Tuning the Properties of Xylan/Chitosan-Based Films by Temperature and Citric Acid Crosslinking Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Xylan
2.2. Chitosan
2.3. Citric Acid and Sodium Hypophosphite
2.4. Preparation of Xyl/Ch PEC Suspensions in the Presence of Citric Acid and Sodium Hypophosphite
2.5. Determination of Particle Size and Z-Potential Values of PECs
2.6. Preparation of Xyl/Ch Films and Crosslinking
2.7. Washing Step
2.8. FTIR Spectroscopy
2.9. Confocal Raman Microscopy
2.10. UV-vis Light Transmittance
2.11. Mechanical Film Properties
2.12. Swelling Behavior, Solubility, and Equilibrium Moisture Content
2.13. Thermal Gravimetric Analysis
2.14. Scanning Electron Microscopy (SEM)
2.15. Contact Angle
2.16. Statistical Analysis
3. Results
3.1. Z-Potential and Average Particle Size
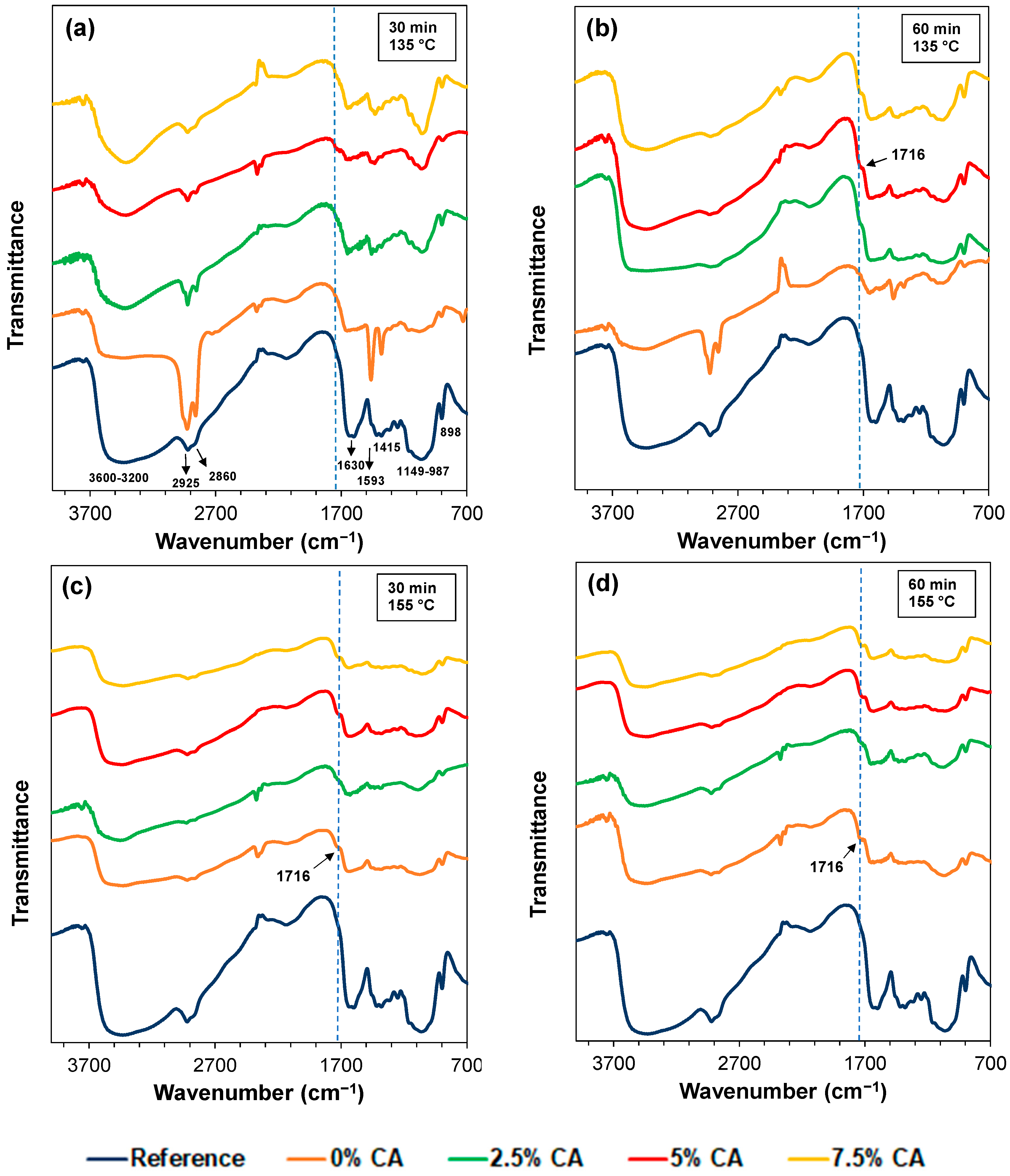
3.2. FTIR Analysis
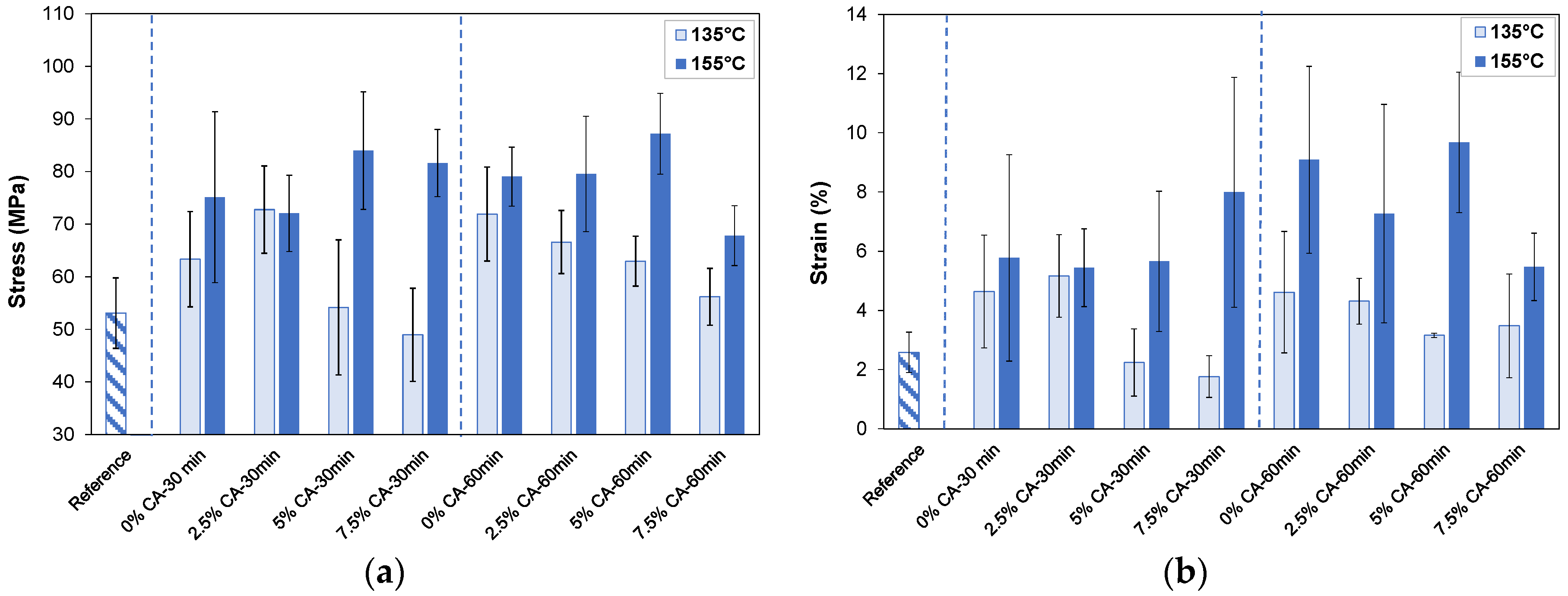
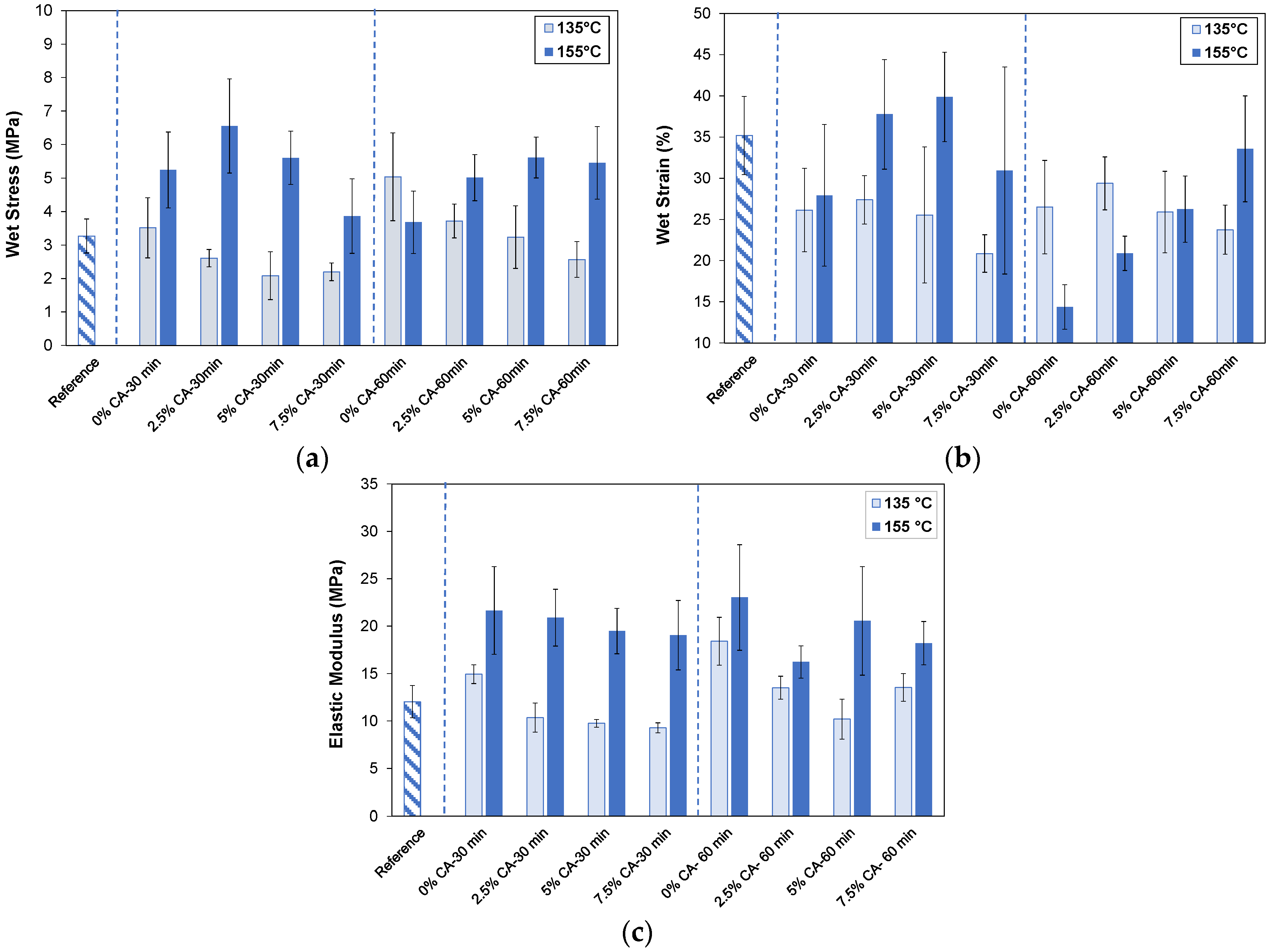
3.3. Mechanical Properties
3.3.1. Dry State
3.3.2. Wet State
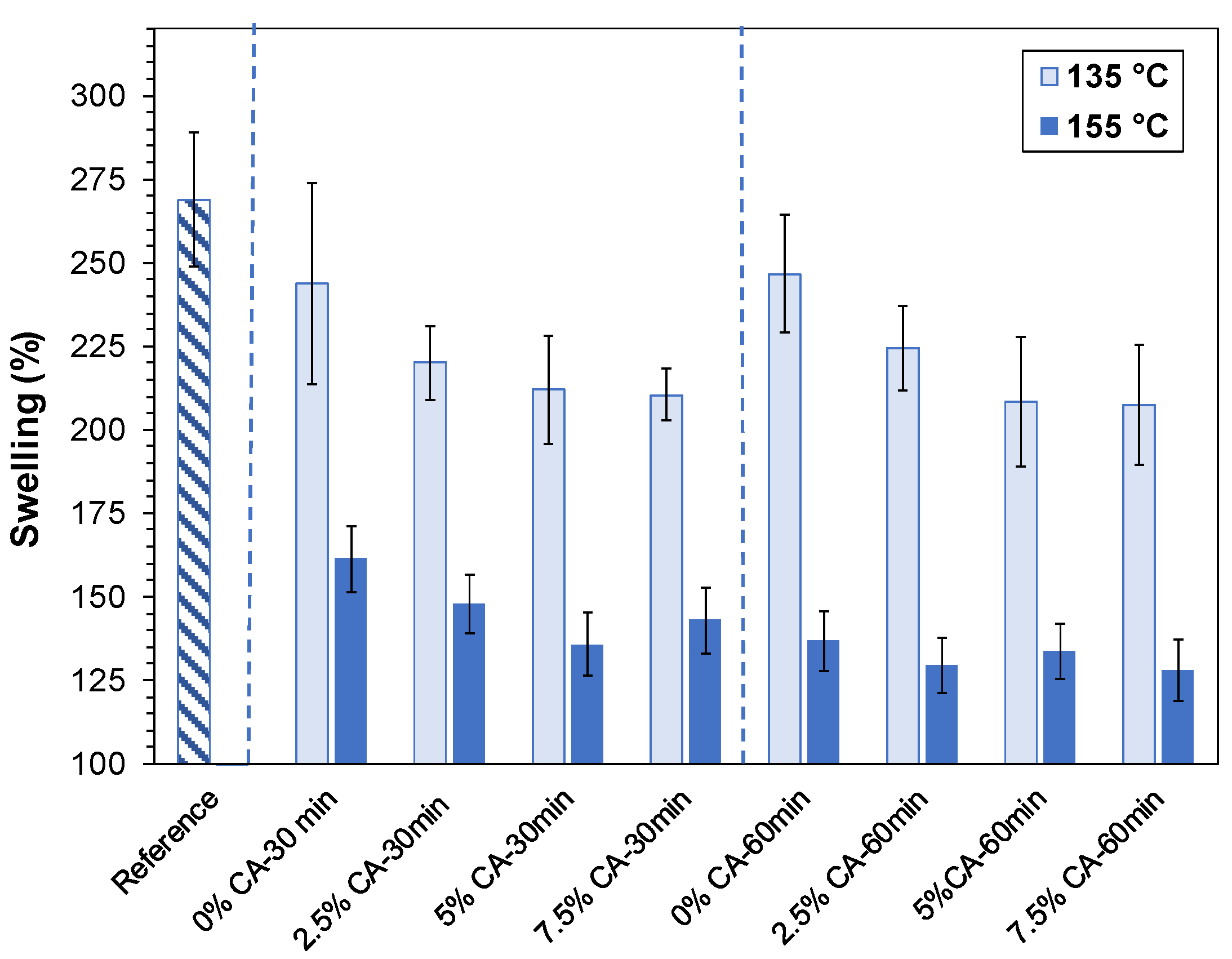
3.4. Swelling and Solubility
3.5. Characterization of Films Thermally Treated at 155 °C
3.5.1. Confocal Raman Microscopy Analysis
3.5.2. UV Barrier and Opacity Properties
3.5.3. SEM Microscopy Analysis
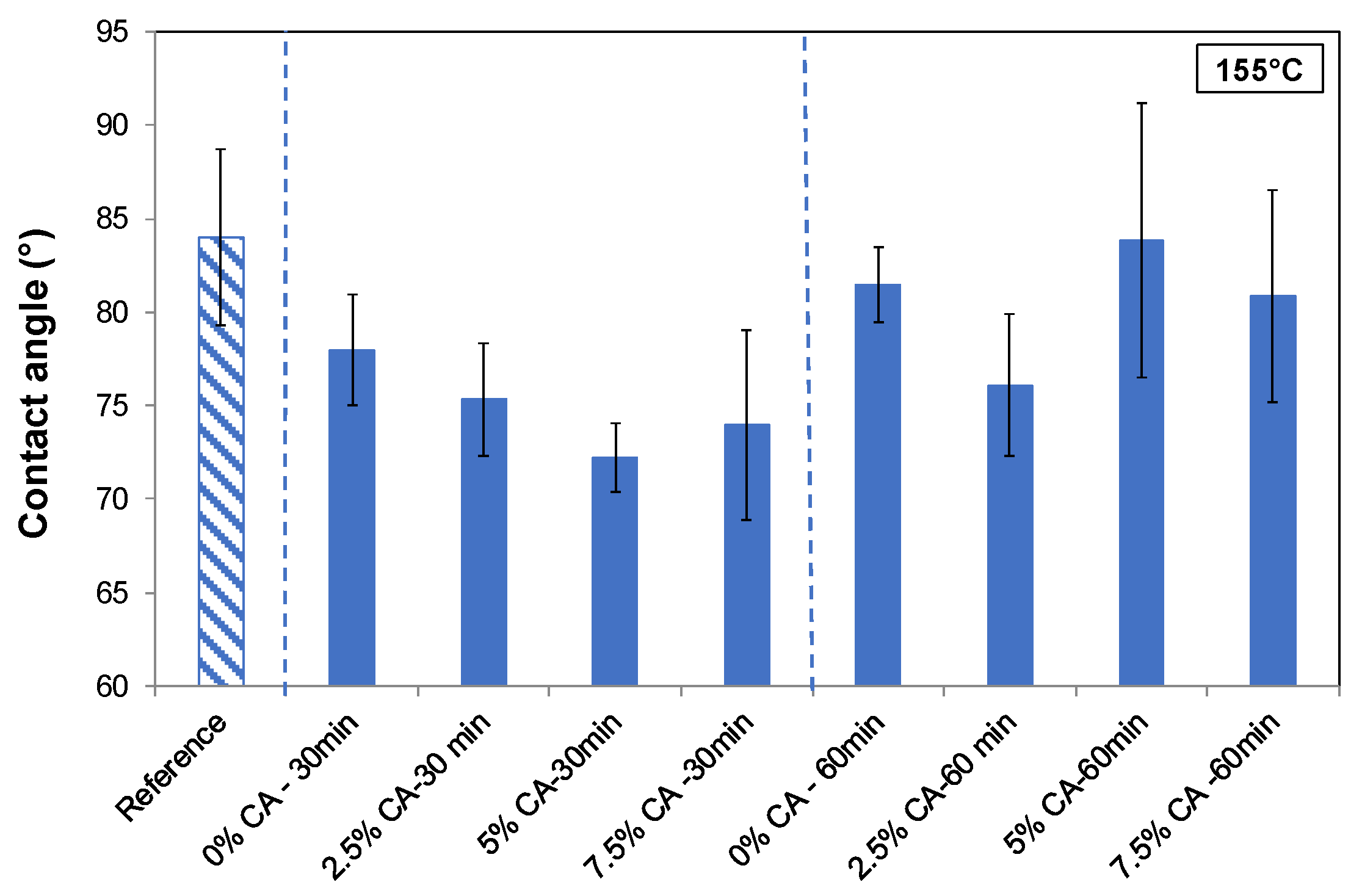
3.5.4. Surface Wettability
3.5.5. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Roy, S.; Assadpour, E.; Cong, X.; Jafari, S.M. Cross-linked biopolymeric films by citric acid for food packaging and preservation. Adv. Colloid Interface Sci. 2023, 314, 102886. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Urruzola, I.; Labidi, J. Xylan-cellulose films: Improvement of hydrophobicity, thermal and mechanical properties. Carbohydr. Polym. 2014, 112, 56–62. [Google Scholar] [CrossRef]
- Schnell, C.N.; Galván, M.V.; Peresin, M.S.; Inalbon, M.C.; Vartiainen, J.; Zanuttini, M.A.; Mocchiutt, P. Films from xylan/chitosan complexes: Preparation and characterization. Cellulose 2017, 24, 4393–4403. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-acorn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Thermomechanical-induced polyelectrolyte complexation between chitosan and carboxymethyl cellulose enabling unexpected hydrolytic stability. Compos. Sci. Technol. 2020, 189, 108031. [Google Scholar] [CrossRef]
- Berger, J.; Reista, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalent and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Schnell, C.N.; Galván, M.V.; Solier, Y.N.; Inalbon, M.C.; Zanuttini, M.A.; Mocchiutti, P. High strength biobased films prepared from xylan/chitosan polyelectrolyte complexes in the presence of ethanol. Carbohydr. Polym. 2021, 273, 118602. [Google Scholar] [CrossRef] [PubMed]
- Schnell, C.N.; Inalbon, M.C.; Minari, R.J.; Mocchiutti, P. Use of micro/nano- and nanofibrillated cellulose to improve the mechanical properties and wet performance of xylan/chitosan films: A comparison. Appl. Polym. Mater. 2023, 5, 7867–7877. [Google Scholar] [CrossRef]
- Solier, Y.N.; Schnell, C.N.; Galván, M.V.; Mocchiutti, P.; Zanuttini, M.A.; Inalbon, M.C. Fast preparation of flexible wet-resistant and biodegradable films from a stable suspension of xylan/chitosan polyelectrolyte complexes. J. Polym. Environ. 2022, 30, 114–124. [Google Scholar] [CrossRef]
- Dastidar, T.G.; Netravali, A.N. ‘Green’ crosslinking of native starches with malonic acid and their properties. Carbohydr. Polym. 2012, 90, 1620–1628. [Google Scholar] [CrossRef]
- Menzel, C.; Olsson, E.; Plivelic, T.S.; Andersson, R.; Johansson, C.; Kuktaite, R.; Järnström, L.; Koch, K. Molecular structure of citric acid cross-linked starch films. Carbohydr. Polym. 2013, 96, 270–276. [Google Scholar] [CrossRef]
- Mansilla, A.Y.; Albertengo, L.; Rodríguez, M.S.; Debbaudt, A.; Zúñiga, A.; Casalongué, C.A. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Menzel, C.; Johansson, C.; Andersson, R.; Koch, K.; Järnström, L. The effect of pH on hydrolysis, cross-linking and barrier properties of starch barriers containing citric acid. Carbohydr. Polym. 2013, 98, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Rena, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. Hemicellulose-based films: Potential green films for food packaging. Polymers 2020, 12, 1775. [Google Scholar] [CrossRef]
- McMurry, J. Aldehydes and Ketones: Nucleophilic Addition Reactions. In Organic Chemistry, 9th ed.; Cengage Learning: Boston, MA, USA, 2016; pp. 605–640. [Google Scholar]
- Tasselli, F.; Mirmohseni, A.; Seyed Dorraji, M.S.; Figoli, A. Mechanical, swelling and adsorptive properties of dry-wet spun chitosan hollow fibers crosslinked with glutaraldehyde. Reactive Funct. Polym. 2013, 73, 218–223. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Mun, G.A.; Dubolazov, A.V.; Khutoryanskiy, V.V. pH effects on the complexation, miscibility and radiation-induced crosslinking in poly(acrylic acid)-poly(vinyl alcohol) blends. Macromol. Biosci. 2005, 5, 424–432. [Google Scholar] [CrossRef]
- Rhim, J.W.; Sohn, M.Y.; Joo, H.J.; Lee, K.H. Pervaporation separation of binary organic-aqueous liquid mixtures using crosslinked PVA membranes. I. Characterization of the reaction between PVA and PAA. J. Appl. Polym. Sci. 1993, 50, 679–684. [Google Scholar] [CrossRef]
- Blasques Bueno, V.; Bentini, R.; Catalani, L.H.; Freitas Siqueira Petri, D. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Zoldners, J.; Kiseleva, T. Modification of hemicelluloses with polycarboxylic acids. Holzforschung 2013, 67, 567–571. [Google Scholar] [CrossRef]
- Cegnar, M.; Kerč, J. Self-assembled polyelectrolyte nanocomplexes of alginate, chitosan and ovalbumin. Acta Chim. Slov. 2010, 57, 431–441. [Google Scholar] [PubMed]
- Wing, R.E. Starch citrate: Preparation and ion exchange properties. Starch/Stärke 1996, 48, 275–279. [Google Scholar] [CrossRef]
- Shen, L.; Xu, H.; Kong, L.; Yang, Y. Non-toxic crosslinking of starch using polycarboxylic acids: Kinetic study and quantitative correlation of mechanical properties and crosslinking degrees. J. Polym. Environ. 2015, 23, 588–594. [Google Scholar] [CrossRef]
- Solier, Y.N.; Sznaider, F.; Navarro, D.A.; Mocchiutti, P.; Inalbon, M.C. Sulfation of poplar wood xylan using a deep eutectic solvent: Effects of the degree of substitution on the properties of biobased hydrogels. Cellulose 2024, 31, 1189–1203. [Google Scholar] [CrossRef]
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of bio-nanocomposite films based on gelatin/polyvinyl alcohol blend reinforced with bacterial cellulose nanowhiskers for food packaging applications. Food Hydrocoll. 2021, 113, 106454. [Google Scholar] [CrossRef]
- ASTM D 882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2002.
- ISO 3781; Paper and Board–Determination of Tensile Strength after Immersion in Water. ISO: Genève, Switzerland, 1983.
- Allasia, M.; Aguirre, M.; Gugliotta, L.M.; Minari, R.J.; Leiza, J.R. High biobased content waterborne latexes stabilized with casein. Prog. Org. Coat. 2022, 168, 106870. [Google Scholar] [CrossRef]
- Schnell, C.N.; Galván, M.V.; Zanuttini, M.A.; Mocchiutti, P. Hydrogels from xylan/chitosan complexes for the controlled release of diclofenac sodium. Cellulose 2020, 27, 1465–1481. [Google Scholar] [CrossRef]
- Krukowski, S.; Karasiewicz, M.; Kolodziejski, W. Convenient UV-spectrophotometric determination of citrates in aqueous solutions with applications in the pharmaceutical analysis of oral electrolyte formulations. J. Food Drug Anal. 2017, 25, 717–722. [Google Scholar] [CrossRef]
- Yuyu, E.; Qi, Q.; Chang, Z.; Jiang, J.; Yao, X.; Li, P.; Lei, F.; Wang, K. Fabrication of pH-sensitive galactomannan/glycerol bio-composite films for food packaging applications. React. Funct. Polym. 2022, 181, 105465. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Synytsya, A.; Copíková, J.; Matejka, P.; Machovic, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Xu, J.; Xia, R.; Yuan, T.; Sun, R. Use of xylooligosaccharides (XOS) in hemicelluloses/chitosan-based films reinforced by cellulose nanofiber: Effect on physicochemical properties. Food Chem. 2019, 298, 125041. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Tomkinson, J.; Ma, P.L.; Liang, S.F. Comparative study of hemicelluloses from rice straw by alkali and hydrogen peroxide treatments. Carbohydr. Polym. 2000, 42, 111–122. [Google Scholar] [CrossRef]
- Jia, C.; Chen, L.; Shao, Z.; Agarwal, U.P.; Hu, L.; Zhu, J.Y. Using a fully recyclable dicarboxylic acid for producing dispersible and thermally stable cellulose nanomaterials from different cellulosic sources. Cellulose 2017, 24, 2483–2498. [Google Scholar] [CrossRef]
- Luo, J.; Huang, K.; Xu, Y.; Fan, Y. A comparative study of lignocellulosic nanofibrils isolated from celery using oxalic acid hydrolysis followed by sonication and mechanical fibrillation. Cellulose 2019, 26, 5237–5246. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Lipatova, I.M.; Yusova, A.A. Effect of mechanical activation on starch crosslinking with citric acid. Int. J. Biol. Macromol. 2021, 185, 688–695. [Google Scholar] [CrossRef]
- Hernandez, M.S.; Luduena, L.N.; Flores, S.K. Citric acid, chitosan and oregano essential oil impact on physical and antimicrobial properties of cassava starch films. Carbohydr. Polym. Technol. Appl. 2023, 5, 100307. [Google Scholar] [CrossRef]
- Sakkara, S.; Venkatesh, K.; Reddy, R.; Nagananda, G.S.; Meghwal, M.; Patil, J.H.; Reddy, N. Characterization of crosslinked macrotyloma Uniflorum (Horsegram) protein films for packaging and medical applications. Polym. Test. 2020, 91, 106794. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Wang, B.; Yang, Y. Improving wet strength of soy protein films using oxidized sucrose. J. Appl. Polym. Sci. 2015, 132, 41473. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Sun, B.; Zhang, R. Improving the wet strength of hemicelluloses based composite films by citric acid crosslinking. J. Wood Chem. Technol. 2020, 41, 1–9. [Google Scholar] [CrossRef]
- Zou, G.; Qu, J.; Zou, X. Optimization of water absorption of starch/PVA composites. Polym. Compos. 2007, 28, 674–679. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Wehling, B.; Moens, L.; Edwards, H.; De Reu, M.; Van Hooydonk, G. Analysis with micro-Raman spectroscopy of natural organic binding media and varnishes used in art. Anal. Chim. Acta 2000, 407, 261–274. [Google Scholar] [CrossRef]
- Hernández, R.; Sacristán, J.; Mijangos, C. Sol/Gel transition of aqueous alginate solutions induced by Fe2+ cations. Macromol. Chem. Phys. 2010, 211, 1254–1260. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Yao, Y.; Wu, N.; Xu, M.; Yin, Z.; Zhao, Y.; Tu, Y. Effects of citric acid crosslinking on the structure and properties of ovotransferrin and chitosan composite films. Int. J. Biol. Macromol. 2023, 229, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Kadam, A.A.; Sing, S.; Gaikwad, K.K. Chitosan based antioxidant films incorporated with pine needles (Cedrus deodara) extract for active food packaging applications. Food Control 2021, 124, 107877. [Google Scholar] [CrossRef]
- Ryan, B.J.; Poduska, K.M. Roughness effects on contact angle measurements. Am. J. Phys. 2008, 76, 1074–1078. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, C.; Liu, R.; Liu, Q.; Xu, J.; Yao, J. Structure and properties of citric acid cross-linked chitosan/poly(vinyl alcohol) composite films for food packaging applications. Carbohydr. Polym. 2023, 312, 120842. [Google Scholar] [CrossRef]
- Guevara, M.M.S.; Saucedo-Rivalcoba, V.; Rivera-Armenta, J.L.; Elvira-Torales, L.I. Evaluation of a cross-linking agent in the preparation of films based on chitosan and pectin for food packaging applications. Cellulose Chem. Technol. 2022, 56, 1061–1070. [Google Scholar] [CrossRef]


| Identification | Z-Potential (mV) | Average Particle Size (nm) | PDI | meq(+)/g PEC |
|---|---|---|---|---|
| 0% CA | 37.15 ± 0.46 | 1078 ± 24 | 0.271 ± 0.030 | 0.99 |
| 2.5% CA | 35.52 ± 0.76 | 1037 ± 27 | 0.286 ± 0.030 | 0.83 |
| 5% CA | 34.11 ± 0.53 | 921 ± 27 | 0.217 ± 0.030 | 0.68 |
| 7.5% CA | 30.05 ± 0.26 | 876 ± 17 | 0.239 ± 0.040 | 0.54 |
| Identification | Tonset (°C) | Tdeg (°C) | Tendset (°C) | Wresidual (%) |
|---|---|---|---|---|
| Reference | 224 | 246 | 280 | 4.6 |
| 0% CA—30 min | 224 | 247 | 293 | 28.4 |
| 2.5% CA—30 min | 229 | 250 | 285 | 11.0 |
| 5% CA—30 min | 230 | 254 | 289 | 27.2 |
| 7.5% CA—30 min | 230 | 250 | 280 | 13.9 |
| 0% CA—60 min | 231 | 255 | 295 | 29.8 |
| 2.5% CA—60 min | 231 | 255 | 302 | 31.0 |
| 5% CA—60 min | 229 | 246 | 289 | 31.7 |
| 7.5% CA—60 min | 231 | 252 | 282 | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camaño Erhardt, M.; Solier, Y.N.; Inalbon, M.C.; Mocchiutti, P. Tuning the Properties of Xylan/Chitosan-Based Films by Temperature and Citric Acid Crosslinking Agent. Polymers 2024, 16, 2407. https://doi.org/10.3390/polym16172407
Camaño Erhardt M, Solier YN, Inalbon MC, Mocchiutti P. Tuning the Properties of Xylan/Chitosan-Based Films by Temperature and Citric Acid Crosslinking Agent. Polymers. 2024; 16(17):2407. https://doi.org/10.3390/polym16172407
Chicago/Turabian StyleCamaño Erhardt, Martina, Yamil Nahún Solier, María Cristina Inalbon, and Paulina Mocchiutti. 2024. "Tuning the Properties of Xylan/Chitosan-Based Films by Temperature and Citric Acid Crosslinking Agent" Polymers 16, no. 17: 2407. https://doi.org/10.3390/polym16172407






