Advances in Hydrogel Polymers for Microbial Control in Water Systems
Abstract
:1. Introduction
2. Materials and Methods
3. Results
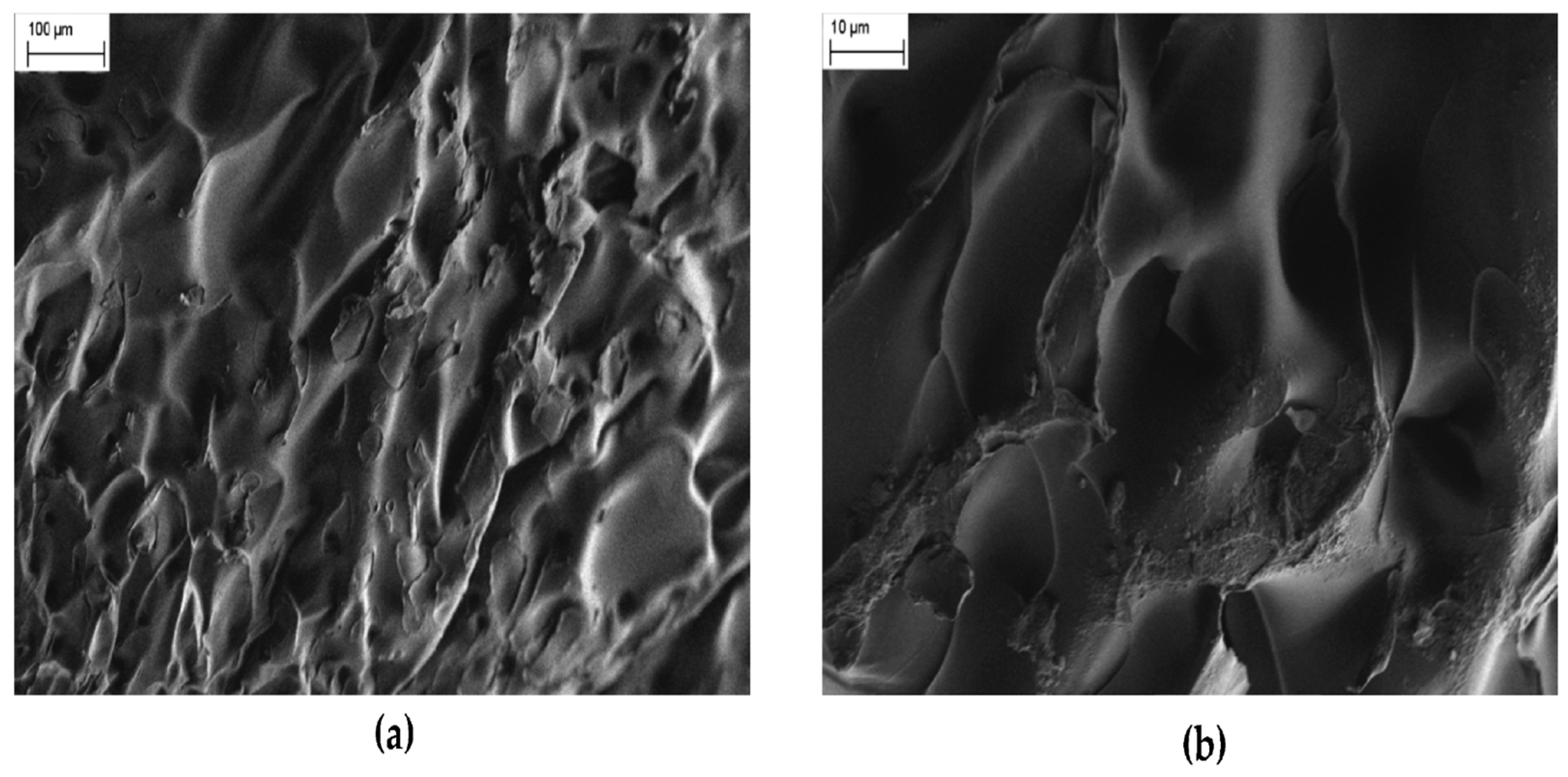
3.1. Fundamentals of Hydrogel Polymers
3.2. Synthesis of Hydrogel Polymers for Microbial Control
3.3. Mechanisms of Microbial Control by Hydrogel Polymers
3.4. Applications of Hydrogel Polymers in Water Systems
| Description | Applications | Examples | Reference | |
|---|---|---|---|---|
| Absorption Capacity | Ability to hold large volumes of water without dissolving, due to porous cross-linked structures. | Water purification, removal of organic and inorganic contaminants | Polyacrylamide (PAAm), Polyethylene glycol (PEG) | [20] |
| Synthesis Methods | Includes solution polymerization, free radical polymerization, and radiation methods. | Tailoring properties for specific water treatment needs | Solution polymerization for large-scale water decontamination | [14] |
| Mechanisms of Action | Encapsulation and immobilization of microorganisms, release of antimicrobial agents. | Controlling spread of pathogens in drinking and wastewater systems | Chitosan-based hydrogels for their antiviral and antibacterial properties | [27,28,31,32] |
| Environmental Impact | Biodegradability, toxicity, and impact on non-target organisms. | Ensuring sustainability and safety in water treatment | Biodegradable chitosan versus synthetic hydrogels | [14,24,31,32] |
| Technological Advances | Smart hydrogels responsive to environmental changes, integration with nanotechnology. | Enhancing effectiveness and application range in water systems | Smart hydrogels for adaptive water purification systems | [48,52,53] |
4. Discussion
4.1. Performance Evaluation and Optimization
4.2. Environmental and Health Impacts
4.3. Future Perspectives and Innovations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Improving Access to Water, Sanitation and Hygiene Can Save 1.4 Million Lives per Year, Says New WHO Report. WHO. 2023. Available online: https://www.who.int/news/item/28-06-2023-improving-access-to-water--sanitation-and-hygiene-can-save-1.4-million-lives-per-year--says-new-who-report (accessed on 23 March 2024).
- Lin, L.; Yang, H.; Xu, X. Effects of water pollution on human health and disease heterogeneity: A review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and consequences of groundwater contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Some, S.; Mondal, R.; Mitra, D.; Jain, D.; Verma, D.; Das, S. Microbial pollution of water with special reference to coliform bacteria and their nexus with environment. Energy Nexus 2021, 1, 100008. [Google Scholar] [CrossRef]
- Cabral, J.P. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.A.; Khan, S.A. Polymer hydrogels for wastewater treatment. In Environmental Chemistry and Recent Pollution Control Approaches; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman FB, A.; Haque, S.; Rashid, T.U.; Kabir, S.F. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Flores-Valenzuela, L.E.; González-Fernández, J.V.; Carranza-Oropeza, M.V. Hydrogel Applicability for the Industrial Effluent Treatment: A Systematic Review and Bibliometric Analysis. Polymers 2023, 15, 2417. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Pourebrahimi, S.; Malloum, A.; Pirooz, M.; Osagie, C.; Ghosh, S.; Dehghani, M.H. Hydrogel-based materials as super adsorbents and antibacterial agents for the remediation of emerging pollutants: A comprehensive review. Emerg. Contam. 2024, 10, 100336. [Google Scholar] [CrossRef]
- Persano, F.; Malitesta, C.; Mazzotta, E. Cellulose-Based Hydrogels for Wastewater Treatment: A Focus on Metal Ions Removal. Polymers 2024, 16, 1292. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural polymer-based hydrogels: From polymer to biomedical applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Khan, S.B. Versatility of hydrogels: From synthetic strategies, classification, and properties to biomedical applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M.; Roy, P.; Bonilla-Petriciolet, A.; Badawi, M.; Ganachari, S.V.; Shetti, N.P.; Aminabhavi, T.M. Polymeric hydrogels-based materials for wastewater treatment. Chemosphere 2023, 331, 138743. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.L.; Vásquez, L.; Athanassiou, A.; Fragouli, D. Polymeric hydrogels—A promising platform in enhancing water security for a sustainable future. Adv. Mater. Interfaces 2021, 8, 2100580. [Google Scholar] [CrossRef]
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent progress of antibacterial hydrogels in wound dressings. Mater. Today Bio 2023, 19, 100582. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Park, G.T.; Han, S.S. Biocompatible, antibacterial, polymeric hydrogels active against multidrug-resistant Staphylococcus aureus strains for food packaging applications. Food Control 2021, 123, 107695. [Google Scholar] [CrossRef]
- Zumbuehl, A.; Ferreira, L.; Kuhn, D.; Astashkina, A.; Long, L.; Yeo, Y.; Kohane, D.S. Antifungal hydrogels. Proc. Natl. Acad. Sci. USA 2007, 104, 12994–12998. [Google Scholar] [CrossRef] [PubMed]
- Pinthong, T.; Yooyod, M.; Daengmankhong, J.; Tuancharoensri, N.; Mahasaranon, S.; Viyoch, J.; Ross, G.M. Development of Natural Active Agent-Containing Porous Hydrogel Sheets with High Water Content for Wound Dressings. Gels 2023, 9, 459. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of polymer hydrogel composites and their applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Yang, J.; Rao, L.; Wang, Y.; Zhao, Y.; Liu, D.; Wang, Z.; Liu, Y. Recent advances in smart hydrogels prepared by ionizing radiation technology for biomedical applications. Polymers 2022, 14, 4377. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Pagador, J.B. Hydrogels for bioprinting: A systematic review of hydrogels synthesis, bioprinting parameters, and bioprinted structures behavior. Front. Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Idumah, C.I.; Nwuzor, I.C.; Odera, R.S. Recent advances in polymer hydrogel nanoarchitectures and applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100143. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel trends in hydrogel development for biomedical applications: A review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.C.; Tham, E.; Liu, X.; Yehl, K.; Rovner, A.J.; Yuk, H.; Lu, T.K. Hydrogel-based biocontainment of bacteria for continuous sensing and computation. Nat. Chem. Biol. 2021, 17, 724–731. [Google Scholar] [CrossRef]
- Salomé Veiga, A.; Schneider, J.P. Antimicrobial hydrogels for the treatment of infection. Pept. Sci. 2013, 100, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in antibacterial hydrogel dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Agili, F.A.; Mohamed, S.F. Synthesis and Characterization of a Self-Crosslinked Organic Copolymer Kappa-Carrageenan/Polyacrylamide/Cetrimide (κ-CAR/PAAm/CI) Hydrogel with Antimicrobial and Anti-Inflammatory Activities for Wound Healing. Chemistry 2023, 5, 2273–2287. [Google Scholar] [CrossRef]
- Lungoci, C.; Rîmbu, C.M.; Motrescu, I.; Serbezeanu, D.; Horhogea, C.E.; Vlad-Bubulac, T.; Ghițău, C.S.; Puiu, I.; Neculai-Văleanu, A.-S.; Robu, T. Evaluation of the Antibacterial Properties of Polyvinyl Alcohol-Pullulan Scaffolds Loaded with Nepeta racemosa Lam. Essential Oil and Perspectives for Possible Applications. Plants 2023, 12, 898. [Google Scholar] [CrossRef] [PubMed]
- Ermawati, D.E.; Surya, A.P.; Setyawati, R.; Niswah, S.U. The effect of glycerin and polyethylene glycol 400 as humectant on stability and antibacterial activity of nanosilver biosynthetic peel-off mask. J. Appl. Pharm. Sci. 2022, 12, 080–089. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and antimicrobial activity of some novel cross-linked chitosan hydrogels. Int. J. Mol. Sci. 2012, 13, 11194–11209. [Google Scholar] [CrossRef] [PubMed]
- Aydin, G.; Zorlu, E.B. Characterisation and antibacterial properties of novel biodegradable films based on alginate and roselle (Hibiscus sabdariffa L.) extract. Waste Biomass Valorization 2022, 13, 2991–3002. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Y.P.; de Almeida Campos, L.A.; Alves Agreles, M.A.; Galembeck, A.; Macário Ferro Cavalcanti, I. Advanced hydrogels combined with silver and gold nanoparticles against antimicrobial resistance. Antibiotics 2023, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Functionalization and antibacterial applications of cellulose-based composite hydrogels. Polymers 2022, 14, 769. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Li, Z.; Cao, Z.; Hao, H.; Bi, J.; Hou, H.; Wu, H.; Zhang, G. Antibacterial film based on κ-carrageenan with benzyl isothiocyanate-β-cyclodextrin inclusion complex: Characterization and application in chicken preservation. Food Hydrocoll. 2023, 145, 109063. [Google Scholar] [CrossRef]
- Dorterler, O.C.; Akgun, B.; Alper, M.; Ayhan, F. Improving Antimicrobial Properties of GelMA Biocomposite Hydrogels for Regenerative Endodontic Treatment. Polymers 2024, 16, 1675. [Google Scholar] [CrossRef] [PubMed]
- Salma-Ancane, K.; Sceglovs, A.; Tracuma, E.; Wychowaniec, J.K.; Aunina, K.; Ramata-Stunda, A.; Loca, D. Effect of crosslinking strategy on the biological, antibacterial and physicochemical performance of hyaluronic acid and ɛ-polylysine based hydrogels. Int. J. Biol. Macromol. 2022, 208, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Luna, V.H.; González-Reynoso, O. Encapsulation of biological agents in hydrogels for therapeutic applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J.M. Chitosan Hydrogels for Water Purification Applications. Gels 2023, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Poore, C. Solar-Powered Gel Produces Enough Clean Water to Meet Daily Needs. Princeton University. 2023. Available online: https://research.princeton.edu/news/solar-powered-gel-produces-enough-clean-water-meet-daily-needs (accessed on 23 March 2024).
- Xu, X.; Guillomaitre, N.; Christie, K.S.; Bay, R.K.; Bizmark, N.; Datta, S.S.; Priestley, R.D. Quick-release antifouling hydrogels for solar-driven water purification. ACS Cent. Sci. 2023, 9, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Kandel, D.R.; Kim, J.T.; Siengchin, S.; Lee, J. Recent advances in cellulose-and alginate-based hydrogels for water and wastewater treatment: A review. Carbohydr. Polym. 2023, 323, 121339. [Google Scholar] [CrossRef] [PubMed]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent achievements in polymer bio-based flocculants for water treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Hossain, M.M.; Khatun, M.K.; Hossain, K.R. Hydrogel-based super adsorbents for efficient removal of heavy metals in industrial wastewater treatment and environmental conservation. Environ. Funct. Mater. 2023, 2, 142–158. [Google Scholar] [CrossRef]
- Feng, C.; Yang, P.; Liu, H.; Mao, M.; Liu, Y.; Xue, T.; Liu, K. Bilayer porous polymer for efficient passive building cooling. Nano Energy 2021, 85, 105971. [Google Scholar] [CrossRef]
- Rehman, T.U.; Shah, L.A. Rheological investigation of polymer hydrogels for industrial application: A review. Int. J. Polym. Anal. Charact. 2022, 27, 430–445. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Hydrogels: Characteristics and application as delivery systems of phenolic and aroma compounds. Foods 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Uva, M.; Atrei, A. Surface morphology at the microscopic scale, swelling/deswelling, and the magnetic properties of PNIPAM/CMC and PNIPAM/CMC/Fe3O4 hydrogels. Gels 2016, 2, 30. [Google Scholar] [CrossRef]
- Clasen, T.F.; Thao, D.H.; Boisson, S.; Shipin, O. Microbiological effectiveness and cost of boiling to disinfect drinking water in rural Vietnam. Environ. Sci. Technol. 2008, 42, 4255–4260. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, S.; Oladejo, T.J.; Muhammed, N.M.; Saka, A.A.; Oluwabunmi, A.A.; Abdulkabir, M.; Joel, O.O. Fabrication of porous ceramic pot filters for adsorptive removal of pollutants in tannery wastewater. Sci. Afr. 2021, 11, e00705. [Google Scholar] [CrossRef]
- Karikari, A.Y.; Ampofo, J.A. Chlorine treatment effectiveness and physico-chemical and bacteriological characteristics of treated water supplies in distribution networks of Accra-Tema Metropolis, Ghana. Appl. Water Sci. 2013, 3, 535–543. [Google Scholar] [CrossRef]
- Rice, E.W.; Rose, L.J.; Johnson, C.H.; Boczek, L.A.; Arduino, M.J.; Reasoner, D.J. Boiling and Bacillus spores. Emerg. Infect. Dis. 2004, 10, 1887. [Google Scholar] [CrossRef] [PubMed]
- Wysowska, E.; Wiewiórska, I.; Kicińska, A. The impact of different stages of water treatment process on the number of selected bacteria. Water Resour. Ind. 2021, 26, 100167. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2021, 751, 141673. [Google Scholar] [CrossRef] [PubMed]
- Tariq, Z.; Iqbal, D.N.; Rizwan, M.; Ahmad, M.; Faheem, M.; Ahmed, M. Significance of biopolymer-based hydrogels and their applications in agriculture: A review in perspective of synthesis and their degree of swelling for water holding. RSC Adv. 2023, 13, 24731–24754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, W.; Hu, X.; Li, K.; Luo, P.; Li, X.; Duan, C. Evaluating the efficacy of point-of-use water treatment systems using the water quality index in rural southwest China. Water 2020, 12, 867. [Google Scholar] [CrossRef]
- Kandemir, N.; Vollmer, W.; Jakubovics, N.S.; Chen, J. Mechanical interactions between bacteria and hydrogels. Sci. Rep. 2018, 8, 10893. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wollensak, K.; Rau, S.; Guevara Solarte, D.L.; Paschke, S.; Lienkamp, K.; Staszewski, O. How do polymer coatings affect the growth and bacterial population of a biofilm formed by total human salivary bacteria?—A study by 16S-rna sequencing. Microorganisms 2021, 9, 1427. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment sensitive hydrogels for drug delivery applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, A.; Constantinidou, C.; Arvanitis, T.N.; Haddleton, D.M.; Charmet, J.; Hand, R.A. Multifunctional composite hydrogels for bacterial capture, growth/elimination, and sensing applications. ACS Appl. Mater. Interfaces 2022, 14, 47323–47344. [Google Scholar] [CrossRef] [PubMed]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D. Biodegradability of bio-based and synthetic hydrogels as sustainable soil amendments: A review. J. Appl. Polym. Sci. 2023, 140, e53655. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Manna, K.; Pal, S. Recent advances in various stimuli-responsive hydrogels: From synthetic designs to emerging healthcare applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Hu, C.; Yang, L.; Wang, Y. Recent advances in smart-responsive hydrogels for tissue repairing. MedComm–Biomater. Appl. 2022, 1, e23. [Google Scholar] [CrossRef]
- Quazi, M.Z.; Park, N. Nanohydrogels: Advanced polymeric nanomaterials in the era of nanotechnology for robust functionalization and cumulative applications. Int. J. Mol. Sci. 2022, 23, 1943. [Google Scholar] [CrossRef] [PubMed]

| Hydrogel Polymer | Bacteria | Diameter of Inhibition Zone (mm) | References |
|---|---|---|---|
| Poly(acrylamide) Hydrogel | Escherichia coli | 13 | [31] |
| Poly(vinyl alcohol) Hydrogel | Staphylococcus aureus | 20 | [32] |
| Poly(ethylene glycol) Hydrogel | Pseudomonas aeruginosa | 18 | [33] |
| Chitosan Hydrogel | Bacillus subtilis | 22.4 | [34] |
| Alginate Hydrogel | Klebsiella pneumoniae | 14 | [35] |
| Polyvinyl Alcohol/Carboxymethyl Cellulose (PVA/CMC) Hydrogel | Proteus vulgaris | 19 | [36] |
| WO3NPs-hydroxyethyl Cellulose Hydrogel | Bacillus cereus | 17 | [37] |
| Benzyl Isothiocyanate-β-cyclodextrin-κ-carrageenan (BITC- βCD-KC) Hydrogel | Listeria monocytogenes | 16.7 | [38] |
| Gelatin methacryloyl/Hyaluronic acid Hydrogel | Enterococcus faecalis | 5.5 | [39] |
| Hyaluronic Acid Hydrogel | Escherichia coli | 20.7 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinsemolu, A.A.; Onyeaka, H. Advances in Hydrogel Polymers for Microbial Control in Water Systems. Polymers 2024, 16, 2205. https://doi.org/10.3390/polym16152205
Akinsemolu AA, Onyeaka H. Advances in Hydrogel Polymers for Microbial Control in Water Systems. Polymers. 2024; 16(15):2205. https://doi.org/10.3390/polym16152205
Chicago/Turabian StyleAkinsemolu, Adenike A., and Helen Onyeaka. 2024. "Advances in Hydrogel Polymers for Microbial Control in Water Systems" Polymers 16, no. 15: 2205. https://doi.org/10.3390/polym16152205







