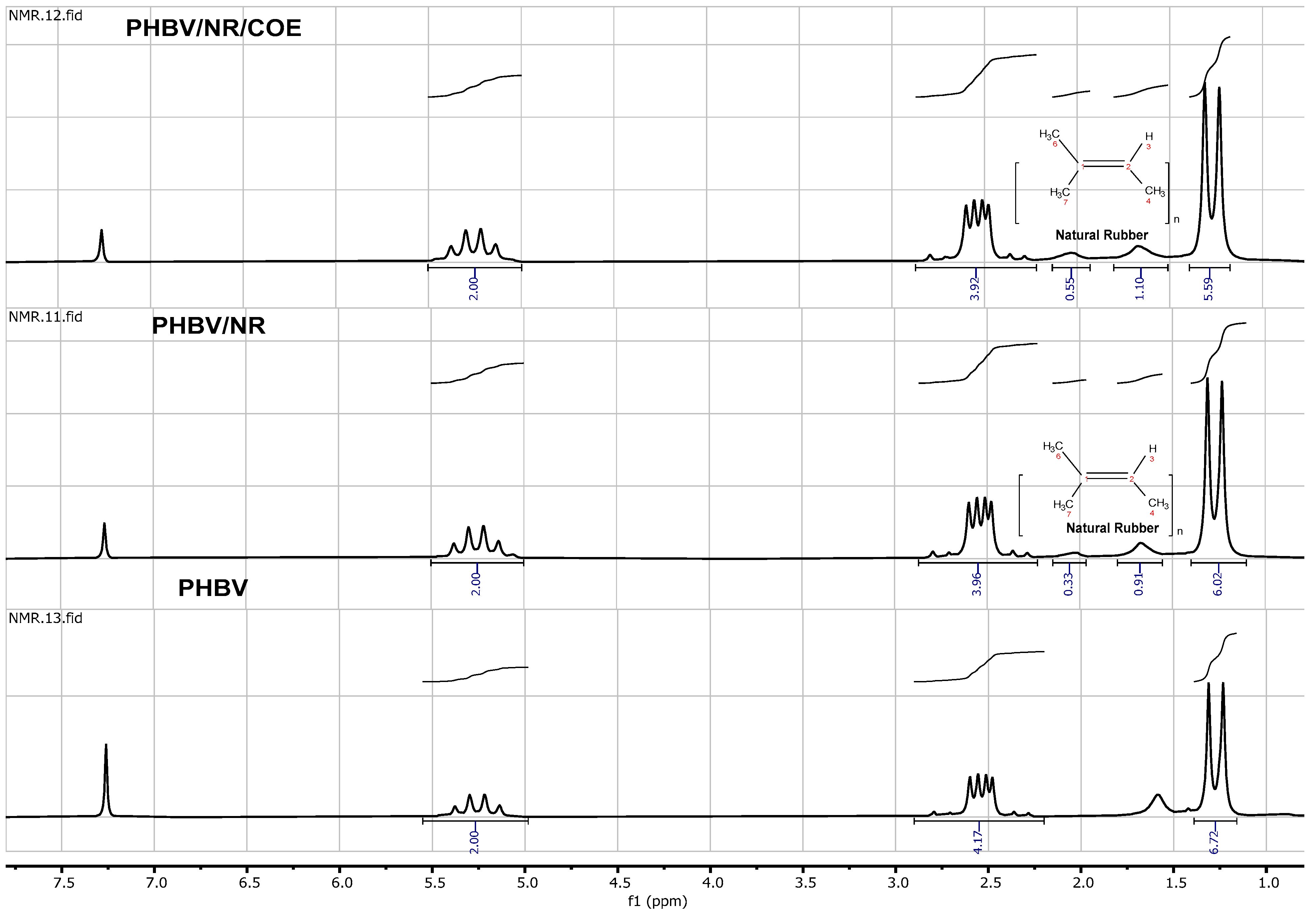
3.6. Nuclear Magnetic Resonance (NMR)
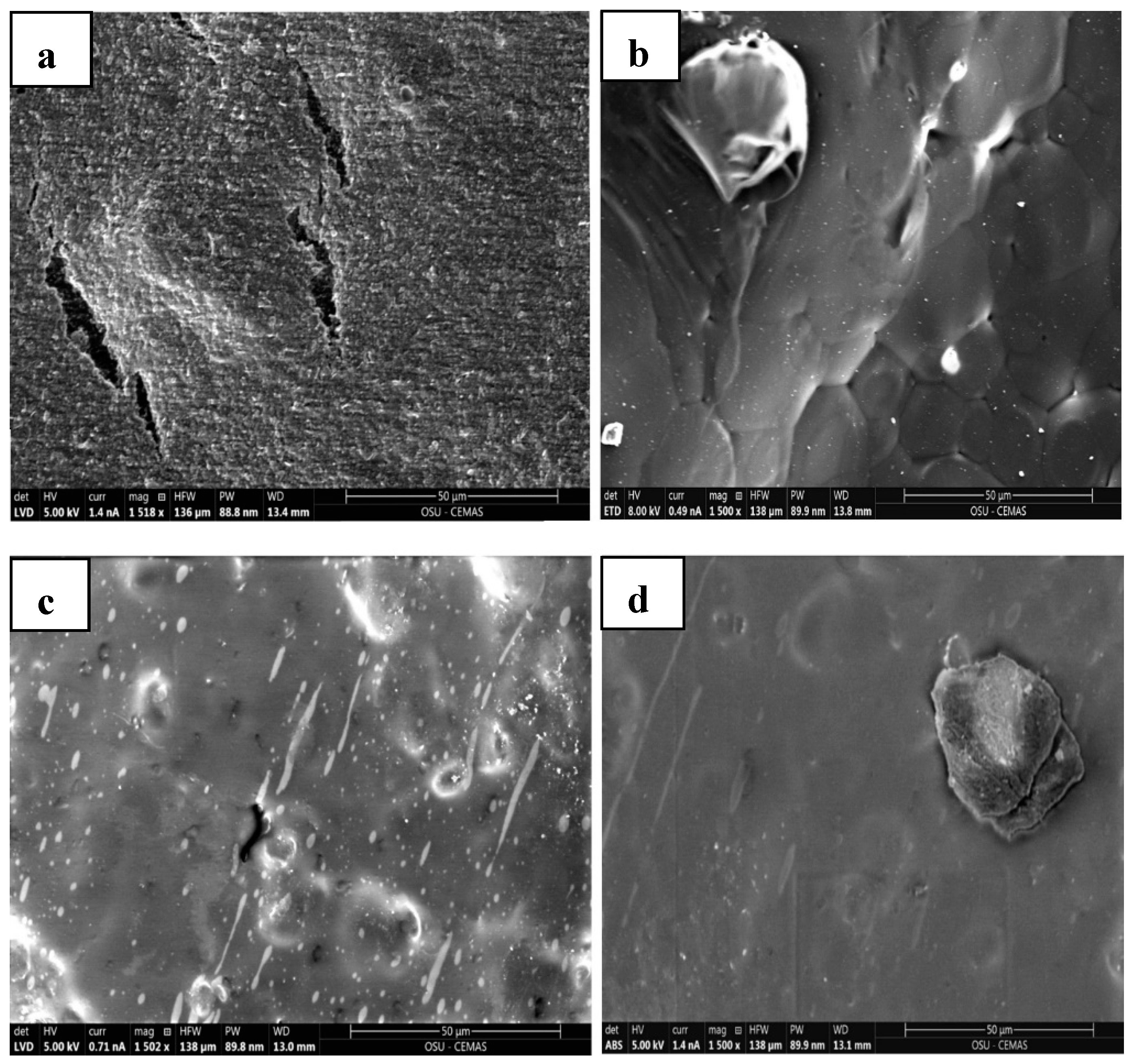
3.7. Scanning Electron Microscopy (SEM) and EDAX
4. Results and Discussion
4.1. Effect of Plasticizer on Crystallization and Melting Behaviors
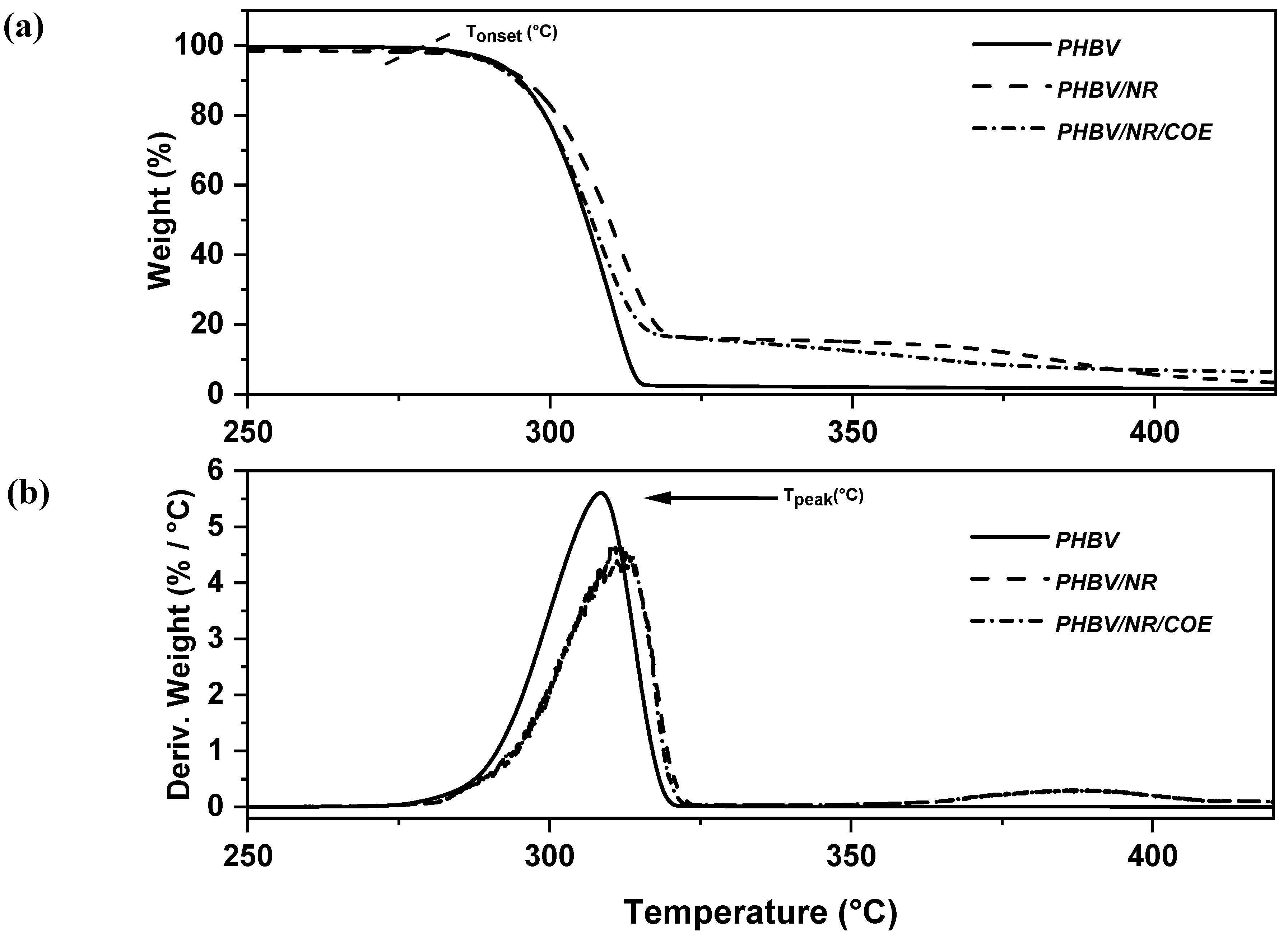
4.2. Effect of Plasticizer on Thermal Stability of PHBV/NR Blended Sheets
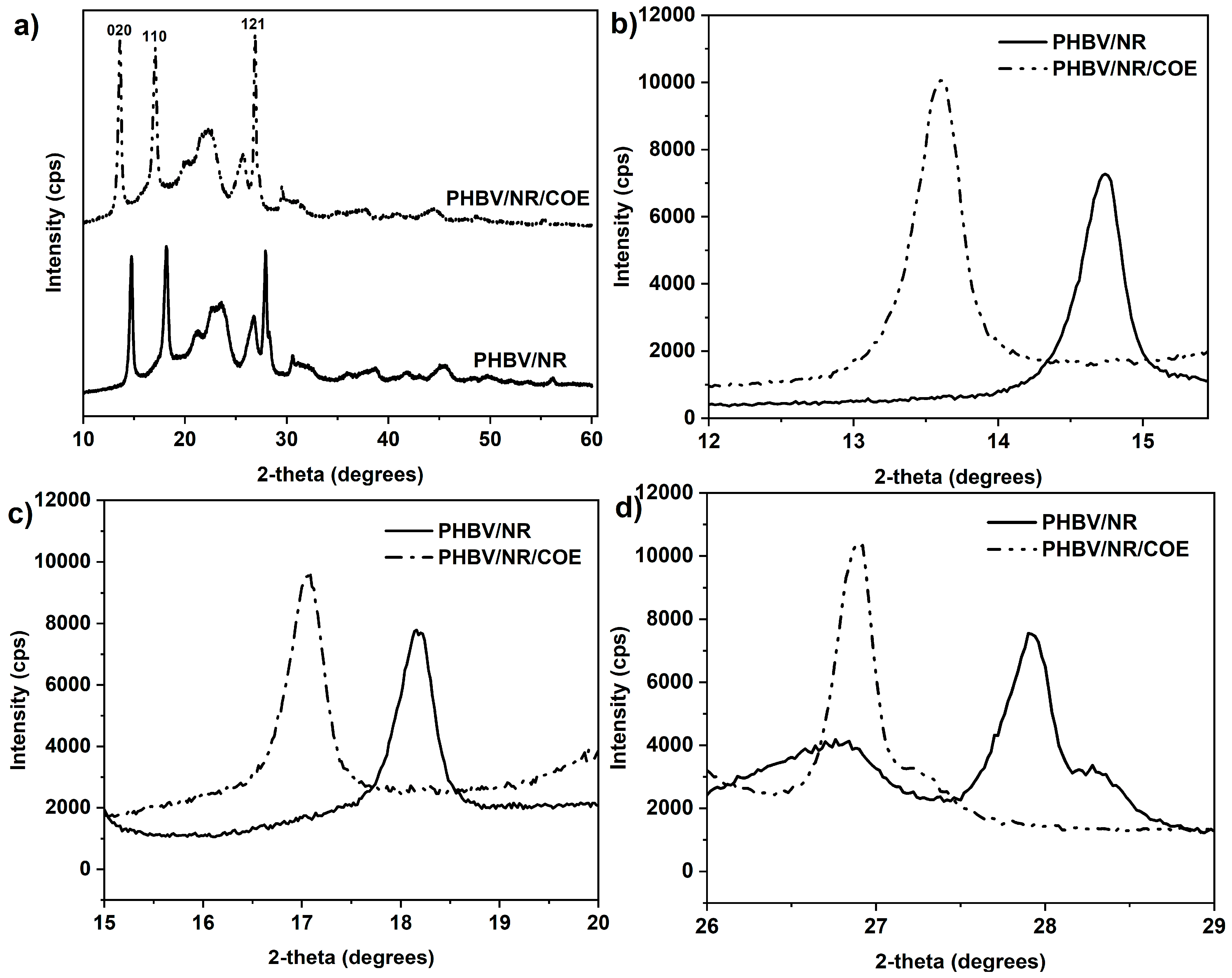
4.3. Effect of Plasticizer on Crystalline Structure of PHBV/NR Blended Sheets
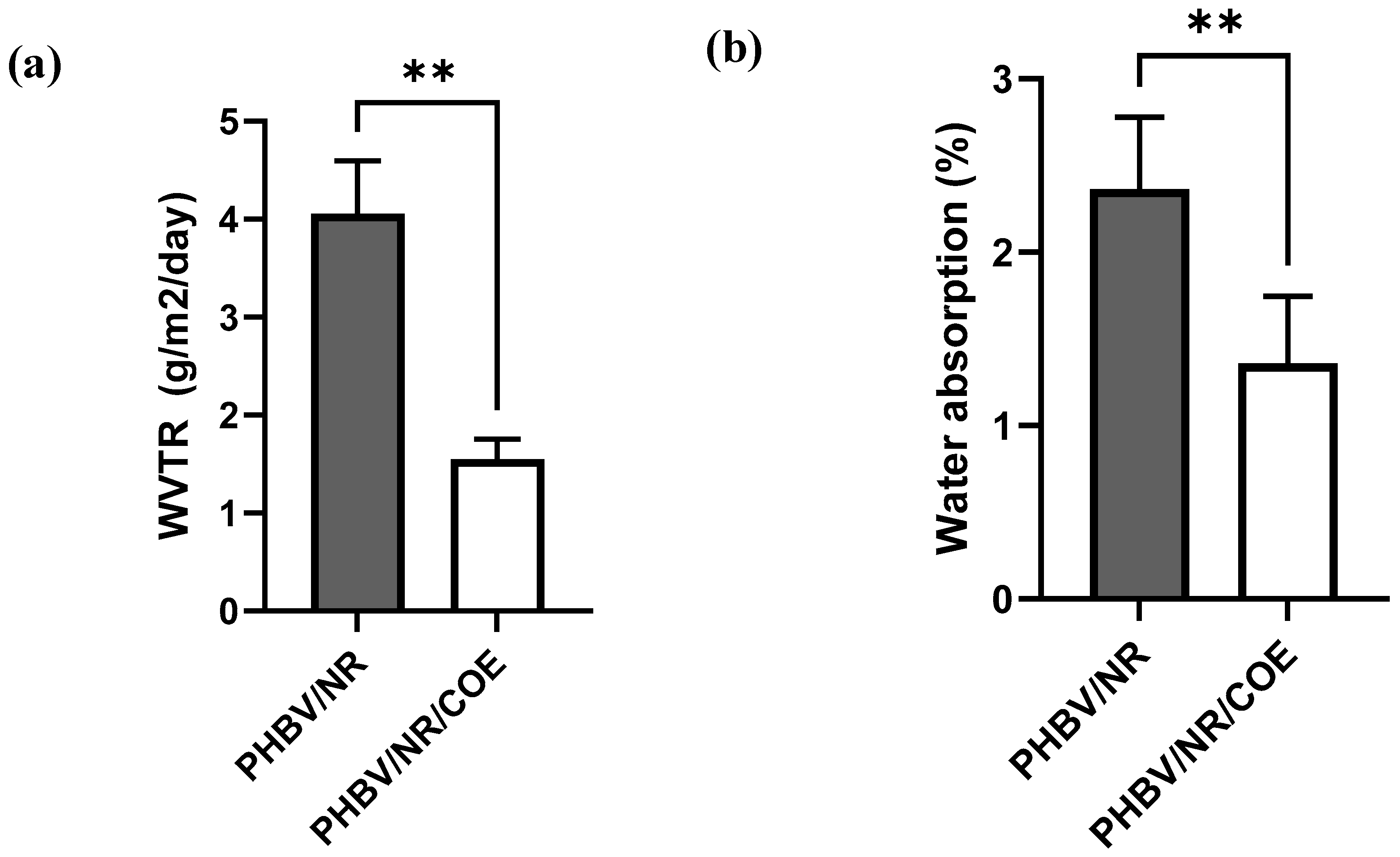
4.4. Effect of Plasticizer on Water Permeability of the PHBV/NR Blend
4.5. Water Absorption Analysis of PHBV/NR and PHBV/NR/COE Blends
4.6. Impact of Plasticizer on the Barrier Properties of the PHBV/NR Blends
4.7. Surface Roughness Measurements
4.8. Effect of Plasticizers on the Molecular Interactions of the PHBV/NR Blends
4.9. Morphological Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.; Moore, C.; Andrady, A.; Gregory, M.; Takada, H.; Weisberg, S. New Directions in Plastic Debris. Science 2005, 310, 1117. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Ogunola, O.S.; Onada, O.A.; Falaye, A.E. Mitigation Measures to Avert the Impacts of Plastics and Microplastics in the Marine Environment (a Review). Environ. Sci. Pollut. Res. 2018, 25, 9293–9310. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, Water Vapor Barrier and Antimicrobial Properties of PLA/Nanoclay Composite Films. LWT-Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Yuzay, I.E.; Auras, R.; Selke, S. Poly(Lactic Acid) and Zeolite Composites Prepared by Melt Processing: Morphological and Physical–Mechanical Properties. J. Appl. Polym. Sci. 2010, 115, 2262–2270. [Google Scholar] [CrossRef]
- Dash, K.; Deka, P.; Bangar, S.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of Inorganic Nanoparticles in Food Packaging: A Comprehensive Review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in Food Packaging: Biodegradability and Potential Migration to Food—A Review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Ishida, K.; Wang, Y.; Inoue, Y. Comonomer Unit Composition and Thermal Properties of Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate)s Biosynthesized by Ralstonia e Utropha. Biomacromolecules 2001, 2, 1285–1293. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Pal, A.K.; Cisneros-López, E.O.; Misra, M.; Mohanty, A.K. The Effect of Natural Fillers on the Marine Biodegradation Behaviour of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV). Sci. Rep. 2021, 11, 911. [Google Scholar] [CrossRef]
- Li, F.; Yu, H.-Y.; Wang, Y.-Y.; Zhou, Y.; Zhang, H.; Yao, J.-M.; Abdalkarim, S.Y.H.; Tam, K.C. Natural Biodegradable Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Nanocomposites with Multifunctional Cellulose Nanocrystals/Graphene Oxide Hybrids for High-Performance Food Packaging. J. Agric. Food Chem. 2019, 67, 10954–10967. [Google Scholar] [CrossRef]
- Dedieu, I.; Aouf, C.; Gaucel, S.; Peyron, S. Mechanical Recyclability of Biodegradable Polymers Used for Food Packaging: Case Study of Polyhydroxybutyrate-Co-Valerate (PHBV) Plastic. Food Addit. Contam. Part A 2022, 39, 1878–1892. [Google Scholar] [CrossRef]
- Pilla, S. (Ed.) Handbook of Bioplastics and Biocomposites Engineering Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An Overview of Biodegradable Packaging in Food Industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of Recent Advances in the Biodegradability of Polyhydroxyalkanoate (PHA) Bioplastics and Their Composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Rodriguez-Uribe, A.; Wang, T.; Pal, A.K.; Wu, F.; Mohanty, A.K.; Misra, M. Injection Moldable Hybrid Sustainable Composites of BioPBS and PHBV Reinforced with Talc and Starch as Potential Alternatives to Single-Use Plastic Packaging. Compos. Part C Open Access 2021, 6, 100201. [Google Scholar] [CrossRef]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic Flexible Films Waste Management—A State of Art Review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef]
- Yu, H.; Yan, C.; Yao, J. Fully Biodegradable Food Packaging Materials Based on Functionalized Cellulose Nanocrystals/Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Nanocomposites. RSC Adv. 2014, 4, 59792–59802. [Google Scholar] [CrossRef]
- Phromma, W.; Magaraphan, R. Fabrication of Admicelled Natural Rubber by Polycaprolactone for Toughening Poly(Lactic Acid). J. Polym. Environ. 2018, 26, 2268–2280. [Google Scholar] [CrossRef]
- Han, C.C.; Ismail, J.; Kammer, H.-W. Melt Reaction in Blends of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Epoxidized Natural Rubber. Polym. Degrad. Stab. 2004, 85, 947–955. [Google Scholar] [CrossRef]
- Zhang, J.; McCarthy, S.; Whitehouse, R. Reverse Temperature Injection Molding of BiopolTM and Effect on Its Properties. J. Appl. Polym. Sci. 2004, 94, 483–491. [Google Scholar] [CrossRef]
- Zhao, X.; Venoor, V.; Koelling, K.; Cornish, K.; Vodovotz, Y. Bio-based Blends from Poly(3-hydroxybutyrate-Co-3-hydroxyvalerate) and Natural Rubber for Packaging Applications. J. Appl. Polym. Sci. 2019, 136, 47334. [Google Scholar] [CrossRef]
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal Mechanical Properties of Biodegradable Natural Rubber-Toughened PHBV Bioplastics Intended for Food Packaging Applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- Tomano, N.; Boondamnoen, O.; Aumnate, C.; Potiyaraj, P. Enhancing Impact Resistance and Biodegradability of PHBV by Melt Blending with ENR. Sci. Rep. 2022, 12, 22633. [Google Scholar] [CrossRef]
- George, J. Dynamically Vulcanised Thermoplastic Elastomer Blends of Polyethylene and Nitrile Rubber. Polymer 2000, 41, 1507–1517. [Google Scholar] [CrossRef]
- Pham, T.; Gahleitner, M. Interfacial Strengthening of High-Impact Polypropylene Compounds by Reactive Modification. Compos. Interfaces 2005, 12, 707–723. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in Bio-Based Plastics and Plasticizing Modifications. J. Mater. Chem. A 2013, 1, 13379. [Google Scholar] [CrossRef]
- Slongo, M.D.; Brandolt, S.D.F.; Daitx, T.S.; Mauler, R.S.; Giovanela, M.; Crespo, J.S.; Carli, L.N. Comparison of the Effect of Plasticizers on PHBV—And Organoclay—Based Biodegradable Polymer Nanocomposites. J. Polym. Environ. 2018, 26, 2290–2299. [Google Scholar] [CrossRef]
- Montilla-Buitrago, C.E.; Gómez-López, R.A.; Solanilla-Duque, J.F.; Serna-Cock, L.; Villada-Castillo, H.S. Effect of Plasticizers on Properties, Retrogradation, and Processing of Extrusion-Obtained Thermoplastic Starch: A Review. Starch 2021, 73, 2100060. [Google Scholar] [CrossRef]
- Emaimo, A.J.; Olkhov, A.A.; Iordanskii, A.L.; Vetcher, A.A. Polyhydroxyalkanoates Composites and Blends: Improved Properties and New Applications. J. Compos. Sci. 2022, 6, 206. [Google Scholar] [CrossRef]
- Nosal, H.; Moser, K.; Warzała, M.; Holzer, A.; Stańczyk, D.; Sabura, E. Selected Fatty Acids Esters as Potential PHB-V Bioplasticizers: Effect on Mechanical Properties of the Polymer. J. Polym. Environ. 2021, 29, 38–53. [Google Scholar] [CrossRef]
- Xu, Z.; Xiong, X.; Zhao, Y.; Xiang, W.; Wu, C. Pollutants Delivered Every Day: Phthalates in Plastic Express Packaging Bags and Their Leaching Potential. J. Hazard. Mater. 2020, 384, 121282. [Google Scholar] [CrossRef]
- Andjelković, T.; Bogdanović, D.; Kostić, I.; Kocić, G.; Nikolić, G.; Pavlović, R. Phthalates Leaching from Plastic Food and Pharmaceutical Contact Materials by FTIR and GC-MS. Environ. Sci. Pollut. Res. 2021, 28, 31380–31390. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the Plasticizer Di(2-Ethylhexyl)Phthalate (DEHP) from Plastic Containers and the Question of Human Exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef]
- Randles, S.J. Environmentally Considerate Ester Lubricants for the Automotive and Engineering Industries. J. Synth. Lubr. 1992, 9, 145–161. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Yang, S.; Chen, H.; Wang, D. The Study of Epoxidized Rapeseed Oil Used as a Potential Biodegradable Lubricant. J. Americ. Oil Chem. Soc. 2000, 77, 561–563. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z. Epoxidized Soybean Oil as a Potential Source of High-Temperature Lubricants. Ind. Crops Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Johansson, L.E.; Lundin, S.T. Copper Catalysts in the Selective Hydrogenation of Soybean and Rapeseed Oils: I. The Activity of the Copper Chromite Catalyst. J. Am. Oil Chem. Soc. 1979, 56, 974–980. [Google Scholar] [CrossRef]
- Vijayarajan, S.; Selke, S.E.M.; Matuana, L.M. Continuous Blending Approach in the Manufacture of Epoxidized Soybean-P Lasticized Poly(Lactic Acid) Sheets and Films. Macro. Mater. Eng. 2014, 299, 622–630. [Google Scholar] [CrossRef]
- Karak, N. Vegetable Oil-Based Epoxies. In Vegetable Oil-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Caillol, S.; Desroches, M.; Boutevin, G.; Loubat, C.; Auvergne, R.; Boutevin, B. Synthesis of New Polyester Polyols from Epoxidized Vegetable Oils and Biobased Acids. Euro. J. Lipid Sci. Technol. 2012, 114, 1447–1459. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Choi, M.S.; Rehman, S.U.; Kim, H.; Han, S.B.; Lee, J.; Hong, J.; Yoo, H.H. Migration of Epoxidized Soybean Oil from Polyvinyl Chloride/Polyvinylidene Chloride Food Packaging Wraps into Food Simulants. Environ. Sci. Pollut. Res. 2018, 25, 5033–5039. [Google Scholar] [CrossRef]
- Samoggia, A.; Del Prete, M.; Argenti, C. Functional Needs, Emotions, and Perceptions of Coffee Consumers and Non-Consumers. Sustainability 2020, 12, 5694. [Google Scholar] [CrossRef]
- Carvalho Neto, D.P.D.; Gonot-Schoupinsky, X.P.; Gonot-Schoupinsky, F.N. Coffee as a Naturally Beneficial and Sustainable Ingredient in Personal Care Products: A Systematic Scoping Review of the Evidence. Front. Sustain. 2021, 2, 697092. [Google Scholar] [CrossRef]
- Daglia, M.; Racchi, M.; Papetti, A.; Lanni, C.; Govoni, S.; Gazzani, G. In Vitro and Ex Vivo Antihydroxyl Radical Activity of Green and Roasted Coffee. J. Agric. Food Chem. 2004, 52, 1700–1704. [Google Scholar] [CrossRef]
- Speer, K.; Kölling-Speer, I. The Lipid Fraction of the Coffee Bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef]
- García-García, D.; Carbonell, A.; Samper, M.D.; García-Sanoguera, D.; Balart, R. Green Composites Based on Polypropylene Matrix and Hydrophobized Spend Coffee Ground (SCG) Powder. Compos. Part B Eng. 2015, 78, 256–265. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil Extracted from Spent Coffee Grounds as a Renewable Source for Fatty Acid Methyl Ester Manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Robertson, M.L.; Chang, K.; Gramlich, W.M.; Hillmyer, M.A. Toughening of Polylactide with Polymerized Soybean Oil. Macromolecules 2010, 43, 1807–1814. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, W.H. Effect of Biodegradable Plasticizers on Thermal and Mechanical Properties of Poly(3-Hydroxybutyrate). Polym. Test. 2004, 23, 455–460. [Google Scholar] [CrossRef]
- Ferri, M.; Papchenko, K.; Degli Esposti, M.; Tondi, G.; De Angelis, M.G.; Morselli, D.; Fabbri, P. Fully Biobased Polyhydroxyalkanoate/Tannin Films as Multifunctional Materials for Smart Food Packaging Applications. ACS Appl. Mater. Interfaces 2023, 15, 28594–28605. [Google Scholar] [CrossRef] [PubMed]
- Ponjavic, M.; Malagurski, I.; Lazic, J.; Jeremic, S.; Pavlovic, V.; Prlainovic, N.; Maksimovic, V.; Cosovic, V.; Atanase, L.I.; Freitas, F.; et al. Advancing PHBV Biomedical Potential with the Incorporation of Bacterial Biopigment Prodigiosin. Int. J. Mol. Sci. 2023, 24, 1906. [Google Scholar] [CrossRef] [PubMed]
- Williamson, K.; Banker, T.; Zhao, X.; Ortega-Anaya, J.; Jimenez-Flores, R.; Vodovotz, Y.; Hatzakis, E. Spent Coffee Ground Oil as a Valuable Source of Epoxides and Epoxidation Derivatives: Quantitation and Characterization Using Low-Field NMR. LWT 2022, 165, 113719. [Google Scholar] [CrossRef]
- Modi, S.J.; Cornish, K.; Koelling, K.; Vodovotz, Y. Fabrication and Improved Performance of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) for Packaging by Addition of High Molecular Weight Natural Rubber. J. Appl. Polym. Sci. 2016, 133, app.43937. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Synergistic Mechanisms Underlie the Peroxide and Coagent Improvement of Natural-Rubber-Toughened Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Mechanical Performance. Polymers 2019, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- McNally, T.; McShane, P.; Nally, G.M.; Murphy, W.R.; Cook, M.; Miller, A. Rheology, Phase Morphology, Mechanical, Impact and Thermal Properties of Polypropylene/Metallocene Catalysed Ethylene 1-Octene Copolymer Blends. Polymer 2002, 43, 3785–3793. [Google Scholar] [CrossRef]
- ASTM D 3985-17; Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM: West Conshohocken, PA, USA, 2024. [CrossRef]
- De Beukelaer, H.; Hilhorst, M.; Workala, Y.; Maaskant, E.; Post, W. Overview of the Mechanical, Thermal and Barrier Properties of Biobased and/or Biodegradable Thermoplastic Materials. Polym. Test. 2022, 116, 107803. [Google Scholar] [CrossRef]
- Ng, J.S.; Kiew, P.L.; Lam, M.K.; Yeoh, W.M.; Ho, M.Y. Preliminary evaluation of the properties and biodegradability of glycerol- and sorbitol-plasticized potato-based bioplastics. Int. J. Environ. Sci. Technol. 2022, 19, 1545–1554. [Google Scholar] [CrossRef]
- Zhao, X.; Pelfrey, A.; Pellicciotti, A.; Koelling, K.; Vodovotz, Y. Synergistic Effects of Chain Extenders and Natural Rubber on PLA Thermal, Rheological, Mechanical and Barrier Properties. Polymer 2023, 269, 125712. [Google Scholar] [CrossRef]
- Dangtungee, R.; Tengsuthiwat, J.; Boonyasopon, P.; Siengchin, S. Sisal natural fiber/clay-reinforced poly(hydroxybutyrate- co -hydroxyvalerate) hybrid composites. J. Thermoplast. Compos. Mater. 2015, 28, 879–895. [Google Scholar] [CrossRef]
- Montanheiro, T.L.D.A.; Montagna, L.S.; Patrulea, V.; Jordan, O.; Borchard, G.; Ribas, R.G.; Campos, T.M.B.; Thim, G.P.; Lemes, A.P. Enhanced Water Uptake of PHBV Scaffolds with Functionalized Cellulose Nanocrystals. Polym. Test. 2019, 79, 106079. [Google Scholar] [CrossRef]
- Xu, Y.; Rather, A.M.; Yao, Y.; Fang, J.-C.; Mamtani, R.S.; Bennett, R.K.A.; Atta, R.G.; Adera, S.; Tkalec, U.; Wang, X. Liquid Crystal–Based Open Surface Microfluidics Manipulate Liquid Mobility and Chemical Composition on Demand. Sci. Adv. 2021, 7, eabi7607. [Google Scholar] [CrossRef] [PubMed]
- Martino, L.; Berthet, M.; Angellier-Coussy, H.; Gontard, N. Understanding External Plasticization of Melt Extruded PHBV –Wheat Straw Fibers Biodegradable Composites for Food Packaging. J. Appl. Polym. Sci. 2015, 132, app.41611. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M. Mechanisms of plasticizers action. In Handbook of Plasticizers; Elsevier: Amsterdam, The Netherlands, 2012; pp. 119–133. [Google Scholar]
- Azuma, Y.; Yoshie, N.; Sakurai, M.; Inoue, Y.; Chûjô, R. Thermal behaviour and miscibility of poly(3-hydroxybutyrate)/poly(vinyl alcohol) blends. Polymer 1992, 33, 4763–4767. [Google Scholar] [CrossRef]
- Ramli, S.A.; Othman, N.; Bakar, A.A.; Hassan, A. Plasticizing Effects of Epoxidized Palm Oil on Mechanical and Thermal Properties of Poly(3-hydroxybutyrate-co-hydroxyvalerate)/Poly(caprolactone) Blends. Chem. Eng. Trans. 2021, 83, 83559–83564. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, Z.; Sun, X.; Turng, L.S.; Peng, X. Morphology and Properties of Injection Molded Solid and Microcellular Polylactic Acid/Polyhydroxybutyrate-Valerate (PLA/PHBV) Blends. Ind. Eng. Chem. Res. 2013, 52, 2569–2581. [Google Scholar] [CrossRef]
- Branciforti, M.C.; Corrêa, M.C.S.; Pollet, E.; Agnelli, J.A.M.; de Paula Nascente, P.A.; Avérous, L. Crystallinity study of nano-biocomposites based on plasticized poly(hydroxybutyrate-co-hydroxyvalerate) with organo-modified montmorillonite. Polym. Test. 2013, 32, 1253–1260. [Google Scholar] [CrossRef]
- Pongtanayut, K.; Thongpin, C.; Santawitee, O. The Effect of Rubber on Morphology, Thermal Properties and Mechanical Properties of PLA/NR and PLA/ENR Blends. Energy Procedia 2013, 34, 888–897. [Google Scholar] [CrossRef]
- Farrag, Y.; Barral, L.; Gualillo, O.; Moncada, D.; Montero, B.; Rico, M.; Bouza, R. Effect of Different Plasticizers on Thermal, Crystalline, and Permeability Properties of Poly(3–hydroxybutyrate–co–3–hydroxyhexanoate) Films. Polymers 2022, 14, 3503. [Google Scholar] [CrossRef]
- Kanda, G.S.; Al-Qaradawi, I.; Luyt, A.S. Morphology and property changes in PLA/PHBV blends as function of blend composition. J. Polym. Res. 2018, 25, 196. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(Lactic Acid) Crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Jost, V.; Langowski, H.-C. Effect of Different Plasticisers on the Mechanical and Barrier Properties of Extruded Cast PHBV Films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- Kim, M.; Kang, J.; Yun, S.I. Alginate-reinforced poly(3-hydroxybutyrate)/poly(hydroxybutyrate-co-hydroxyvalerate) aerogel monoliths fabricated by phase separation as environmental floating adsorbents. Int. J. Biol. Macromol. 2022, 217, 956–968. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Akhila, K.; Ramakanth, D.; Rao, L.L.; Gaikwad, K.K. UV-blocking biodegradable film based on flaxseed mucilage/pectin impregnated with titanium dioxide and calcium chloride for food packaging applications. Int. J. Biol. Macromol. 2023, 239, 124335. [Google Scholar] [CrossRef] [PubMed]
- Baraka, A. Adsorptive removal of tartrazine and methylene blue from wastewater using melamine-formaldehyde-tartaric acid resin (and a discussion about pseudo second order model). Desalination Water Treat. 2012, 44, 128–141. [Google Scholar] [CrossRef]
- Eissenberger, K.; Ballesteros, A.; De Bisschop, R.; Bugnicourt, E.; Cinelli, P.; Defoin, M.; Demeyer, E.; Fürtauer, S.; Gioia, C.; Gómez, L.; et al. Approaches in Sustainable, Biobased Multilayer Packaging Solutions. Polymers 2023, 15, 1184. [Google Scholar] [CrossRef] [PubMed]
- Donhowe, I.G.; Fennema, O. The effects of plasticizers on crystallinity, permeability, and mechanical properties of methylcellulose films. J. Food Process. Preserv. 1993, 17, 247–257. [Google Scholar] [CrossRef]
- Attallah, O.A.; Mojicevic, M.; Garcia, E.L.; Azeem, M.; Chen, Y.; Asmawi, S.; Brenan Fournet, M. Macro and Micro Routes to High Performance Bioplastics: Bioplastic Biodegradability and Mechanical and Barrier Properties. Polymers 2021, 13, 2155. [Google Scholar] [CrossRef]
- Patel, M.K.; Zaccone, M.; De Brauwer, L.; Nair, R.; Monti, M.; Martinez-Nogues, V.; Frache, A.; Oksman, K. Improvement of Poly(lactic acid)-Poly(hydroxy butyrate) Blend Properties for Use in Food Packaging: Processing, Structure Relationships. Polymers 2022, 14, 5104. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Leontiou, A.; Karabagias, I.K.; Georgopoulos, S.; Karydis-Messinis, A.; Zaharioudakis, K.; Andritsos, N.; Kehayias, G.; et al. Thymol@activated Carbon Nanohybrid for Low-Density Polyethylene-Based Active Packaging Films for Pork Fillets’ Shelf-Life Extension. Foods 2023, 12, 2590. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Zhang, W.; Liu, C.; Huang, F. Composition and Oil-Water Interfacial Tension Studies in Different Vegetable Oils. Food Biophys. 2020, 15, 229–239. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Contact Angle Hysteresis Explained. Langmuir 2006, 22, 6234–6237. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Qiang, Z.; Ren, J. In Situ Grafting Approach for Preparing PLA/PHBV Degradable Blends with Improved Mechanical Properties. Polym. Bull. 2022, 79, 9543–9562. [Google Scholar] [CrossRef]
- Kennouche, S.; Le Moigne, N.; Kaci, M.; Quantin, J.-C.; Caro-Bretelle, A.-S.; Delaite, C.; Lopez-Cuesta, J.-M. Morphological Characterization and Thermal Properties of Compatibilized Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV)/Poly(Butylene Succinate) (PBS)/Halloysite Ternary Nanocomposites. Eur. Polym. J. 2016, 75, 142–162. [Google Scholar] [CrossRef]

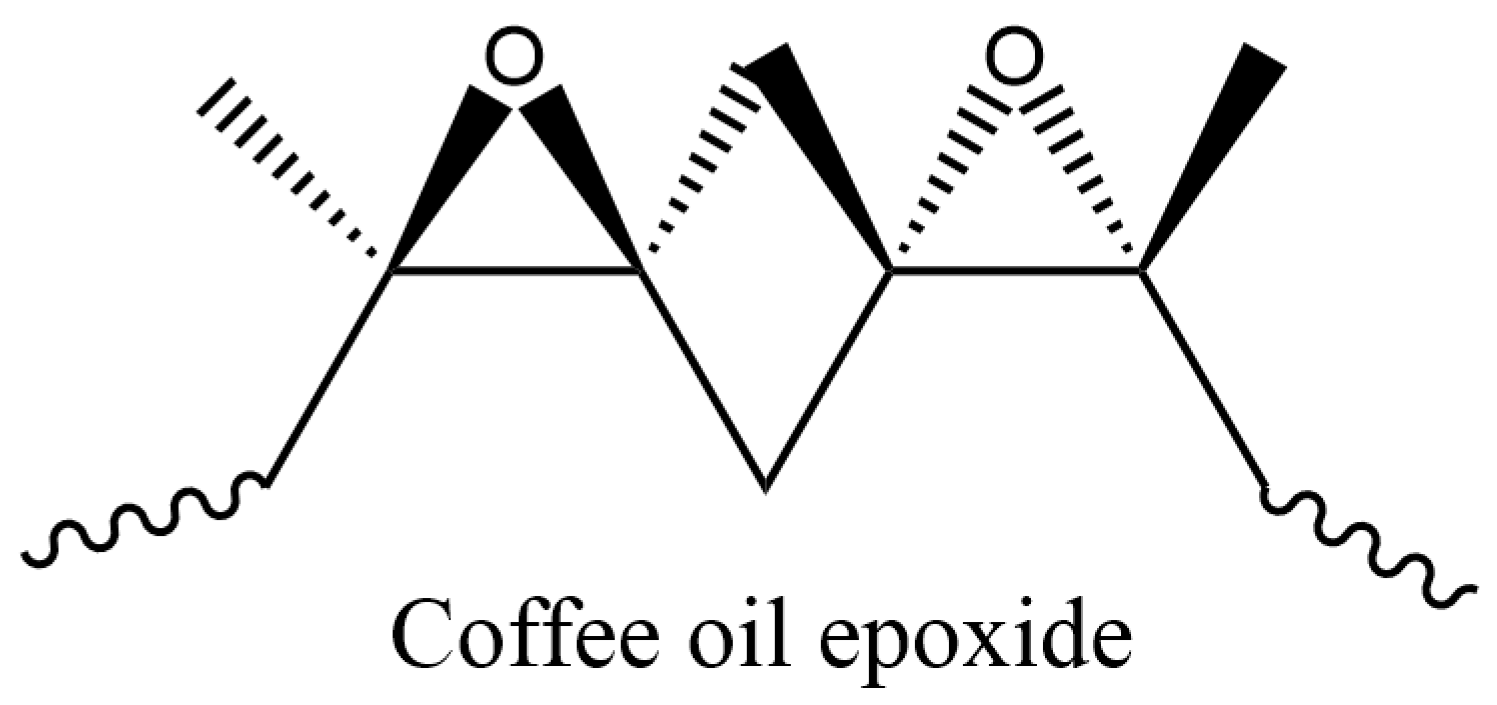




| Zone | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | Die |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 180 | 180 | 178 | 178 | 175 | 175 | 167 | 165 | 165 | 162 |
| Sample | ΔHm (J/g) | Tm onset (°C) | Tm peak (°C) | ΔHc (J/g) | Tc onset (°C) | Tc peak (°C) | Xc | Tg of PHBV | Tg of Rubber |
|---|---|---|---|---|---|---|---|---|---|
| PHBV | 92.5 ± 0.3 | 163.6 ± 0.9 | 170.4 ± 0.9 | 93.6 ± 0.4 | 124.5 ± 0.3 | 120.9 ± 0.4 | 68.5 ± 0.5 | 2.6 ± 0.4 | − |
| PHBV/NR | 79.6 ± 1.2 | 160.8 ± 0.4 | 165.4 ± 0.2 | 78.3 ± 1.4 | 120.1 ± 0.2 | 116.4 ± 0.2 | 54.5 ± 0.8 | 1.8 ± 0.3 | (−) 62.6 ± 1.3 |
| PHBV/NR/COE | 78.9 ± 1.0 | 160.7 ± 0.1 | 166.4 ± 0.2 | 77.3 ± 1.2 | 119.7 ± 0.1 | 115.9 ± 0.2 | 53.5 ± 0.4 | 1.7 ± 0.3 | (−) 63.6 ± 1.4 |
| Thermal Degradation Temperature | Natural Rubber | |||
|---|---|---|---|---|
| Samples | T Onset (°C) | T Peak (°C) | T Onset (°C) | T Peak (°C) |
| PHBV | 294.6 ± 3.1 | 304.0 ± 4.5 | - | - |
| PHBV/NR | 298.8 ± 0.3 | 310.7 ± 0.9 | 328.0 ± 0.9 | 367.9 ± 0.6 |
| PHBV/NR/COE | 297.9 ± 2.4 | 308.6 ± 2.5 | 327.0 ± 3.0 | 369.0 ± 1.1 |
| Sample | 2θ (°) | hkl | d-Spacing (Å) | Xc (%) | FWHM (°) | D-Value (nm) |
|---|---|---|---|---|---|---|
| PHBV/NR | 14.74 | 020 | 5.86 | 63.52 | 0.35 | 22.67 |
| 18.16 | 110 | 4.76 | 0.45 | 17.66 | ||
| 27.92 | 121 | 3.11 | 1.69 | 4.82 | ||
| PHBV/NR/COE | 13.58 | 020 | 6.51 | 68.85 | 0.39 | 20.32 |
| 17.08 | 110 | 5.18 | 0.63 | 12.72 | ||
| 26.92 | 121 | 3.31 | 0.34 | 23.73 |
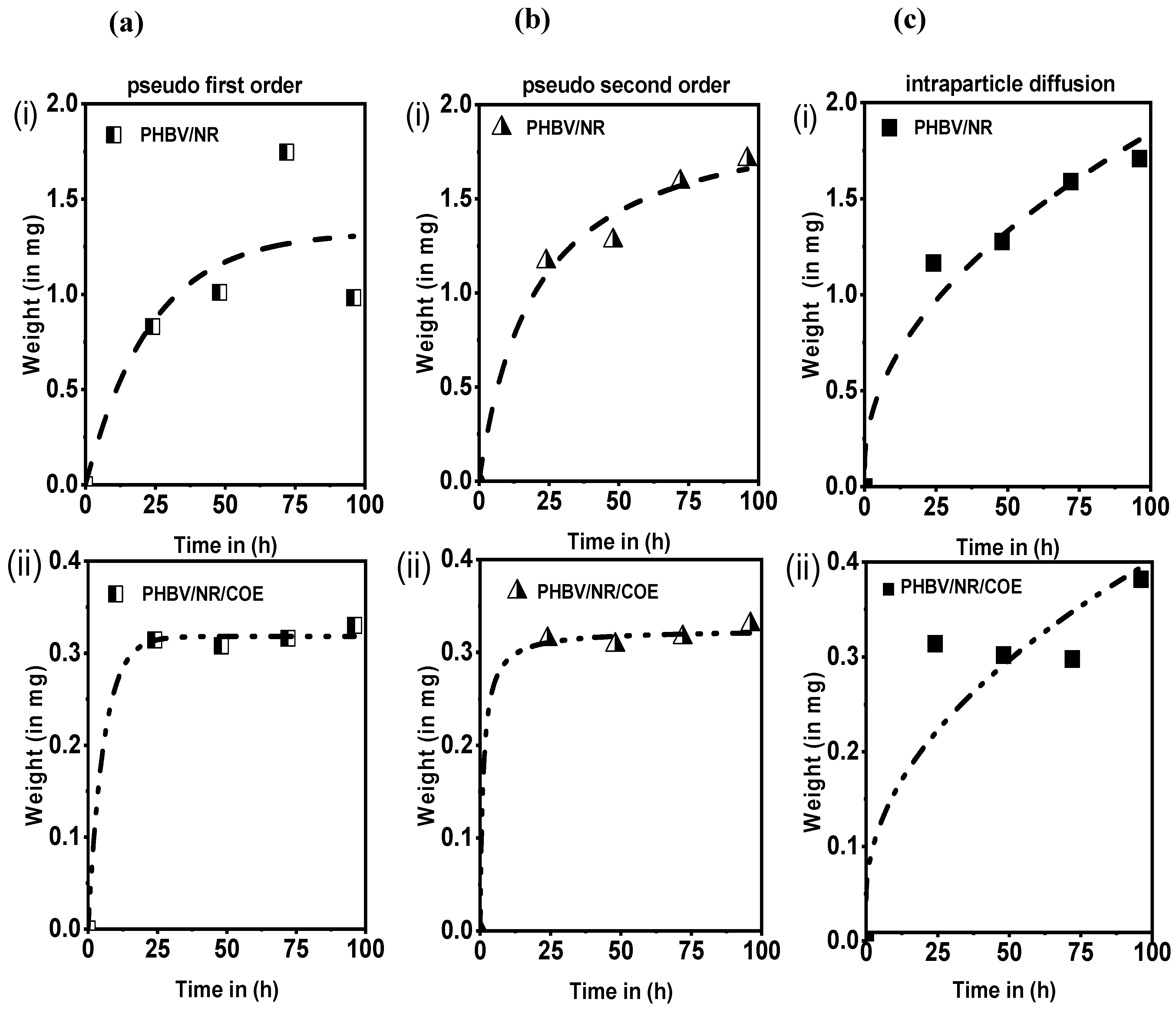
| Samples | Pseudo-First-Order Model | Pseudo-Second-Order Model | Intraparticle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | Kin | C | R2 | |
| PHBV/NR | 0.073 | 1.385 | 0.994 | 0.084 | 1.526 | 0.998 | 0.147 | 0.163 | 0.862 |
| PHBV/NR/COE | 0.023 | 0.382 | 0.999 | 0.032 | 0.543 | 0.989 | 0.035 | 9.89 × 10−26 | 0.976 |
| Samples | WVTR | OTR |
|---|---|---|
| g/m2.24 h | cc/m2/day | |
| PHBV/NR | 4.05 ± 0.54 | 2025.29 ± 35.69 |
| PHBV/NR/COE | 1.55 ± 0.20 | 21.51 ± 1.68 |
| Samples | Element | Weight % | Atomic % |
|---|---|---|---|
| PHBV | C | 82.26 | 86.26 |
| O | 17.18 | 13.52 | |
| Ca | 0.29 | 0.09 | |
| Al | 0.28 | 0.13 | |
| PHBV/NR | C | 60.55 | 54.61 |
| O | 3.67 | 2.49 | |
| Ca | 0.05 | 0.01 | |
| Al | 0.07 | 0.03 | |
| Be | 35.65 | 42.85 | |
| PHBV/NR/COE | C | 69.64 | 75.71 |
| O | 29.17 | 23.81 | |
| Ca | 0.59 | 0.19 | |
| Al | 0.59 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, R.; Zhao, X.; Vodovotz, Y. Addition of Coffee Waste-Derived Plasticizer Improves Processability and Barrier Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-Natural Rubber Bioplastic. Polymers 2024, 16, 2164. https://doi.org/10.3390/polym16152164
Ghosh R, Zhao X, Vodovotz Y. Addition of Coffee Waste-Derived Plasticizer Improves Processability and Barrier Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-Natural Rubber Bioplastic. Polymers. 2024; 16(15):2164. https://doi.org/10.3390/polym16152164
Chicago/Turabian StyleGhosh, Rinky, Xiaoying Zhao, and Yael Vodovotz. 2024. "Addition of Coffee Waste-Derived Plasticizer Improves Processability and Barrier Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-Natural Rubber Bioplastic" Polymers 16, no. 15: 2164. https://doi.org/10.3390/polym16152164
APA StyleGhosh, R., Zhao, X., & Vodovotz, Y. (2024). Addition of Coffee Waste-Derived Plasticizer Improves Processability and Barrier Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-Natural Rubber Bioplastic. Polymers, 16(15), 2164. https://doi.org/10.3390/polym16152164







