Rapid Construction of PVA@CDs/SiO2 Fluorescent/Structural Color Dual-Mode Anti-Counterfeiting Labels via Spray-Coating Method
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Multicolor Fluorescent CDs
2.3. Preparation of SiO2 Microspheres
2.4. Preparation of Dual-Mode Anti-Counterfeiting Labels
2.5. Characterization
3. Results and Discussion
3.1. Characterization of CDs
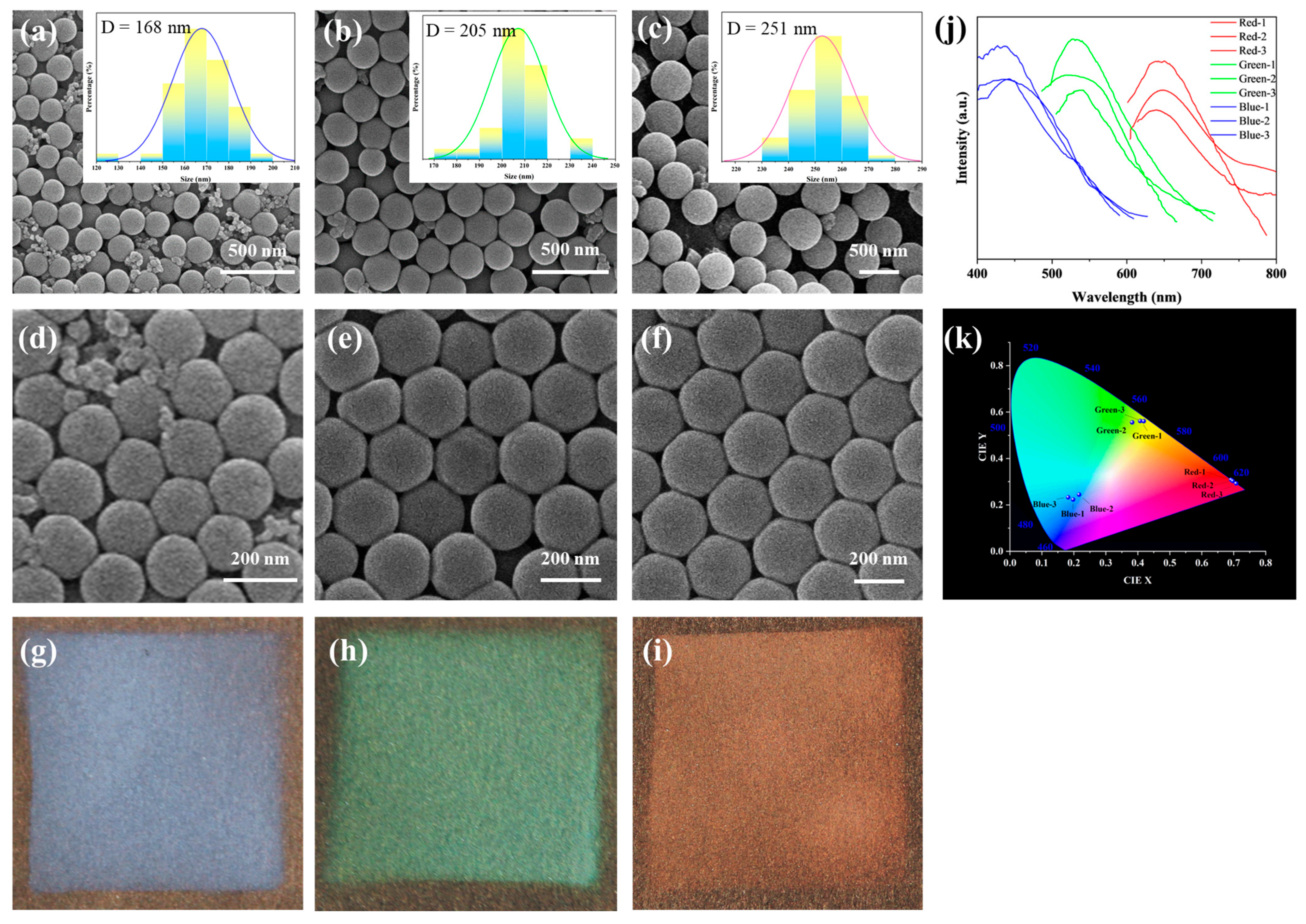
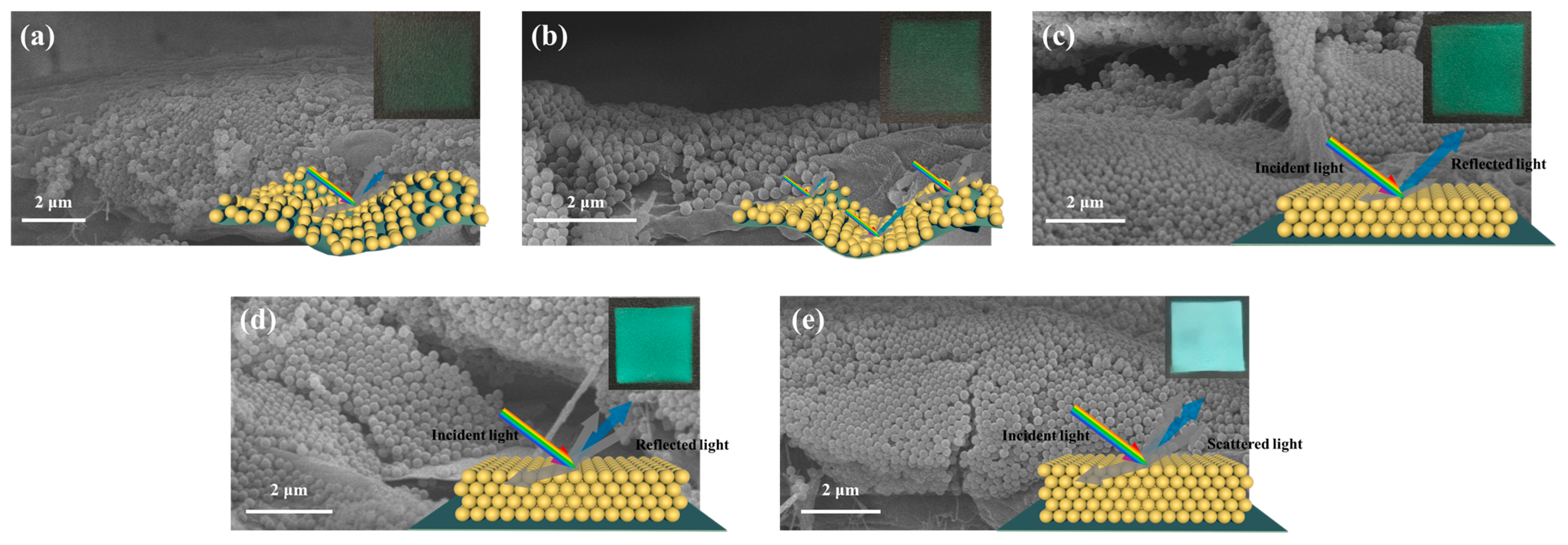
3.2. Characterization of Photonic Crystals
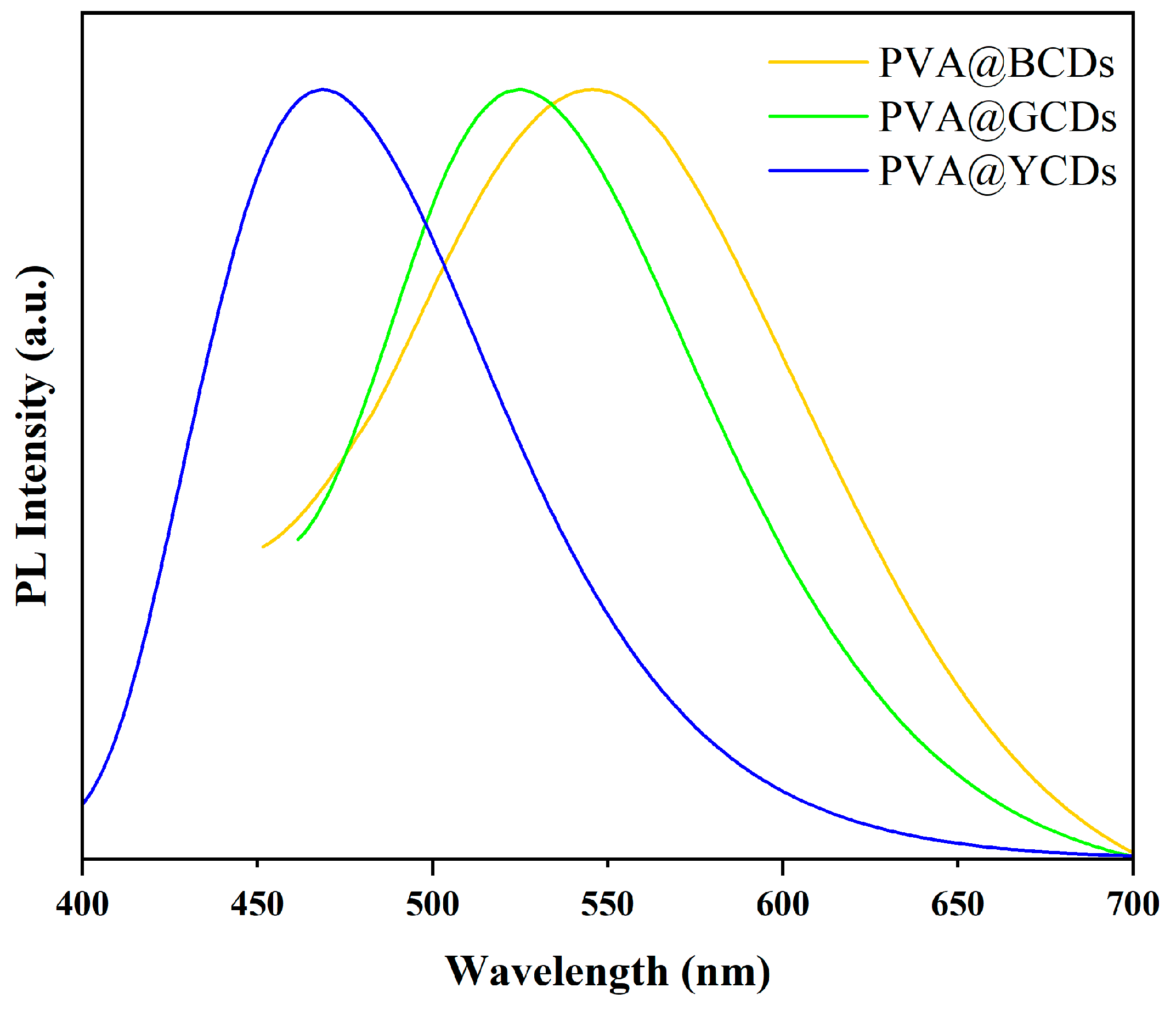
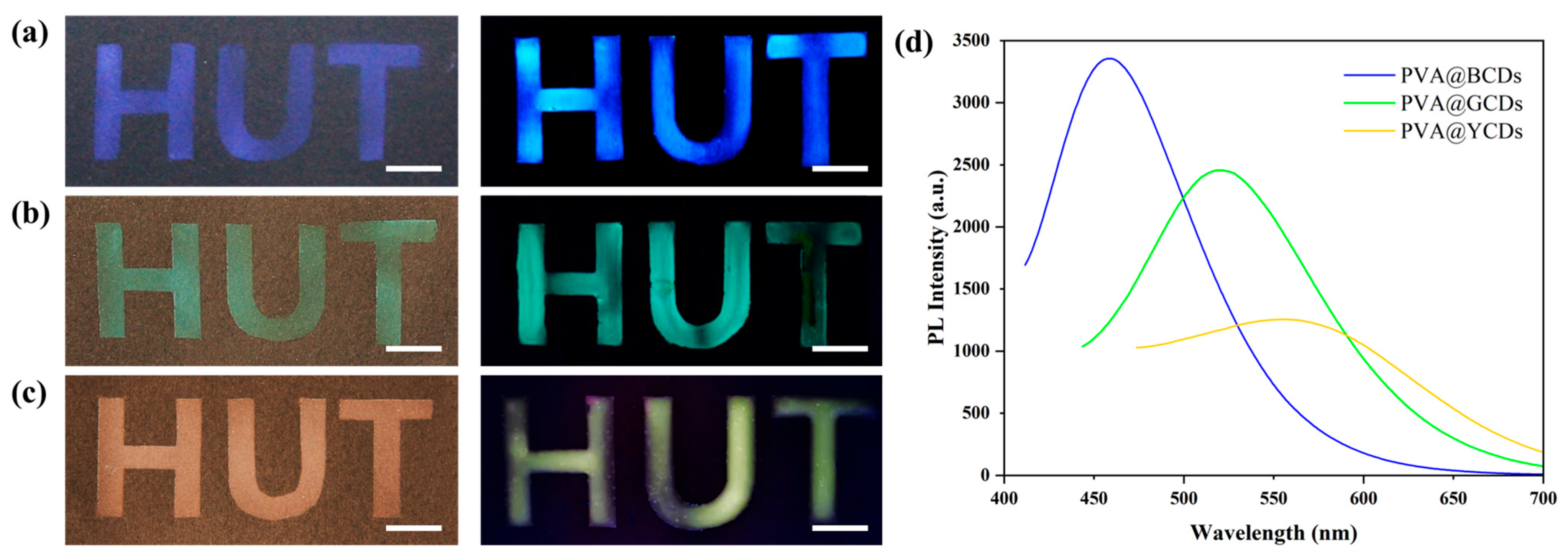
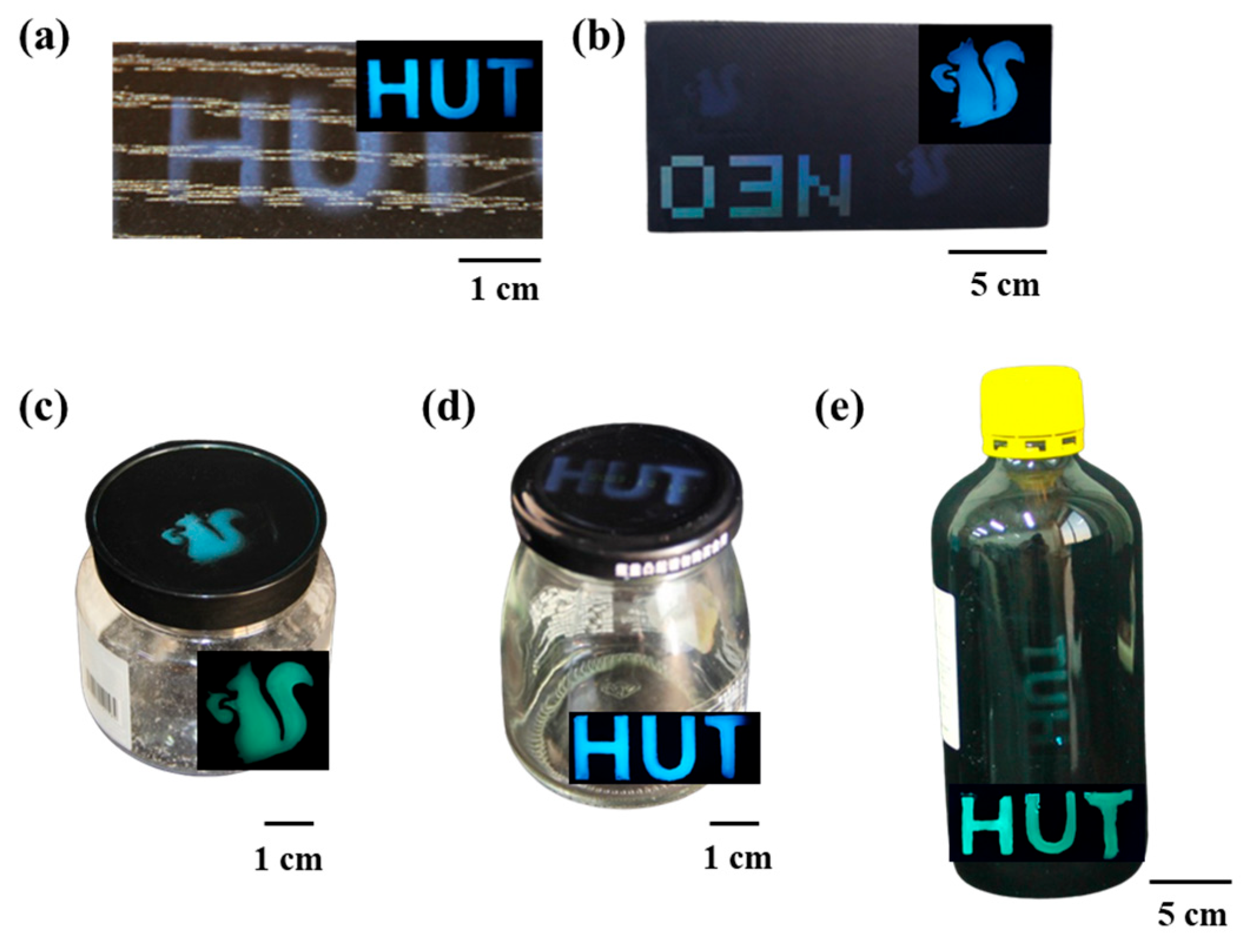
3.3. Multicolor PVA@CDs/SiO2 for Dual-Mode Anti-Counterfeiting Labels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Xia, X.; Guo, Y.; Sun, C.; Ma, J.; Wang, Q. Carbon Dots/SiO2 fluorescent photonic crystals for anti-counterfeiting. ACS Appl. Nano Mater. 2024, 7, 6547–6555. [Google Scholar] [CrossRef]
- Li, Y.; Mao, Y.; Wang, J.; Liu, Z.; Jia, P.; Wu, N.; Yu, H.; Wang, J.; Song, Y.; Zhou, J. Cracking enabled unclonability in colloidal crystal patterns authenticated with computer vision. Nanoscale 2022, 14, 8833–8841. [Google Scholar] [CrossRef]
- Fang, Y.; Yan, P.; Zhang, Q.; Liu, X. Preparation of Janus structural colors with different hydrophilicity by spraying hydrophobic P(HFBMA-co-GMA) microspheres on polydopamine modified cotton fabrics. Colloid Surface A 2024, 686, 133386. [Google Scholar] [CrossRef]
- Chen, T.; Du, X.-Y.; Peng, G. Construction of dendrimer-induced quantum dots loaded colloids towards fluorescent photonic crystal patterns via direct writing. Mater. Lett. 2022, 307, 130942. [Google Scholar] [CrossRef]
- Kuo, W.-K.; Weng, H.-P.; Hsu, J.-J.; Yu, H. A bioinspired color-changing polystyrene microarray as a rapid qualitative sensor for methanol and ethanol. Mater. Chem. Phys. 2016, 173, 285–290. [Google Scholar] [CrossRef]
- Ganesh, N.; Zhang, W.; Mathias, P.C.; Chow, E.; Soares, J.; Malyarchuk, V.; Smith, A.D.; Cunningham, B. Enhanced fluorescence emission from quantum dots on a photonic crystal surface. Nat. Nanotech. 2007, 2, 515–520. [Google Scholar] [CrossRef]
- Mastour, N.; Ben Hamed, Z.; Benchaabane, A.; Sanhoury, M.A.; Kouki, F. Effect of ZnSe quantum dot concentration on the fluorescence enhancement of polymer P3HT film. Org. Electron. 2013, 14, 2093–2100. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Wang, J.-X.; Ji, Z.-Y.; Hu, W.-P.; Jiang, L.; Song, Y.-L.; Zhu, D.-B. Solid-state fluorescence enhancement of organic dyes by photonic crystals. J. Mater. Chem. 2007, 17, 90–94. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Lin, H.; Xu, L.; Xu, W.; Wang, R.; Song, Y.; Zhu, D. Amplification of fluorescent contrast by photonic crystals in optical storage. Adv. Mater. 2010, 11, 1237–1241. [Google Scholar] [CrossRef]
- Yin, Z.; Li, H.; Xu, W.; Cui, S.; Zhou, D.; Chen, X.; Zhu, Y.; Qin, G.; Song, H. Local field modulation induced three-order upconversion enhancement: Combining surface plasmon effect and photonic crystal effect. Adv. Mater. 2016, 28, 2518–2525. [Google Scholar] [CrossRef]
- Zhou, P.; Zhou, D.; Tao, L.; Zhu, Y.; Xu, W.; Xu, S.; Cui, S.; Xu, L.; Song, H. 320-fold luminescence enhancement of [Ru(dpp)3] Cl2 dispersed on PMMA opal photonic crystals and highly improved oxygen sensing performance. Light Sci. Appl. 2014, 3, e209. [Google Scholar] [CrossRef]
- Li, M.; Lyu, Q.; Peng, B.; Chen, X.; Zhang, L.; Zhu, J. Bioinspired colloidal photonic composites: Fabrications and emerging applications. Adv. Mater. 2022, 34, 2110488. [Google Scholar] [CrossRef]
- Xiong, R.; Luan, J.; Kang, S.; Ye, C.; Singamaneni, S.; Tsukruk, V.V. Biopolymeric photonic structures: Design, fabrication, and emerging applications. Chem. Soc. Rev. 2020, 49, 983–1031. [Google Scholar] [CrossRef]
- Kaddour, S.; Ridene, S. Effect of defects and doping on the transmission spectrum enhancement of photonic crystal based on 2D-MoS2. J. Nonlinear Opt. Phys. Mater. 2023, 33, 2340009. [Google Scholar] [CrossRef]
- Chen, X.; Ren, P.; Li, M.; Lyu, Q.; Zhang, L.; Zhu, J. Dynamic regulation of photoluminescence based on mechanochromic photonic elastomers. Chem. Eng. J. 2021, 426, 131259. [Google Scholar] [CrossRef]
- Li, H.; Han, B.; Ma, H.; Li, R.; Hou, X.; Zhang, Y.; Wang, J.-J. A “turn-on” inverse opal photonic crystal fluorescent sensing film for detection of cysteine and its bioimaging of living cells. Microchim. Acta 2023, 190, 49. [Google Scholar] [CrossRef]
- Kuo, W.-K.; Weng, H.-P.; Hsu, J.-J.; Yu, H. Photonic Crystal-Based Sensors for Detecting Alcohol Concentration. Appl. Sci. 2016, 6, 67. [Google Scholar] [CrossRef]
- Yu, T.; Wang, B.; Yu, L.P. Dual-mode color-changing pH sensor based on fluorescent MOF embedded photonic crystal hydrogel. J. Chin. Chem. Soc. 2022, 69, 831–839. [Google Scholar] [CrossRef]
- Wang, C.; Lin, X.; Schäfer, C.G.; Hirsemann, S.; Ge, J. Spray synthesis of photonic crystal based automotive coatings with bright and angular-dependent structural colors. Adv. Funct. Mater. 2020, 31, 2008601. [Google Scholar] [CrossRef]
- Xu, L.; Qian, Y.; Bao, L.; Wang, W.; Deng, N.; Zhang, L.; Wang, G.; Fu, X.; Fu, W. Nitrogen-doped carbon quantum dots for fluorescence sensing, anti-counterfeiting and logic gate operations. New J. Chem. 2024, 48, 155–161. [Google Scholar] [CrossRef]
- Xu, L.; Qian, Y.; Bao, L.; Wang, W.; Deng, N.; Zhang, L.; Wang, G.; Fu, X.; Fu, W. Highly bright carbon quantum dots for flexible anti-counterfeiting. J. Mater. Chem. C 2022, 10, 11338–11346. [Google Scholar]
- Ma, H.; Guan, L.; Chen, M.; Zhang, Y.; Wu, Y.; Liu, Z.; Wang, D.; Wang, F.; Li, X. Synthesis and enhancement of carbon quantum dots from Mopan persimmons for Fe3+ sensing and anti-counterfeiting applications. Chem. Eng. J. 2023, 453, 139906. [Google Scholar] [CrossRef]
- Kong, J.; Wei, Y.; Zhou, F.; Shi, L.; Zhao, S.; Wan, M.; Zhang, X. Carbon quantum dots: Properties, preparation, and applications. Molecules 2024, 29, 2002. [Google Scholar] [CrossRef]
- Gao, W.; Rigout, M.; Owens, H. Facile control of silica nanoparticles using a novel solvent varying method for the fabrication of artificial opal photonic crystals. J. Nanopart. Res. 2016, 18, 387. [Google Scholar] [CrossRef]
- Zhi, B.; Gallagher, M.J.; Frank, B.P.; Lyons, T.Y.; Qiu, T.A.; Da, J.; Mensch, A.C.; Hamers, R.J.; Rosenzweig, Z.; Fairbrother, D.H.; et al. Investigation of phosphorous doping effects on polymeric carbon dots: Fluorescence, photostability, and environmental impact. Carbon 2018, 129, 438–449. [Google Scholar] [CrossRef]
- Mei, S.; Wei, X.; Hu, Z.; Wei, C.; Su, D.; Yang, D.; Zhang, G.; Zhang, W.; Guo, R. Amphipathic carbon dots with solvent-dependent optical properties and sensing application. Opt. Mater. 2019, 89, 224–230. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Tang, M.; Qiu, H. Tailored fabrication of carbon dot composites with full-color ultralong room-temperature phosphorescence for multidimensional encryption. Adv. Sci. 2022, 9, 2103833. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, C.; Chen, X.; Zhang, Y.; Colvin, V.L.; Rice, Q.; Seo, J.; Feng, S.; Wang, S.; William, W.Y. Excitation wavelength independent visible color emission of carbon dots. Nanoscale 2017, 9, 1909–1915. [Google Scholar] [CrossRef]
- Ma, W.; Da, B.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Facile preparation of robust microgrooves based photonic crystals film for anti-counterfeiting. Polymer 2023, 276, 125889. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, W.; Yang, S.; Liu, J.; Hasan, M.S.; Zhang, Z.; Chen, H. From silica colloidal particles to photonic crystals: Progress in fabrication and application of structurally colored materials. Text. Res. J. 2023, 93, 2877–2893. [Google Scholar] [CrossRef]





| Test Number | Concentration | Spray Distance | Spray Duration | Drying Temperature |
|---|---|---|---|---|
| 1 | 5% | 3 cm | 1 s | 10 °C |
| 2 | 10% | 5 cm | 3 s | 20 °C |
| 3 | 15% | 7 cm | 5 s | 30 °C |
| 4 | 20% | 10 cm | 7 s | 40 °C |
| 5 | 30% | 15 cm | 10 s | 50 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Yang, L.; Li, T.; Liu, Y.; Tang, Z.; Hu, S.; Wang, X.; Tan, H. Rapid Construction of PVA@CDs/SiO2 Fluorescent/Structural Color Dual-Mode Anti-Counterfeiting Labels via Spray-Coating Method. Polymers 2024, 16, 2211. https://doi.org/10.3390/polym16152211
Liu W, Yang L, Li T, Liu Y, Tang Z, Hu S, Wang X, Tan H. Rapid Construction of PVA@CDs/SiO2 Fluorescent/Structural Color Dual-Mode Anti-Counterfeiting Labels via Spray-Coating Method. Polymers. 2024; 16(15):2211. https://doi.org/10.3390/polym16152211
Chicago/Turabian StyleLiu, Wenjuan, Ling Yang, Ting Li, Yuejun Liu, Zengming Tang, Shuyang Hu, Xionggang Wang, and Haihu Tan. 2024. "Rapid Construction of PVA@CDs/SiO2 Fluorescent/Structural Color Dual-Mode Anti-Counterfeiting Labels via Spray-Coating Method" Polymers 16, no. 15: 2211. https://doi.org/10.3390/polym16152211
APA StyleLiu, W., Yang, L., Li, T., Liu, Y., Tang, Z., Hu, S., Wang, X., & Tan, H. (2024). Rapid Construction of PVA@CDs/SiO2 Fluorescent/Structural Color Dual-Mode Anti-Counterfeiting Labels via Spray-Coating Method. Polymers, 16(15), 2211. https://doi.org/10.3390/polym16152211







