Zn-Al Ferrite/Polypyrrole Nanocomposites: Structure and Dielectric and Magnetic Properties for Microwave Applications
Abstract
1. Introduction
2. Experimental Techniques and Procedures
3. Results and Discussion
3.1. XRD Analysis
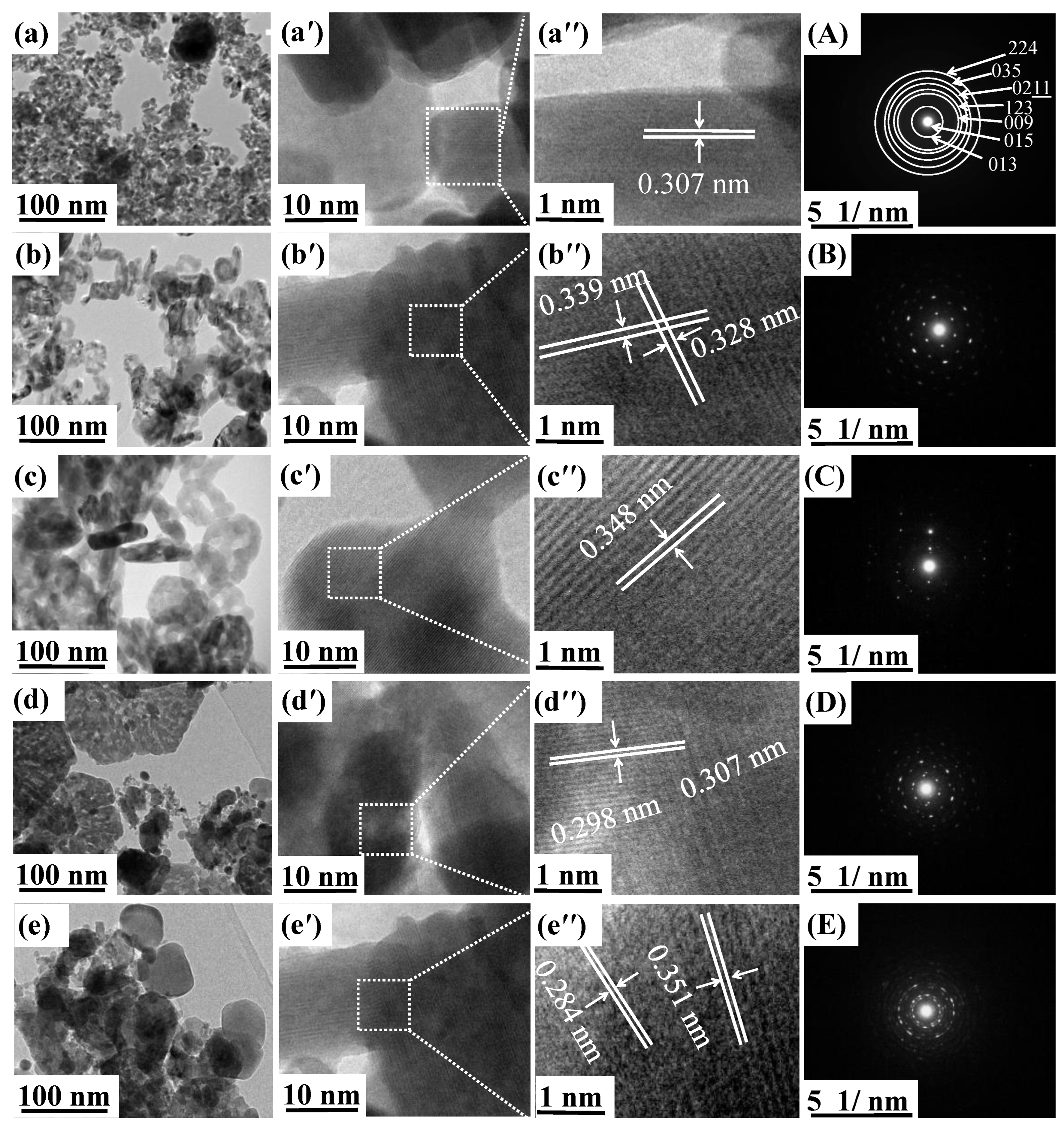
3.2. Morphology
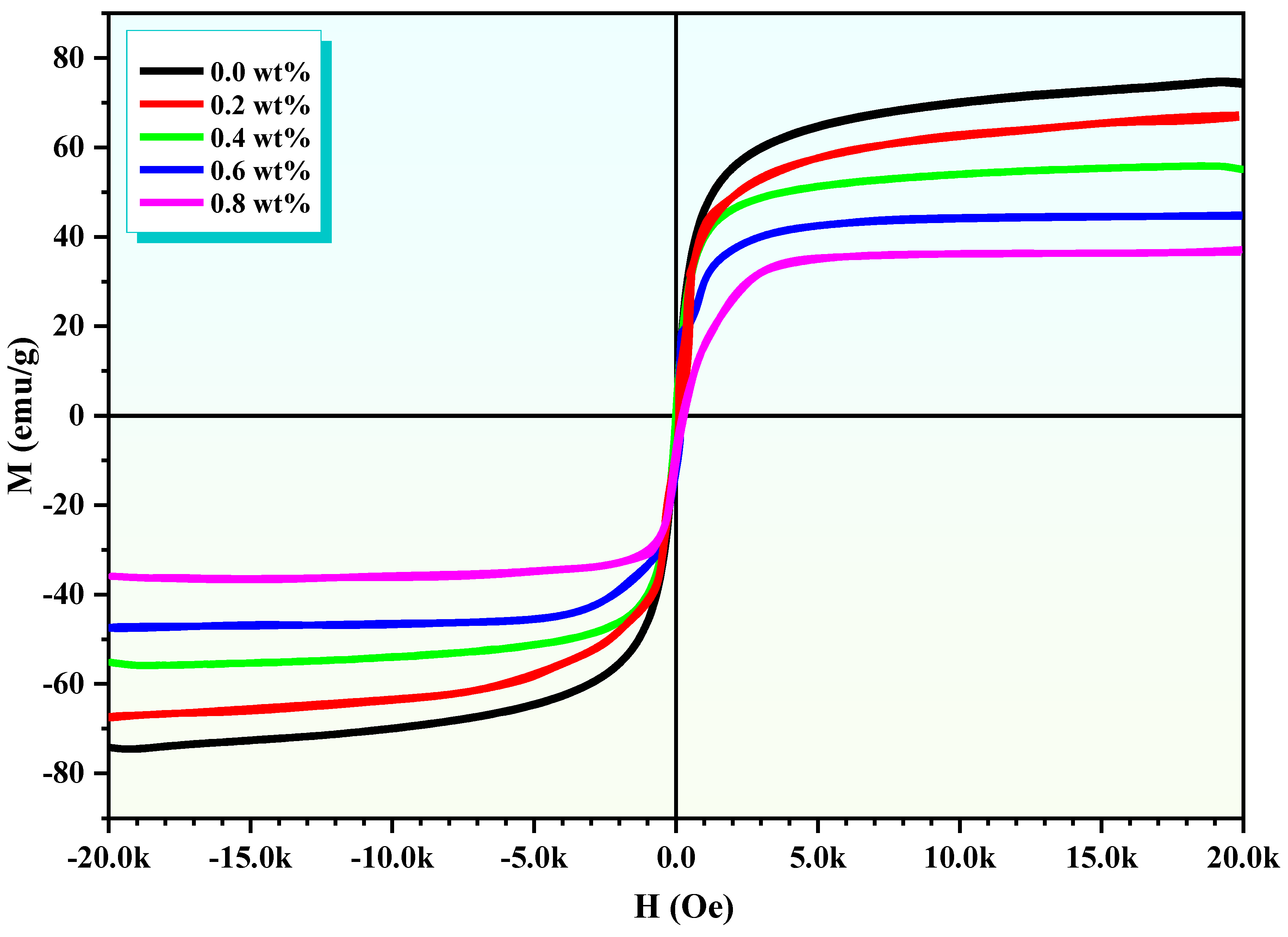
3.3. Magnetic Properties
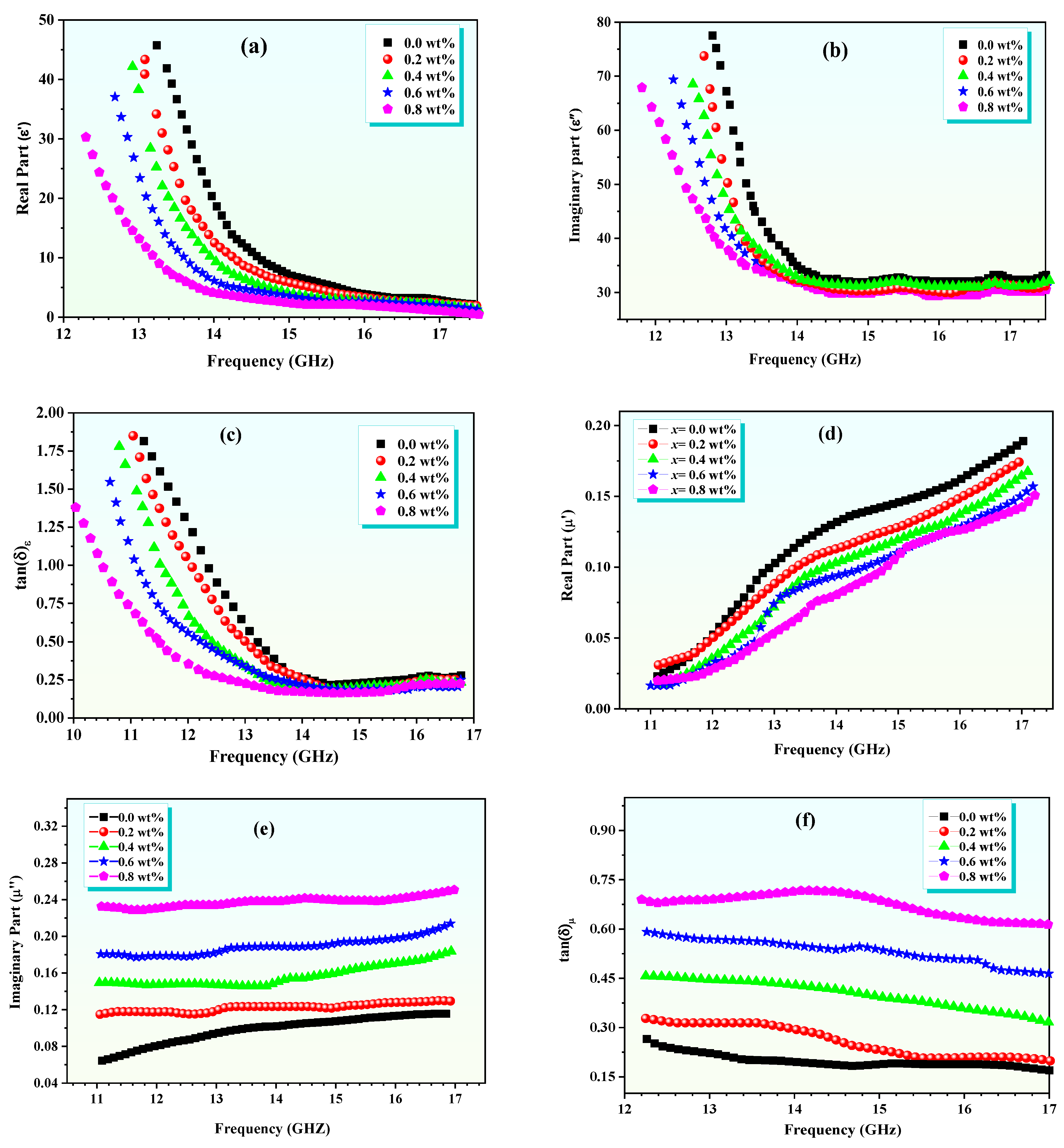
4. Dielectric and Microwave Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmahaishi, M.F.; Azis, R.S.; Ismail, I.; Muhammad, F.D. A review on electromagnetic microwave absorption properties: Their materials and performance. J. Mater. Res. Technol. 2022, 20, 2188–2220. [Google Scholar] [CrossRef]
- Klimov, A.S.; Bakeev, I.Y.; Dolgova, A.V.; Kazakov, A.V.; Korablev, N.S.; Zenin, A.A. Features of Electron Beam Processing of Mn-Zn Ferrites in the Fore-Vacuum Pressure Range in Continuous and Pulse Modes. Coatings 2023, 13, 1766. [Google Scholar] [CrossRef]
- Vissurkhanova, Y.A.; Ivanova, N.M.; Soboleva, Y.A.; Muldakhmetov, Z.M.; Abulyaissova, L.K.; Minaev, B.F. Fe-Cu composites preparation using Cu-Zn ferrite and their electrocatalytic application. Mater. Lett. 2023, 333, 133521. [Google Scholar] [CrossRef]
- Joulaei, M.; Hedayati, K.; Ghanbari, D. Investigation of magnetic, mechanical and flame retardant properties of polymeric nanocomposites: Green synthesis of MgFe2O4 by lime and orange extracts. Compos. Part B Eng. 2019, 176, 107345. [Google Scholar] [CrossRef]
- Hajlaoui, M.E.; Dhahri, R.; Hnainia, N.; Benchaabane, A.; Dhahri, E.; Khirouni, K. Conductivity and giant permittivity study of Zn0.5Ni0.5Fe2O4 spinel ferrite as a function of frequency and temperature. RSC Adv. 2019, 9, 32395–32402. [Google Scholar] [CrossRef]
- Ahmad, M.; Amin, K.; Rehman, A.U.; Wahab, A.; Wabaidur, S.M. Detailed investigation of Mn-substituted Zn ferrites for microwave applications up to 6 GHz. Mater. Sci. Technol. 2024, 40, 479–492. [Google Scholar] [CrossRef]
- Ajeel, K.I.; Kareem, Q.S. Synthesis and Characteristics of Polyaniline (PANI) Filled by Graphene (PANI/GR) nano-Films. J. Phys. Conf. Ser. 2019, 1234, 012020. [Google Scholar] [CrossRef]
- Rybicki, T.; Stempien, Z.; Karbownik, I. EMI Shielding and Absorption of Electroconductive Textiles with PANI and PPy Conductive Polymers and Numerical Model Approach. Energies 2021, 14, 7746. [Google Scholar] [CrossRef]
- Mamatha, G.M.; Pradipkumar Dixit RHari Krishna Girish Kumar, S. Polymer based composites for electromagnetic interference (EMI) shielding: The role of magnetic fillers in effective attenuation of microwaves, a review. Hybrid Adv. 2024, 6, 100200. [Google Scholar]
- Khalil, H.F.; Malidarreh, R.B.; Alabsy, M.T.; Hassan, A.M.; El-Khatib, A.M.; Issa, S.A.; Zakaly, H.M. Structural, Morphological, and γ-ray Attenuation properties of m-type hexaferrite BaFe12O19 doped with V2O5, Ce2O3 and Bi2O3 for Radiation Shielding applications. Ceram. Int. 2024, 50, 33771–33780. [Google Scholar] [CrossRef]
- Devi, R.; Patra, J.; Tapadia, K.; Chang, J.-K.; Maharana, T. Arrangement of ZnFe2O4@PPy nanoparticles on carbon cloth for highly efficient symmetric supercapacitor. J. Taiwan Inst. Chem. Eng. 2022, 138, 104474. [Google Scholar] [CrossRef]
- Elahi, A.; Shakoor, A.; Irfan, M.; Mahmood, K.; Niaz, N.A.; Awan, M.S.; Bashir, T. Polypyrrole and its nanocomposites with Zn0.5Ni0.4Cr0.1Fe2O4 ferrite: Preparation and electromagnetic properties. J. Mater. Sci. Mater. Electron. 2016, 27, 6964–6973. [Google Scholar] [CrossRef]
- Khan, M.Z.; Gul, I.H.; Baig, M.M.; Khan, A.N. Comprehensive study on structural, electrical, magnetic and photocatalytic degradation properties of Al3+ ions substituted nickel ferrites nanoparticles. J. Alloys Compd. 2020, 848, 155795. [Google Scholar] [CrossRef]
- Carrara, S. Towards New Efficient Nanostructured Hybrid Materials for ECL Applications. Ph.D. Thesis, Université de Strasbourg, Strasbourg, France, 2017. [Google Scholar]
- Khafagy, R.M. Synthesis, characterization, magnetic and electrical properties of the novel conductive and magnetic Polyani-line/MgFe2O4 nanocomposite having the core–shell structure. J. Alloys Compd. 2021, 509, 9849–9857. [Google Scholar] [CrossRef]
- Ben Farhat, L.; Ben Ahmed, S.; Ezzine, S.; Amami, M. Particle size dependent structural, magnetic and electrical properties of Cr-doped lead-free multiferroic AlFeO3 prepared by co-precipitation and solid state method. Mater. Chem. Phys. 2020, 255, 123631. [Google Scholar] [CrossRef]
- Srinivasulu, T.; Saritha, K.; Reddy, K.R. Synthesis and characterization of Fe-doped ZnO thin films deposited by chemical spray pyrolysis. Mod. Electron. Mater. 2017, 3, 76–85. [Google Scholar] [CrossRef]
- Nigam, A.; Pawar, S. Structural, magnetic, and antimicrobial properties of zinc doped magnesium ferrite for drug delivery applications. Ceram. Int. 2020, 46, 4058–4064. [Google Scholar] [CrossRef]
- Soin, N. Magnetic Nanoparticles—Piezoelectric Polymer Nanocomposites for Energy Harvesting, Magnetic Nanostructured Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–322. [Google Scholar]
- Wan, C.; Bowen, C.R. Multiscale-structuring of polyvinylidene fluoride for energy harvesting: The impact of molecular-, micro- and macro-structure. J. Mater. Chem. A 2017, 5, 3091–3128. [Google Scholar] [CrossRef]
- Wu, Y.; Hsu, S.L.; Honeker, C.; Bravet, D.J.; Williams, D.S. The Role of Surface Charge of Nucleation Agents on the Crystallization Behavior of Poly(vinylidene fluoride). J. Phys. Chem. B 2012, 116, 7379–7388. [Google Scholar] [CrossRef]
- Ma, Y.; Tong, W.; Wang, W.; An, Q.; Zhang, Y. Montmorillonite/PVDF-HFP-based energy conversion and storage films with enhanced piezoelectric and dielectric properties. Compos. Sci. Technol. 2018, 168, 397–403. [Google Scholar] [CrossRef]
- Zhang, S.; Tong, W.; Wang, J.; Wang, W.; Wang, Z.; Zhang, Y. Modified sepiolite/PVDF-HFP composite film with enhanced piezoelectric and dielectric properties. J. Appl. Polym. Sci. 2019, 137, 48412. [Google Scholar] [CrossRef]
- Kusuma, D.Y.; Nguyen, C.A.; Lee, P.S. Enhanced ferroelectric switching characteristics of P(VDF-TrFE) for organic memory devices. J. Phys. Chem. B 2010, 114, 13289–13293. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Hu, N.; Wu, L.; Alamusi; Ning, H.; Wang, Y.; Jin, Z.; Liu, Y. Improvement of the piezoe-lectricity of PVDF-HFP by CoFe2O4 nanoparticles. Nano Mater. Sci. 2023, 6, 201–210. [Google Scholar] [CrossRef]
- Durgaprasad, P.; Hemalatha, J. Magnetoelectric investigations on poly (vinylidene fluoride)/CoFe2O4 flexible electrospun membranes. J. Magn. Magn. Mater. 2018, 448, 94–99. [Google Scholar] [CrossRef]
- Rothwell, E.J.; Frasch, J.L.; Ellison, S.M.; Chahal, P.; Ouedraogo, R.O. Analysis of the Nicol-son-Ross-Weir Method for Characterizing the Electromagnetic Properties of Engineered Materials. Prog. Electromagn. Res. 2016, 157, 31–47. [Google Scholar] [CrossRef]
- Costa, F.; Borgese, M.; Degiorgi, M.; Monorchio, A. Electromagnetic Characterisation of Materials by Using Transmission/Reflection (T/R) Devices. Electronics 2017, 6, 95. [Google Scholar] [CrossRef]
- Barroso, J.J.; de Paula, A.L. Retrieval of Permittivity and Permeability of Homogeneous Materials from Scattering Parameters. J. Electromagn. Waves Appl. 2010, 24, 1563–1574. [Google Scholar] [CrossRef]
- Indrakanti, R.; Brahmaji Rao, V.; Udaya Kiran, C. Optical parameters of gallium nitride doped ferrite–polypyrrole nano-composites. J. Mater. Sci. Mater. Electron. 2020, 31, 3238–3244. [Google Scholar] [CrossRef]
- Martins, P.; Costa, C.M.; Lanceros-Mendez, S. Nucleation of electroactive β-phase poly(vinilidene fluoride) with CoFe2O4 and NiFe2O4 nanofillers: A new method for the preparation of multiferroic nanocomposites. Appl. Phys. A 2010, 103, 233–237. [Google Scholar] [CrossRef]
- Manohar, A.; Geleta, D.D.; Krishnamoorthi, C.; Lee, J. Synthesis, characterization and magnetic hyperthermia properties of nearly monodisperse CoFe2O4 nanoparticles. Ceram. Int. 2020, 46, 28035–28041. [Google Scholar] [CrossRef]
- Kolhar, P.; Sannakki, B. Investigation of the electrical properties of synthesised polyaniline-magnesium ferrite compo-sites. Mater. Today Proc. 2023, 422, 2214–7853. [Google Scholar] [CrossRef]
- Ge, Y.; Li, C.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. ZnFe2O4@SiO2@Polypyrrole nanocompo-sites with efficient electromagnetic wave absorption properties in the K and Ka band regions. Ceram. Int. 2021, 47, 1728–1739. [Google Scholar] [CrossRef]
- Li, F.; Zhuang, L.; Zhan, W.; Zhou, M.; Sui, G.; Zho, A.; Bai, G.; Xia, W.; Yang, X. Desirable micro-wave absorption performance of ZnFe2O4@ZnO@rGO nanocomposites based on controllable permittivity and permeability. Ceram. Int. 2020, 46, 21744–21751. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, X.; Li, X.; Chen, J.; Hu, P.; Han, J. SiC whiskers-reduced graphene oxide composites decorated with MnO nanoparticles for tunable microwave absorption. Chem. Eng. J. 2020, 392, 123817. [Google Scholar] [CrossRef]
- Liu, P.-J.; Yao, Z.-J.; Ng, V.M.H.; Zhou, J.-T.; Yang, Z.-H.; Kong, L.-B. Enhanced Microwave Absorption Properties of Double-Layer Absorbers Based on Spherical NiO and Co0.2Ni0.4Zn0.4Fe2O4 Ferrite Composites. Acta Met. Sin. 2018, 31, 171–179. [Google Scholar] [CrossRef]
- Choi, M.; Lee, S.; Kim, J. Clustering effect on the frequency-dependent magnetic properties of Fe–Co micro hollow fiber composites. IEEE Trans. Magn. 2017, 53, 1–5. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wu, R.-Z.; Feng, J.; Zhang, J.-H.; Hou, L.-G.; Liu, M.-D. Recent advances of electromagnetic interference shielding Mg matrix materials and their processings: A review. Trans. Nonferrous Met. Soc. China 2022, 32, 1385–1404. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Wu, R.; Xu, L.; Zhang, Z.; Feng, J.; Zhang, J.; Hou, L.; Jiao, Y. X-band shielding properties of Mg−9Li matrix composite containing Ni0.4Zn0.4Co0.2Fe2O4 fabricated by multi-layer composite rolling. J. Alloys Compd. 2020, 843, 156053. [Google Scholar] [CrossRef]
- Kruželák, J.; Kvasničáková, A.; Hložeková, K.; Hudec, I. Progress in polymers and polymer composites used as efficient materials for EMI shielding. Nanoscale Adv. 2021, 3, 123–172. [Google Scholar] [CrossRef]
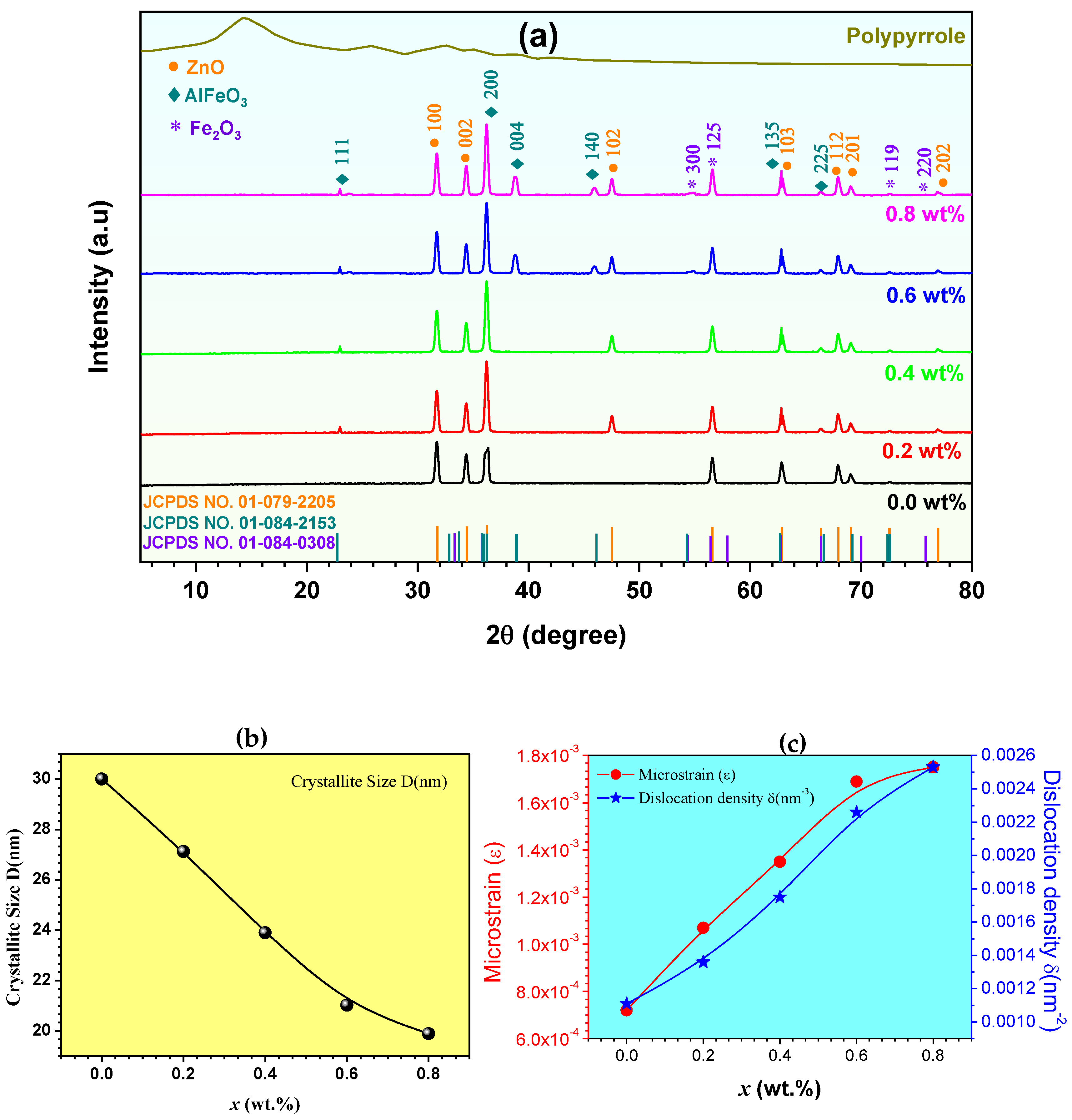


| x (wt.%) | D (nm) | ε | δ (nm−3) |
|---|---|---|---|
| 0.0 | 30.01 | 0.00072 | 0.00111 |
| 0.2 | 27.13 | 0.00107 | 0.00136 |
| 0.4 | 23.90 | 0.00135 | 0.00175 |
| 0.6 | 21.02 | 0.00169 | 0.00226 |
| 0.8 | 19.89 | 0.00175 | 0.00253 |
| x (wt.%) | Ms (emu/g) | Mr (emu/g) | Hc (Oe) | Mr/Ms | μB |
|---|---|---|---|---|---|
| 0.0 | 75 | 2.26 | 38.91 | 0.030 | 10.79 |
| 0.2 | 67 | 3.87 | 38.52 | 0.057 | 9.17 |
| 0.4 | 55 | 2.19 | 38.86 | 0.039 | 7.15 |
| 0.6 | 45 | 1.48 | 39.17 | 0.032 | 5.54 |
| 0.8 | 36 | 2.00 | 39.64 | 0.055 | 4.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, H.F.; Elsharkawy, S.G.; AL-Harby, N.F.; El-Batouti, M. Zn-Al Ferrite/Polypyrrole Nanocomposites: Structure and Dielectric and Magnetic Properties for Microwave Applications. Polymers 2024, 16, 2432. https://doi.org/10.3390/polym16172432
Khalil HF, Elsharkawy SG, AL-Harby NF, El-Batouti M. Zn-Al Ferrite/Polypyrrole Nanocomposites: Structure and Dielectric and Magnetic Properties for Microwave Applications. Polymers. 2024; 16(17):2432. https://doi.org/10.3390/polym16172432
Chicago/Turabian StyleKhalil, Huda F., Sherif G. Elsharkawy, Nouf F. AL-Harby, and Mervette El-Batouti. 2024. "Zn-Al Ferrite/Polypyrrole Nanocomposites: Structure and Dielectric and Magnetic Properties for Microwave Applications" Polymers 16, no. 17: 2432. https://doi.org/10.3390/polym16172432
APA StyleKhalil, H. F., Elsharkawy, S. G., AL-Harby, N. F., & El-Batouti, M. (2024). Zn-Al Ferrite/Polypyrrole Nanocomposites: Structure and Dielectric and Magnetic Properties for Microwave Applications. Polymers, 16(17), 2432. https://doi.org/10.3390/polym16172432









