Experimental Study on the Chlorine-Induced Corrosion and Blister Formation of Steel Pipes Coated with Modified Polyethylene Powder
Abstract
:1. Introduction
2. Research Significance
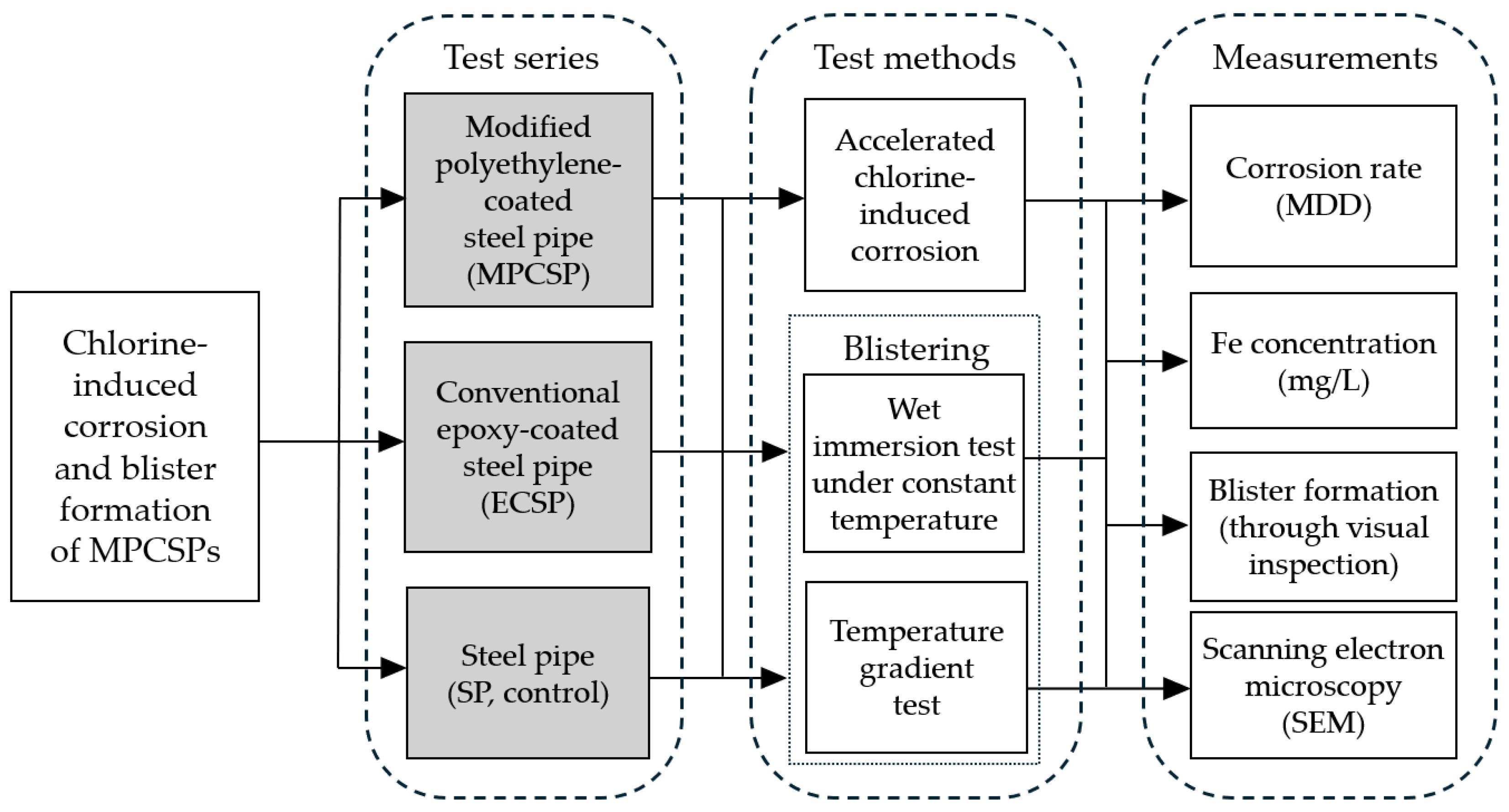
3. Experimental Design
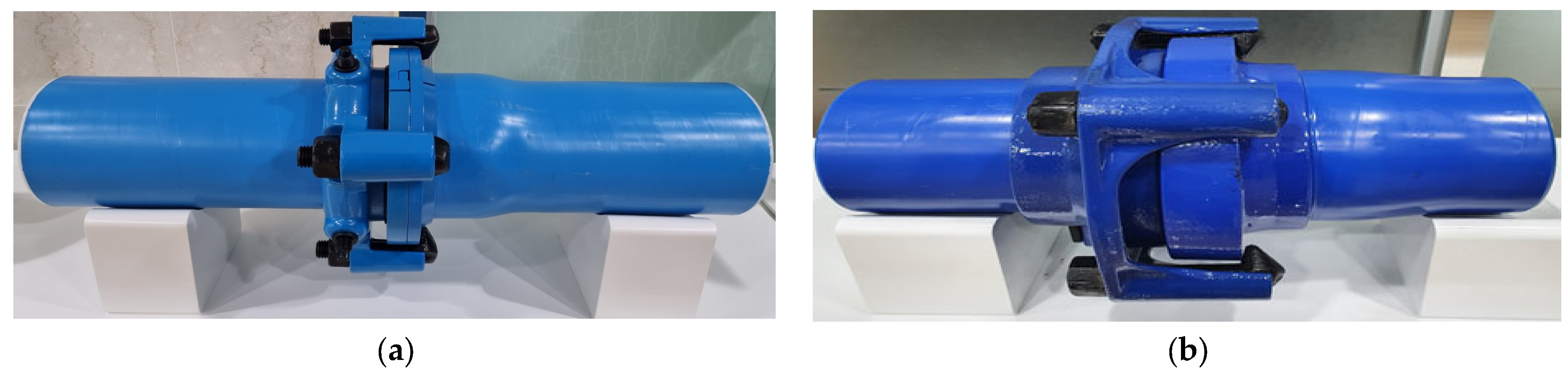
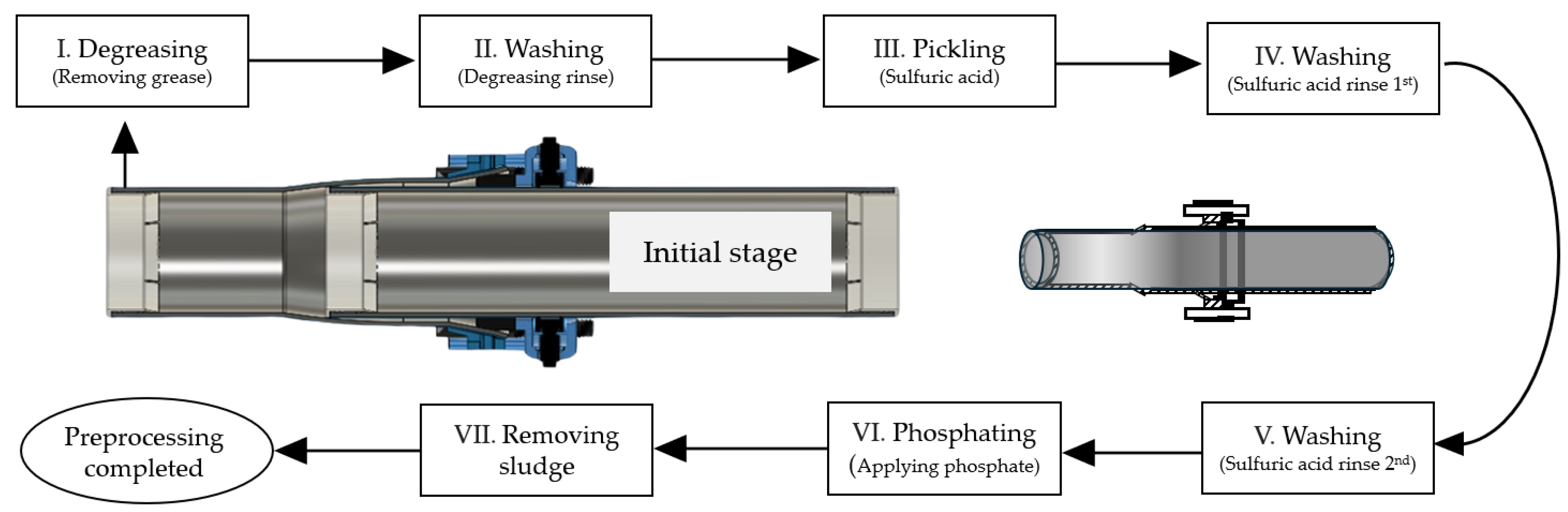
3.1. Test Specimens and Coating Processes
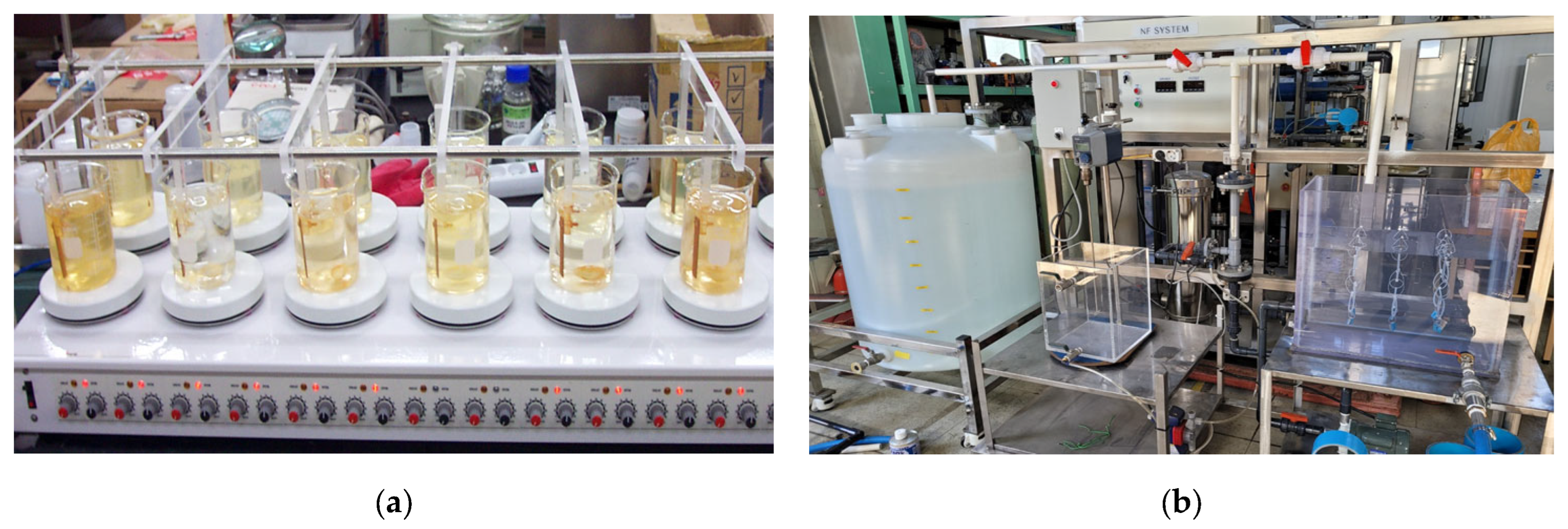
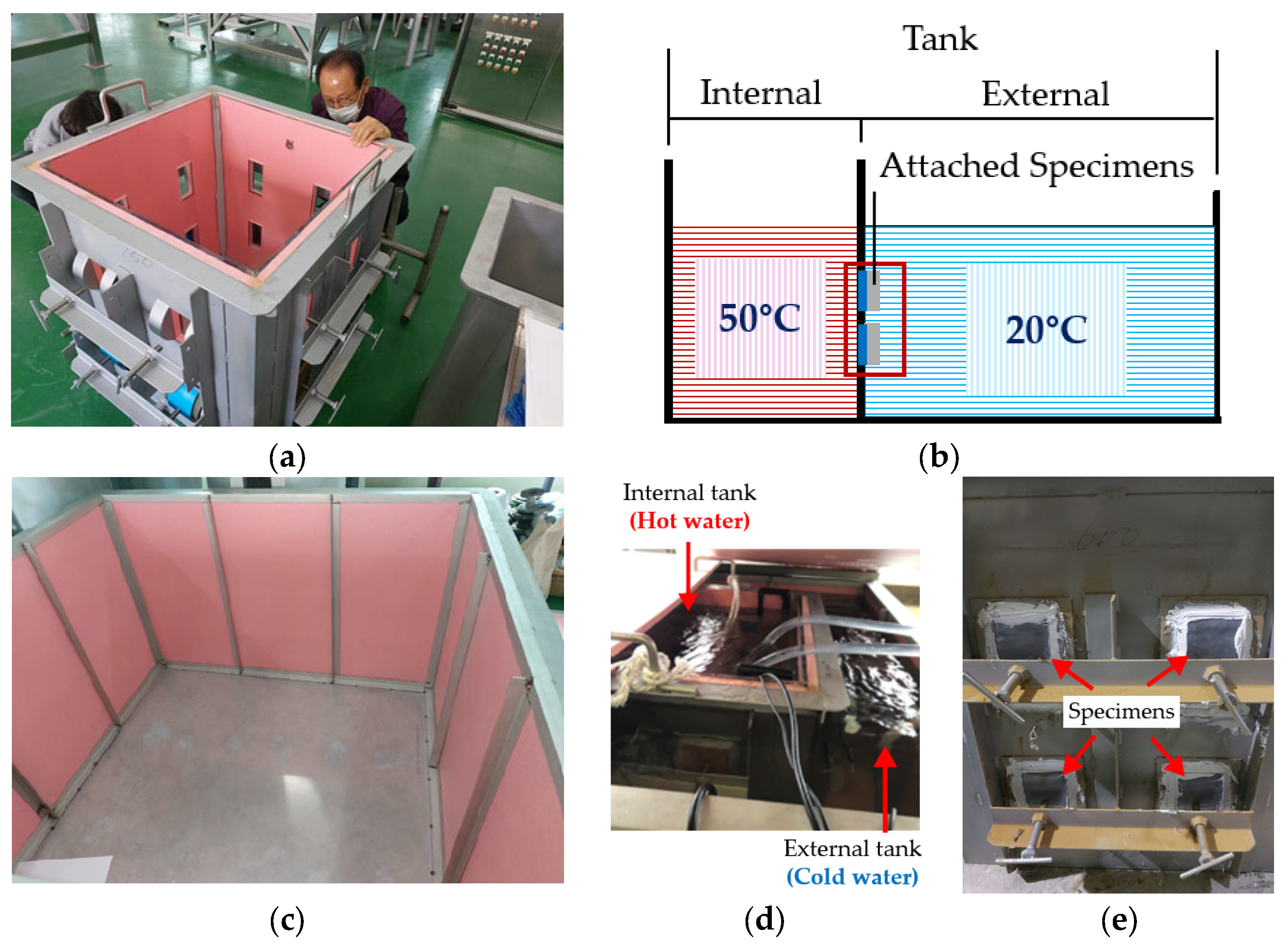
3.2. Chlorine-Induced Corrosion Test
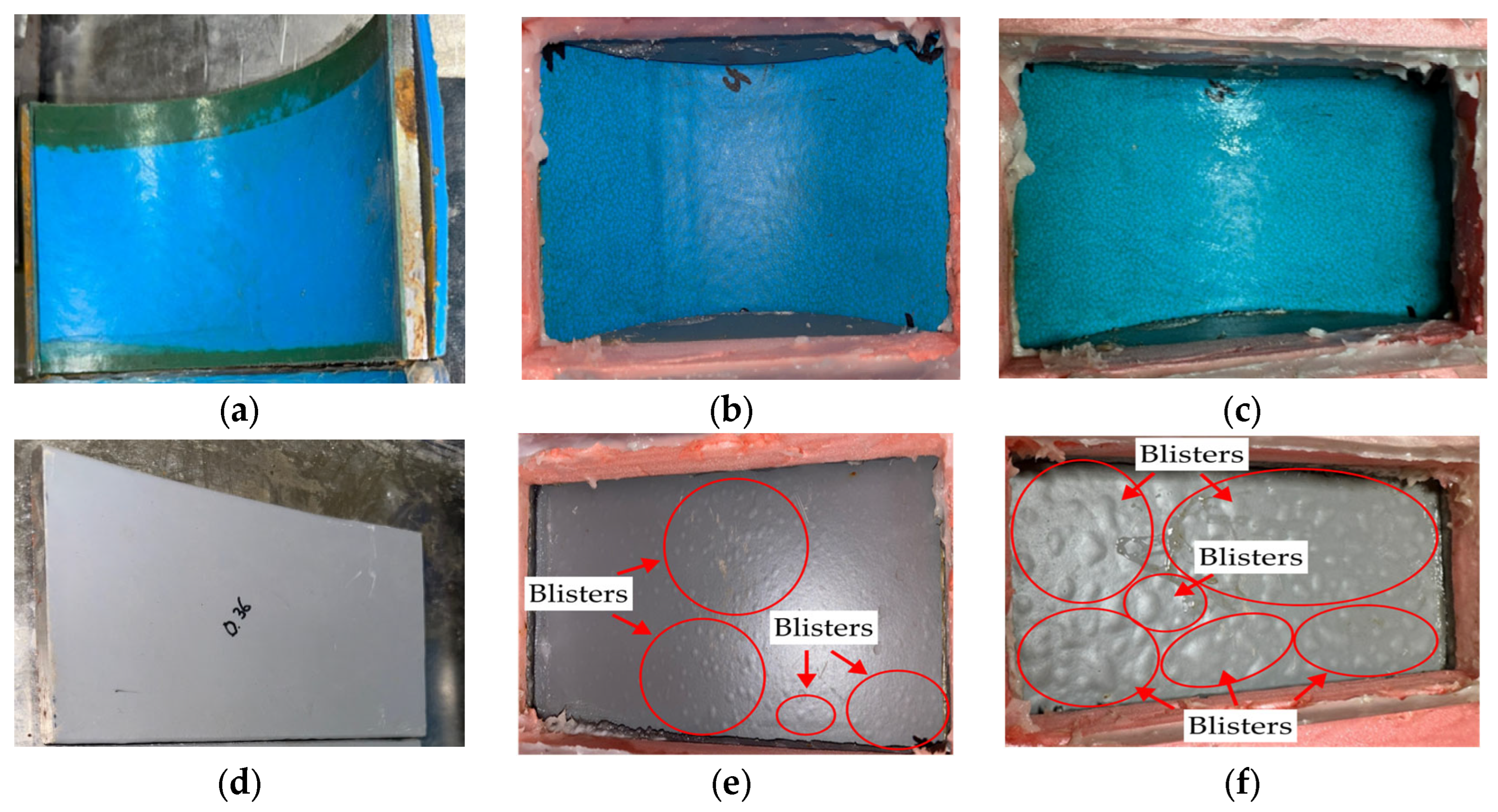
3.3. Blistering Test
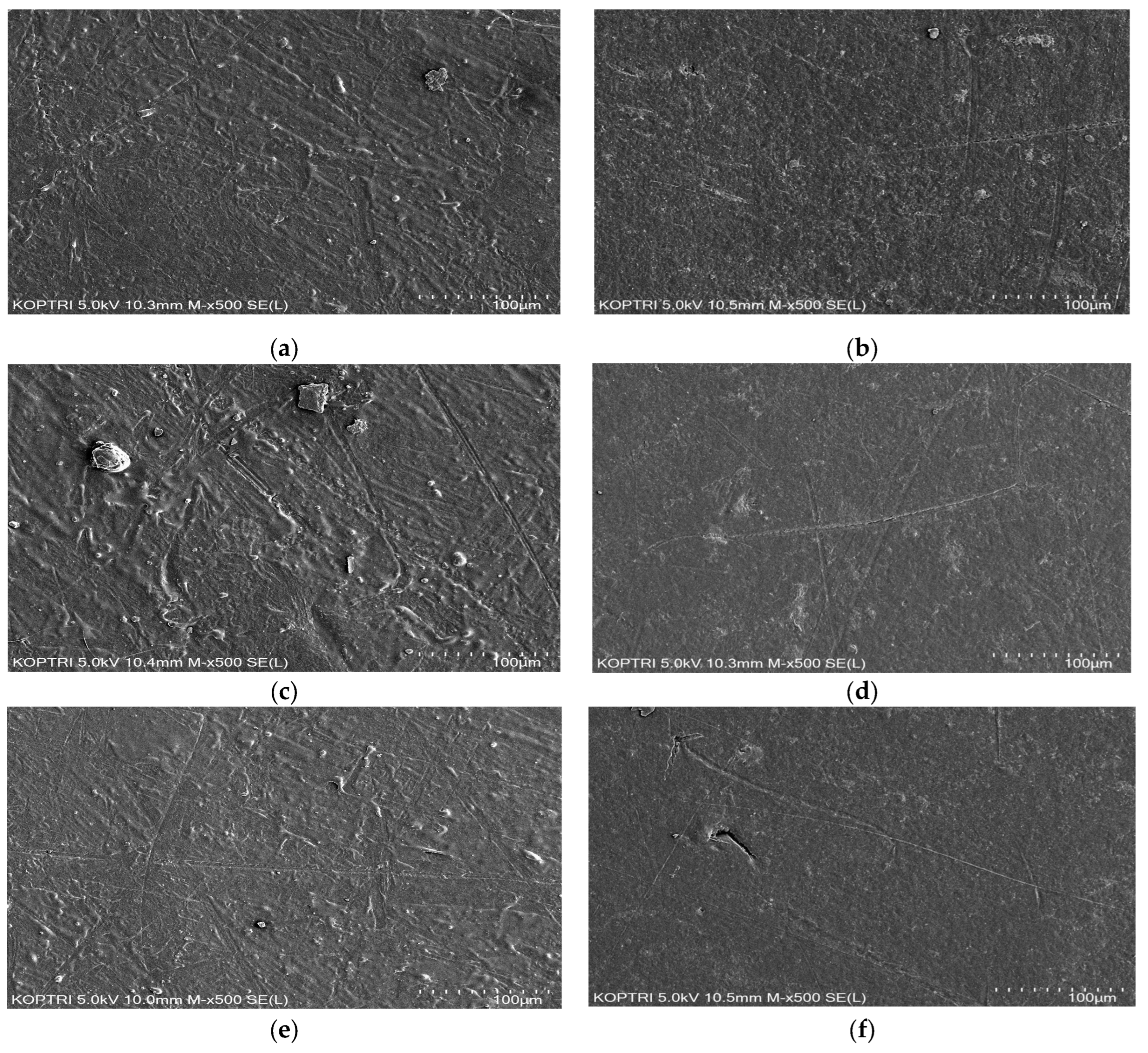
3.4. Measurements and Surface Analysis
4. Test Results
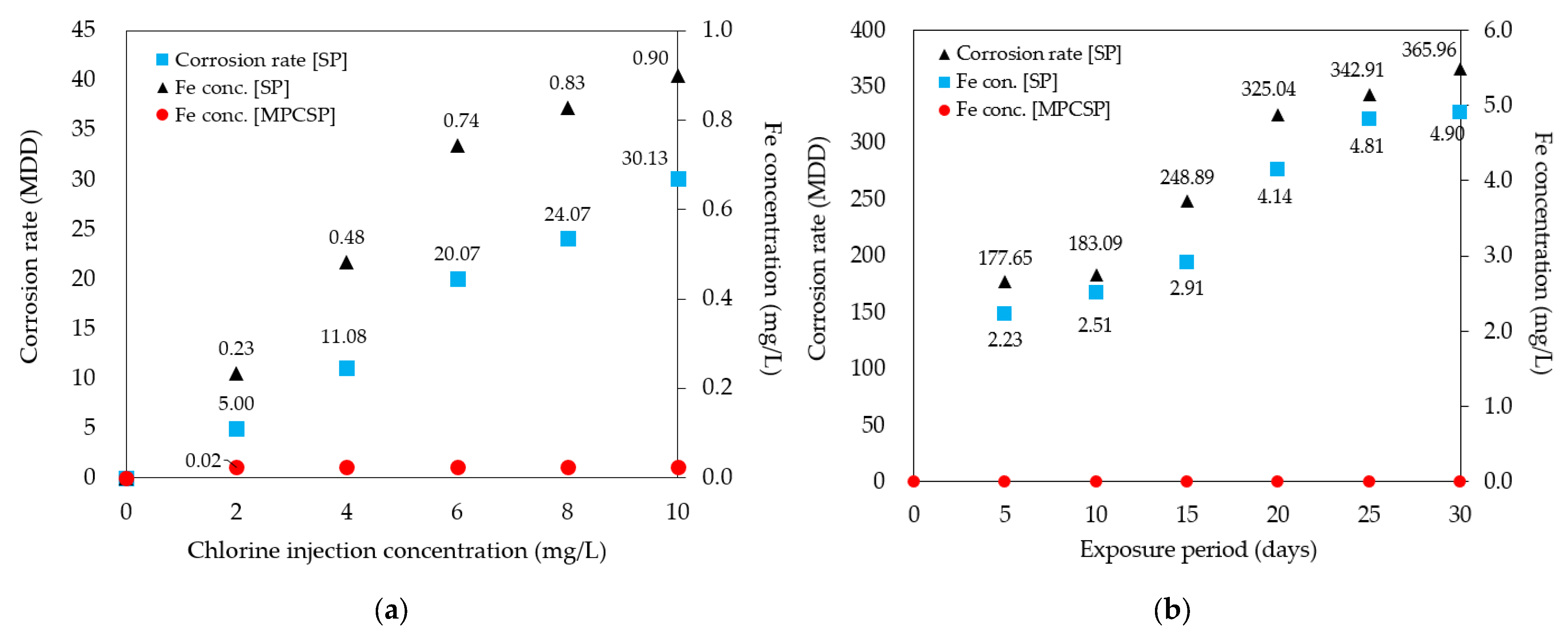
4.1. Effect of Coating Type on Measured Corrosion Rate and Fe Concentration
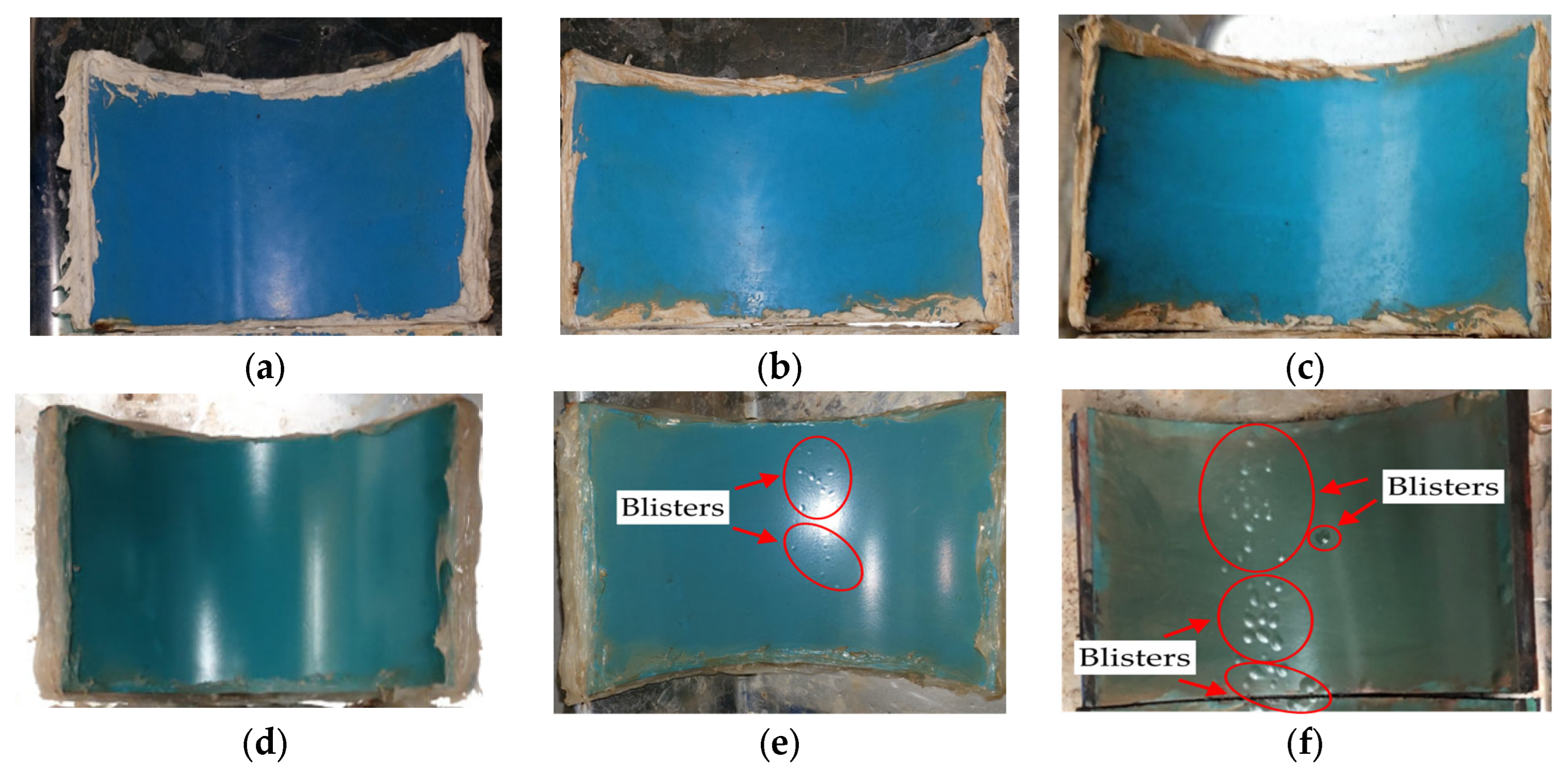
4.2. Effect of Coating Type on Blister Formation
5. Limitations and Future Studies
6. Conclusions
- MPCSPs demonstrated improved corrosion resistance compared to standard steel pipes (SPs), effectively preventing iron leaching and maintaining structural integrity under chlorine exposure.
- The modified polyethylene coating exhibited robust resistance to environmental stressors such as moisture and temperature variations, ensuring stable performance without significant degradation.
- MPCSPs did not develop blisters even after 100 days of immersion, whereas ECSPs began showing blisters as early as 50 days.
- The three-layer coating system effectively prevented blister formation, outperforming conventional epoxy resin coatings.
- The study highlights the potential of modified polyethylene to enhance both the chemical and physical properties of coatings, promoting more advanced and sustainable solutions.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomson, I.; Saithala, J.R. Review of pipeline coating systems from an operator’s perspective. Corros. Eng. Sci. Technol. 2016, 51, 118–135. [Google Scholar] [CrossRef]
- Chiong, S.; Goh, P.; Ismail, A. Novel hydrophobic PVDF/APTES-GO nanocomposite for natural gas pipelines coating. J. Nat. Gas Sci. Eng. 2017, 42, 190–202. [Google Scholar] [CrossRef]
- Samimi, A. Use of Polyurethane Coating to Prevent Corrosion in Oil and Gas Pipelines Transfer. Int. J. Innov. Appl. Stud. 2012, 1, 186–193. [Google Scholar]
- Sridhar, N.; Dunn, D.; Anderko, A.; Lencka, M.; Schutt, H. Effects of water and gas compositions on the internal corrosion of gas pipelines-modeling and experimental studies. Corrosion 2001, 57, 221–235. [Google Scholar] [CrossRef]
- García-Ávila, F.; Del Pino, L.F.; Bonifaz, G.A.; Zhindòn-Arévalo, C.; Ramos-Fernàndez, L.; Garcìa-Altamirano, D.; Sanchez, C. Effect of chlorine residual on copper pipes in drinking water systems. J. Eng. Sci. Technol. Rev. 2019, 12, 119–126. [Google Scholar] [CrossRef]
- Cantor, A.F.; Park, J.K.; Vaiyavatjamai, P. The effect of chlorine on corrosion in drinking water systems. J. Am. Water Work. Assoc. AWWA 2003, 95, 112–123. [Google Scholar] [CrossRef]
- Guidetti, G.P.; Rigosi, G.L.; Marzola, R. The use of polypropylene in pipeline coatings. Prog. Org. Coat. 1996, 27, 79–85. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Shi, A.; Luo, X.; Lin, J.; Zheng, R.; Fan, H.; Selasie, S.V.; Lin, H. A Facile Method to Modify Polypropylene Membrane by Polydopamine Coating via Inkjet Printing Technique for Superior Performance. J. Colloid Interface Sci. 2019, 552, 719–727. [Google Scholar] [CrossRef]
- Himma, N.F.; Anisah, S.; Prasetya, N.; Wenten, I.G. Advances in Preparation, Modification, and Application of Polypropylene Membrane. J. Polym. Eng. 2016, 36, 329–362. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. External corrosion of oil and gas pipelines: A review of failure mechanisms and predictive preventions. J. Nat. Gas Sci. Eng. 2022, 100, 104467. [Google Scholar] [CrossRef]
- Sani, F.M.; Brown, B.; Nesic, S. An Electrochemical Study of the Effect of High Salt Concentration on Uniform Corrosion of Carbon Steel in Aqueous CO2 Solutions. J. Electrochem. Soc. 2021, 168, 051501. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Z.; Lu, Y.; Sun, P. Cause analysis and preventive measures of pipeline corrosion and leakage accident in alkylation unit. Eng. Fail. Anal. 2021, 128, 105623. [Google Scholar] [CrossRef]
- Ashrafriahi, A.; Carcea, A.G.; Newman, R.C. An Inhibitive Effect of Aeration on the Pitting Corrosion of Steels in Ethanolic Environments. Corrosion 2022, 78, 181–188. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, X.; Dong, L.; Liu, G.; Huang, Y.; Xu, Y. On the cavitation erosion-corrosion of pipeline steel at different locations of Venturi pipe. Eng. Fail. Anal. 2022, 138, 106333. [Google Scholar] [CrossRef]
- Ormellese, M.; Beretta, S.; Brugnetti, F.; Brenna, A. Effects of non-stationary stray current on carbon steel buried pipelines under cathodic protection. Constr. Build. Mater. 2021, 281, 122645. [Google Scholar] [CrossRef]
- Kang, S.J.; Hong, M.S.; Kim, J.G. Method for Mitigating Stray Current Corrosion in Buried Pipelines Using Calcareous Deposits. Materials 2021, 14, 7905. [Google Scholar] [CrossRef]
- Han, K.-S.; Park, J.-H.; Park, Y.-B.; Kim, S.-J.; Kim, H.-D.; Choi, Y.-J.; Choi, I.-c.; Hong, S.-H. Effect of residual chlorine concentration on water pipe corrosion and corrosion control plan. Corros. Sci. Technol. 2018, 17, 12–19. [Google Scholar]
- Kim, K.; Kang, H.; Kim, T.; Iseley, D.T.; Choi, J.; Koo, J. Influencing factors analysis for drinking water steel pipe pitting corrosion using artificial neural network. Urban Water J. 2023, 20, 550–563. [Google Scholar] [CrossRef]
- Shein, A. Corrosion-electrochemical behavior of iron family silicides in various electrolytes. Prot. Met. Phys. Chem. 2010, 46, 479–488. [Google Scholar] [CrossRef]
- Kong, L.; Qi, D.; Li, H.; Ding, N.; Ge, P.; Xu, Y.; Zhang, C.; Pan, C.; Fan, X. Aging of polyethylene of raised temperature resistance pipe liner after a four-year service in a crude oil gathering system. J. Fail. Anal. Prev. 2021, 21, 1323–1330. [Google Scholar] [CrossRef]
- Yin, X.; Liang, J.; Gao, Y.; Lin, Z.; Chen, S.; Liu, C.; Tian, K.; Zhang, H.; Tang, G. Effects of LaB6 on the high-temperature oxidation behavior of TiC+TiBx reinforced titanium matrix composite coatings fabricated by laser cladding. Surf. Coat. Technol. 2021, 421, 127445. [Google Scholar] [CrossRef]
- Du, J.; Li, F.; Li, Y.; Lu, H.; Qi, X.; Yang, B.; Li, C.; Yu, P.; Wang, J.; Gao, L. The influence of nano-CeO2 on tribological properties and microstructure evolution of Cr3C2-NiCrCoMo composite coatings at high temperature. Surf. Coat. Technol. 2021, 428, 127913. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Hao, H.; Gao, Q.; Cao, Y.-B.; Han, R.-H.; Qi, H.-B. Strengthening mechanism and high-temperature properties of H13 + WC/Y2O3 laser-cladding coatings. Surf. Coat. Technol. 2021, 405, 126544. [Google Scholar] [CrossRef]
- Rajasärkkä, J.; Pernica, M.; Kuta, J.; Lašňák, J.; Šimek, Z.; Bláha, L. Drinking water contaminants from epoxy resin-coated pipes: A field study. Water Res. 2016, 103, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; el Gouri, M.; Sherif, E.-S.M.; Ebenso, E.E. Epoxy Resins as Anticorrosive Polymeric Materials: A Review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Samimi, A.; Dokhani, S.; Neshat, N.; Almasinia, B.; Setoudeh, M. The application and new mechanism of universal produce the 3-layer polyethylene coating. Int. J. Adv. Sci. Tech. Res. India 2012, 2, 465–473. [Google Scholar]
- Sastri, V.S.; Ghali, E.; Elboujdaini, M. Corrosion Prevention and Protection; Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Fu, A.Q.; Cheng, Y.F. Characterization of the permeability of a high performance composite coating to cathodic protection and its implications on pipeline integrity. Prog. Org. Coat. 2011, 72, 423–428. [Google Scholar] [CrossRef]
- Suárez-Vega, A.; Berriozabal, G.; Urbegain, A.; Minudri, D.; Somers, A.; Forsyth, M.; Marinova, N. Exploring Sustainable Coating Solutions for Applications in Highly Corrosive Environments. Coatings 2024, 14, 521. [Google Scholar] [CrossRef]
- Papavinasam, S.; Attard, M.; Revie, R.W. Evolution of External Pipeline Coatings for Corrosion Protection—A Review. Corros. Rev. 2008, 26, 373–438. [Google Scholar] [CrossRef]
- Samimi, A.; Zarinabadi, S. An Analysis of Polyethylene Coating Corrosion in Oil and Gas Pipelines. Am. J. Sci. 2011, 7, 103201036. [Google Scholar]
- Branch, M.; Mahshahr, I. Study an Analysis and Suggest new Mechanism of 3 layer polyethylene coating corrosion cooling water pipeline in oil refinery in Iran. Int. J. Innov. Appl. Stud. 2012, 1, 216–225. [Google Scholar]
- Lee, S.; Oh, W.; Kim, J. Acceleration and quantitative evaluation of degradation for corrosion protective coatings on buried pipeline: Part II. Application to the evaluation of polyethylene and coal-tar enamel coatings. Prog. Org. Coat. 2013, 76, 784–789. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Park, J.-Y.; Koo, Y.-H. Adhesion property and high-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Lee, M.K.; Cho, S.; Kim, M.O. Experimental Study on the Adhesion and Performance Evaluation of Joints for Modified Polyethylene Coated Steel Pipes. Compos. Res. 2024, 37, 238–245. [Google Scholar]
- Singh, R. Pipeline Integrity: Management and Risk Evaluation; Gulf Professional Publishing: Houston, TX, USA, 2017. [Google Scholar]
- Wang, L.L.; Chen, H.J.; Hao, L.; Lin, A.; Gan, F.X. Electrochemical corrosion behavior of electroless Ni-P coating in NaCl and H2SO4 solutions. Mater. Corros. 2010, 62, 1003–1007. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Mahdi, E.-S.; Alfantazi, A. Electrochemical evaluation of the corrosion behaviour of API-X100 pipeline steel in aerated bicarbonate solutions. Corros. Sci. 2012, 58, 181–191. [Google Scholar] [CrossRef]
- Kumar, S.A.; Alagar, M.; Mohan, V. Studies on corrosion-resistant behavior of siliconized epoxy interpenetrating coatings over mild steel surface by electrochemical methods. J. Mater. Eng. Perform. 2002, 11, 123–129. [Google Scholar] [CrossRef]
- Won, B.; Kim, M.O.; Park, S.; Yi, J.H. Effects of Water Exposure on the Interfacial Bond between an Epoxy Resin Coating and a Concrete Substrate. Materials 2019, 12, 3715. [Google Scholar] [CrossRef]
- Kim, S.; Hong, H.; Han, T.H.; Kim, M.O. Early-age tensile bond characteristics of epoxy coatings for underwater applications. Coatings 2019, 9, 757. [Google Scholar] [CrossRef]
- Kim, S.; Hong, H.; Park, J.K.; Park, S.; Choi, S.I.; Kim, M.O. Effect of exposure conditions on the interfacial bond properties of SS400 plate coated with various epoxy resins. Coatings 2020, 10, 1159. [Google Scholar] [CrossRef]
- Kim, M.O.; Jeong, Y.; Kang, S.H.; Moon, J.; Yi, J.H. Tensile bond characteristics between underwater coating materials and concrete substrate. J. Korean Soc. Coast. Ocean Eng. 2018, 30, 298–305. [Google Scholar] [CrossRef]
- Zargarnezhad, H.; Asselin, E.; Wong, D.; Lam, C.C. A critical review of the time-dependent performance of polymeric pipeline coatings: Focus on hydration of epoxy-based coatings. Polymers 2021, 13, 1517. [Google Scholar] [CrossRef]

| Location | 150A | 600A | 900A | 1200A |
|---|---|---|---|---|
| a * | 1.04 | 4.15 | 6.23 | 8.31 |
| b * | 24.88 | 99.54 | 149.30 | 199.08 |
| Elements | Unit | No Chlorine Injection | Chlorine Injection |
|---|---|---|---|
| C (Carbon) | wt % | 75.925 | 89.225 |
| O (Oxygen) | 16.975 | 8.925 | |
| Na (Sodium) | 0.275 | 0.17 | |
| Al (Aluminum) | 0.075 | 0.085 | |
| Si (Silicon) | 5.1 | 0.325 | |
| Cl (Chlorine) | 0.1 | 0.17 | |
| K (Potassium) | 0.15 | 0.1 | |
| Ca (Calcium) | 0.125 | 0.13 | |
| Ti (Titanium) | 0.6 | 0.5 | |
| Fe (Iron) | 0.725 | 0.53 | |
| Total | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.K.; Kim, D.; Kim, M.O. Experimental Study on the Chlorine-Induced Corrosion and Blister Formation of Steel Pipes Coated with Modified Polyethylene Powder. Polymers 2024, 16, 2415. https://doi.org/10.3390/polym16172415
Lee MK, Kim D, Kim MO. Experimental Study on the Chlorine-Induced Corrosion and Blister Formation of Steel Pipes Coated with Modified Polyethylene Powder. Polymers. 2024; 16(17):2415. https://doi.org/10.3390/polym16172415
Chicago/Turabian StyleLee, Myung Kue, Dongchan Kim, and Min Ook Kim. 2024. "Experimental Study on the Chlorine-Induced Corrosion and Blister Formation of Steel Pipes Coated with Modified Polyethylene Powder" Polymers 16, no. 17: 2415. https://doi.org/10.3390/polym16172415







