The Effect of Rubber–Metal Interactions on the Mechanical, Magneto–Mechanical, and Electrical Properties of Iron, Aluminum, and Hybrid Filler-Based Styrene–Butadiene Rubber Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
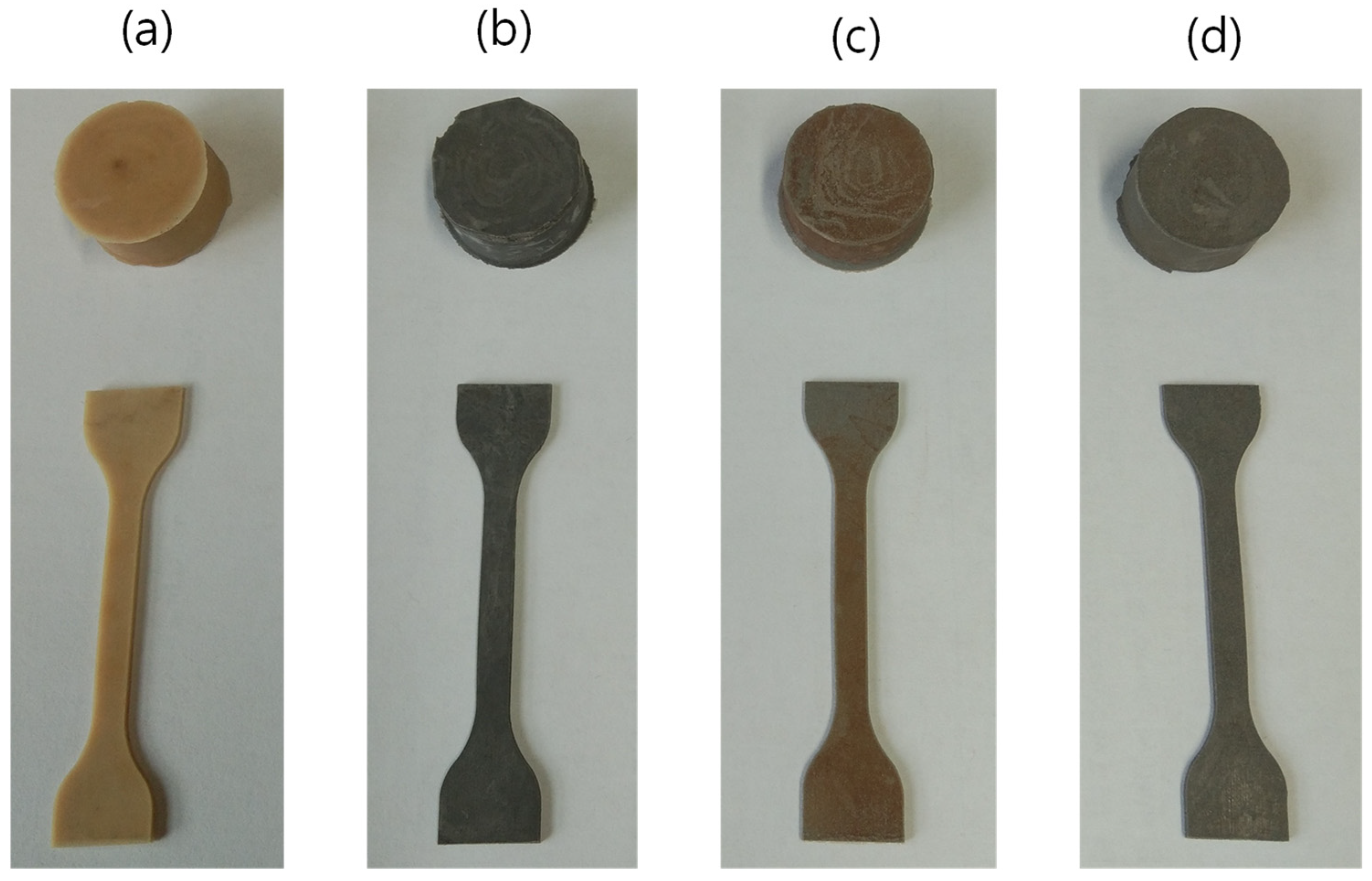
2.2. Fabrication of Rubber Composites
2.3. Measurements of the Mechanical Properties
2.4. Magneto-Mechanical Properties
2.5. Electrical Properties
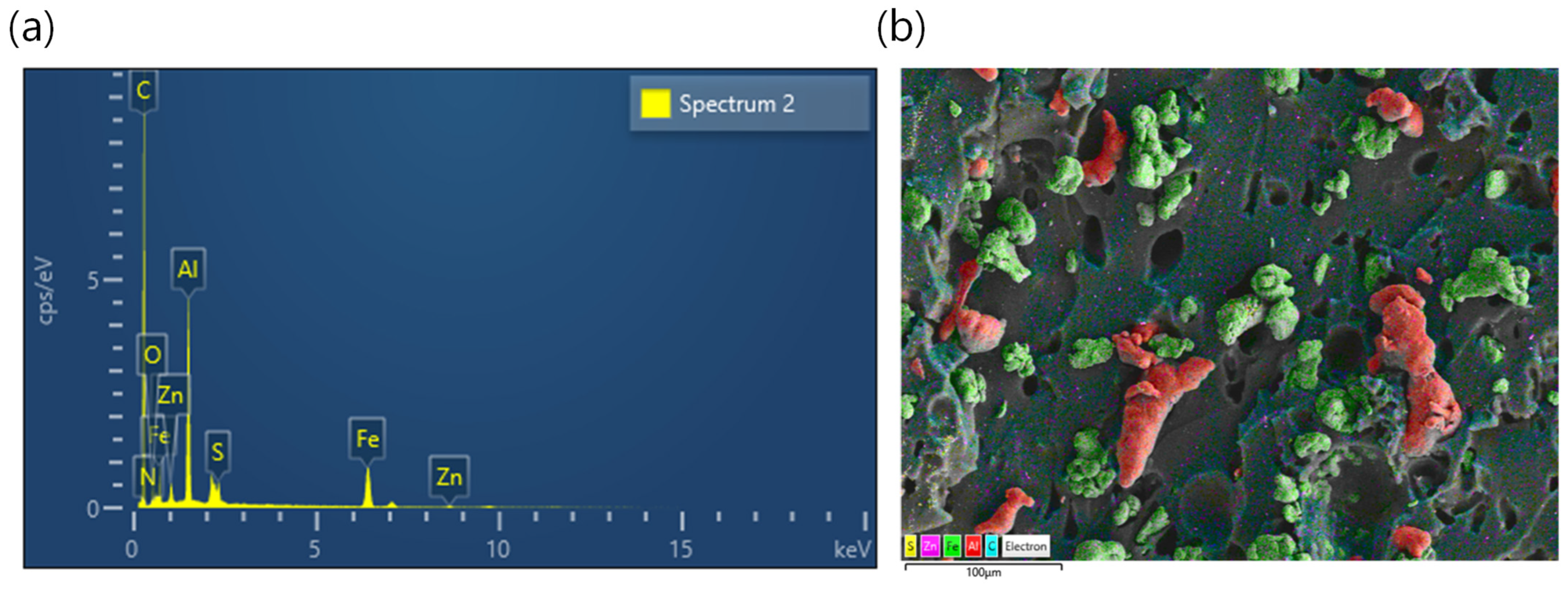
2.6. Filler Dispersion
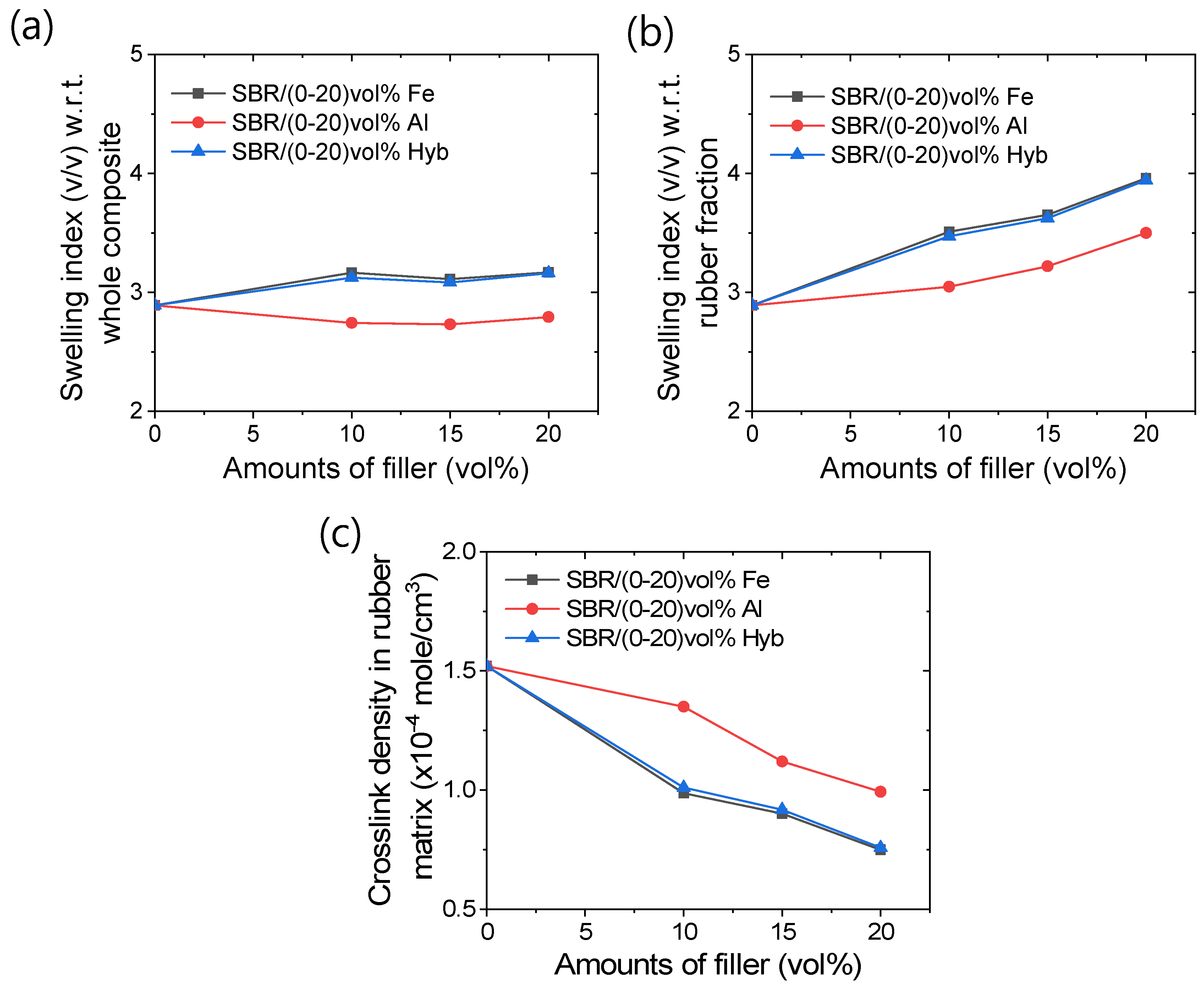
2.7. Swelling Studies and Crosslink Density Measurements
3. Results and Discussion
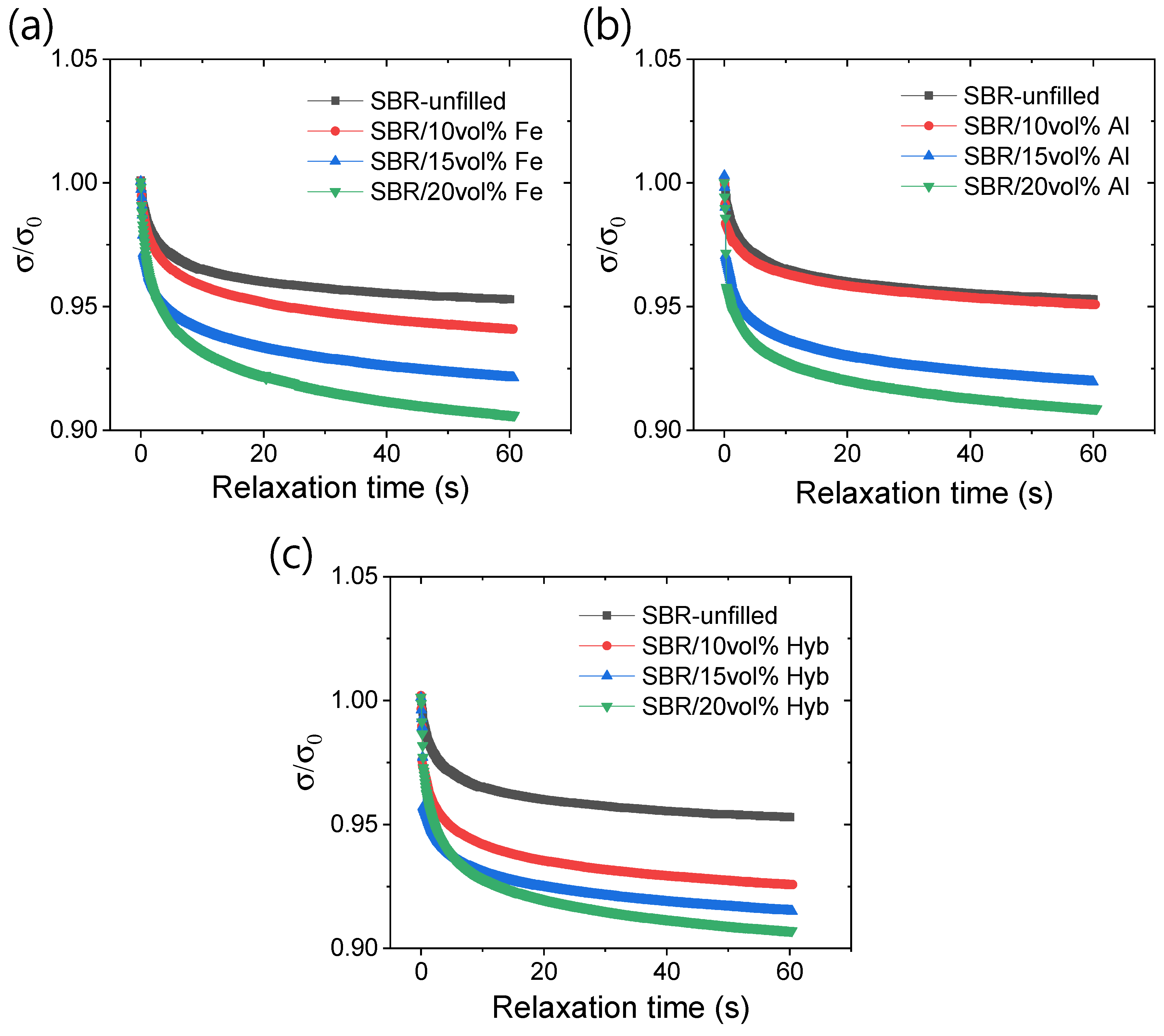
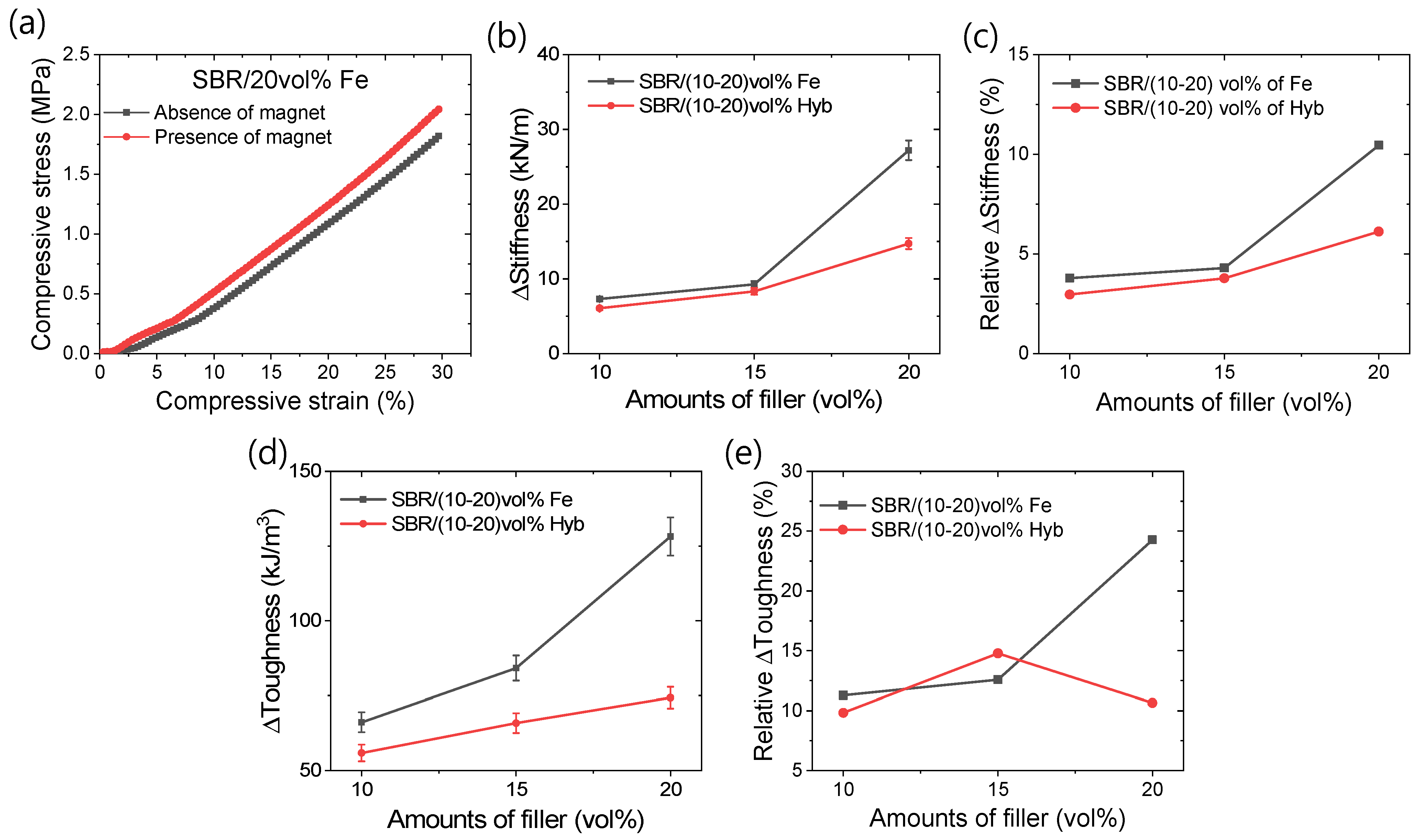
3.1. Mechanical Properties
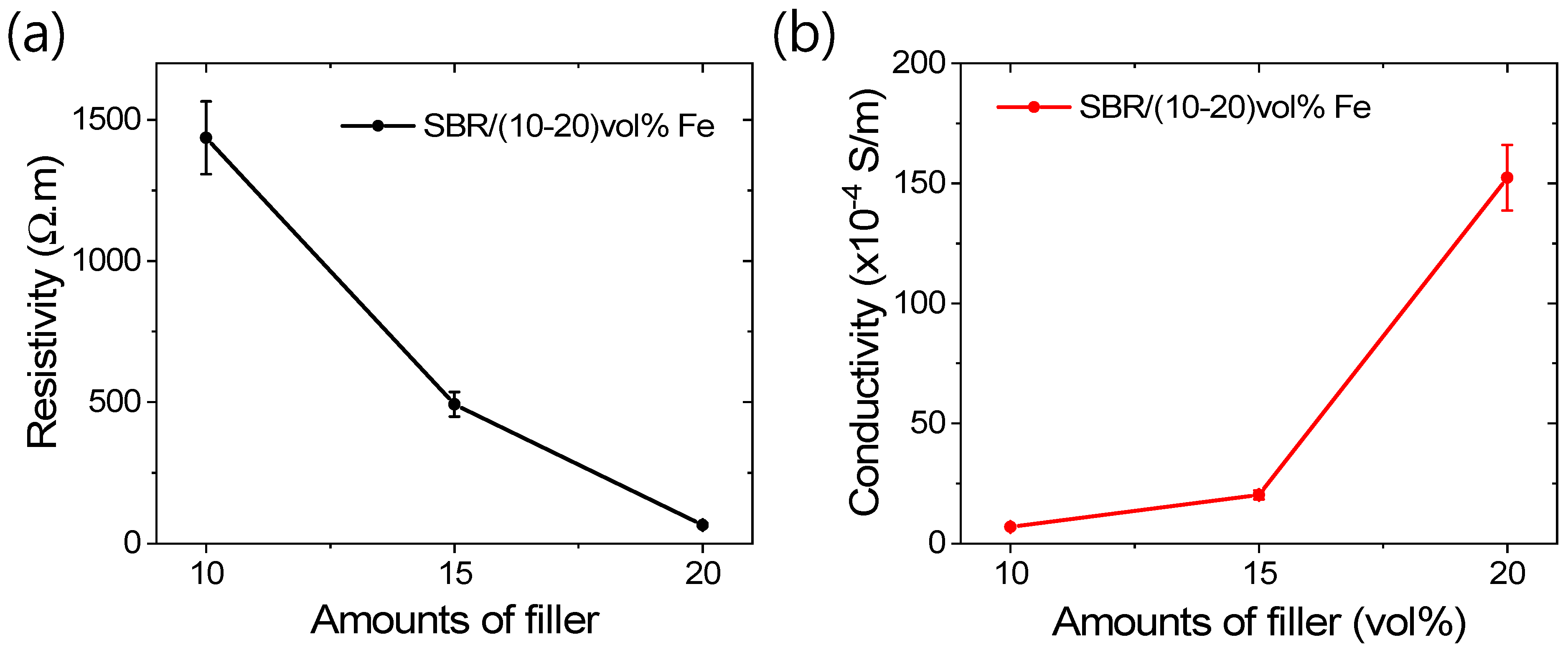
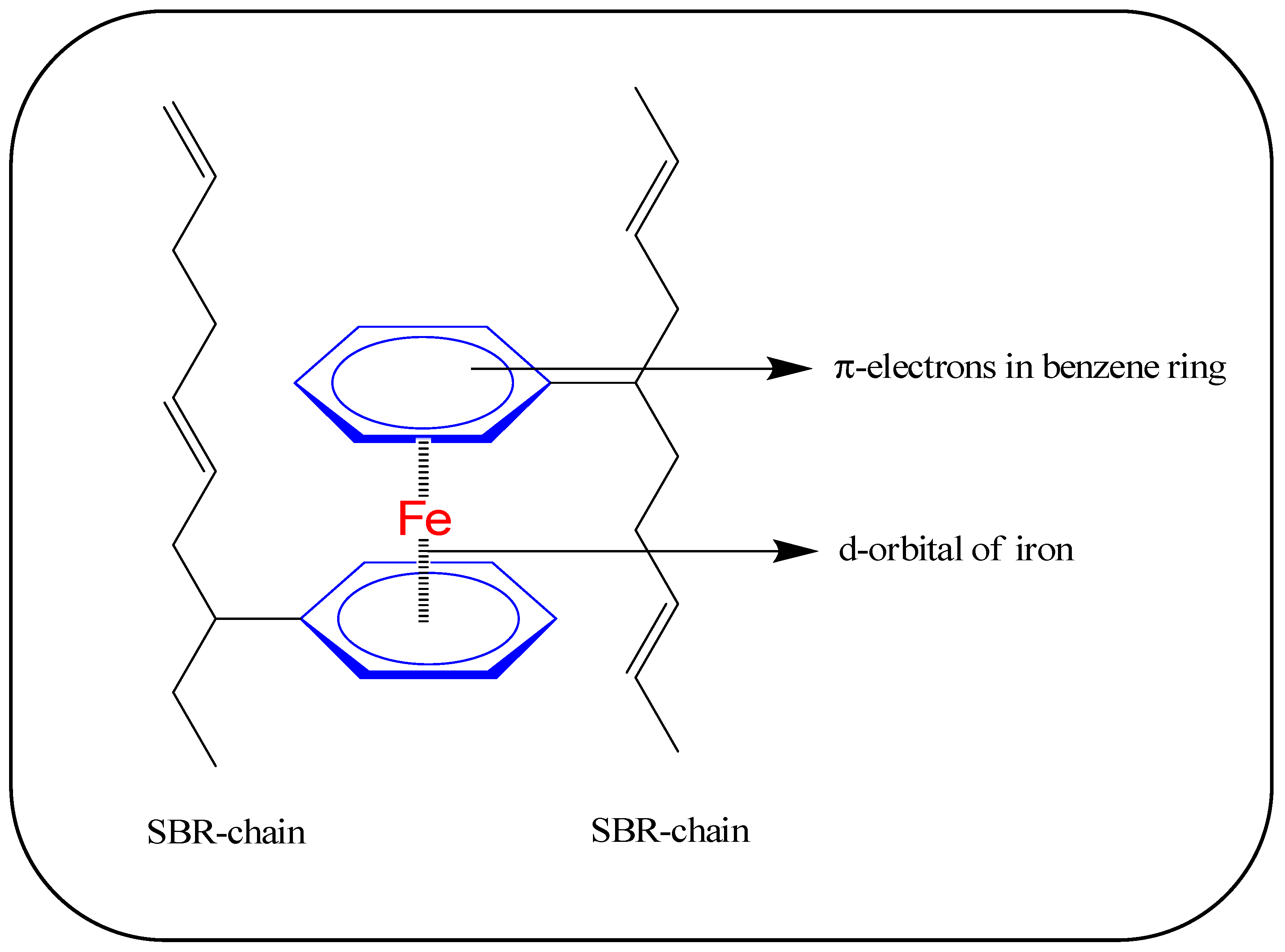
3.2. Magneto-Mechanical and Electrical Properties
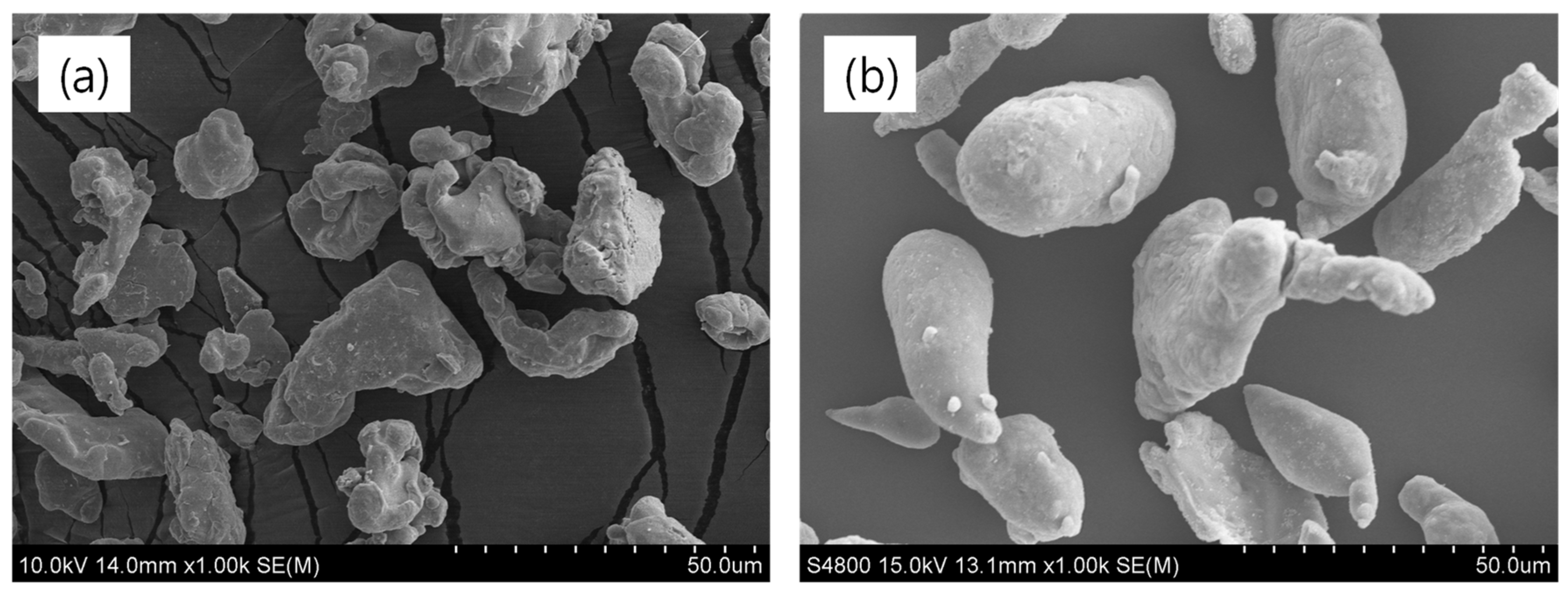
3.3. Morphology of Rubber Composites
3.4. Swelling and Chemical Crosslink Densities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pothen, L.A.; Chan, C.H.; Thomas, S. Natural Rubber Materials, Volume 2-Composites and Nanocomposites; Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Umoren, S.A.; Solomon, M.M. Protective Polymeric Films for Industrial Substrates: A Critical Review on Past and Recent Applications with Conducting Polymers and Polymer Composites/Nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar]
- Al-Sehemi, A.G.; Al-Ghamdi, A.A.; Dishovsky, N.T.; Malinova, P.A.; Atanasov, N.T.; Atanasova, G.L. Analysis of the Electrical and Magnetic Properties of Elastomeric Composites and Their Applicability in Small Flexible Wearable Antennas. Mater. Res. Express 2017, 4, 076304. [Google Scholar] [CrossRef]
- Marin, C.N.; Malaescu, I.; Sfirloaga, P.; Teusdea, A. Electric and Magnetic Properties of a Composite Consisting of Silicone Rubber and Ferrofluid. J. Ind. Eng. Chem. 2021, 101, 405–413. [Google Scholar] [CrossRef]
- Kruželák, J.; Kvasničáková, A.; Džuganová, M.; Dosoudil, R.; Hudec, I.; Krump, H. The Electrical Conductivity, EMI Absorption Shielding Performance, Curing Process, and Mechanical Properties of Rubber Composites. Polymers 2024, 16, 566. [Google Scholar] [CrossRef]
- Boudenne, A.; Mamunya, Y.; Levchenko, V.; Garnier, B.; Lebedev, E. Improvement of Thermal and Electrical Properties of Silicone–Ni Composites Using Magnetic Field. Eur. Polym. J. 2015, 63, 11–19. [Google Scholar] [CrossRef]
- Wu, S.; Hu, W.; Ze, Q.; Sitti, M.; Zhao, R. Multifunctional Magnetic Soft Composites: A Review. Multifunct. Mater. 2020, 3, 042003. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, A.A.; Yunus, N.A.; Mazlan, S.A.; Shabdin, M.K.; Abdul Aziz, S.A.; Nordin, N.A.; Nazmi, N.; Abdul Rahman, M.A. Rheological and Resistance Properties of Magnetorheological Elastomer with Cobalt for Sensor Application. Appl. Sci. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Böse, H.; Gerlach, T.; Ehrlich, J. Magnetorheological Elastomers—An Underestimated Class of Soft Actuator Materials. J. Intell. Mater. Syst. Struct. 2021, 32, 1550–1564. [Google Scholar] [CrossRef]
- Bastola, A.K.; Hossain, M. A Review on Magneto-Mechanical Characterizations of Magnetorheological Elastomers. Compos. Part B Eng. 2020, 200, 108348. [Google Scholar] [CrossRef]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.B.; Pasha, S.K. Recent Advances in Electromagnetic Interference Shielding Properties of Metal and Carbon Filler Reinforced Flexible Polymer Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Wu, W.; Yang, T.; Zhang, Y.; Wang, F.; Nie, Q.; Ma, Y.; Cao, X.; Wang, Z.L.; Wang, N.; Zhang, L. Application of Displacement-Current-Governed Triboelectric Nanogenerator in an Electrostatic Discharge Protection System for the Next-Generation Green Tire. ACS Nano 2019, 13, 8202–8212. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, S.; Lee, S.; Seo, J.; Lee, J.; Son, S.; Cho, H.J.; Algadi, H.; Al-Sayari, S.; Kim, D.E.; et al. Ag Nanowire Reinforced Highly Stretchable Conductive Fibers for Wearable Electronics. Adv. Funct. Mater. 2015, 25, 3114–3121. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, Y.; Wang, S.; Zhou, G.; Zhao, N.; Wong, C.P. A Highly Stretchable and Conductive Composite Based on an Emulsion-Templated Silver Nanowire Aerogel. J. Mater. Chem. A 2020, 8, 1724–1730. [Google Scholar] [CrossRef]
- Yun, G.; Tang, S.Y.; Lu, H.; Zhang, S.; Dickey, M.D.; Li, W. Hybrid-Filler Stretchable Conductive Composites: From Fabrication to Application. Small Sci. 2021, 1, 2000080. [Google Scholar] [CrossRef]
- Vinod, V.S.; Varghese, S.; Kuriakose, B. Degradation Behaviour of Natural Rubber–Aluminium Powder Composites: Effect of Heat, Ozone and High Energy Radiation. Polym. Degrad. Stab. 2002, 75, 405–412. [Google Scholar] [CrossRef]
- Vinod, V.S.; Varghese, S.; Kuriakose, B. Aluminium Powder Filled Nitrile Rubber Composites: Effect of Bonding Agents. Kautsch. Gummi Kunstst. 2004, 57, 641–644. [Google Scholar]
- Anuar, J.; Mariatti, M.; Ismail, H. Properties of Aluminium and Zinc-Filled Natural Rubber Composites. Polym. Plast. Technol. Eng. 2007, 46, 667–674. [Google Scholar] [CrossRef]
- Nassar, A.; Yehia, A.A.; El-Sabbagh, S.H. Evaluation of the Physico-Mechanical and Electrical Properties of Styrene-Butadiene Rubber/Aluminum Powder and Styrene-Butadiene Rubber/Cerium Sulfate Composites. Polimery 2015, 60, 100–108. [Google Scholar] [CrossRef]
- Kang, S.S.; Choi, K.; Nam, J.D.; Choi, H.J. Magnetorheological Elastomers: Fabrication, Characteristics, and Applications. Materials 2020, 13, 4597. [Google Scholar] [CrossRef] [PubMed]
- Mamunya, Y.P.; Davydenko, V.V.; Pissis, P.; Lebedev, E.V. Electrical and Thermal Conductivity of Polymers Filled with Metal Powders. Eur. Polym. J. 2002, 38, 1887–1897. [Google Scholar] [CrossRef]
- Shen, X.Z.; Xie, S.M.; Guo, J.; Liu, Z.C. Microwave Absorbing Properties of Ternary Linear Low-Density Polyethylene/Carbonyl Iron Powder/Carbon Black Composites. J. Appl. Polym. Sci. 2009, 114, 3434–3439. [Google Scholar] [CrossRef]
- Song, Z.; Xie, J.; Zhou, P.; Wang, X.; Liu, T.; Deng, L. Toughened Polymer Composites with Flake Carbonyl Iron Powders and Their Electromagnetic/Absorption Properties. J. Alloys Compd. 2013, 551, 677–681. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Qiu, T.; Tang, C. Preparation and Characterization of Carbonyl Iron Powder/Millable Polyurethane Elastomer Microwave Absorbing Patch. Polym. Compos. 2014, 35, 1318–1324. [Google Scholar] [CrossRef]
- Amutha Jeevakumari, S.A.; Indhumathi, K.; Arun Prakash, V.R. Role of cobalt nanowire and graphene nanoplatelet on microwave shielding behavior of natural rubber composite in high frequency bands. Polym. Compos. 2020, 41, 4362–4371. [Google Scholar] [CrossRef]
- Darwish, M.S.; Al-Harbi, L.M. The Influence of Self-Heating Iron on the Thermal, Mechanical, and Swelling Properties of PDMS Composites for Organic Solvents Removal. Polymers 2021, 13, 4231. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Kumar, V.; Jo, C.R.; Ryu, S.R.; Lee, D.J.; Park, S.S. Mechanical and magneto-mechanical properties of styrene-butadiene-rubber-based magnetorheological elastomers conferred by novel filler-polymer interactions. Compos. Sci. Technol. 2022, 229, 109669. [Google Scholar] [CrossRef]
- Alam, M.N.; Kumar, V.; Ryu, S.R.; Ko, T.J.; Lee, D.J.; Choi, J. Synergistic magnetorheological NR–NBR elastomer blend with electrolytic iron particles. Rubber Chem. Technol. 2021, 94, 642–656. [Google Scholar] [CrossRef]
- ISO-37; Rubber, Vulcanized or Thermoplastic-Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- Flory, P.J.; Rehner, J., Jr. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler–filler and filler–elastomer interaction on rubber reinforcement. Compos. Part A Appl. Sci. Manuf. 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Youn, I.S.; Kim, D.Y.; Singh, N.J.; Park, S.W.; Youn, J.; Kim, K.S. Intercalation of transition metals into stacked benzene rings: A model study of the intercalation of transition metals into bilayered graphene. J. Chem. Theory Comput. 2012, 8, 99–105. [Google Scholar] [CrossRef]
- Demircan, C.A.; Bozkaya, U. Transition metal cation−π interactions: Complexes formed by Fe2+, Co2+, Ni2+, Cu2+, and Zn2+ binding with benzene molecules. J. Phys. Chem. A 2017, 121, 6500–6509. [Google Scholar] [CrossRef]
- Liao, G.J.; Gong, X.L.; Xuan, S.H.; Kang, C.J.; Zong, L.H. Development of a real-time tunable stiffness and damping vibration isolator based on magnetorheological elastomer. J. Intell. Mater. Syst. Struct. 2012, 23, 25–33. [Google Scholar] [CrossRef]
- Alam, M.N.; Kumar, V.; Lee, D.J.; Choi, J. Magnetically active response of acrylonitrile-butadiene-rubber-based magnetorheological elastomers with different types of iron fillers and their hybrid. Compos. Commun. 2021, 24, 100657. [Google Scholar] [CrossRef]
- Chen, L.; Gong, X.L.; Li, W.H. Damping of magnetorheological elastomers. Chin. J. Chem. Phys. 2008, 21, 581–585. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Li, W.; Du, H. A state-of-the-art review on magnetorheological elastomer devices. Smart Mater. Struct. 2014, 23, 123001. [Google Scholar] [CrossRef]
- Yang, J.; Gong, X.; Deng, H.; Qin, L.; Xuan, S. Investigation on the Mechanism of Damping Behavior of Magnetorheological Elastomers. Smart Mater. Struct. 2012, 21, 125015. [Google Scholar] [CrossRef]
- Alam, M.N.; Debnath, S.C.; Choi, J. Nitrosamine-safe thiuram disulfide and benzothiazole sulfenamide as a synergistic pair of accelerators for the vulcanization of rubber. J. Polym. Sci. 2021, 28, 141. [Google Scholar] [CrossRef]
- Alam, M.N.; Kumar, V.; Jeong, M.; Park, S.S. MgO as a cure modifier for reducing the conventional rubber vulcanization temperature at water’s boiling point with improved vulcanization kinetics. J. Polym. Res. 2024, 31, 317. [Google Scholar] [CrossRef]



| Formulation | Amount of Masterbatch Rubber (vol%) | Amount of Iron (vol%) | Amount of Aluminum (vol%) | Hybrid (1:1 v/v) | |
|---|---|---|---|---|---|
| Amount of Iron (vol%) | Amount of Aluminum (vol%) | ||||
| SBR-unfilled | 23.5 (100) * | - | - | - | |
| SBR/10vol% Fe | 21.15 (90) | 19.68 (10) | - | - | |
| SBR/15vol% Fe | 19.98 (85) | 29.51 (15) | - | - | |
| SBR/20vol% Fe | 18.8 (80) | 39.35 (20) | - | - | |
| SBR/10vol% Al | 21.15 (90) | - | 6.75 (10) | - | |
| SBR/15vol% Al | 19.98 (85) | - | 10.13 (15) | - | |
| SBR/20vol% Al | 18.8 (80) | - | 13.5 (20) | - | |
| SBR/10vol% Hyb | 21.15 (90) | - | - | 9.84 (5) | 3.38 (5) |
| SBR/15vol% Hyb | 19.98 (85) | - | - | 14.76 (7.5) | 5.07 (7.5) |
| SBR/20vol% Hyb | 18.8 (80) | 19.68 (10) | 6.75 (10) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.N.; Kumar, V.; Jeong, S.-U.; Park, S.-S. The Effect of Rubber–Metal Interactions on the Mechanical, Magneto–Mechanical, and Electrical Properties of Iron, Aluminum, and Hybrid Filler-Based Styrene–Butadiene Rubber Composites. Polymers 2024, 16, 2424. https://doi.org/10.3390/polym16172424
Alam MN, Kumar V, Jeong S-U, Park S-S. The Effect of Rubber–Metal Interactions on the Mechanical, Magneto–Mechanical, and Electrical Properties of Iron, Aluminum, and Hybrid Filler-Based Styrene–Butadiene Rubber Composites. Polymers. 2024; 16(17):2424. https://doi.org/10.3390/polym16172424
Chicago/Turabian StyleAlam, Md Najib, Vineet Kumar, Seok-U Jeong, and Sang-Shin Park. 2024. "The Effect of Rubber–Metal Interactions on the Mechanical, Magneto–Mechanical, and Electrical Properties of Iron, Aluminum, and Hybrid Filler-Based Styrene–Butadiene Rubber Composites" Polymers 16, no. 17: 2424. https://doi.org/10.3390/polym16172424






