Polymeric Protective Films as Anticorrosive Coatings—Environmental Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment and Methods
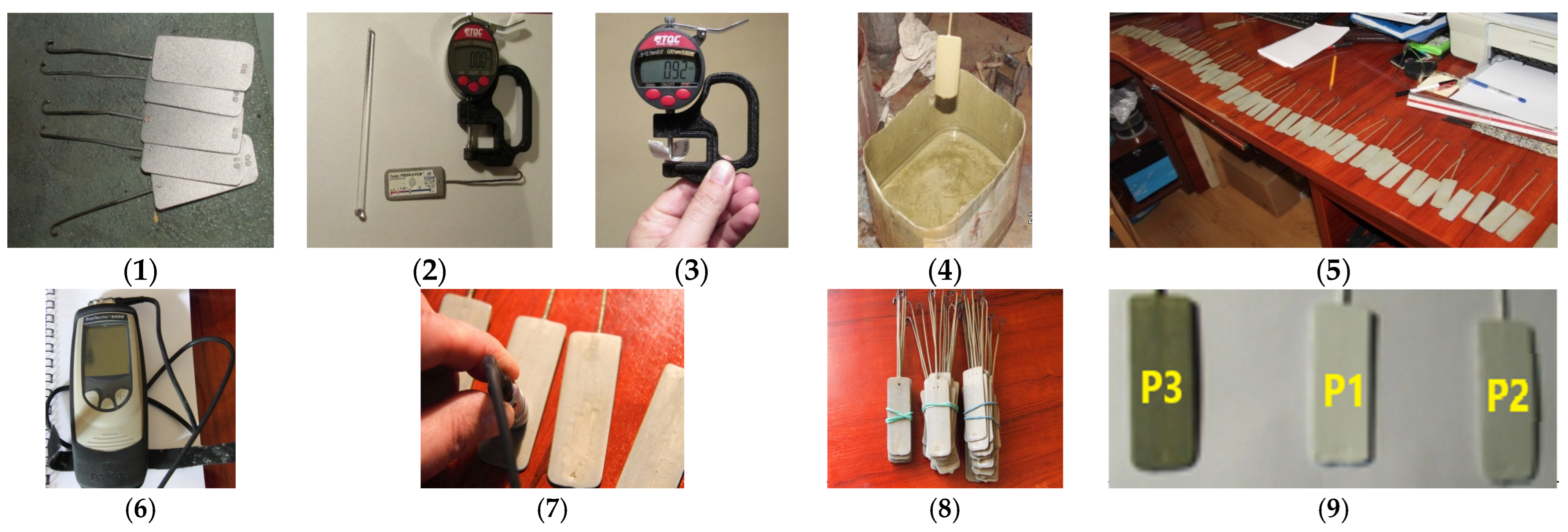
2.2.1. Material Characterization
Dielectric Characterization
Dry Heat
- -
- A temperature of 100 °C for 240 h, 480 h, 720 h, and 960 h, with intermediate tg δ measurements being taken after each cycle;
- -
- A temperature of 200 °C for 72 h, 144 h, 216 h, and 288 h, with intermediate tg δ measurements being taken after each cycle;
- -
- A temperature of 250 °C for 24 h, 48 h, 72 h, and 96 h, with intermediate tg δ measurements being taken after each cycle.
Salt Fog
UV Radiation and Humidity
Alpine and Marine Atmosphere
Mechanical Characterization
3. Results and Discussion
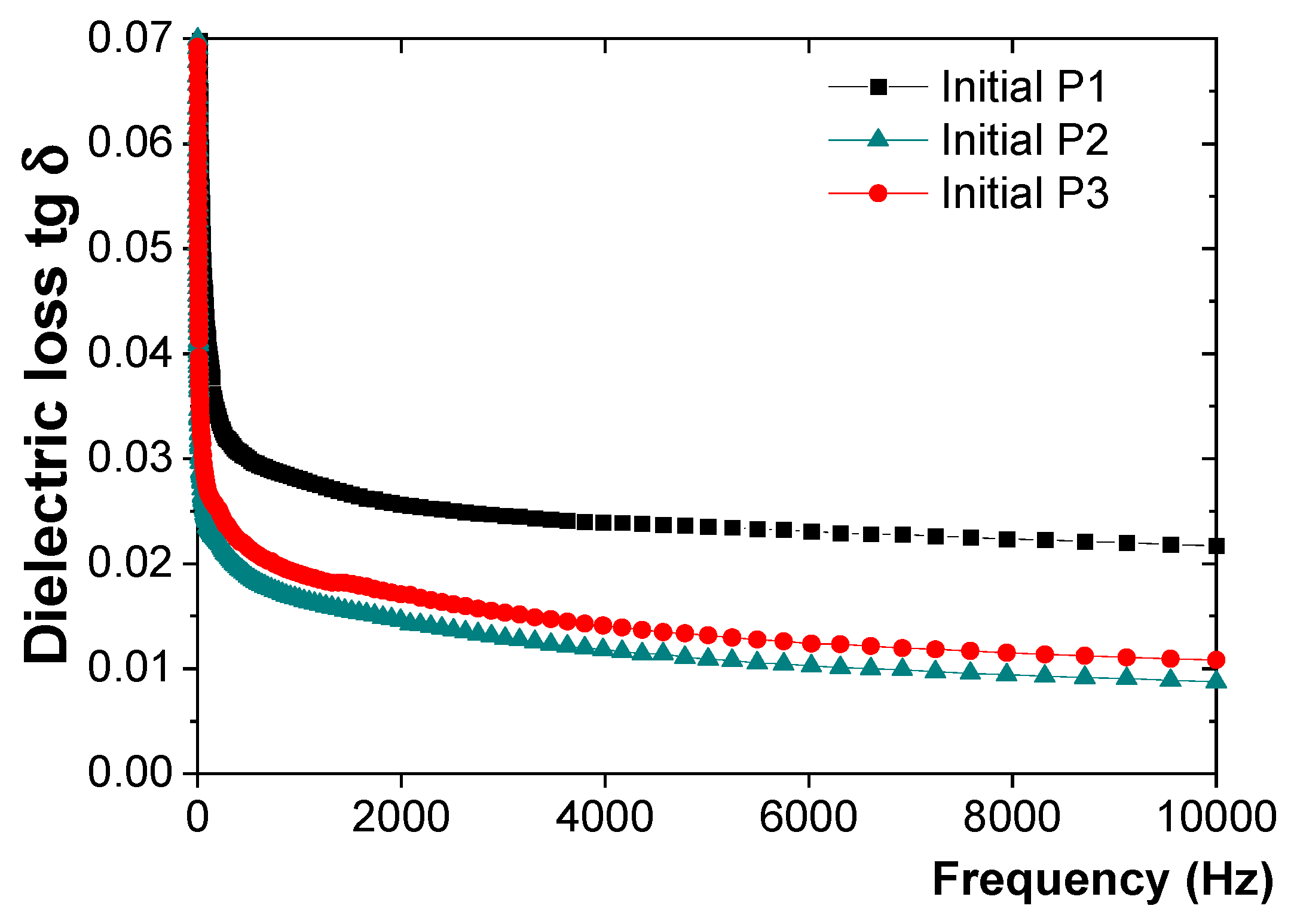
3.1. Initial Characterization of the P1, P2, and P3 Coatings
3.2. Dry Heat
3.2.1. Characterization of the Polymeric Coatings to the Action of Dry Heat at 100 °C
- -
- For the P1 coating (Figure 3a), the tg δ values exhibited a relatively constant decreasing trend, averaging about 74% reduction, up to 720 h of exposure to 100 °C. This trend likely reflects the failure of crosslinking reactions, leading to the epoxy resin transitioning into a three-dimensional state. Between 720 h and 960 h of exposure, a significant increase in tg δ values was observed, with values higher by about 63% compared to those recorded at 720 h. However, these values did not exceed the initial value, indicating that the degradation of the polymer coating has not yet occurred.
- -
- For the P2 coating (Figure 3b), the tg δ values tended to decrease relatively constantly up to 960 h of exposure to 100 °C. This trend suggested the completion of polymerization and crosslinking reactions, leading to the polyurethane resin transitioning into a three-dimensional (3D) state. Moreover, no signs of degradation of the polyurethane resin coating (P2) were observed, unlike the epoxy coating (P1).
- -
- For the P3 coating (Figure 3c), since only the protective epoxy primer coating was present, the measured tg δ values exhibited greater dispersion, with fluctuations up to 720 h of exposure to 100 °C. These variations likely originated from pores and voids in the polymer material directly exposed on the metal plates, consistent with comparative measurements taken prior to exposure to dry heat. After 960 h of exposure, an increase of about 45% was observed compared to the value at 720 h and about 100% compared to the initial value of tg δ, indicating the onset of polymer coating degradation.
3.2.2. Characterization of the Polymeric Coatings to the Action of Dry Heat at 200 °C
- -
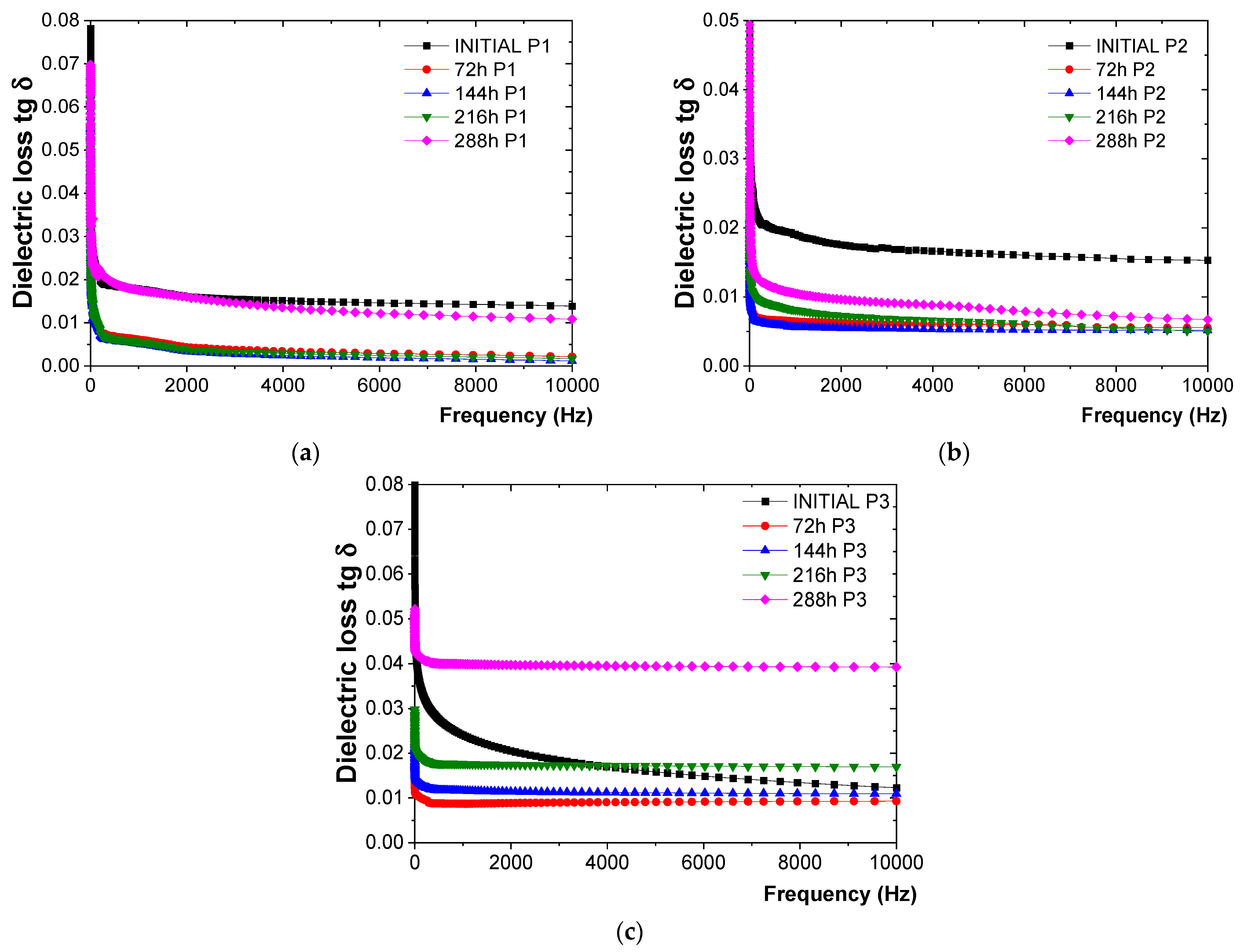
- For the P1 coating (Figure 4a), the tg δ values tended to decrease relatively constantly up to 216 h of exposure to 200 °C. This trend was likely due to the completion of crosslinking reactions, resulting in the epoxy resin transitioning into a three-dimensional (3D) state. After 216 h of exposure, a constant increase in tg δ values was observed, with an increase of 40% compared to the values obtained after 216 h of exposure, although it did not exceed the initial value of tg δ.
- -
- For the P2 coating (Figure 4b), the tg δ values decreased up to 144 h of exposure to 200 °C, also likely due to the completion of crosslinking reactions leading to a three-dimensional (3D) state of the polyurethane resin. Subsequently, an increase of approximately 62% was observed at 288 h of exposure compared to the previous cycle (216 h), without exceeding the initial value of tg δ.
- -
- For the P3 coating (Figure 4c), the tg δ values decreased up to 72 h, after which they started to increase slightly. After 288 h of continuous exposure, an increase of 111% was observed compared to the previous cycle (216 h), along with an increase of about 10% compared to the initial value of tg δ, indicating the beginning of coating degradation.
3.2.3. Characterization of the Polymeric Coatings to the Action of Dry Heat at 250 °C
- -
- For the P1 coating (Figure 5a), a continuous decrease in tg δ values was exhibited up to 48 h of exposure to 250 °C. This trend was likely due to the completion of crosslinking reactions, resulting in the epoxy resin transitioning into a three-dimensional (3D) state. However, from 48 h to 72 h, coating degradation began, as evidenced by a sudden increase in tg δ values of about 35% at 72 h compared to those recorded after 48 h of exposure.
- -
- For the P2 coating (Figure 5b), a decrease in tg δ values was observed up to 48 h of exposure to 250 °C. After 48 h, the tg δ values began to increase continuously, indicating that the polymeric coating degradation started after 96 h of exposure.
- -
- For the P3 coating (Figure 5c), greater dispersion in tg δ values was measured, with fluctuations up to 48 h of exposure to 250 °C. These variations were likely due to pores and voids in the polymeric material directly applied to the metal plates, consistent with comparative measurements made before exposure to dry heat. After 48 h of exposure, a constant increase in tg δ values was observed, indicating that the polymeric coating degraded after 96 h of exposure.
3.2.4. Determining the Remaining Lifetime Until the Replacement of the Polymeric Protective Coatings
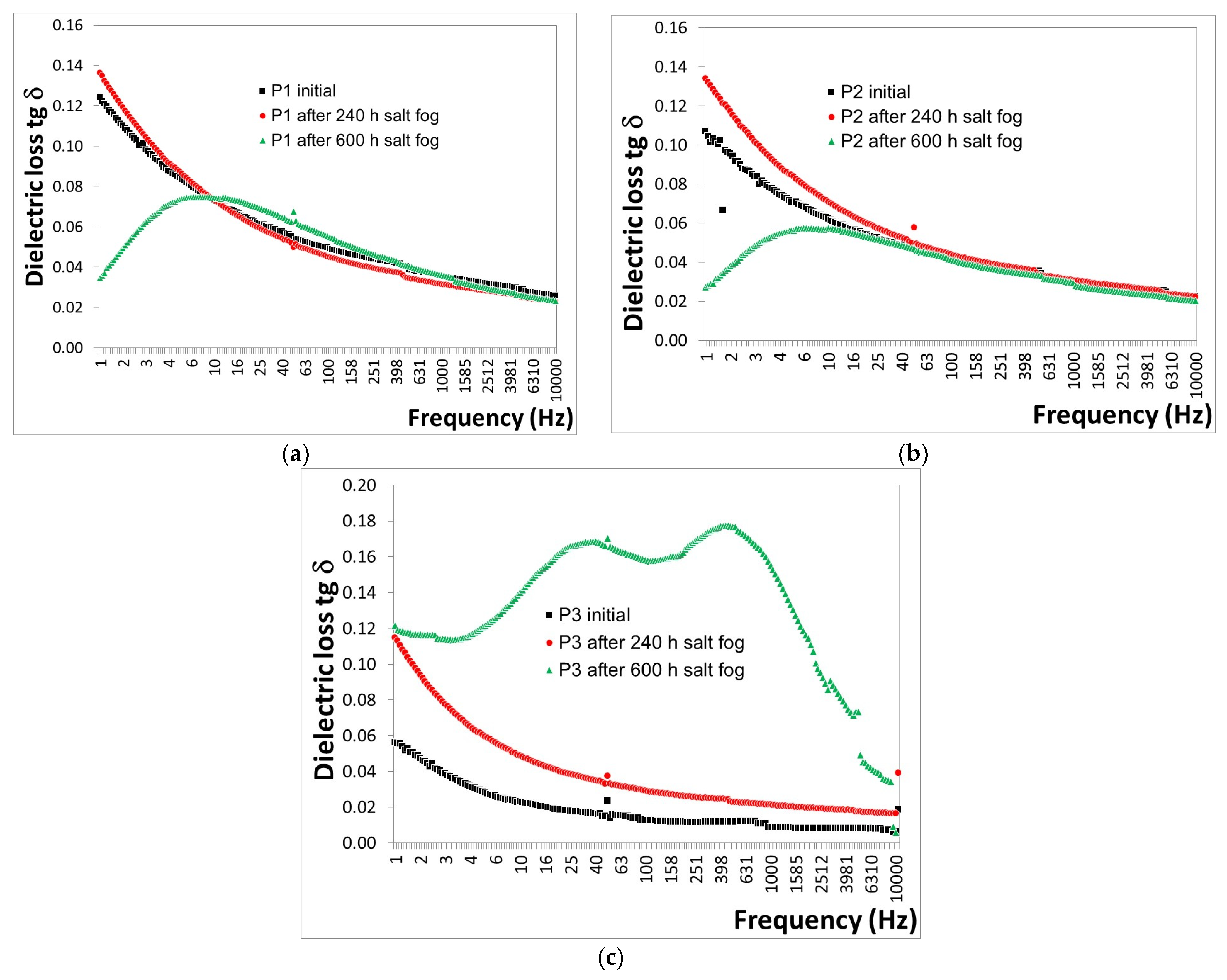
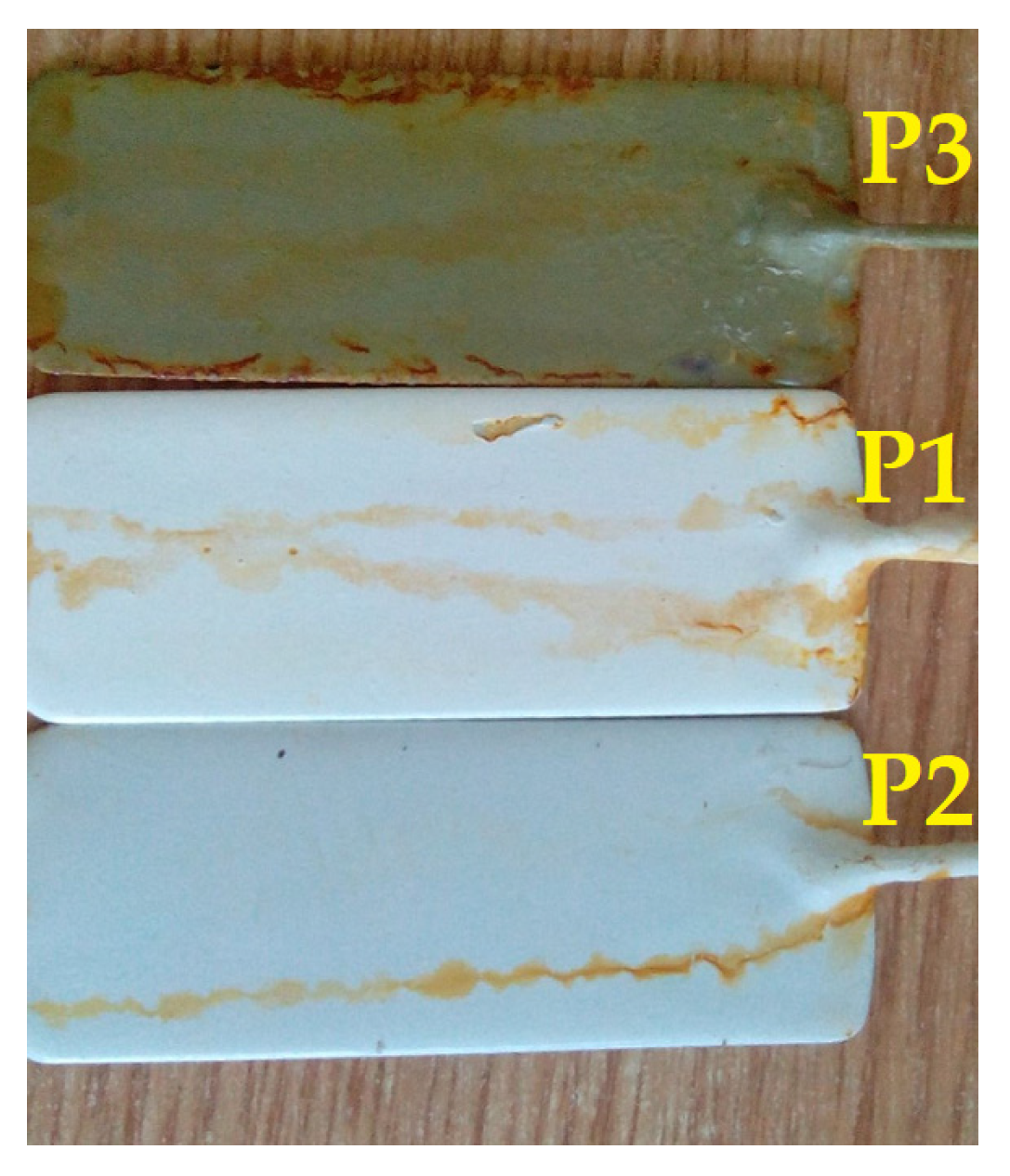
3.3. Salt Fog
- -
- For the P1 coating (Figure 7a), a decrease in tg δ values of approximately 5.24% was observed after 240 h, followed by a slight increase of approximately 0.7% after 600 h of exposure to salt fog.
- -
- For the P2 coating (Figure 7b), a decrease in tg δ values of approximately 15.4% was observed after 240 h, with a further decrease of approximately 23.5% after 600 h of exposure to salt fog.
- -
- For the P3 coating (Figure 7c), a decrease in tg δ values of approximately 109.8% was observed after 240 h, and a further decrease of approximately 615.7% after 600 h of exposure to salt fog.
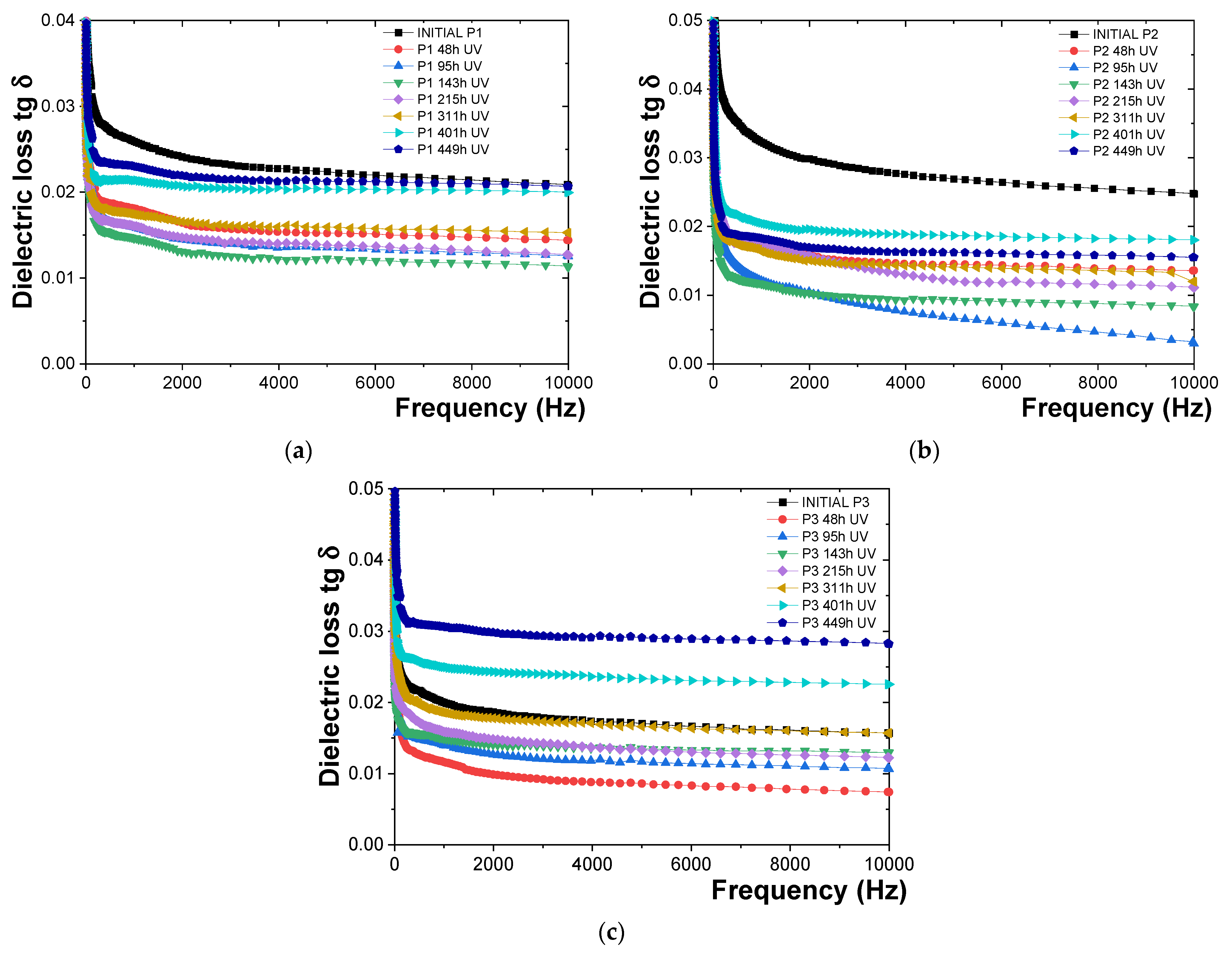
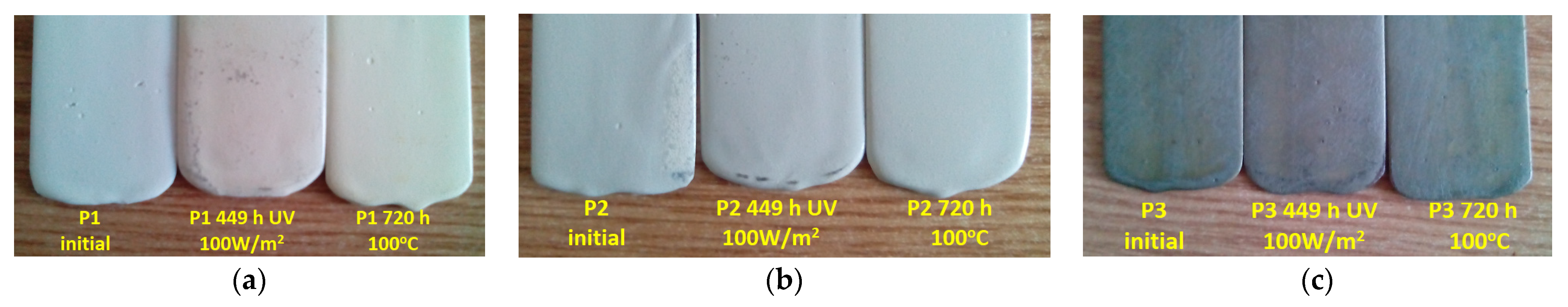
3.4. Characterization of the Behavior of Polymeric Protective Coatings to UV Radiation and Humidity
- -
- For the P1 polymeric coating (Figure 9a), the tg δ values measured after exposure to UV radiation decreased compared to the initial tg δ values by about 28.43% for the 48 h dose, 30.48% for the 95 h dose, 36.69% for the 143 h dose, 37.86% for the 215 h dose, 29.09% for the 311 h dose, 26.48% for the 401 h dose, and 15.86% for the 449 h dose. It was observed that the protective epoxy coating (P1) did not degrade after 449 h of exposure to UV radiation and humidity. The onset of degradation of the epoxy resin coating (P1) was identified by a sharp and continuous increase in tg δ values.
- -
- For the P2 polymeric coating (Figure 9b), it was found that the tg δ values decreased compared to the initial tg δ values by about 41.29% for the 48 h dose, 45.28% for the 95 h dose, 52.7% for the 143 h dose, 35.50% for the 215 h dose, 40.64% for the 311 h dose, 39.80% for the 401 h dose, and 39.75% for the 449 h dose. The same behavior in terms of tg δ values as observed for the epoxy resin-based polymeric coating (P1) was indicated by these results. It was concluded that the onset of degradation for the polyurethane resin was not reached after 449 h of exposure to UV radiation and humidity, as evidenced by the absence of a sharp and continuous increase in tg δ values.
- -
- For the P3 polymeric coating (Figure 9c), it was observed that the tg δ values initially decreased compared to the initial tg δ values upon exposure to UV radiation, then increased. Specifically, tg δ values were reduced by approximately 6.44% for the 48 h dose, 5.71% for the 95 h dose, 30.35% for the 143 h dose, and 23.95% for the 215 h dose. Subsequently, a slight increase was recorded, with tg δ values rising by about 2.56% for the 311 h dose, 3.17% for the 401 h dose, and an increase of about 48.69% for the 449 h dose, indicating that the epoxy primer coating has begun to deteriorate.
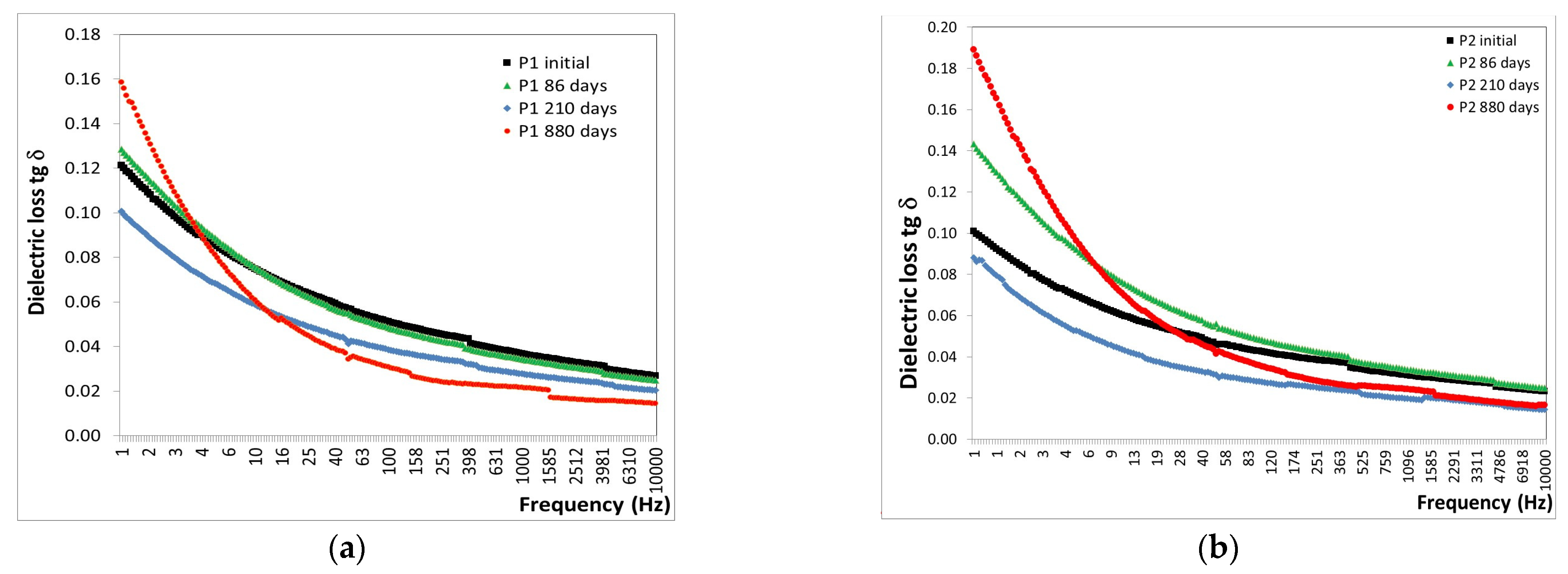
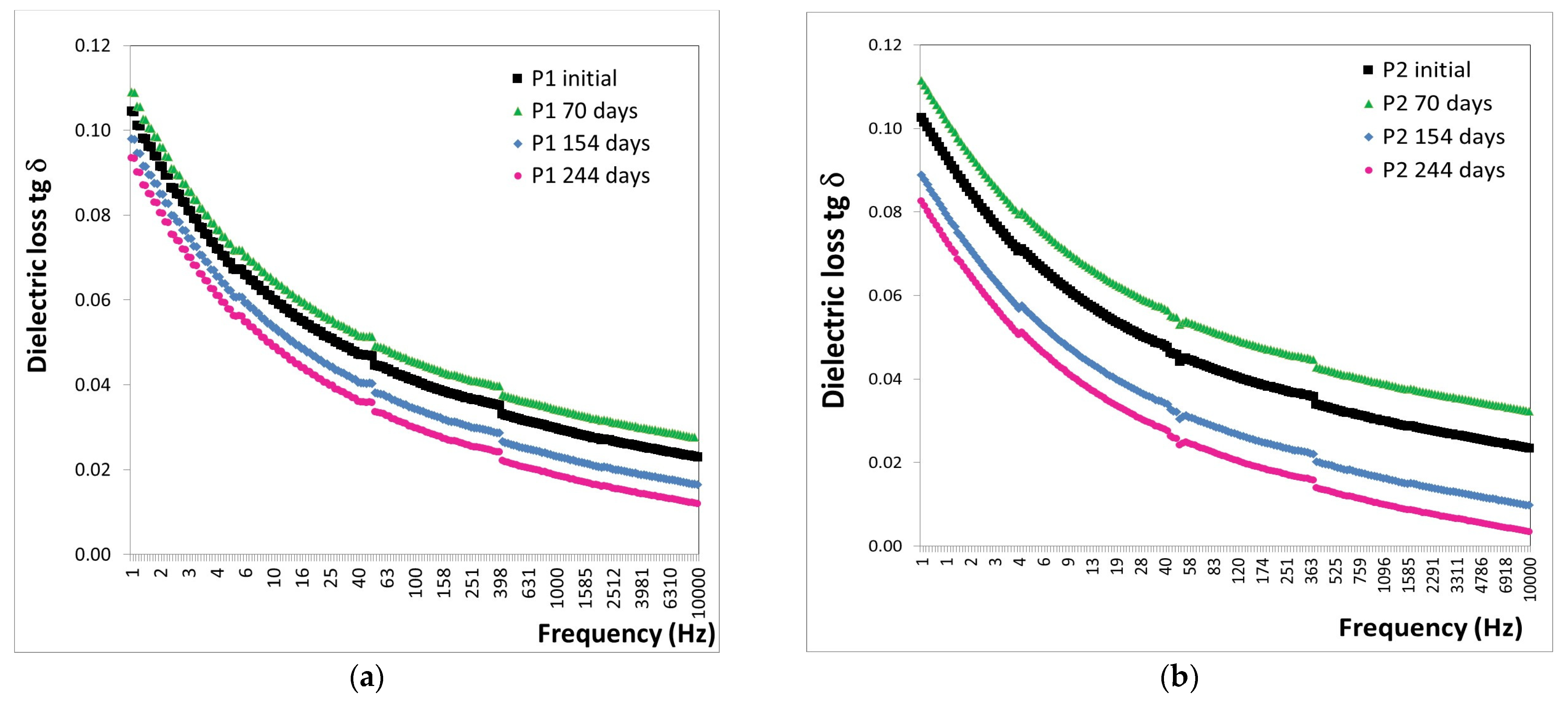
3.5. Characterization of the Behavior of the Protective Coatings in Extreme Conditions—Alpine and Marine Atmosphere
3.5.1. Alpine Atmosphere
- -
- After 86 days of exposure to the alpine atmosphere, a slight decrease in tg δ values was observed, with a reduction of approximately 1% for both P1 and P2 coatings.
- -
- After 210 days of exposure to the alpine atmosphere, a continued decrease in tg δ values was noted, with reductions of approximately 25% for the P1 coating and 39% for the P2 coating.
- -
- After 880 days of exposure to the alpine atmosphere, a decrease in tg δ values of 19% was recorded for the P1 coating and 13% for the P2 coating.
3.5.2. Marine Atmosphere
- -
- After 70 days of exposure to the marine atmosphere, significant increases were observed in the tg δ values, with an 88% increase for the P1 coating and a 91% increase for the P2 coating.
- -
- After 154 days of exposure to the marine atmosphere, average decreases in tg δ values were noted, approximately 14% for the P1 coating and 27% for the P2 coating.
- -
- After 244 days of exposure to the marine atmosphere, average decreases in tg δ values were observed, approximately 45% for the P1 coating and 54% for the P2 coating.
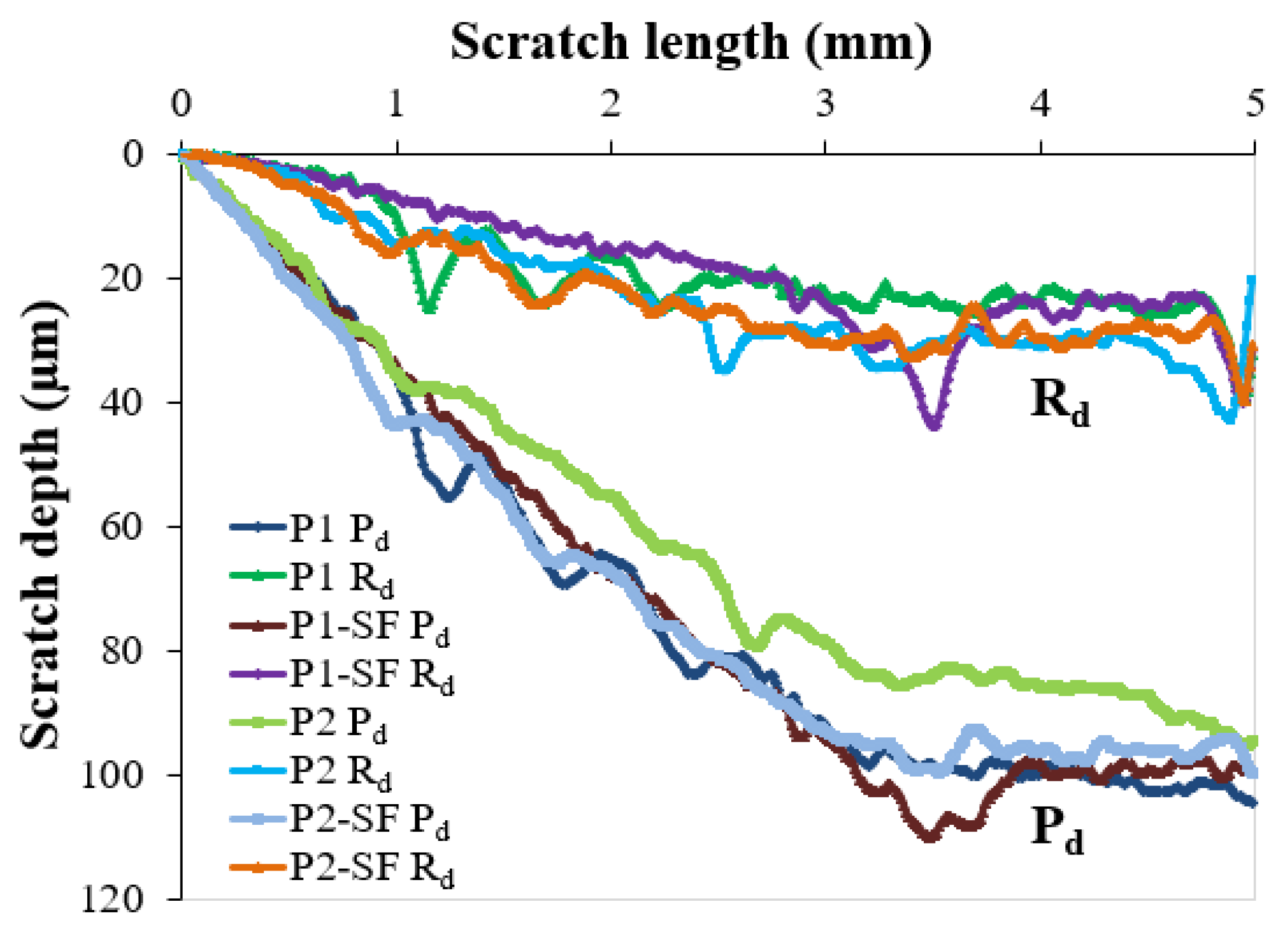
3.6. Mechanical Characterization
4. Conclusions
- -
- Dry heat: The coatings underwent stages of complete crosslinking and degradation. At 100 °C, only the P3 coating degraded after 1968 h. At 200 °C, the P1 and P2 coatings began degrading after 648 h, with degradation completing at 250 °C. The P2 coating demonstrated the highest temperature resistance among the coatings tested.
- -
- Remaining lifetime: The analysis of the variation in tg δ and electrical resistivity with frequency revealed that a longer service life was provided by the polyurethane coating (P2) compared to the epoxy coating (P1) after exposure to 100 °C for 1680 h.
- -
- Salt spray (fog) resistance: Slight degradation onset was shown by the P1 coating after 600 h of exposure to salt fog (36.5 °C, 5 ± 0.5 wt.% NaCl, and pH = 6.5–7.2), indicated by a minor increase in tg δ values, whereas this early degradation was not exhibited by the P2 coating. Significant degradation was shown by the P3 coating after 600 h. Thus, more resistance to salt fog was exhibited by the P2 coating compared to the P1 and P3 coatings.
- -
- UV and moisture resistance: After 449 h of exposure to UV radiation of 100 W/m2 and a relative humidity of 60%, degradation was not reached by any of the polymeric top coatings (P1 and P2), as indicated by tg δ values. Furthermore, slower degradation was observed in the P2 coating compared to the P1 coating, indicating higher resistance to UV radiation and moisture. However, deterioration of the P3 coating began after 449 h of exposure.
- -
- Extreme conditions: Alpine atmosphere tests conducted for 880 days at an altitude of 1246 m and marine atmosphere tests conducted for 244 days in the Black Sea water revealed no degradation for both P1 and P2 coatings under these conditions, as indicated by the decreasing tendency in tg δ values.
- -
- Mechanical properties: Nanoindentation and microscratch tests revealed that lower mechanical characteristics were exhibited by all coatings compared to the steel substrate. Slightly greater hardness (HIT of 0.168 ± 0.011 GPa), a lower elastic modulus (EIT of 3.867 ± 0.168 GPa), and superior wear and scratch resistance were shown by the P1 coating compared to the P2 coating (HIT of 0.149 ± 0.006 GPa, and EIT of 4.185 ± 0.100 GPa), under the tested conditions. These differences remained within a narrow range even after 600 h of exposure to salt fog.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Sarno, L.; Majidian, A.; Karagiannakis, G. The Effect of Atmospheric Corrosion on Steel Structures: A State-of-the-Art and Case-Study. Buildings 2021, 11, 571. [Google Scholar] [CrossRef]
- Medupin, R.O.; Ukoba, K.O.; Yoro, K.O.; Jen, T.-C. Sustainable Approach for Corrosion Control in Mild Steel Using Plant-Based Inhibitors: A Review. Mater. Today Sustain. 2023, 22, 100373. [Google Scholar] [CrossRef]
- Ramezanpour, J.; Ramezanzadeh, B.; Samani, N.A. Progress in Bio-Based Anti-Corrosion Coatings; A Concise Overview of the Advancements, Constraints, and Advantages. Prog. Org. Coat. 2024, 194, 108556. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Frankel, G.S. The Carbon Footprint of Steel Corrosion. NPJ Mater. Degrad. 2022, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- SSPC/NACE Joint Standard Practice, SSPC CPC-1/NACE SP21412-2020 Corrosion Prevention and Control Planning. Available online: https://store.ampp.org/sp21412-2020-sspc-cpc-1-corrosion-prevention-and-control-planning (accessed on 24 May 2024).
- SSPC Coating Application Guide 5—Guide to Maintenance Coating of Steel Structures in Atmospheric Service. Available online: https://store.ampp.org/guide-to-maintenance-coating-of-steel-structures-in-atmospheric-service (accessed on 24 May 2024).
- Aljibori, H.S.; Alamiery, A.; Kadhum, A.A.H. Advances in Corrosion Protection Coatings: A Comprehensive Review. Int. J. Corros. Scale Inhib. 2023, 12, 1476–1520. [Google Scholar] [CrossRef]
- Helsel, J.L.; Lanterman, R. Expected Service Life and Cost Considerations for Maintenance and New Construction Protective Coating Work. In Proceedings of the AMPP Annual Conference + Expo, San Antonio, TX, USA, 6–10 March 2022; p. D031S033R002. [Google Scholar]
- Corrosion Prevention and Control Planning Guidebook for Military Systems and Equipment. Office of DoD Corrosion Policy and Oversight; Spiral: 4. Release Date: 4 February 2014. Available online: https://www.dau.edu/sites/default/files/Migrated/CopDocuments/CPC%20Planning%20Guidebook%204%20Feb%2014.pdf (accessed on 24 May 2024).
- Bhat, S.I.; Mobin, M.; Islam, S.; Zehra, S.; Shahid-ul-Islam. Recent Advances in Anticorrosive Coatings Based on Sustainable Polymers: Challenges and Perspectives. Surf. Coat. Technol. 2024, 480, 130596. [Google Scholar] [CrossRef]
- Sun, W.; Xing, C.; Tang, X.; Zuo, Y.; Tang, Y.; Zhao, X. Comparative Study on the Degradation of a Zinc-Rich Epoxy Primer/Acrylic Polyurethane Coating in Different Simulated Atmospheric Solutions. J. Coat. Technol. Res. 2021, 18, 397–413. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Ravi, R.P.; Udoh, I.I.; Mathew, G.M.; Rajan, T.P.D. Polymer-Based Coating for Steel Protection, Highlighting Metal–Organic Framework as Functional Actives: A Review. Corros. Mater. Degrad. 2023, 4, 284–316. [Google Scholar] [CrossRef]
- Trentin, A.; Samiee, R.; Pakseresht, A.H.; Duran, A.; Castro, Y.; Galusek, D. Influence of Pre-Treatments on Adhesion, Barrier and Mechanical Properties of Epoxy Coatings: A Comparison between Steel, AA7075 and AA2024. Appl. Surf. Sci. Adv. 2023, 18, 100479. [Google Scholar] [CrossRef]
- Panahi, P.; Khorasani, S.N.; Mensah, R.A.; Das, O.; Neisiany, R.E. A Review of the Characterization Methods for Self-Healing Assessment in Polymeric Coatings. Prog. Org. Coat. 2024, 186, 108055. [Google Scholar] [CrossRef]
- Deryushev, V.V.; Zaitseva, M.M.; Kosenko, E.E.; Kosenko, V.V. The Quality Analysis of the Anti-Corrosion Coatings Metal Structures Operating in Difficult Conditions. IOP Conf. Ser. Mater. Sci. Eng. 2020, 913, 042059. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, J.; Qu, S.; Quan, L.; Zhao, X.; Zuo, Y. Degradation of a High Build Epoxy Primer/Polyurethane Composite Coatings under Cyclic Wet–Dry Conditions. Int. J. Electrochem. Sci. 2018, 13, 3874–3887. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Fan, W.; Tang, H.; Yang, J.; Ding, R.; Zhao, X. Anticorrosion Performance of an Eco-Friendly Coating System Including an Epoxy Tie Primer with Aluminum Tripolyphosphates and a Polyurethane Topcoat for Marine Aluminum Alloy. Prog. Org. Coat. 2023, 174, 107294. [Google Scholar] [CrossRef]
- Verma, S.; Mohanty, S.; Nayak, S.K. A Review on Protective Polymeric Coatings for Marine Applications. J. Coat. Technol. Res. 2019, 16, 307–338. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Accelerated Ageing of Organic Coating Systems by Thermal Treatment. Corros. Sci. 2001, 43, 1337–1343. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Effect of Environmental Temperature Variations on Protective Properties of Organic Coatings. Prog. Org. Coat. 2003, 46, 49–54. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Bergo, A.; Fanicchia, M. Evaluation of Accelerated Aging Procedures of Painted Galvanised Steels by EIS. Electrochim. Acta 2006, 51, 1864–1872. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Bergo, A.; Deflorian, F.; Valentinelli, L. Assessment of Protective Properties of Organic Coatings by Thermal Cycling. Prog. Org. Coat. 2003, 48, 271–280. [Google Scholar] [CrossRef]
- Miszczyk, A.; Szociński, M.; Darowicki, K. Interlayer Defect Evolution in an Organic Coating System on Steel under Hydromechanical Loading. J. Appl. Electrochem. 2007, 37, 353–358. [Google Scholar] [CrossRef]
- Bastos, A.C.; Simões, A.M.P. Effect of Uniaxial Strain on the Protective Properties of Coil-Coatings. Prog. Org. Coat. 2003, 46, 220–227. [Google Scholar] [CrossRef]
- Rossi, S.; Deflorian, F.; Fontanari, L.; Cambruzzi, A.; Bonora, P.L. Electrochemical Measurements to Evaluate the Damage Due to Abrasion on Organic Protective System. Prog. Org. Coat. 2005, 52, 288–297. [Google Scholar] [CrossRef]
- Kotnarowska, D. Influence of Ageing with UV Radiation on Physicochemical Properties of Acrylic-Polyurethane Coatings. JSEMAT 2018, 8, 95–109. [Google Scholar] [CrossRef]
- Momber, A.W.; Fröck, L.; Marquardt, T. Effects of Accelerated Ageing on the Mechanical Properties of Adhesive Joints between Stainless Steel and Polymeric Top Coat Materials for Marine Applications. Int. J. Adhes. Adhes. 2020, 103, 102699. [Google Scholar] [CrossRef]
- Bierwagen, G.; Tallman, D.; Li, J.; He, L.; Jeffcoate, C. EIS Studies of Coated Metals in Accelerated Exposure. Prog. Org. Coat. 2003, 46, 149–158. [Google Scholar] [CrossRef]
- Hinderliter, B.R.; Croll, S.G.; Tallman, D.E.; Su, Q.; Bierwagen, G.P. Interpretation of EIS Data from Accelerated Exposure of Coated Metals Based on Modeling of Coating Physical Properties. Electrochim. Acta 2006, 51, 4505–4515. [Google Scholar] [CrossRef]
- Oliveira, J.L.; Skilbred, A.W.B.; Loken, A.; Henriques, R.R.; Soares, B.G. Effect of Accelerated Ageing Procedures and Flash Rust Inhibitors on the Anti-Corrosive Performance of Epoxy Coatings: EIS and Dynamic-Mechanical Analysis. Prog. Org. Coat. 2021, 159, 106387. [Google Scholar] [CrossRef]
- Da Silva Lopes, T.; Lopes, T.; Martins, D.; Carneiro, C.; Machado, J.; Mendes, A. Accelerated Aging of Anticorrosive Coatings: Two-Stage Approach to the AC/DC/AC Electrochemical Method. Prog. Org. Coat. 2020, 138, 105365. [Google Scholar] [CrossRef]
- Song, J.; Liu, X.; Hu, W.; Zhu, T.; Xiao, K.; Gao, J. Corrosion and Aging Behavior of Epoxy/Polyurethane Coatings on Carbon Steel in a Subhumid Environment. Int. J. Electrochem. Sci. 2022, 17, 22063. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Ferreira, M.G.S. Ranking High-Quality Paint Systems Using EIS. Part I: Intact Coatings. Corros. Sci. 2003, 45, 123–138. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Ferreira, M.G.S. Ranking High-Quality Paint Systems Using EIS. Part II: Defective Coatings. Corros. Sci. 2003, 45, 139–147. [Google Scholar] [CrossRef]
- Van der Weijde, D.H.; Van Westing, E.P.M.; Ferrari, G.M.; De Wit, J.H.W. Lifetime Prediction of Organic Coatings with Impedance Spectroscopy. In Proceedings of the Polymeric Materials Science and Engineering Spring Meeting 1996, San Francisco, CA USA, 8–12 April 1996; Volume 74, pp. 12–13. [Google Scholar]
- ISO 12944-5:2019; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems, Part 5: Protective Paint Systems. International Organization for Standardization: Geneva, Switzerland, 2019.
- Rus, T.; Caramitu, A.; Mitrea, S.; Lingvay, I. Comparative Study about the Thermal Stability and UV Resistance of Some Paint Layers for Electro-Energetic Equipment. In Proceedings of the 2016 Diagnostic of Electrical Machines and Insulating Systems in Electrical Engineering (DEMISEE), Papradno, Slovakia, 20–22 June 2016; pp. 60–65. [Google Scholar] [CrossRef]
- Caramitu, A.R.; Ion, I.; Bors, A.M.; Ciobanu, C.R.; Schreiner, C.; Aradoaei, M.; Caramitu, A.-M.D. Physico-Chemical Characterization of Paint Films with Electromagnetic Properties. Mater. Plast. 2022, 59, 9–23. [Google Scholar] [CrossRef]
- Rus, T.; Bors, A.M.; Caramitu, A.R.; Lingvay, I.; Vaireanu, D.I. Comparative Studies on the Thermal Ageing of Some Painting Materials. Mater. Plast. 2018, 55, 168–175. [Google Scholar] [CrossRef]
- Rus, T.; Caramitu, A.R.; Ungureanu, L.C.; Marinescu, V.; Bors, A.M.; Vaireanu, D.I. Ageing of Some Painting Materials Subjected to Thermal Treatment in Natural Esters. Mater. Plast. 2018, 55, 28–32. [Google Scholar] [CrossRef]
- Rus, T.; Lingvay, I.; Caramitu, A.R.; BORS, A.M.; Vaireanu, D.I. Comparative Studies on the UV Radiations Resistance of Some Painting Materials. Mater. Plast 2017, 54, 720–725. [Google Scholar] [CrossRef]
- ISO 19840:2012; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Measurement of, and Acceptance Criteria for, the Thickness of Dry Films on Rough Surfaces. International Organization for Standardization: Geneva, Switzerland, 2012.
- Almeida, E.; Santos, D.; Fragata, F.; De La Fuente, D.; Morcillo, M. Anticorrosive Painting for a Wide Spectrum of Marine Atmospheres: Environmental-Friendly versus Traditional Paint Systems. Prog. Org. Coat. 2006, 57, 11–22. [Google Scholar] [CrossRef]
- Pei, W.; Pei, X.; Xie, Z.; Wang, J. Research Progress of Marine Anti-Corrosion and Wear-Resistant Coating. Tribol. Int. 2024, 198, 109864. [Google Scholar] [CrossRef]
- Gao, J.; Hu, W.; Wang, R.; Li, X. Study on a Multifactor Coupling Accelerated Test Method for Anticorrosive Coatings in Marine Atmospheric Environments. Polym. Test. 2021, 100, 107259. [Google Scholar] [CrossRef]
- Lawal, S.L.; Afolalu, S.A.; Jen, T.C.; Akinlabi, E.T. Corrosion Control and Its Application in Marine Environment—A Review. SSP 2024, 355, 61–73. [Google Scholar] [CrossRef]
- Vera, R.; Bagnara, M.; Henríquez, R.; Muñoz, L.; Rojas, P.; Díaz-Gómez, A. Performance of Anticorrosive Paint Systems for Carbon Steel in the Antarctic Marine Environment. Materials 2023, 16, 5713. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Popa, I.; Muresanu, A.M. Study of a New Possibility to Predict the Behavior of High-Performance Anticorrosive Protections Applied on Steel After Their Exposure in Natural Aggressive Environments and in Laboratory Accelerated Conditions, Respectively. Constructii 2014, 15, 40. [Google Scholar]
- Caramitu, A.R.; Mitrea, S.; Stancu, N.; Butoi, N.; Luchian, A.-M. Device for Determining the State of Degradation and Method for Evaluating the Life Time of Paint Layers. RO Patent No. 133364 B1, 30 August 2023. [Google Scholar]
- ASTM G151-19; Standard Practice for Exposing Nonmetallic Materials in Accelerated Test Devices That Use Laboratory Light Sources. ASTM International: West Conshohocken, PA, USA, USA, 2019.
- ASTM G155-21; Standard Practice for Operating Xenon Arc Lamp Apparatus for Exposure of Materials. ASTM International: West Conshohocken, PA, USA, USA, 2021.
- ASTM D2565-23; Standard Practice for Xenon-Arc Exposure of Plastics Intended for Outdoor Applications. ASTM International: West Conshohocken, PA, USA, USA, 2023.
- Kovacs, M.; Pécskay, Z.; Fülöp, A.; Jurje, M.; Edelstein, O. Geochronology of the Neogene Intrusive Magmatism of the Oaș—Gutâi Mountains, Eastern Carpathians (NW Romania). Geol. Carpath. 2013, 64, 483–496. [Google Scholar] [CrossRef]
- Kovacs, M.; Fülöp, A. Baia Mare Geological and Mining Park—A Potential New Geopark in the Northwestern Part of Romania. Studia UBB, Geologia 2009, 54, 27–32. [Google Scholar] [CrossRef]
- Dobrot, O.-M.; Mocanu, C.-I. Study of the Destruction Influence of Elements from Offshore Platform Leg on Stress and Vibration Modes. Constanta Marit. Univ. Ann. 2010, 14, 25–30. [Google Scholar]
- Mihailov, M.-E.; Tomescu-Chivu, M.-I.; Dima, V. Black Sea Water Dynamics on the Romanian Littoral–Case Study: The Upwelling Phenomena. Rom. Rep. Phys. 2012, 64, 232–245. [Google Scholar]
- Onea, F.; Rusu, L. A Long-Term Assessment of the Black Sea Wave Climate. Sustainability 2017, 9, 1875. [Google Scholar] [CrossRef]
- Krivoguz, D.; Semenova, A.; Mal’ko, S. Spatial Analysis of Seasonal Patterns in Sea Surface Temperature and Salinity Distribution in the Black Sea (1992–2017). IOP Conf. Ser. Earth Environ. Sci. 2021, 937, 032013. [Google Scholar] [CrossRef]
- Caramitu, A.R.; Lungu, M.V.; Ciobanu, R.C.; Ion, I.; Marin, M.; Marinescu, V.; Pintea, J.; Aradoaei, S.; Schreiner, O.D. Recycled Polypropylene/Strontium Ferrite Polymer Composite Materials with Electromagnetic Shielding Properties. Polymers 2024, 16, 1129. [Google Scholar] [CrossRef] [PubMed]
- Manea, C.A.; Geambazu, L.E.; Tălpeanu, D.; Marinescu, V.; Sbârcea, G.B.; Pătroi, D.; Udrea, R.M.; Lungu, M.V.; Lucaci, M. CoxCrFeNiTi High-Entropy Alloys Prepared via Mechanical Alloying and Spark Plasma Sintering for Magnetron Sputtering Coatings. Materials 2023, 16, 6386. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Shen, L.; Wang, L.; Liu, T.; He, C. Nanoindentation and Morphological Studies of Epoxy Nanocomposites. Macro Mater. Eng. 2006, 291, 1358–1366. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Anis, A.; Alam, M.; Ubaidullah, M.; Al-Zahrani, S.M. Effects of SiO2 and ZnO Nanoparticles on Epoxy Coatings and Its Performance Investigation Using Thermal and Nanoindentation Technique. Polymers 2021, 13, 1490. [Google Scholar] [CrossRef]
- SR EN 60216-3:2007; Electroinsulating Materials. Thermal Endurance Properties. Part 3: Instructions for the Calculation of Thermal Endurance Characteristics. ASRO: Tokyo, Japan, 2007.
- Lekatou, A.G.; Emmanouilidou, S.; Dimitriadis, K.; Baikousi, M.; Karakassides, M.A.; Agathopoulos, S. Simulating Porcelain Firing Effect on the Structure, Corrosion and Mechanical Properties of Co–Cr–Mo Dental Alloy Fabricated by Soft Milling. Odontology 2024, 112, 372–389. [Google Scholar] [CrossRef]
- Cagliero, R.; Barbato, G.; Maizza, G.; Genta, G. Measurement of Elastic Modulus by Instrumented Indentation in the Macro-Range: Uncertainty Evaluation. Int. J. Mech. Sci. 2015, 101–102, 161–169. [Google Scholar] [CrossRef]
- Maganty, S.; Roma, M.P.C.; Meschter, S.J.; Starkey, D.; Gomez, M.; Edwards, D.G.; Ekin, A.; Elsken, K.; Cho, J. Enhanced Mechanical Properties of Polyurethane Composite Coatings through Nanosilica Addition. Prog. Org. Coat. 2016, 90, 243–251. [Google Scholar] [CrossRef]
- Ghermezcheshme, H.; Mohseni, M.; Yahyaei, H. Use of Nanoindentation and Nanoscratch Experiments to Reveal the Mechanical Behavior of POSS Containing Polyurethane Nanocomposite Coatings: The Role of Functionality. Tribol. Int. 2015, 88, 66–75. [Google Scholar] [CrossRef]
- Momber, A.W.; Irmer, M.; Marquardt, T. Effects of Polymer Hardness on the Abrasive Wear Resistance of Thick Organic Offshore Coatings. Prog. Org. Coat. 2020, 146, 105720. [Google Scholar] [CrossRef]
- Lungu, M.V.; Sobetkii, A.; Sobetkii, A.A.; Pătroi, D.; Prioteasa, P.; Ion, I.; Negrilă, C.C.; Chifiriuc, M.C. Functional Properties Improvement of Ag-ZnO Thin Films Using Inconel 600 Interlayer Produced by Electron Beam Evaporation Technique. Thin Solid Films 2018, 667, 76–87. [Google Scholar] [CrossRef]


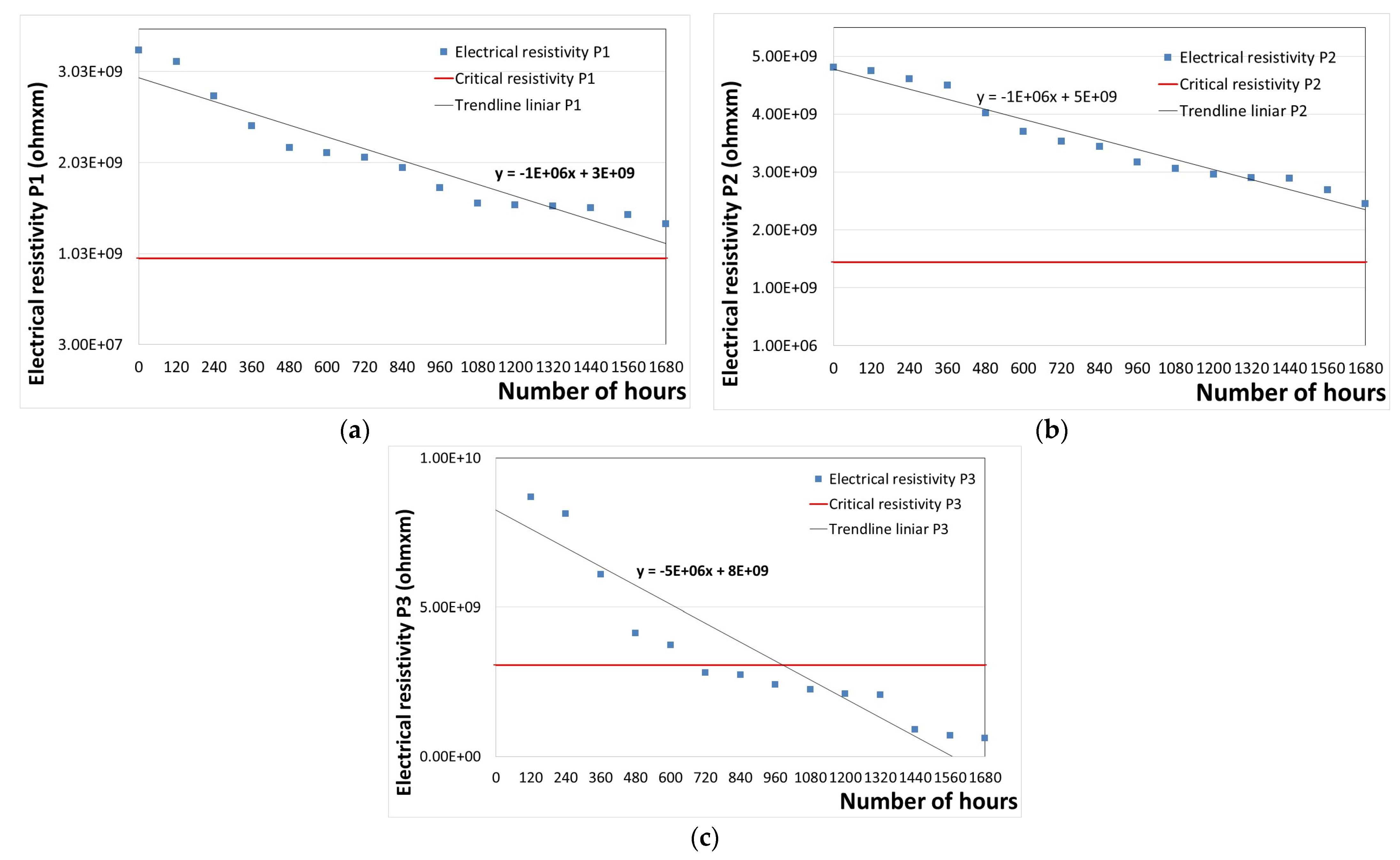


| Sample | Equation | y | b | a | Lifetime until Complete Degradation (h) |
|---|---|---|---|---|---|
| P1 | y = −1 × 106x + 3 × 109 | 9.80 × 108 | 3.00 × 109 | −1.0 × 106 | 2020 |
| P2 | y = −1 × 106x + 5 × 109 | 1.44 × 109 | 5.00 × 109 | −1.0 × 106 | 3558 |
| P3 | y = −5 × 106x + 8 × 109 | 3.06 × 109 | 8.00 × 109 | −5.0 × 106 | 988 |
| Sample | Mean HIT ± SD (GPa) | Mean HV ± SD | Mean EIT ± SD (GPa) | HIT/EIT | HIT3/EIT2 (GPa) |
|---|---|---|---|---|---|
| P1 coating | 0.168 ± 0.011 | 15.5 ± 1.0 | 3.867 ± 0.168 | 0.0434 | 0.00032 |
| P1-SF coating | 0.156 ± 0.012 | 14.4 ± 1.1 | 3.624 ± 0.171 | 0.0430 | 0.00029 |
| P2 coating | 0.149 ± 0.006 | 13.8 ± 0.6 | 4.185 ± 0.100 | 0.0356 | 0.00019 |
| P2-SF coating | 0.121 ± 0.009 | 11.2 ± 0.8 | 3.591 ± 0.111 | 0.0337 | 0.00014 |
| Steel substrate | 2.261 ± 0.088 | 209.4 ± 8.2 | 128.328 ± 2.518 | 0.0176 | 0.00070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caramitu, A.R.; Ciobanu, R.C.; Lungu, M.V.; Lungulescu, E.-M.; Scheiner, C.M.; Aradoaei, M.; Bors, A.M.; Rus, T. Polymeric Protective Films as Anticorrosive Coatings—Environmental Evaluation. Polymers 2024, 16, 2192. https://doi.org/10.3390/polym16152192
Caramitu AR, Ciobanu RC, Lungu MV, Lungulescu E-M, Scheiner CM, Aradoaei M, Bors AM, Rus T. Polymeric Protective Films as Anticorrosive Coatings—Environmental Evaluation. Polymers. 2024; 16(15):2192. https://doi.org/10.3390/polym16152192
Chicago/Turabian StyleCaramitu, Alina Ruxandra, Romeo Cristian Ciobanu, Magdalena Valentina Lungu, Eduard-Marius Lungulescu, Cristina Mihaela Scheiner, Mihaela Aradoaei, Adriana Mariana Bors, and Traian Rus. 2024. "Polymeric Protective Films as Anticorrosive Coatings—Environmental Evaluation" Polymers 16, no. 15: 2192. https://doi.org/10.3390/polym16152192
APA StyleCaramitu, A. R., Ciobanu, R. C., Lungu, M. V., Lungulescu, E.-M., Scheiner, C. M., Aradoaei, M., Bors, A. M., & Rus, T. (2024). Polymeric Protective Films as Anticorrosive Coatings—Environmental Evaluation. Polymers, 16(15), 2192. https://doi.org/10.3390/polym16152192








