Preparation of High-Temperature Resistant Aerogels by Incorporating Aluminum Sol into Composite Silica Sources Using Ambient Pressure Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Characterization
3. Results and Discussion
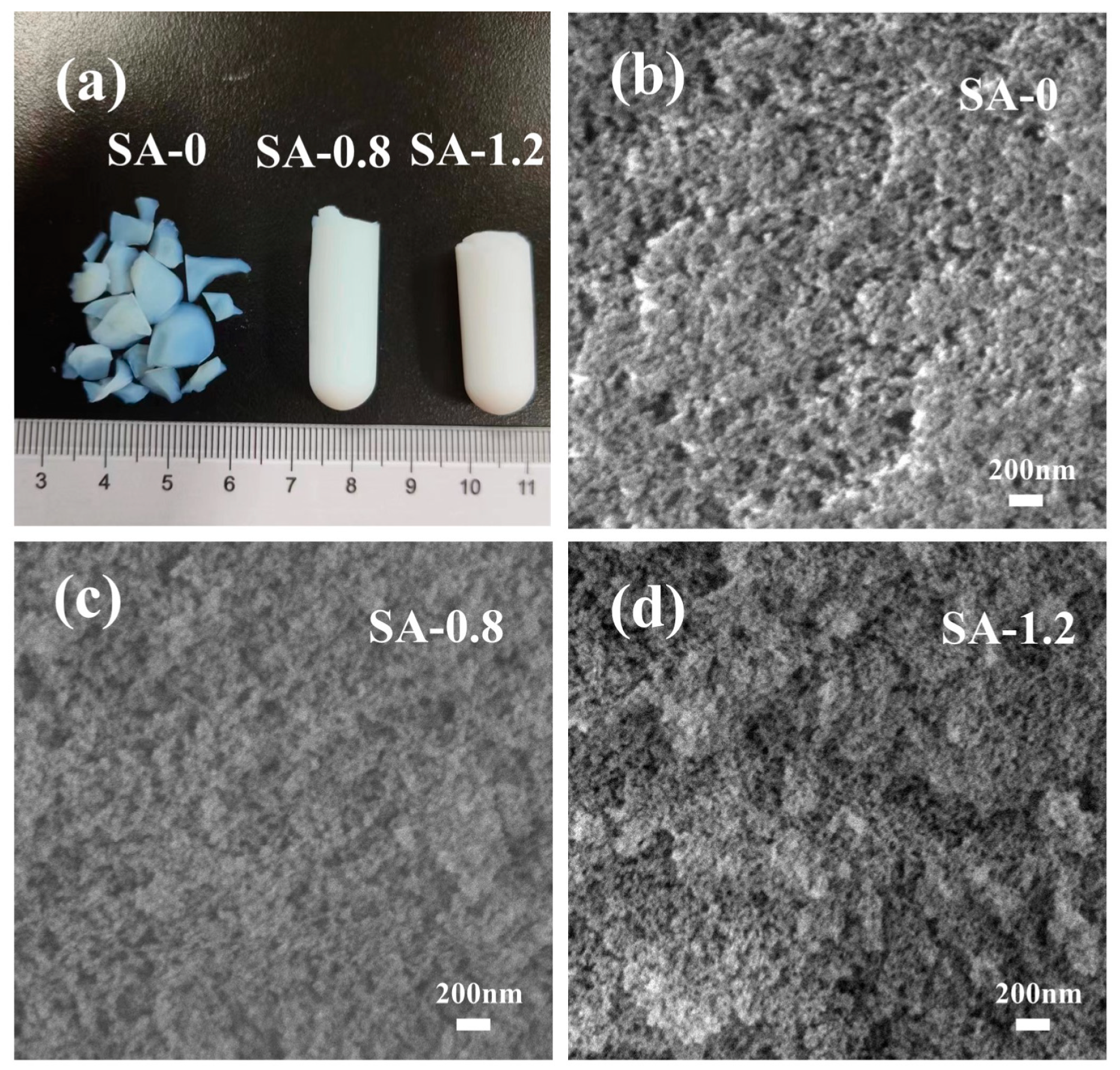
3.1. Physical Properties of the SA
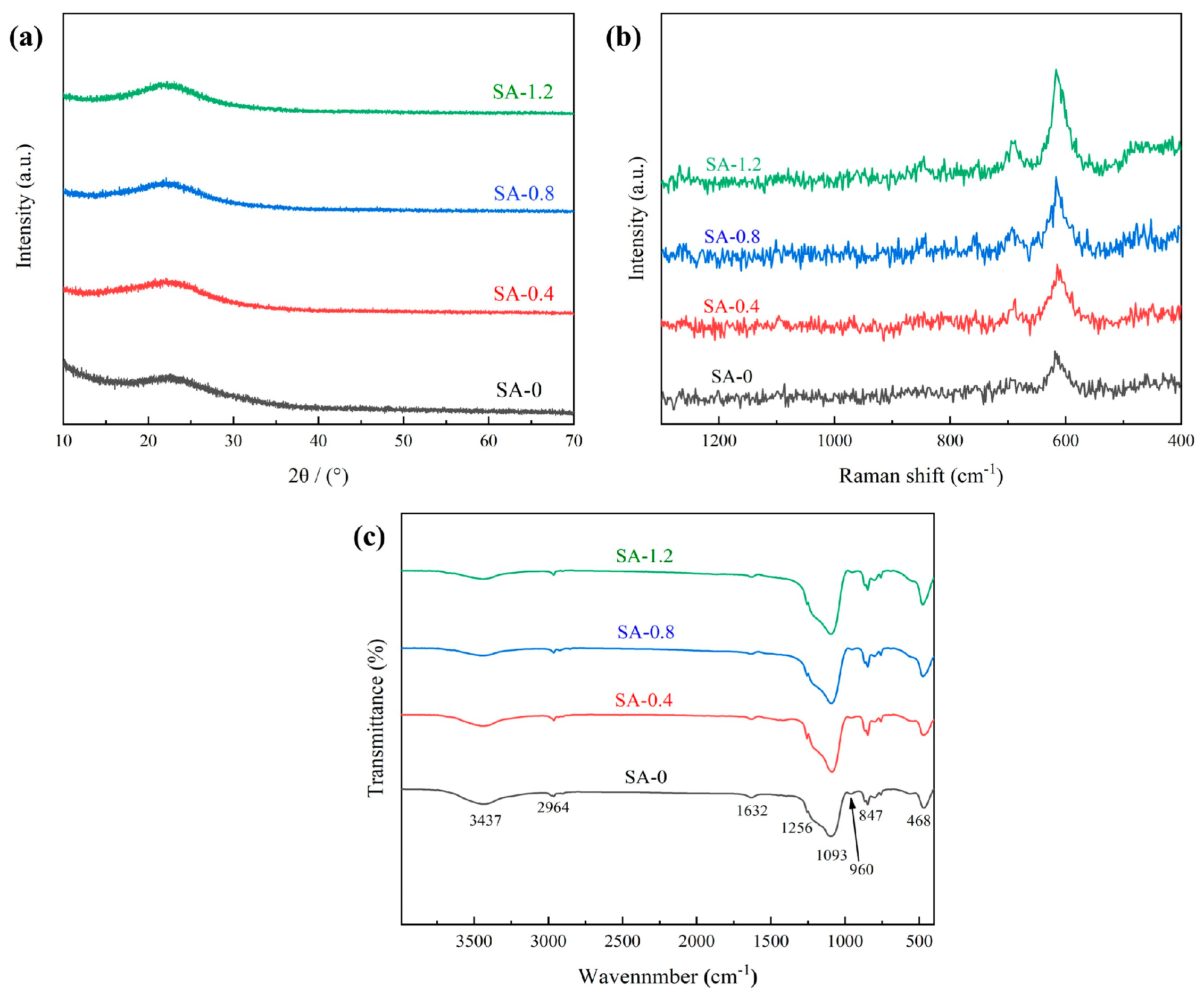
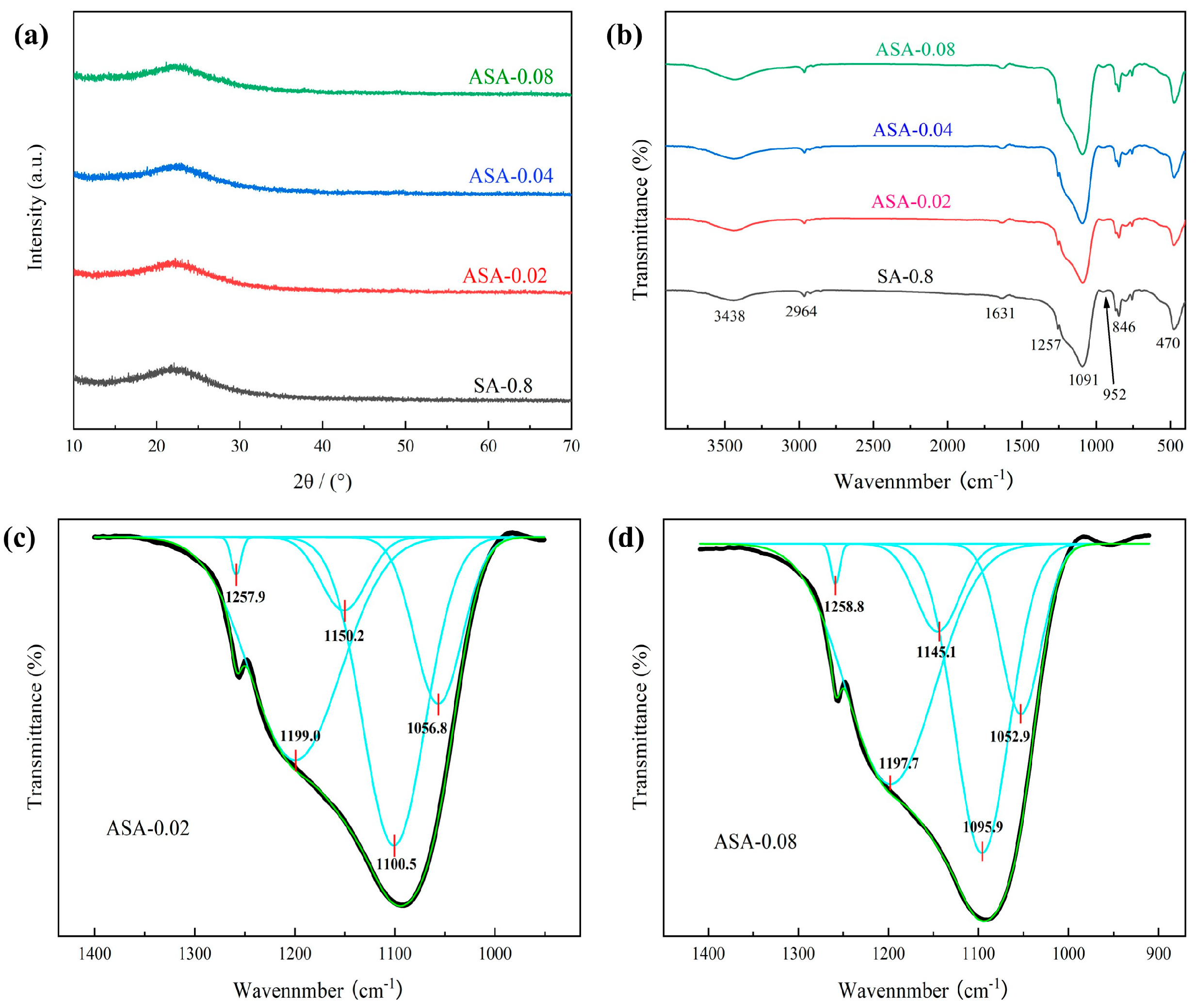
3.2. Morphology, Crystalline and Chemical Structure of the SAs
3.3. Physical Properties of the ASA
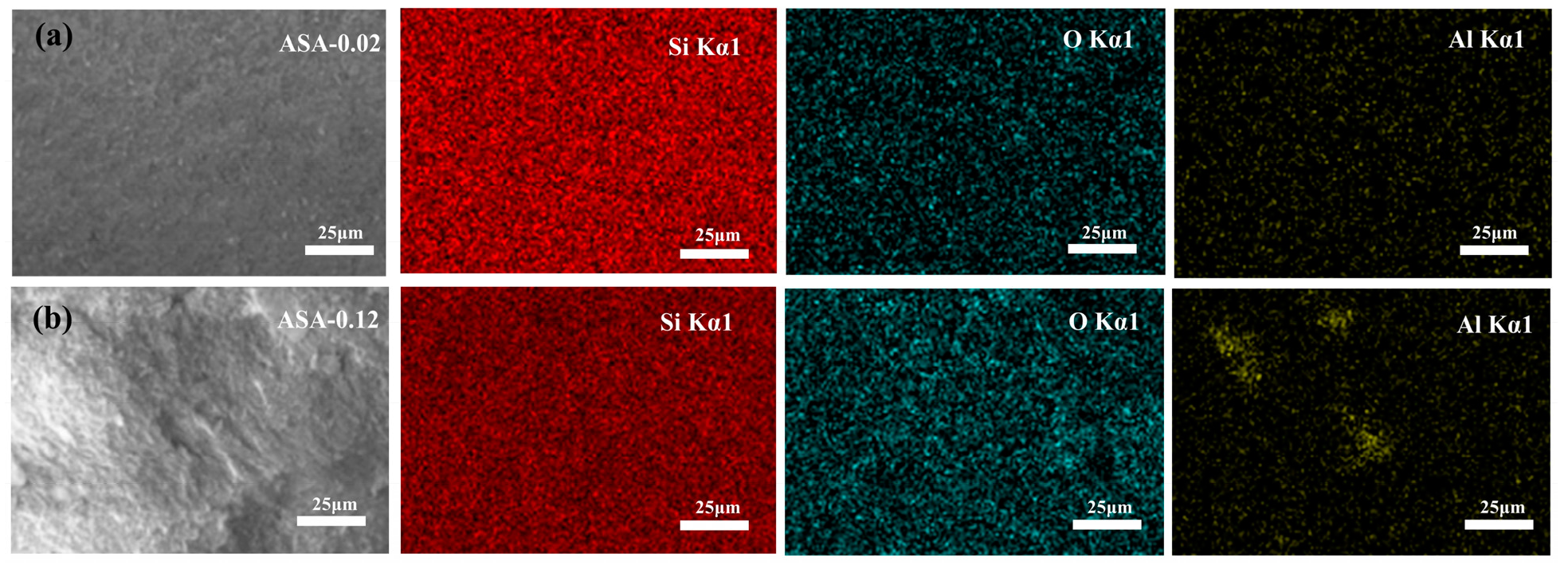
3.4. Morphology, Crystalline, and Chemical Structure of the ASAs
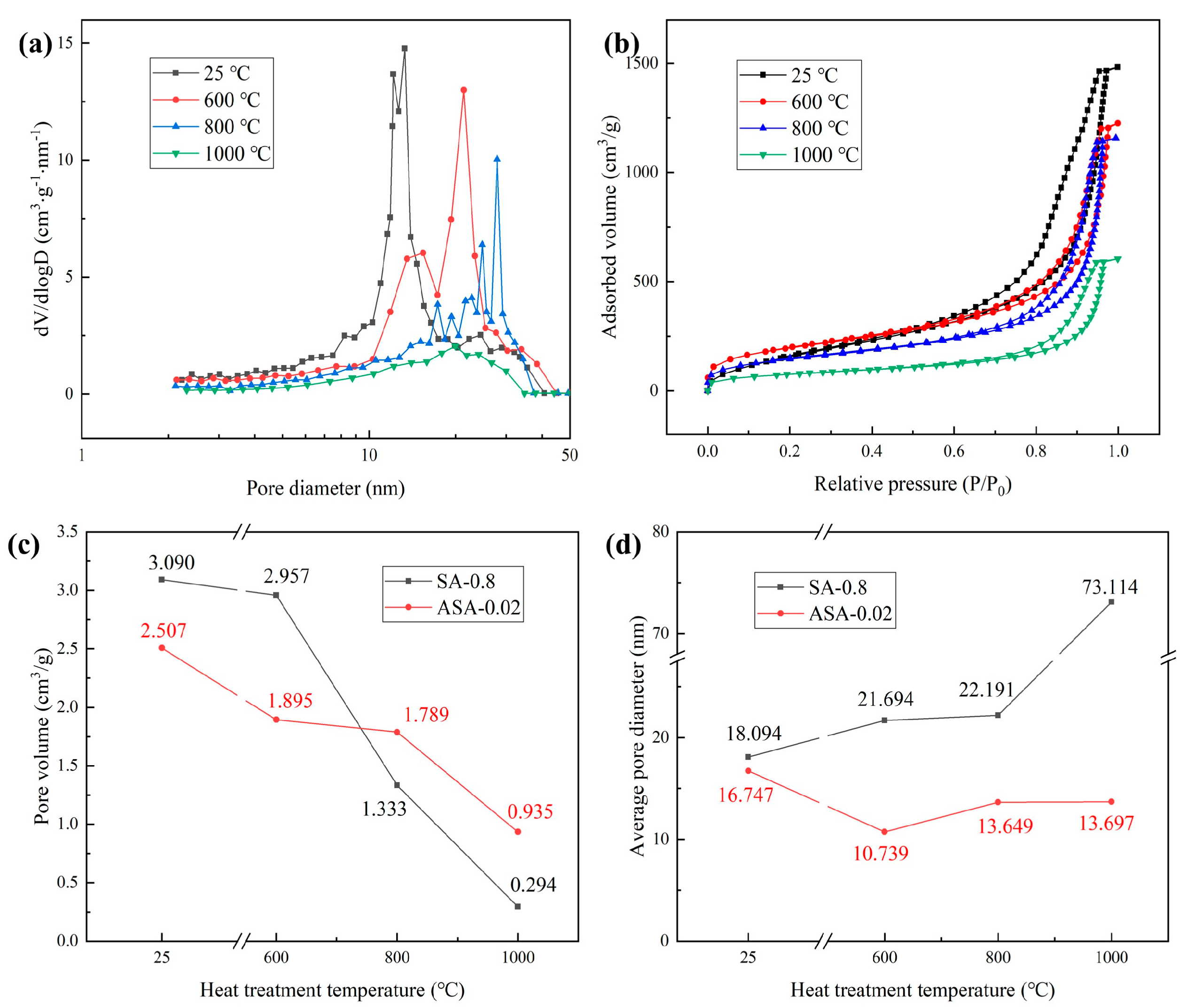
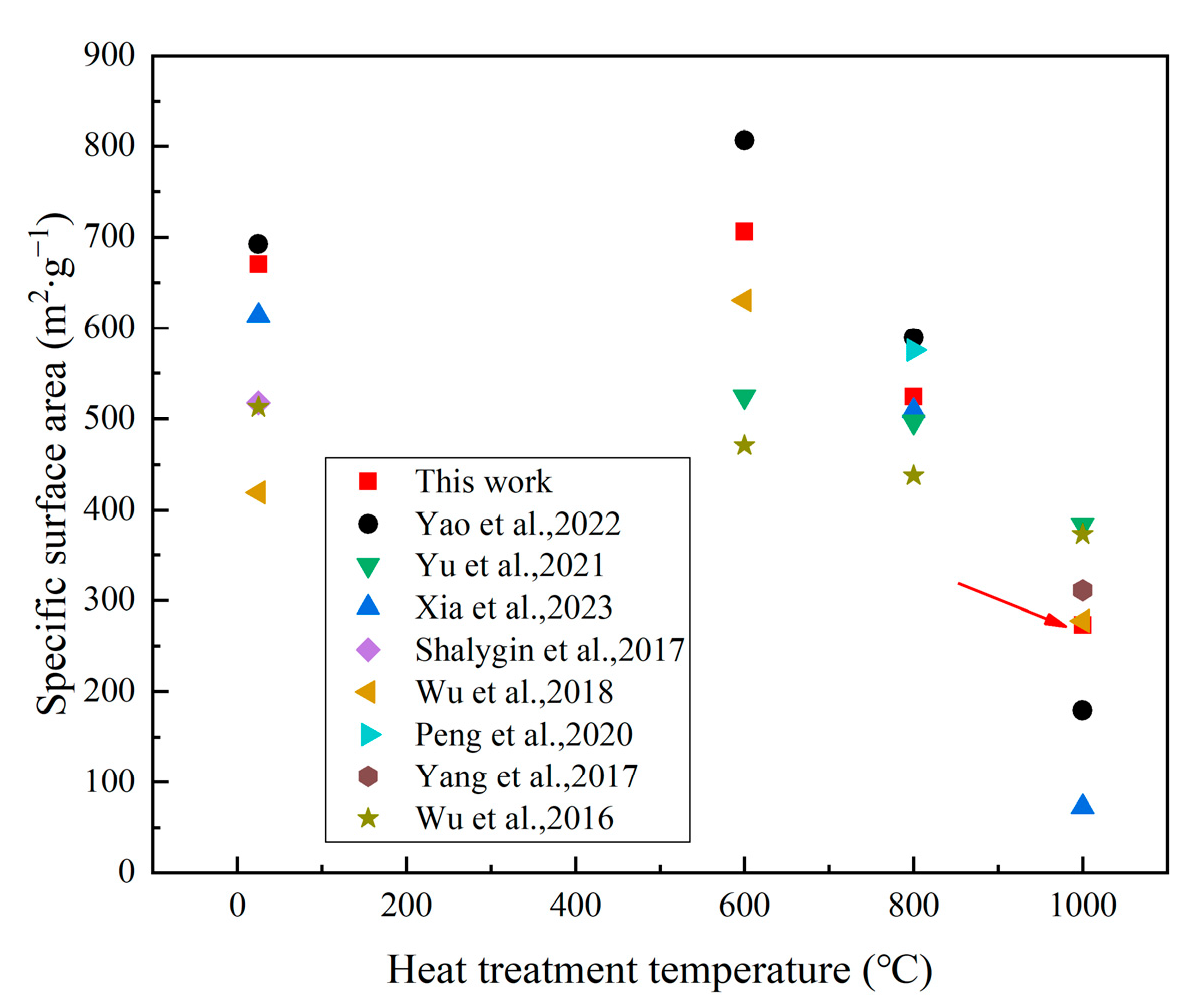
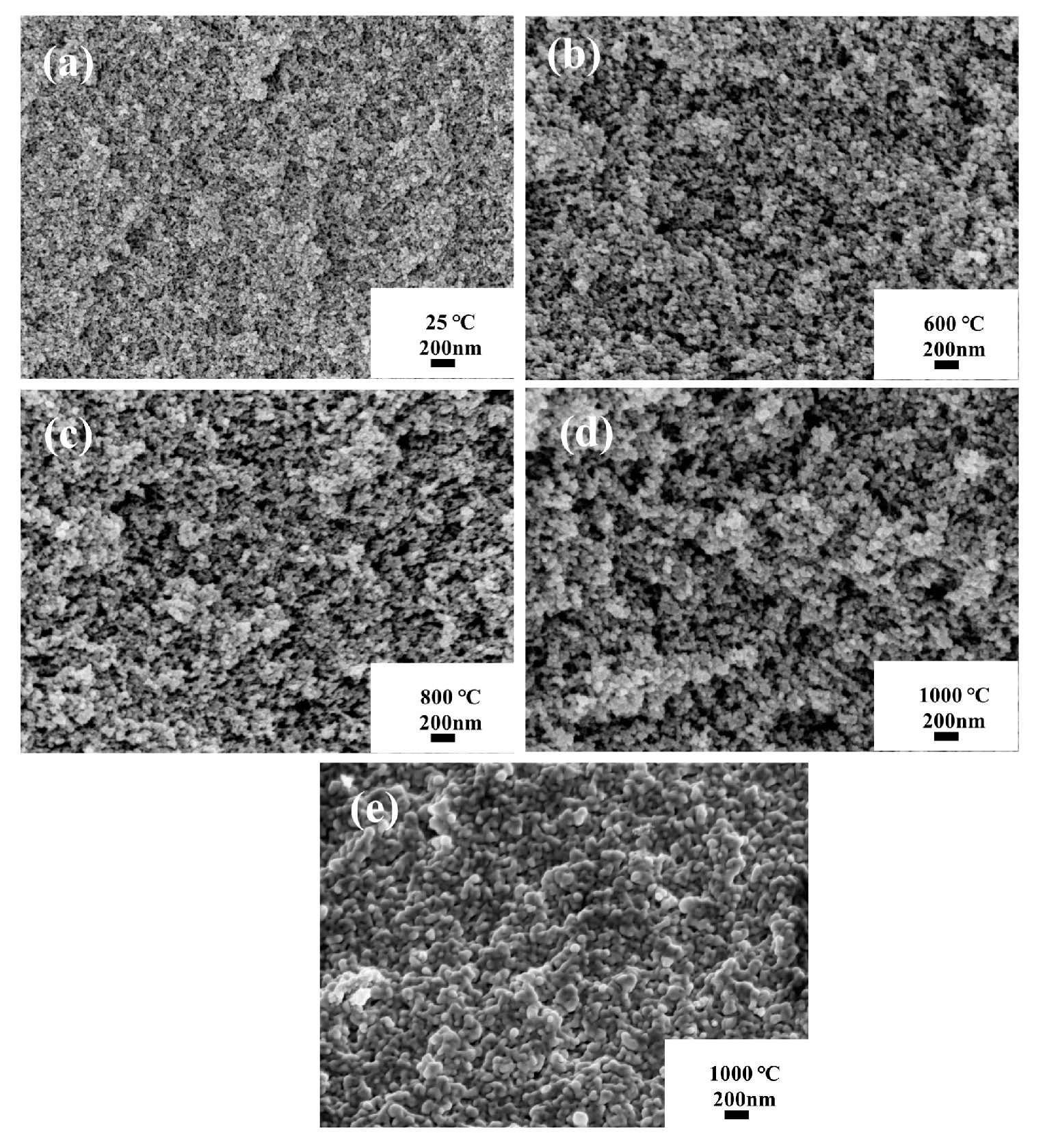
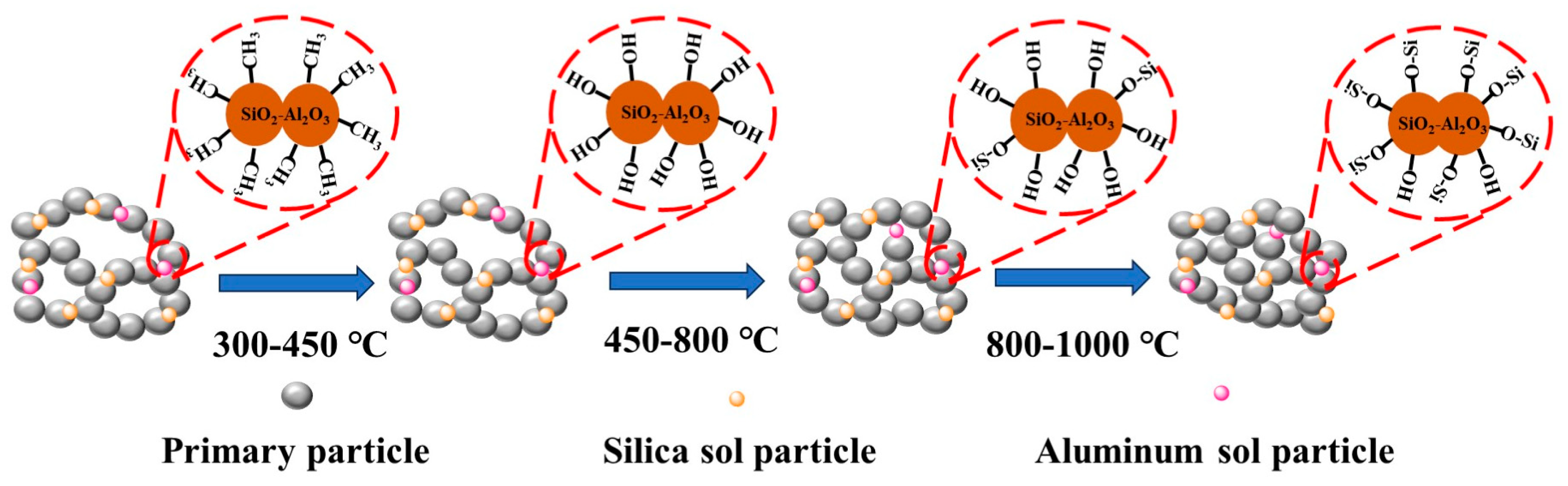
3.5. Thermal Stability of the ASAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, M.; Li, S.-L.; Cheng, J.-B.; Zhang, A.-N.; Wang, Y.-Z.; Zhao, H.-B. Fully Bio-Based, Low Fire-Hazard and Superelastic Aerogel without Hazardous Cross-Linkers for Excellent Thermal Insulation and Oil Clean-up Absorption. J. Hazard. Mater. 2021, 403 Pt 3, 123977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, Y.; Li, C.; Sun, G.; You, B. Superhydrophobic and Multifunctional Aerogel Enabled by Bioinspired Salvinia Leaf-Like Structure. Adv. Funct. Mater. 2022, 32, 2110830. [Google Scholar] [CrossRef]
- Villasmil, W.; Fischer, L.J.; Worlitschek, J. A Review and Evaluation of Thermal Insulation Materials and Methods for Thermal Energy Storage Systems. Renew. Sustain. Energy Rev. 2019, 103, 71–84. [Google Scholar] [CrossRef]
- Wang, L.; Guo, R.; Ren, J.; Song, G.; Chen, G.; Zhou, Z.; Li, Q. Preparation of Superhydrophobic and Flexible Polysiloxane Aerogel. Ceram. Int. 2020, 46, 10362–10369. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhou, Z.-H.; Zhou, C.-G.; Sun, W.-J.; Gao, J.-F.; Dai, K.; Yan, D.-X.; Li, Z.-M. Lightweight and Robust Carbon Nanotube/Polyimide Foam for Efficient and Heat-Resistant Electromagnetic Interference Shielding and Microwave Absorption. ACS Appl. Mater. Interfaces 2020, 12, 8704–8712. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.Y.; Zhang, X.X.; Cheng, X.T.; Ma, Z.M.; Wang, X.Q.; Si, Y.; Yu, J.Y.; Ding, B. Hierarchical Cellular Structured Ceramic Nanofibrous Aerogels with Temperature-Invariant Superelasticity for Thermal Insulation. ACS Appl. Mater. Interfaces 2019, 11, 29056–29064. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Z.; Su, B.L.; Xia, H.S.; Zhao, S.Y.; Gao, C.; Wang, L.K.; Ogbeide, O.; Feng, J.; Hasan, T. Printed Aerogels: Chemistry, Processing, and Applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, X.; Li, Z.; Shi, L.; Zhang, Y.; Liu, Q. Rapid Synthesis and Characterization of Monolithic Ambient Pressure Dried Mtms Aerogels in Pure Water. J. Porous Mater. 2020, 27, 1241–1251. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. Aerogel Based Thermal Insulating Cementitious Composites: A Review. Energy Build. 2021, 245, 111058. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Chen, Z.-Y.; Tang, Y.-H.; Li, Y.-T.; Ma, D.; Zhang, G.-D.; Boukherroub, R.; Cao, C.-F.; Gong, L.-X.; Song, P.; et al. Silicone/Graphene Oxide Co-Cross-Linked Aerogels with Wide-Temperature Mechanical Flexibility, Super-Hydrophobicity and Flame Resistance for Exceptional Thermal Insulation and Oil/Water Separation. J. Mater. Sci. Technol. 2022, 114, 131–142. [Google Scholar] [CrossRef]
- Yao, J.; Gao, X.; Wu, Y.; Zhao, X.; Li, X. High-Temperature Resistant Ambient Pressure-Dried Aluminum Doped Silica Aerogel from Inorganic Silicon and Aluminum Sources. Ceram. Int. 2022, 48, 15006–15016. [Google Scholar] [CrossRef]
- He, F.; Zhou, L.; Zhang, X.; Li, W.; Yang, L.; Zhao, H.; He, X. Synthesis and Anisotropic Properties of Alumina-Silica Aerogels Constructed by Silica Sols Infiltrated into Unidirectional Frozen Alumina Templates. Ceram. Int. 2019, 45, 11963–11970. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Kalekar, A.S.; Harale, N.S.; Patil, S.S.; Dhere, S.L. Rapid Synthesis of Ambient Pressure Dried Tetraethoxysilane Based Silica Aerogels. J. Sol-Gel Sci. Technol. 2021, 97, 5–10. [Google Scholar] [CrossRef]
- Li, Z.; Liao, R.; Jia, R.; Liu, Y.; Xu, X.; Shen, J.; Wang, X.; Wu, G.; Wu, Q.; Shi, J. A Novel Preparation of Superhydrophobic Silica Aerogels Via the Combustion Drying Method. Ceram. Int. 2021, 47, 25274–25280. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Shen, J. Metal Oxide Aerogels for High-Temperature Applications. J. Sol-Gel Sci. Technol. 2023, 106, 360–380. [Google Scholar] [CrossRef]
- Li, M.M.; Chen, X.; Li, X.T.; Dong, J.; Zhao, X.; Zhang, Q.H. Controllable Strong and Ultralight Aramid Nanofiber-Based Aerogel Fibers for Thermal Insulation Applications. Adv. Fiber Mater. 2022, 4, 1267–1277. [Google Scholar] [CrossRef]
- Dingding, Z.; Wenya, B.; Meng, G.; Xia, Y.; Jianyong, Y.; Shichao, Z.; Bin, D. Bubble Templated Flexible Ceramic Nanofiber Aerogels with Cascaded Resonant Cavities for High-Temperature Noise Absorption. ACS Nano 2022, 16, 13740–13749. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Liu, H.; Lu, L.; Wang, T. “Finger Coral-Like” Ceramic Fiber Aerogels with Enhanced High-Temperature Thermal Insulation, Anti-Oxidation, and Mechanical Performance. Compos. Sci. Technol. 2022, 225, 109515. [Google Scholar] [CrossRef]
- Liang, X.; Shao, Z.; Wu, Z. Flexible Sic Nanowire Aerogel with Excellent Thermal Insulation Properties. Ceram. Int. 2022, 48, 22172–22178. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Yu, C.; Liu, L.; Zhang, Z.; Wu, Y.; Shen, J. Organic/Inorganic Double-Precursor Cross-Linked Alumina Aerogel with High Specific Surface Area and High-Temperature Resistance. Ceram. Int. 2022, 48, 17261–17269. [Google Scholar] [CrossRef]
- Kurajica, S.; Mužina, K.; Simčić, I.; Mandić, V. Thermal Evolution of Gels Prepared by Chelation of Aluminum Sec-Butoxide with Ethyl Acetoacetate in Various Amounts. J. Therm. Anal. Calorim. 2023, 148, 1231–1239. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, Q.; Qiu, G.; Yue, C.; Guo, M.; Chen, F.; Zhang, M. Preparation of Monolithic Silica-Based Aerogels with High Thermal Stability by Ambient Pressure Drying. Ceram. Int. 2018, 44, 11923–11931. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.Y.; Xu, D.; Hai, O.; Zheng, W. Low Density and Hydrophobic Silica Aerogels Dried under Ambient Pressure Using a New Co-Precursor Method. J. Non-Cryst. Solids 2016, 452, 187–193. [Google Scholar] [CrossRef]
- Sun, D.; Li, K.; Sui, X.; Zhou, C.; Liu, F. RResearch of Silica Aerogels Prepared by Acidic Silica Sol under the Condition of Atmospheric Pressure Drying. J. Porous Mater. 2018, 25, 341–349. [Google Scholar] [CrossRef]
- Tan, H.B.; Ding, Y.P.; Yang, J.F. Mullite Fibers Prepared from an Inorganic Sol-Gel Precursor. J. Sol-Gel Sci. Technol. 2010, 53, 378–383. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Makki, A.; Hajjar, D.; Sarawade, P.B. Synthesis of Light Weight Recron Fiber-Reinforced Sodium Silicate Based Silica Aerogel Blankets at an Ambient Pressure for Thermal Protection. J. Porous Mater. 2022, 29, 957–969. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, Z.F.; Zhang, J.X.; Ye, X.L.; Cui, S. Hydrophobic Silica Aerogels Prepared by Microwave Irradiation. Chem. Phys. Lett. 2021, 762, 9. [Google Scholar] [CrossRef]
- Cai, H.F.; Jiang, Y.G.; Feng, J.; Zhang, S.Z.; Peng, F.; Xiao, Y.Y.; Li, L.J.; Feng, J.Z. Preparation of Silica Aerogels with High Temperature Resistance and Low Thermal Conductivity by Monodispersed Silica Sol. Mater. Des. 2020, 191, 11. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Lee, Y.K.; Chavan, N.K.; Mahadik, S.A.; Park, H.H. Monolithic and Shrinkage-Free Hydrophobic Silica Aerogels Via New Rapid Supercritical Extraction Process. J. Supercrit. Fluids 2016, 107, 84–91. [Google Scholar] [CrossRef]
- Wu, L.N.; Huang, Y.D.; Wang, Z.J.; Liu, L.; Xu, H.F. Fabrication of Hydrophobic Alumina Aerogel Monoliths by Surface Modification and Ambient Pressure Drying. Appl. Surf. Sci. 2010, 256, 5973–5977. [Google Scholar] [CrossRef]
- Eris, G.; Sanli, D.; Ulker, Z.; Bozbag, S.E.; Jonas, A.; Kiraz, A.; Erkey, C. Three-Dimensional Optofluidic Waveguides in Hydrophobic Silica Aerogels Via Supercritical Fluid Processing. J. Supercrit. Fluids 2013, 73, 28–33. [Google Scholar] [CrossRef]
- Yu, H.; Tong, Z.; Yue, S.; Li, X.; Su, D.; Ji, H. Effect of SiO2 Deposition on Thermal Stability of Al2O3-SiO2 Aerogel. J. Eur. Ceram. Soc. 2021, 41, 580–589. [Google Scholar] [CrossRef]
- Xia, C.; Hao, M.; Liu, W.; Zhang, X.; Miao, Y.; Ma, C.; Gao, F. Synthesis of Al2O3-SiO2 Aerogel from Water Glass with High Thermal Stability and Low Thermal Conductivity. J. Sol-Gel Sci. Technol. 2023, 106, 561–571. [Google Scholar] [CrossRef]
- Shalygin, A.S.; Kozhevnikov, I.V.; Gerasimov, E.Y.; Andreev, A.S.; Lapina, O.B.; Martyanov, O.N. The Impact of Si/Al Ratio on Properties of Aluminosilicate Aerogels. Microporous Mesoporous Mater. 2017, 251, 105–113. [Google Scholar] [CrossRef]
- Wu, X.; Ding, J.; Kong, Y.; Sun, Z.; Shao, G.; Li, B.; Wu, J.; Zhong, Y.; Shen, X.; Cui, S. Synthesis of a Novel Three-Dimensional Na2SO4@ SiO2@ Al2O3-SiO2 Phase Change Material Doped Aerogel Composite with High Thermal Resistance and Latent Heat. Ceram. Int. 2018, 44, 21855–21865. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Li, L.; Cai, H.; Feng, J. A Facile Method to Fabricate Monolithic Alumina–Silica Aerogels with High Surface Areas and Good Mechanical Properties. J. Eur. Ceram. Soc. 2020, 40, 2480–2488. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Wang, T.; Liang, Y. Facile One-Step Precursor-to-Aerogel Synthesis of Silica-Doped Alumina Aerogels with High Specific Surface Area at Elevated Temperatures. J. Porous Mater. 2017, 24, 889–897. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Cui, S.; Wang, L.; Shen, X. Synthesis of a Novel Al2O3-SiO2 Composite Aerogel with High Specific Surface Area at Elevated Temperatures Using Inexpensive Inorganic Salt of Aluminum. Ceram. Int. 2016, 42, 874–882. [Google Scholar] [CrossRef]
- Osaki, T.; Nagashima, K.; Watari, K.; Tajiri, K. Silica-Doped Alumina Cryogels with High Thermal Stability. J. Non-Cryst. Solids 2007, 353, 2436–2442. [Google Scholar] [CrossRef]

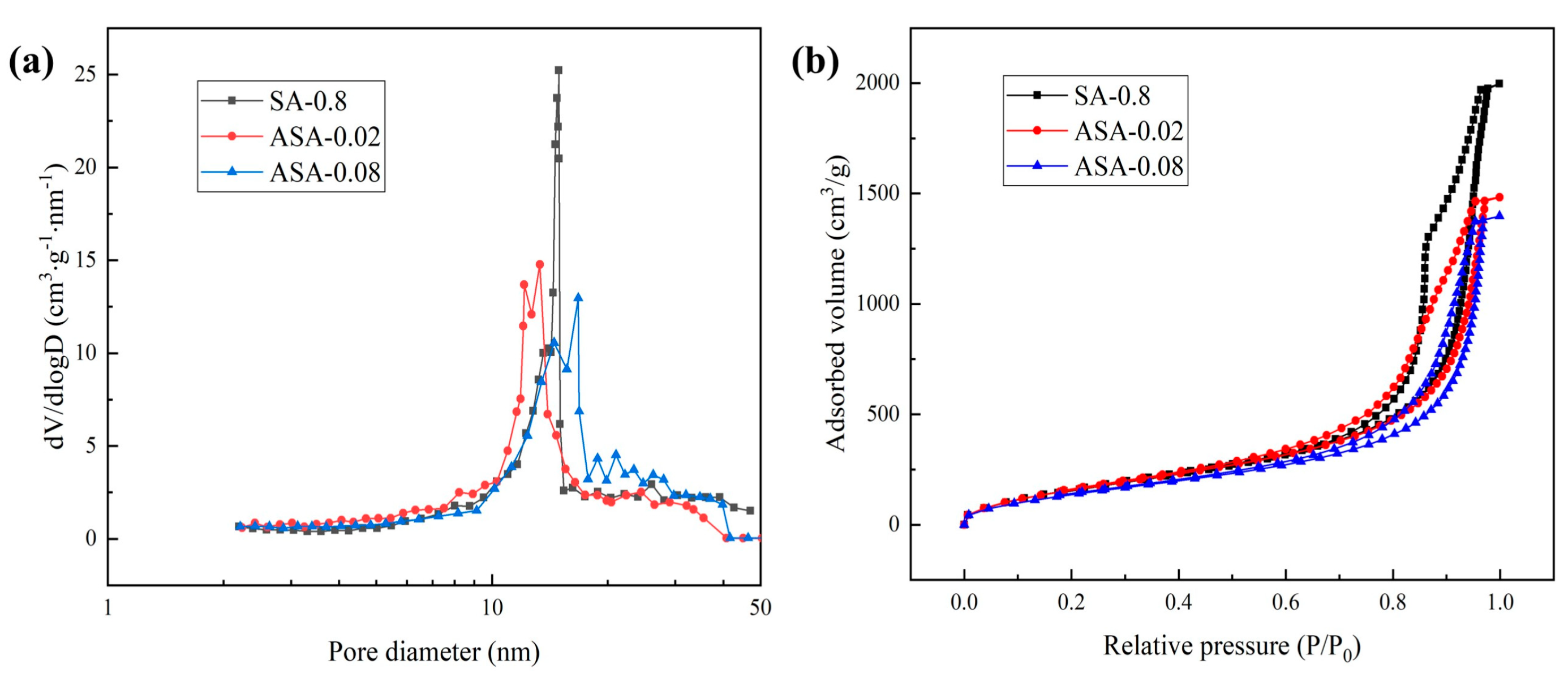



| Sample | Gelation Time (min) | Bulk Density (g·cm−3) | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| SA-0 | 73 | 0.117 | 579.540 | 3.110 | 21.456 |
| SA-0.2 | 61 | 0.125 | 706.659 | 2.612 | 14.787 |
| SA-0.4 | 56 | 0.132 | 743.898 | 2.766 | 14.873 |
| SA-0.6 | 51 | 0.135 | 754.243 | 2.806 | 14.880 |
| SA-0.8 | 55 | 0.138 | 683.204 | 3.090 | 18.094 |
| SA-1.0 | 65 | 0.159 | 585.139 | 3.140 | 21.467 |
| SA-1.2 | 81 | 0.178 | 540.383 | 2.648 | 19.598 |
| Sample | Gelation Time (min) | Bulk Density (g·cm−3) | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| SA-0.8 | 55 | 0.138 | 683.204 | 3.090 | 18.094 |
| ASA-0.02 | 67 | 0.142 | 670.432 | 2.507 | 16.747 |
| ASA-0.04 | 68 | 0.147 | 624.340 | 2.304 | 14.761 |
| ASA-0.06 | 79 | 0.154 | 598.462 | 2.188 | 14.624 |
| ASA-0.08 | 92 | 0.166 | 580.844 | 2.162 | 14.890 |
| ASA-0.1 | 115 | 0.187 | 570.316 | 1.397 | 11.016 |
| ASA-0.12 | 125 | 0.223 | 526.521 | 1.620 | 12.307 |
| Sample | Specific Surface Area (25 °C)/m2·g−1 | Specific Surface Area (600 °C)/m2·g−1 | Specific Surface Area (800 °C)/m2·g−1 | Specific Surface Area (1000 °C)/m2·g−1 |
|---|---|---|---|---|
| SA-0.8 | 683.204 | 545.145 | 240.195 | 16.082 |
| ASA-0.02 | 670.432 | 705.956 | 524.267 | 273.099 |
| ASA-0.04 | 624.340 | 686.276 | 519.568 | 277.328 |
| ASA-0.06 | 598.462 | 670.238 | 517.435 | 236.236 |
| ASA-0.08 | 580.844 | 675.452 | 519.191 | 220.475 |
| ASA-0.1 | 570.316 | 557.016 | 518.883 | 203.088 |
| ASA-0.12 | 526.521 | 489.963 | 480.081 | 209.856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Cao, Z.; Liu, K.; Liu, S.; Pang, W.; Jiang, H. Preparation of High-Temperature Resistant Aerogels by Incorporating Aluminum Sol into Composite Silica Sources Using Ambient Pressure Drying. Polymers 2024, 16, 2296. https://doi.org/10.3390/polym16162296
Gao S, Cao Z, Liu K, Liu S, Pang W, Jiang H. Preparation of High-Temperature Resistant Aerogels by Incorporating Aluminum Sol into Composite Silica Sources Using Ambient Pressure Drying. Polymers. 2024; 16(16):2296. https://doi.org/10.3390/polym16162296
Chicago/Turabian StyleGao, Shuai, Zeqi Cao, Kai Liu, Shuning Liu, Wanjun Pang, and Hongyi Jiang. 2024. "Preparation of High-Temperature Resistant Aerogels by Incorporating Aluminum Sol into Composite Silica Sources Using Ambient Pressure Drying" Polymers 16, no. 16: 2296. https://doi.org/10.3390/polym16162296
APA StyleGao, S., Cao, Z., Liu, K., Liu, S., Pang, W., & Jiang, H. (2024). Preparation of High-Temperature Resistant Aerogels by Incorporating Aluminum Sol into Composite Silica Sources Using Ambient Pressure Drying. Polymers, 16(16), 2296. https://doi.org/10.3390/polym16162296





