Bio-Composite Films Based on Carboxymethyl Chitosan Incorporated with Calcium Oxide: Synthesis and Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
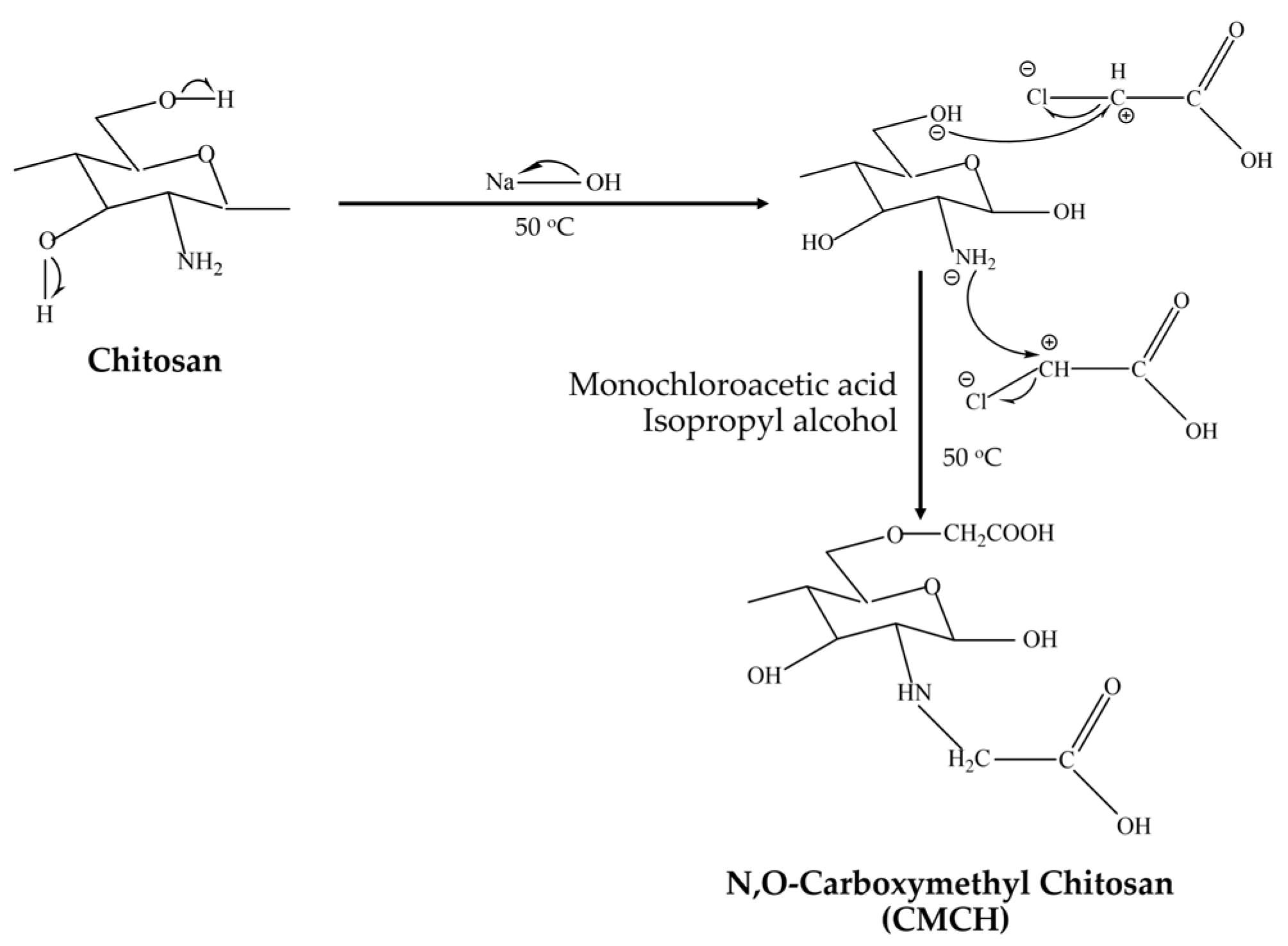
2.2.1. Synthesis of Carboxymethyl Chitosan
2.2.2. Synthesis of Calcium Oxide
2.2.3. Preparation of CMCH-CaO Bio-Composite Films
2.3. Characterizations
2.3.1. Chemical Structure
2.3.2. Morphology and Metal Composition
2.3.3. Crystal Structure
2.3.4. Thermal Stability
2.3.5. Mechanical Properties
2.3.6. Hydrophilic Property
2.3.7. Antimicrobial Activity
3. Results and Discussion
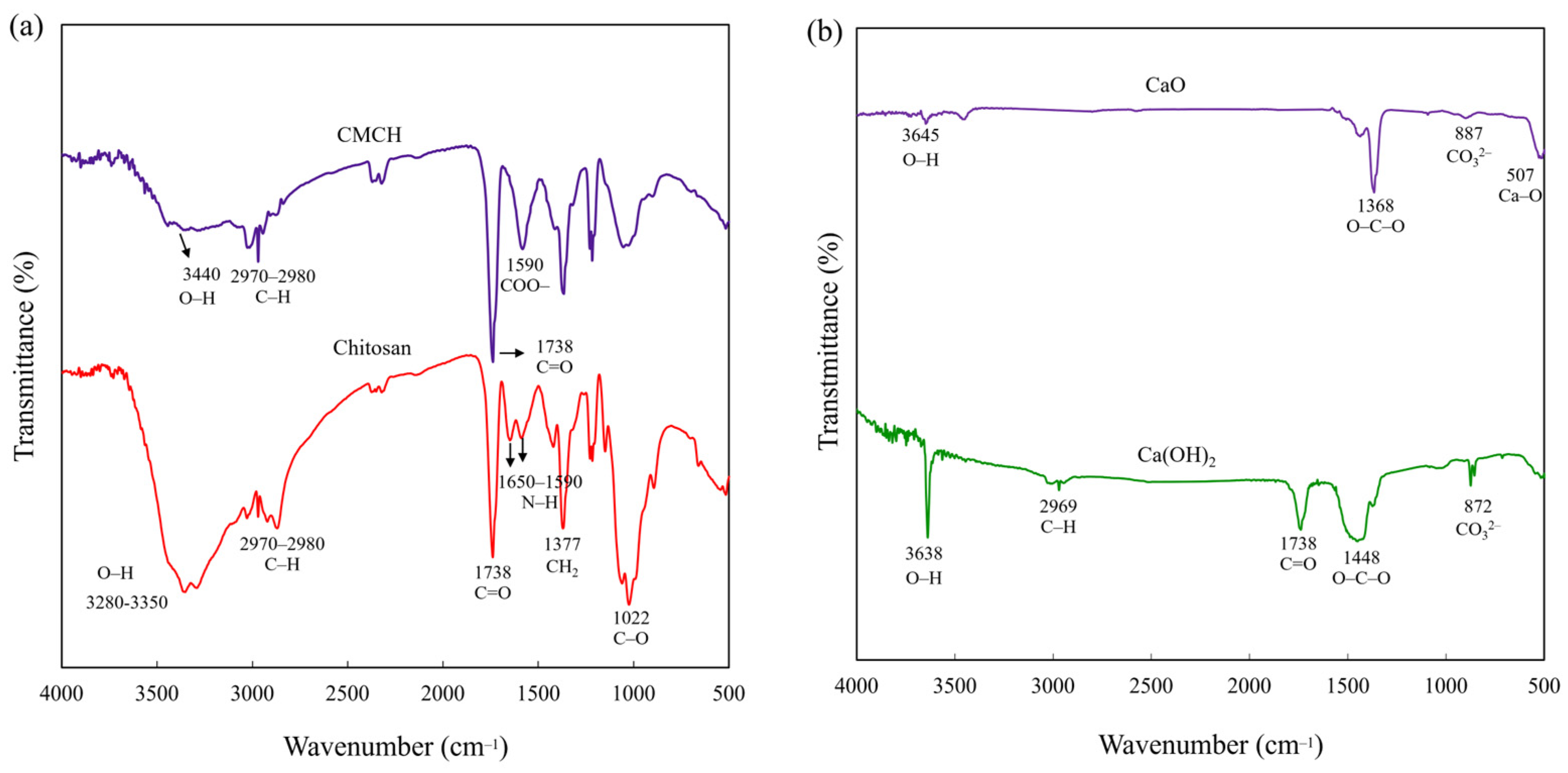
3.1. Chemical Structure of Synthesized CMCH and Synthesized CaO
3.2. Morphology of Synthesized CMCH and Synthesized CaO
3.3. XRD Pattern and Elements of Synthesized CaO
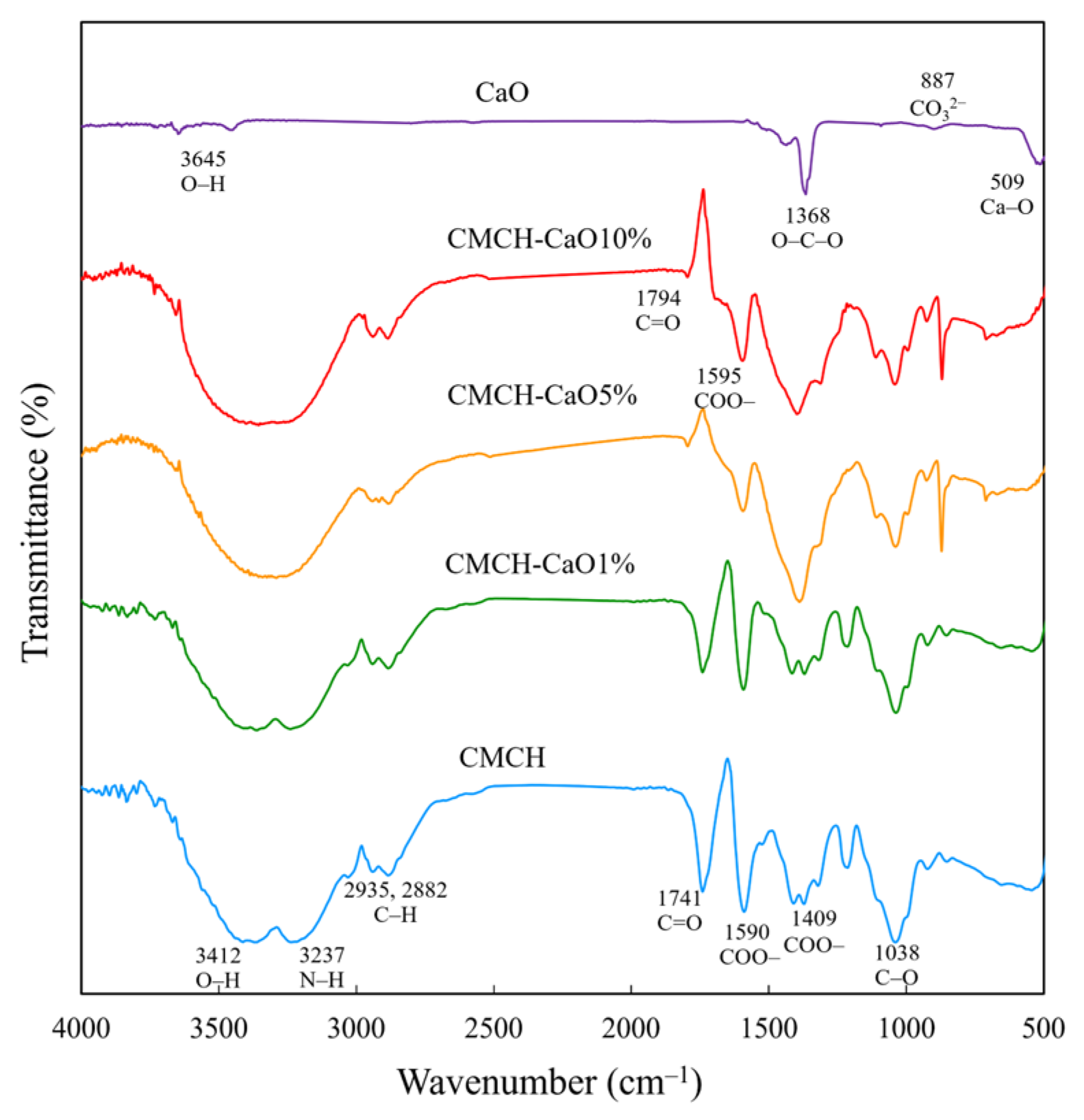
3.4. Chemical Structures of CMCH-CaO Bio-Composite Films
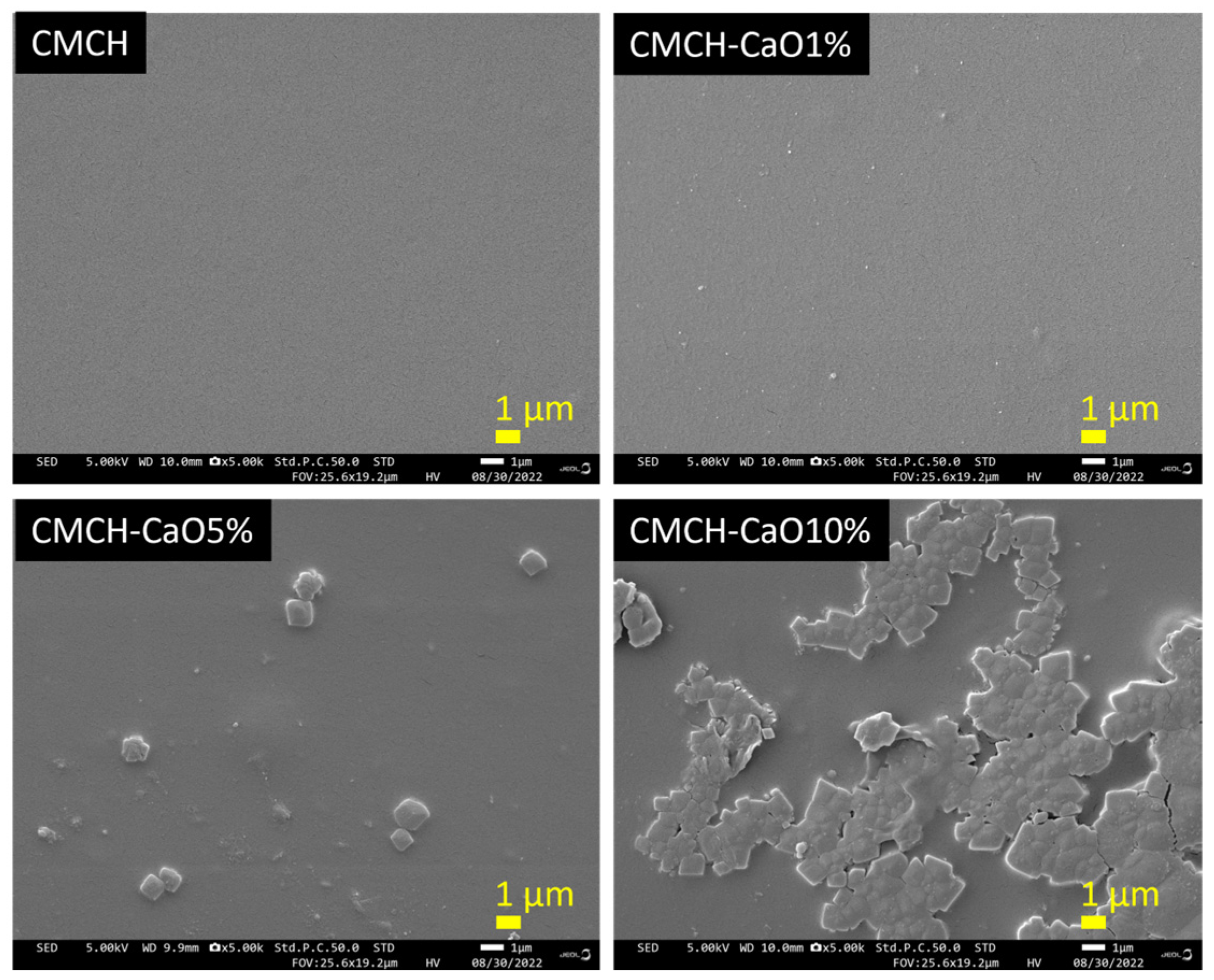
3.5. Morphology of CMCH-CaO Bio-Composite Films
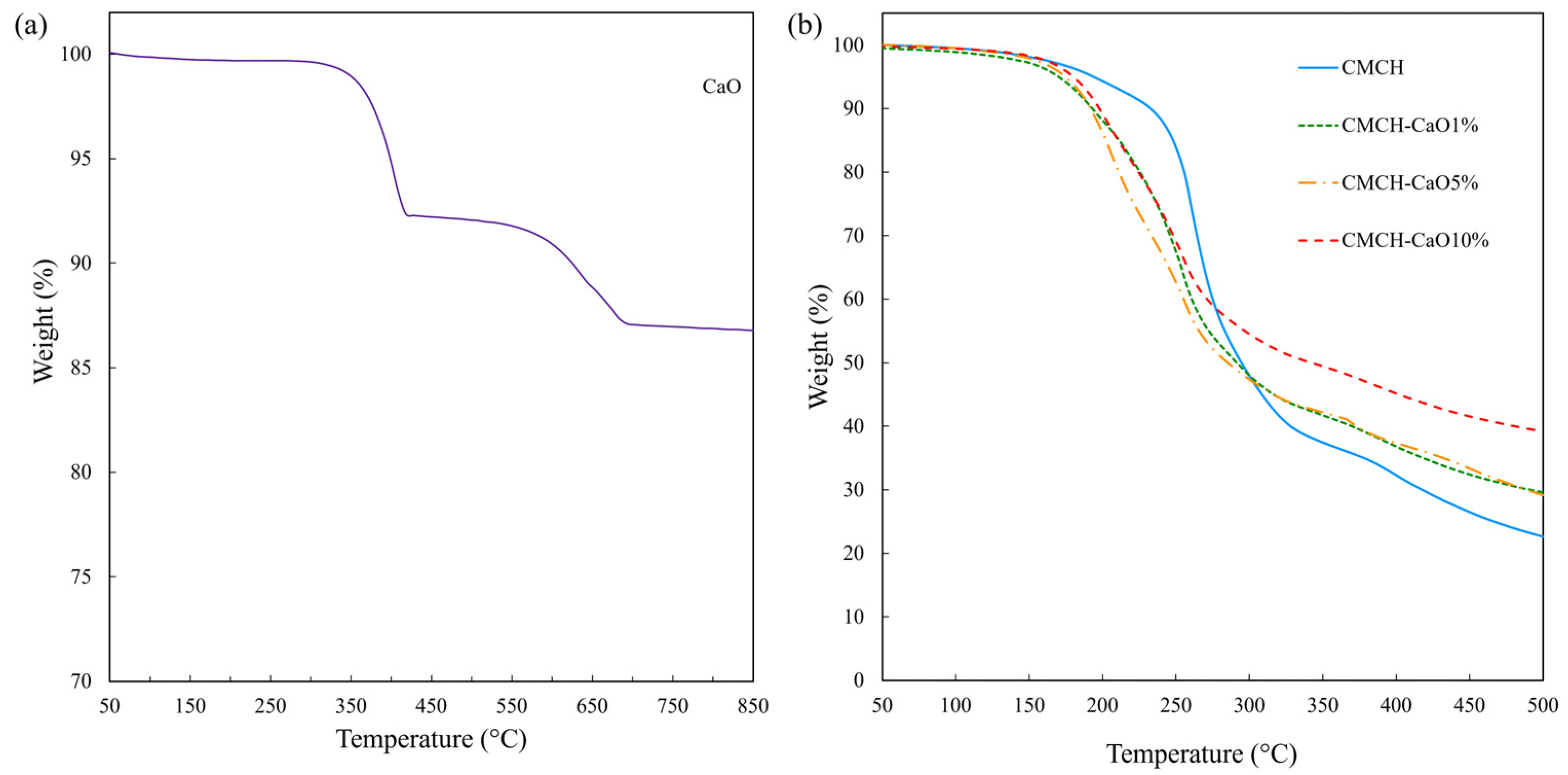
3.6. Thermal Stability of CMCH-CaO Bio-Composite Films
3.7. Mechanical Properties of CMCH-CaO Bio-Composite Films
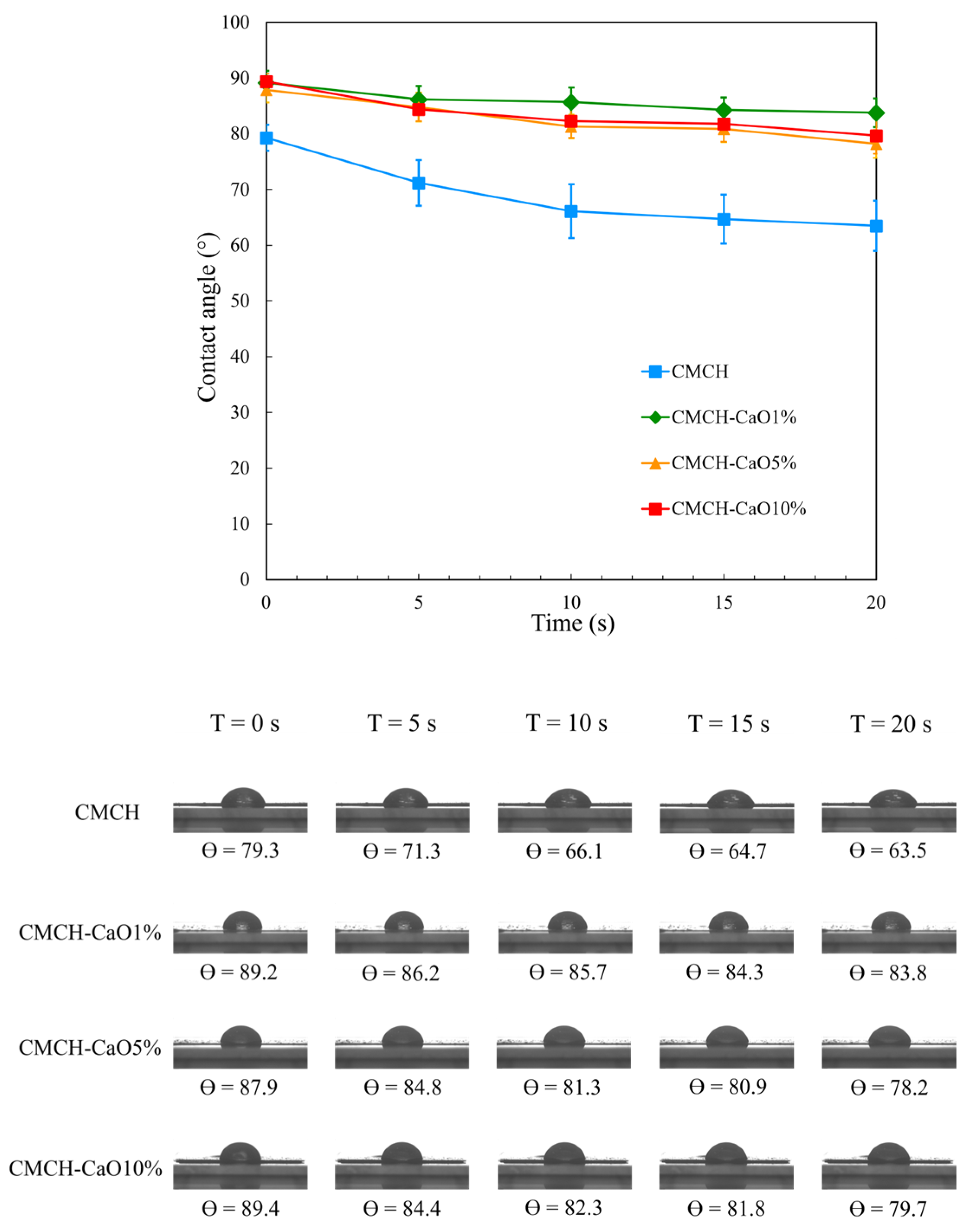
3.8. Hydrophilicity of CMCH-CaO Bio-Composite Films
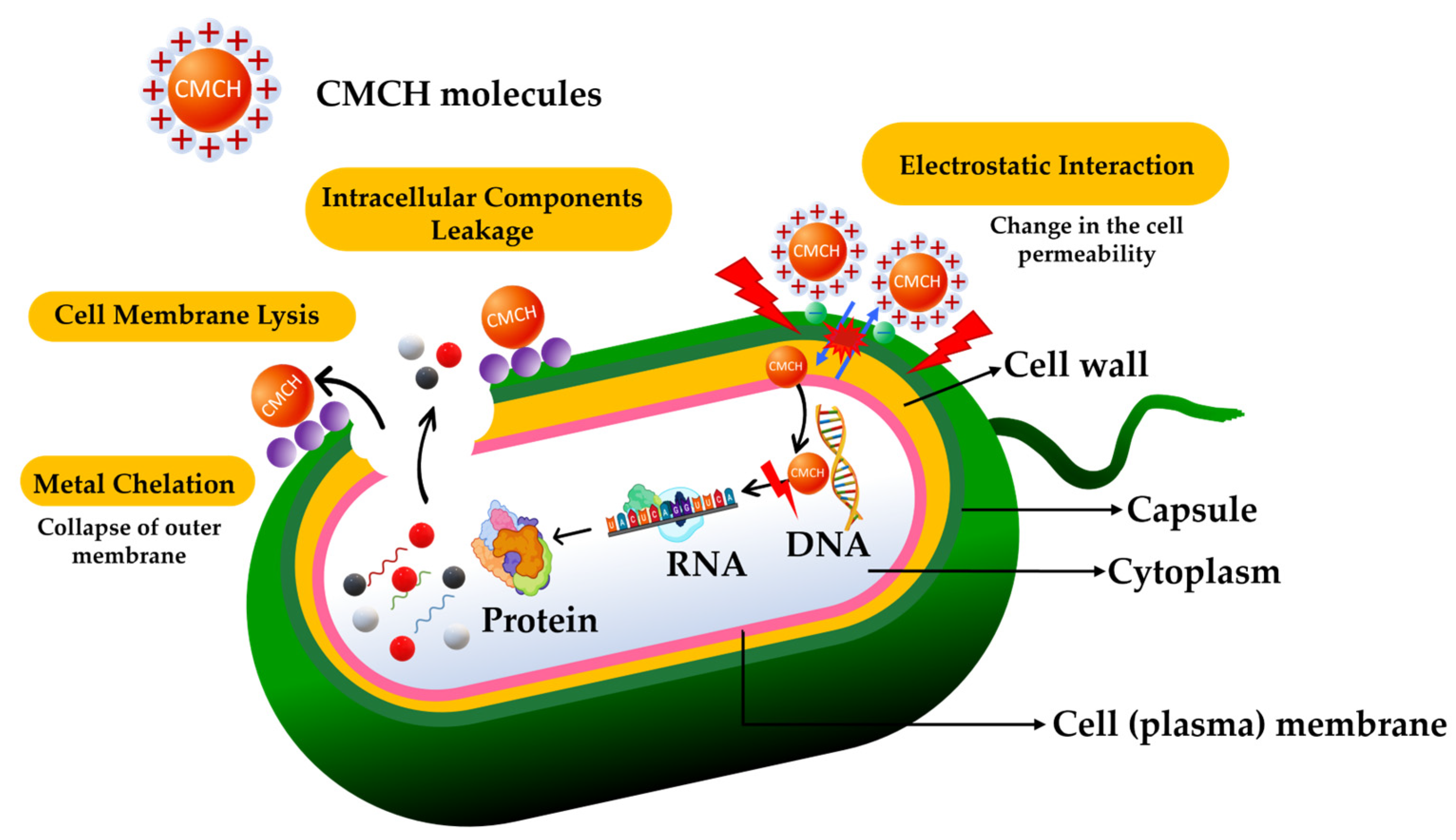
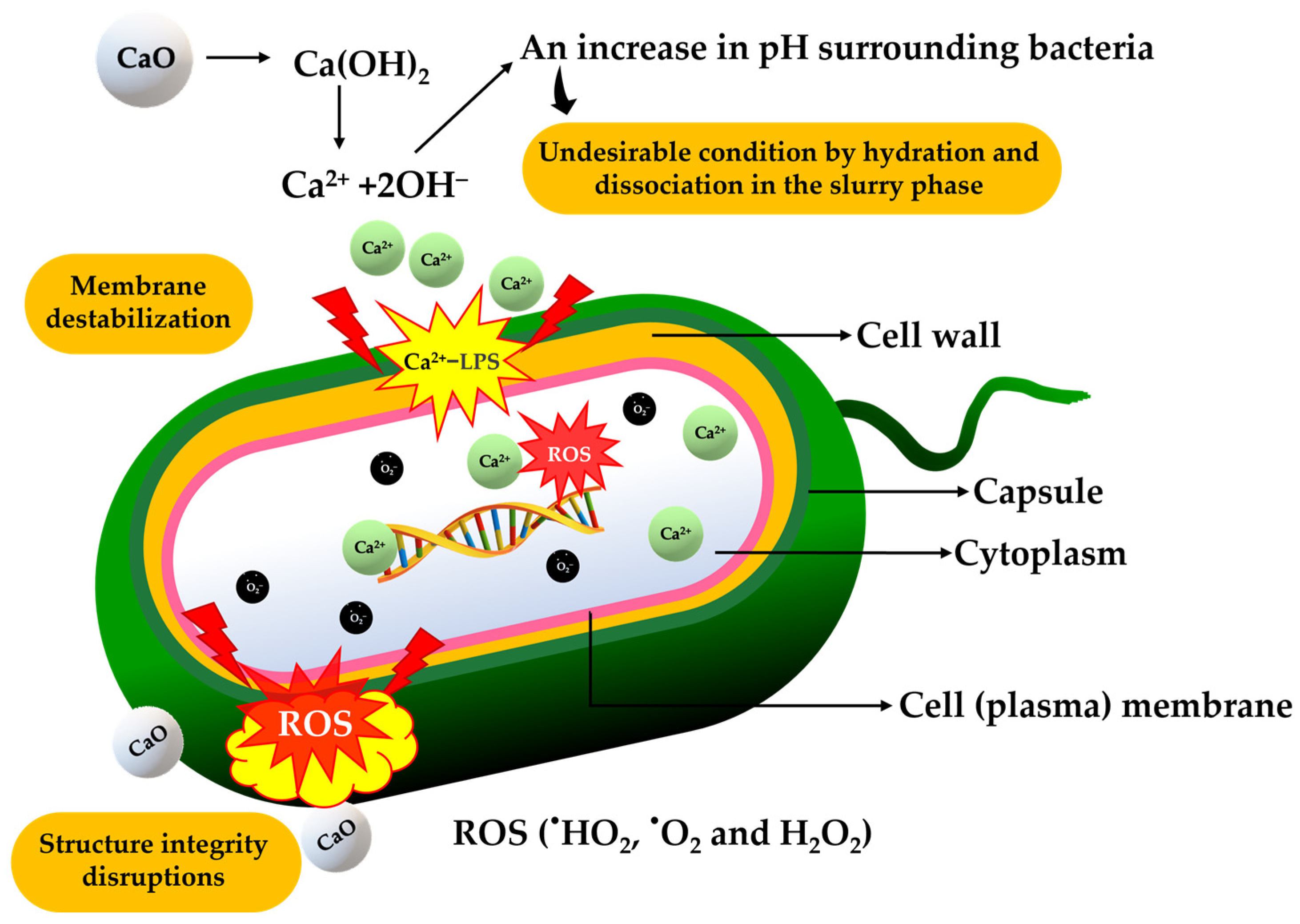
3.9. Antimicrobial Activity of Synthesized CaO and CMCH-CaO Bio-Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Aziz, S.M.; Asker, M.M.; Keera, A.A.; Mahmoud, M.G. Microbial food spoilage: Control strategies for shelf life extension. In Microbes in Food and Health; Garg, N., Abdel-Aziz, S.M., Aeron, A., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2016; pp. 239–264. [Google Scholar]
- Pandey, S.; Sharma, K.; Gundabala, V. Antimicrobial Bio-Inspired Active Packaging Materials for Shelf Life and Safety Development: A Review. Food Biosci. 2022, 48, 101730. [Google Scholar] [CrossRef]
- Mayegowda, S.B.; Bhoomika, S.; Nagshetty, K.; Manjula, N.G. Environmental Adequacy of Green Polymers and Biomaterials. In Polymeric Biomaterials, 1st ed.; Agarwal, P., Tripathy, D.B., Gupta, A., Kuanr, B.K., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 193–214. [Google Scholar]
- Zhou, J. Ultrafast fabrication of self-healing and injectable carboxymethyl chitosan hydrogel dressing for wound healing. ACS Appl. Mater. Interfaces 2021, 13, 24095–24105. [Google Scholar]
- Das, S.S.; Kar, S.; Singh, S.K.; Hussain, A.; Verma, P.R.P.; Beg, S. Carboxymethyl chitosan in advanced drug-delivery applications. In Chitosan in Drug Delivery; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: London, UK, 2022; pp. 323–360. [Google Scholar]
- Sionkowska, A.; Lewandowska, K.; Kurzawa, M. Chitosan-based films containing rutin for potential cosmetic applications. Polymers 2023, 15, 3224. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Rachtanapun, C.; Jantrawut, P.; Thanakkasaranee, S.; Kasi, G.; Tantala, J.; Panraksa, P.; Chaiwarit, T. Carboxymethyl Chitosan-Based Materials in Packaging, Food, Pharmaceutical, and Cosmetics. In Multifaceted Carboxymethyl Chitosan Derivatives: Properties and Biomedical Applications; Jayakumar, R., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 139–203. [Google Scholar]
- Muñoz-Nuñez, C.; Cuervo-Rodríguez, R.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A. Synthesis and characterization of thiazolium chitosan derivative with enhanced antimicrobial properties and its use as component of chitosan based films. Carbohydr. Polym. 2023, 302, 120438. [Google Scholar] [CrossRef] [PubMed]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of mechanical properties and thermal stability of biodegradable rice starch–based films blended with carboxymethyl chitosan. Ind. Crop. Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Mazher, M.; Ishtiaq, M.; Hamid, B.; Haq, S.M.; Mazhar, A.; Bashir, F.; Mazhar, M.; Mahmoud, E.A.; Casini, R.; Alataway, A.; et al. Biosynthesis and characterization of Calcium Oxide nanoparticles from Citrullus colocynthis Fruit extracts; their biocompatibility and bioactivities. Materials 2023, 16, 2768. [Google Scholar] [CrossRef] [PubMed]
- do Amparo Madureira, J.; Marinho, B.M.; Carvalho, S.M.; de Fátima Leite, M.; Borsagli, F.G.M. Sustainable calcium oxide nanoparticles based on agroindustry waste for potential endodontics applications. Mater. Chem. Phys. 2024, 320, 129426. [Google Scholar] [CrossRef]
- Shan, G.N.M.; Rafatullah, M.; Siddiqui, M.R.; Kapoor, R.T.; Qutob, M. Calcium oxide from eggshell wastes for the removal of pharmaceutical emerging contaminant: Synthesis and adsorption studies. J. Indian Chem. Soc. 2024, 101, 101174. [Google Scholar] [CrossRef]
- Rashtbari, S.; Dehghan, G.; Amini, M. An ultrasensitive label-free colorimetric biosensor for the detection of glucose based on glucose oxidase-like activity of nanolayered manganese-calcium oxide. Anal. Chim. Acta 2020, 1110, 98–108. [Google Scholar] [CrossRef]
- Park, K.; Sadeghi, K.; Thanakkasaranee, S.; Park, Y.I.; Park, J.; Nam, K.H.; Han, H.; Seo, J. Effects of calcination temperature on morphological and crystallographic properties of oyster shell as biocidal agent. Int. J. Appl. Ceram. Technol. 2021, 18, 302–311. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głąb, M.; Kędzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical investigations of chitosan-based hydrogels containing aloe vera designed for biomedical use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; Ruksiriwanich, W.; Rose Sommano, S.; Punyodom, W.; et al. High substitution synthesis of carboxymethyl chitosan for properties improvement of carboxymethyl chitosan films depending on particle sizes. Molecules 2021, 26, 6013. [Google Scholar] [CrossRef]
- Stodolak-Zych, E.; Jeleń, P.; Dzierzkowska, E.; Krok-Borkowicz, M.; Zych, Ł.; Boguń, M.; Rapacz-Kmita, A.; Kolesińska, B. Modification of chitosan fibers with short peptides as a model of synthetic extracellular matrix. J. Mol. Struct. 2020, 1211, 128061. [Google Scholar] [CrossRef]
- Sadeghi, K.; Thanakkasaranee, S.; Lim, I.J.; Seo, J. Calcined marine coral powders as a novel ecofriendly antimicrobial agent. Mater. Sci. Eng. C 2020, 107, 110193. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Thanakkasaranee, S.; Auras, R.A.; Chaiwong, N.; Jantanasakulwong, K.; Jantrawut, P.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; et al. Morphology, mechanical, and water barrier properties of carboxymethyl rice starch films: Sodium hydroxide effect. Molecules 2022, 27, 331. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of nano-calcium oxide from waste eggshell by sol-gel method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, S.; Pal, B. Superior adsorption removal of dye and high catalytic activity for transesterification reaction displayed by crystalline CaO nanocubes extracted from mollusc shells. Fuel Process. Technol. 2021, 213, 106707. [Google Scholar] [CrossRef]
- Lee, Y.H.; Eom, H.; Lee, S.M.; Kim, S.S. Effects of pH and metal composition on selective extraction of calcium from steel slag for Ca(OH)2 production. RSC Adv. 2021, 11, 8306–8313. [Google Scholar] [CrossRef]
- Hossain, M.S.; Jahan, S.A.; Ahmed, S. Crystallographic characterization of bio-waste material originated CaCO3, green-synthesized CaO and Ca(OH)2. Results Chem. 2023, 5, 100822. [Google Scholar] [CrossRef]
- Mohamed, F.; Shaban, M.; Aljohani, G.; Ahmed, A.M. Synthesis of novel eco-friendly CaO/C photocatalyst from coffee and eggshell wastes for dye degradation. Mater. Res. Technol. 2021, 14, 3140–3149. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Al-Aufi, R.; Al-Saidi, A.; Al-Samahi, S.; Al-Bulushi, R.; Rajan, G.; Abdelmouleh, M.; Jedidi, I. Effect of compression molding of CaCO3 powder on the kinetics of CO2 capture towards sustainable CO2 capture and sequestration cycle. Environ. Sci. Pollut. Res. Int. 2023, 30, 110981–110994. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, Y.; Zhao, X.; Shao, X.; Levchenko, S.V.; Nilius, N.; Sterrer, M.; Freund, H.J. Interaction of water with the CaO(001) surface. J. Phys. Chem. C 2016, 120, 5565–5576. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos.-A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D.; Hong, Q.; Gui, Y.; Cao, Y.; Ren, G.; Liang, Z. Surface functionalization of metal and metal oxide nanoparticles for dispersion and tribological applications-a review. J. Mol. Liq. 2023, 389, 122821. [Google Scholar] [CrossRef]
- Saleh, T.A. Properties of nanoadsorbents and adsorption mechanisms. In Interface Science and Technology; Saleh, T.A., Ed.; Elsevier: Oxford, UK, 2022; Volume 34, pp. 233–263. [Google Scholar]
- Giammaria, G.; Lefferts, L. Catalytic effect of water on calcium carbonate decomposition. J. CO2 Util. 2019, 33, 341–356. [Google Scholar] [CrossRef]
- Suneetha, M.; Won, S.Y.; Zo, S.M.; Han, S.S. Fungal carboxymethyl chitosan-impregnated bacterial cellulose hydrogel as wound-dressing agent. Gels 2023, 9, 184. [Google Scholar] [CrossRef]
- Chrissafis, K.; Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim. Acta 2011, 523, 1–24. [Google Scholar] [CrossRef]
- Barra, G.; Guadagno, L.; Raimondo, M.; Santonicola, M.G.; Toto, E.; Vecchio Ciprioti, S. A comprehensive review on the thermal stability assessment of polymers and composites for aeronautics and space applications. Polymers 2023, 15, 3786. [Google Scholar] [CrossRef]
- Šupová, M.; Martynková, G.S.; Barabaszová, K. Effect of nanofillers dispersion in polymer matrices: A review. Sci. Adv. Mater. 2011, 3, 1–25. [Google Scholar] [CrossRef]
- Kontou, E.; Christopoulos, A.; Koralli, P.; Mouzakis, D.E. The effect of silica particle size on the mechanical enhancement of polymer nanocomposites. Nanomaterials 2023, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Sängerlaub, S.; Kucukpinar, E.; Kiese, S.; Bauer, K.D.; Müller, K. Desiccant films made of low-density polyethylene with dispersed calcium oxide: Water vapor absorption, permeation and mechanical properties. J. Appl. Polym. Sci. 2019, 136, 47460. [Google Scholar] [CrossRef]
- Silva, C.; Bobillier, F.; Canales, D.; Antonella Sepúlveda, F.; Cament, A.; Amigo, N.; Rivas, L.M.; Ulloa, M.T.; Reyes, P.; Ortiz, J.A.; et al. Mechanical and antimicrobial polyethylene composites with CaO nanoparticles. Polymers 2020, 12, 2132. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Weng, Y.X.; Huang, Z.G.; Jin, Y.J.; Hu, J.; Chou, D.; Shao, S.X. Manufacture of a hydrophobic CaO/polylactic acid composite. Mater. Manuf. Process. 2019, 34, 303–311. [Google Scholar] [CrossRef]
- Bonaccurso, E.; Graf, K. Nanostructuring effect of plasma and solvent treatment on polystyrene. Langmuir 2004, 20, 11183–11190. [Google Scholar] [CrossRef]
- Erdogan, N.; Ozbay, S. Contact Angle Studies on Functional Surfaces Containing Magnetic Particles. In Handbook of Magnetic Hybrid Nanoalloys and their Nanocomposites; Thomas, S., Nochehdehi, A.R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 2, pp. 733–759. [Google Scholar]
- Komalsingsakul, A.; Klaophimai, A.; Srisatjaluk, R.L.; Senawongse, P. Effect of the surface roughness of composite resins on the water contact angle and biofilm formation. Mahidol Den. J. 2019, 39, 75–84. [Google Scholar]
- Thanakkasaranee, S.; Sadeghi, K.; Seo, J. Smart steam release of newly developed temperature-responsive nanocomposite films derived from phase change material. Polymer 2021, 219, 123543. [Google Scholar] [CrossRef]
- Másson, M. Antimicrobial properties of chitosan and its derivatives. In Chitosan for Biomaterials III: Structure-Property Relationships; Jayakumar, R., Prabaharan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 287, pp. 131–168. [Google Scholar]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Gauri, S.S.; Bhattacharya, M.; Bhattacharya, J. Antimicrobial activity of CaO nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 1570–1578. [Google Scholar] [CrossRef]
- Feng, X.; Hou, X.; Cui, C.; Sun, S.; Sadik, S.; Wu, S.; Zhou, F. Mechanical and antibacterial properties of tannic acid-encapsulated carboxymethyl chitosan/polyvinyl alcohol hydrogels. Eng. Regen. 2021, 2, 57–62. [Google Scholar] [CrossRef]
- Tantala, J.; Thongngam, M.; Rachtanapun, P.; Rachtanapun, C. Antimicrobial activity of chitosan and carboxymethyl chitosan from different types and sources of chitosan. Ital. J. Food Sci./Riv. Ital. Di Sci. Degli Aliment. 2012, 24, 97–101. [Google Scholar]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Clifton, L.A.; Skoda, M.W.; Le Brun, A.P.; Ciesielski, F.; Kuzmenko, I.; Holt, S.A.; Lakey, J.H. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 2015, 31, 404–412. [Google Scholar] [CrossRef]
- Schneck, E.; Papp-Szabo, E.; Quinn, B.E.; Konovalov, O.V.; Beveridge, T.J.; Pink, D.A.; Tanaka, M. Calcium ions induce collapse of charged O-side chains of lipopolysaccharides from Pseudomonas aeruginosa. J. R. Soc. Interface 2009, 6 (Suppl. 5), S671–S678. [Google Scholar] [CrossRef]


| Samples | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| CMCH | 5.3 ± 0.4 | 76.0 ± 3.4 |
| CMCH-CaO1% | 15.8 ± 0.8 | 59.2 ± 1.0 |
| CMCH-CaO5% | 13.2 ± 0.8 | 79.1 ± 2.8 |
| CMCH-CaO10% | 4.8 ± 0.2 | 49.1 ± 1.1 |
| Samples | E. coli | S. aureus | ||
|---|---|---|---|---|
| Viable Cells (CFU/mL) | R (%) | Viable Cells (CFU/mL) | R (%) | |
| CaO | N.D. | ≥99.9 | N.D. | ≥99.9 |
| LDPE | 1.06 × 109 | - | 2.23 × 108 | - |
| CMCH | 9.28 × 108 | 12.5 | 2.01 × 108 | 9.82 |
| CMCH-CaO-1% | 1.87 × 108 | 82.4 | 5.56 × 107 | 75.1 |
| CMCH-CaO-5% | 1.86 × 107 | 98.2 | 2.64 × 107 | 88.1 |
| CMCH-CaO-10% | 1.32 × 107 | 98.8 | 1.82 × 108 | 91.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanakkasaranee, S.; Rachtanapun, P.; Rachtanapun, C.; Kanthiya, T.; Kasi, G.; Sommano, S.R.; Jantanasakulwong, K.; Seo, J. Bio-Composite Films Based on Carboxymethyl Chitosan Incorporated with Calcium Oxide: Synthesis and Antimicrobial Activity. Polymers 2024, 16, 2393. https://doi.org/10.3390/polym16172393
Thanakkasaranee S, Rachtanapun P, Rachtanapun C, Kanthiya T, Kasi G, Sommano SR, Jantanasakulwong K, Seo J. Bio-Composite Films Based on Carboxymethyl Chitosan Incorporated with Calcium Oxide: Synthesis and Antimicrobial Activity. Polymers. 2024; 16(17):2393. https://doi.org/10.3390/polym16172393
Chicago/Turabian StyleThanakkasaranee, Sarinthip, Pornchai Rachtanapun, Chitsiri Rachtanapun, Thidarat Kanthiya, Gopinath Kasi, Sarana Rose Sommano, Kittisak Jantanasakulwong, and Jongchul Seo. 2024. "Bio-Composite Films Based on Carboxymethyl Chitosan Incorporated with Calcium Oxide: Synthesis and Antimicrobial Activity" Polymers 16, no. 17: 2393. https://doi.org/10.3390/polym16172393








