Applying pH Modulation to Improve the Thermal Stability of Melamine–Formaldehyde Microcapsules Containing Butyl Stearate as a Phase-Change Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
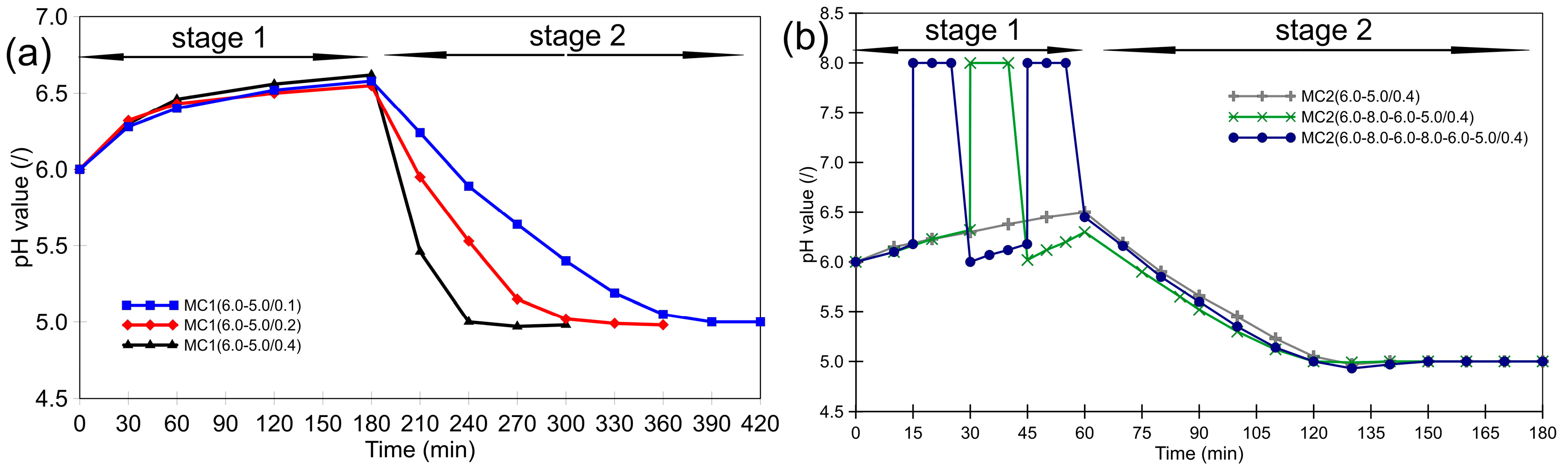
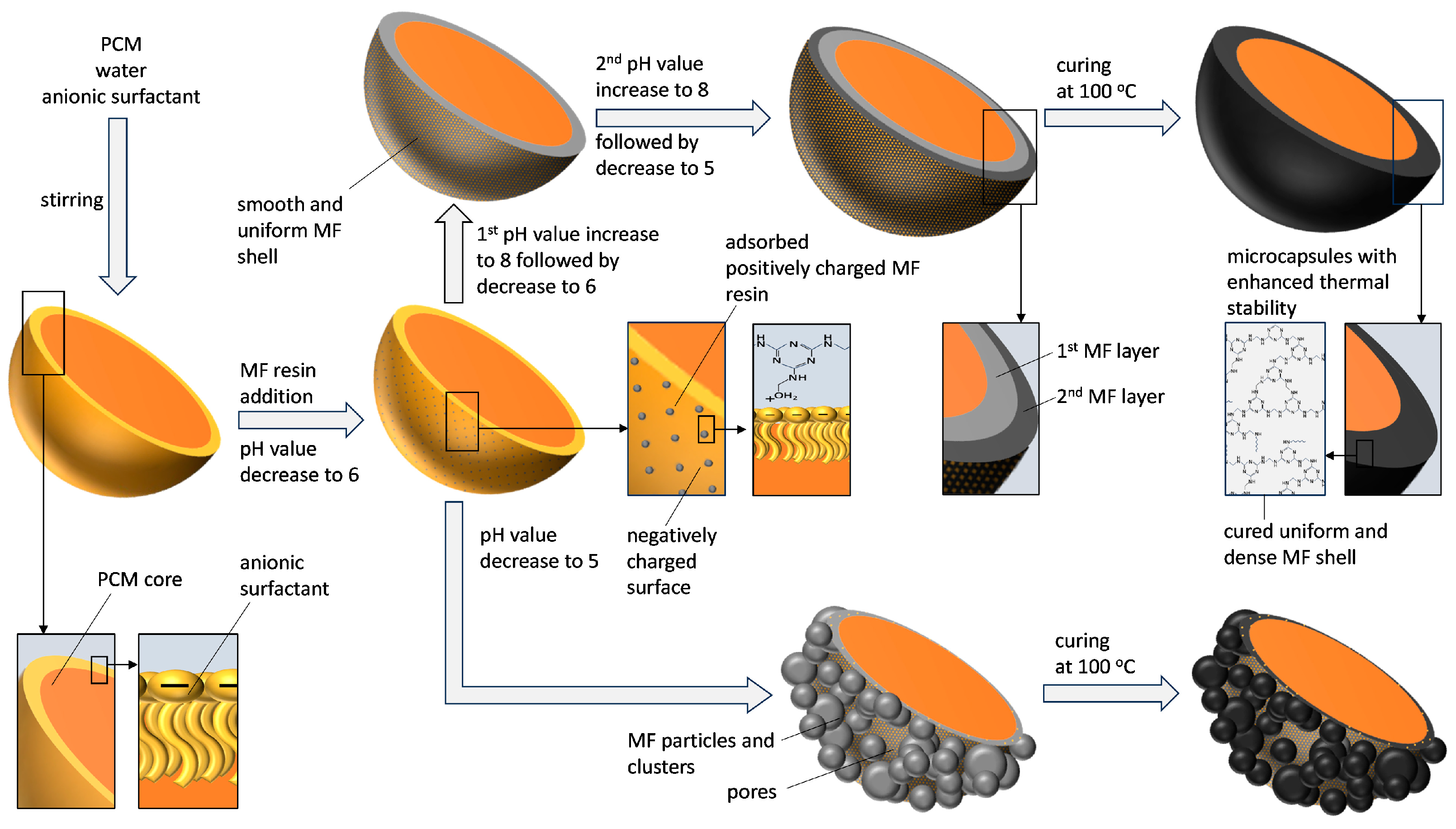
2.2. Preparation of Microcapsules
2.3. Characterization of the Microcapsules
2.3.1. DSC Analysis
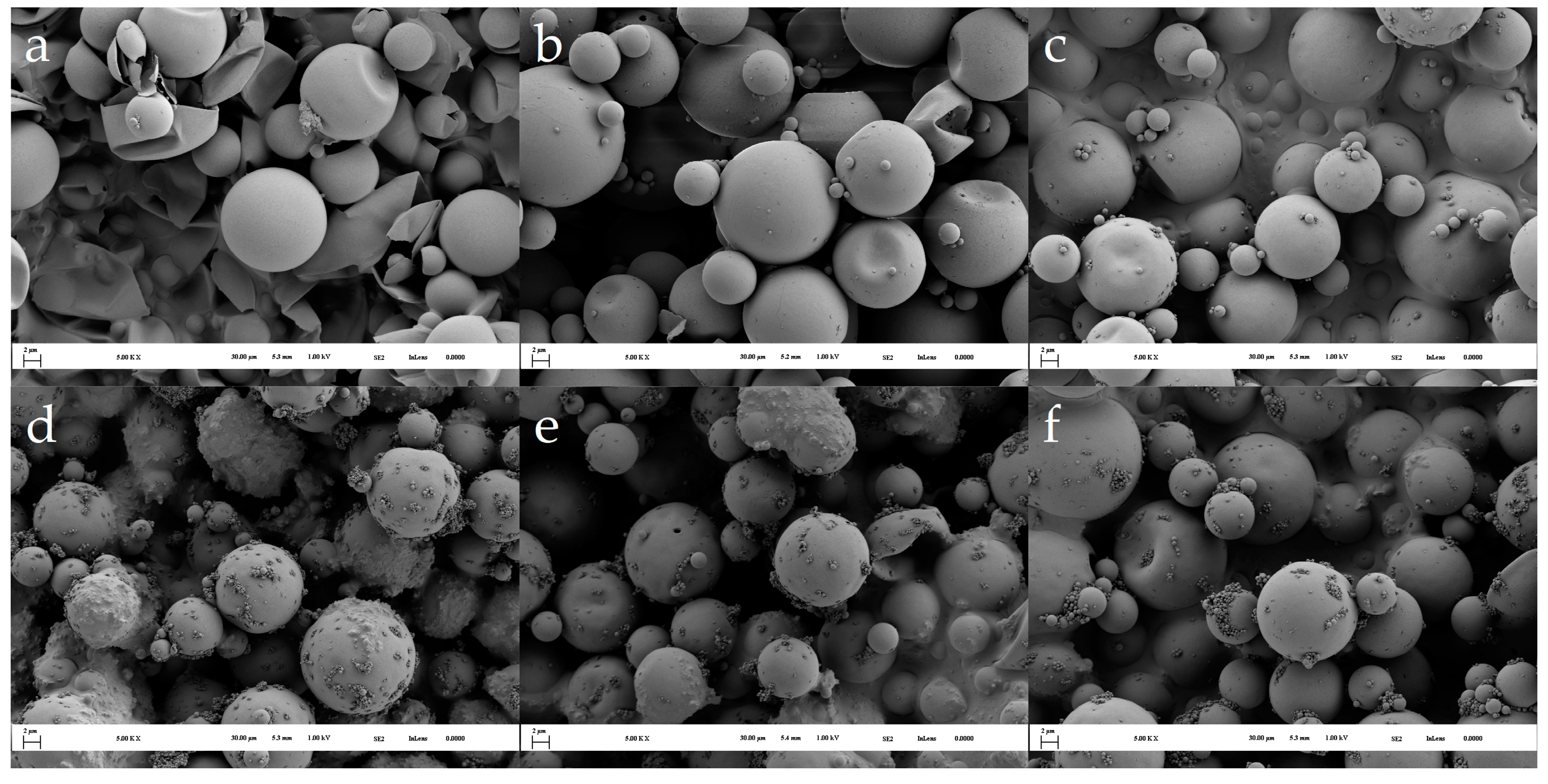
2.3.2. SEM Analysis
2.3.3. Thermogravimetric Analysis
3. Results and Discussion
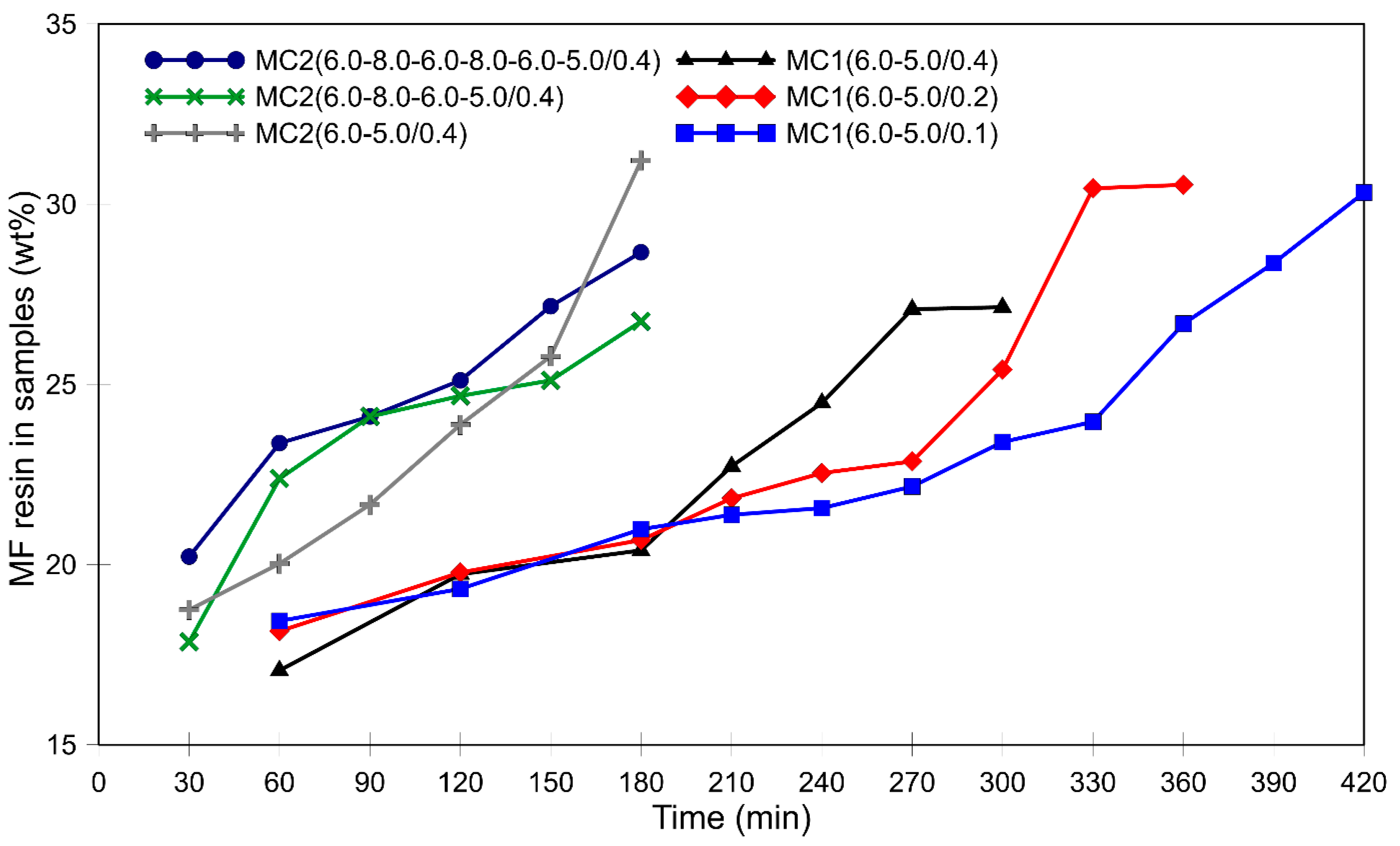
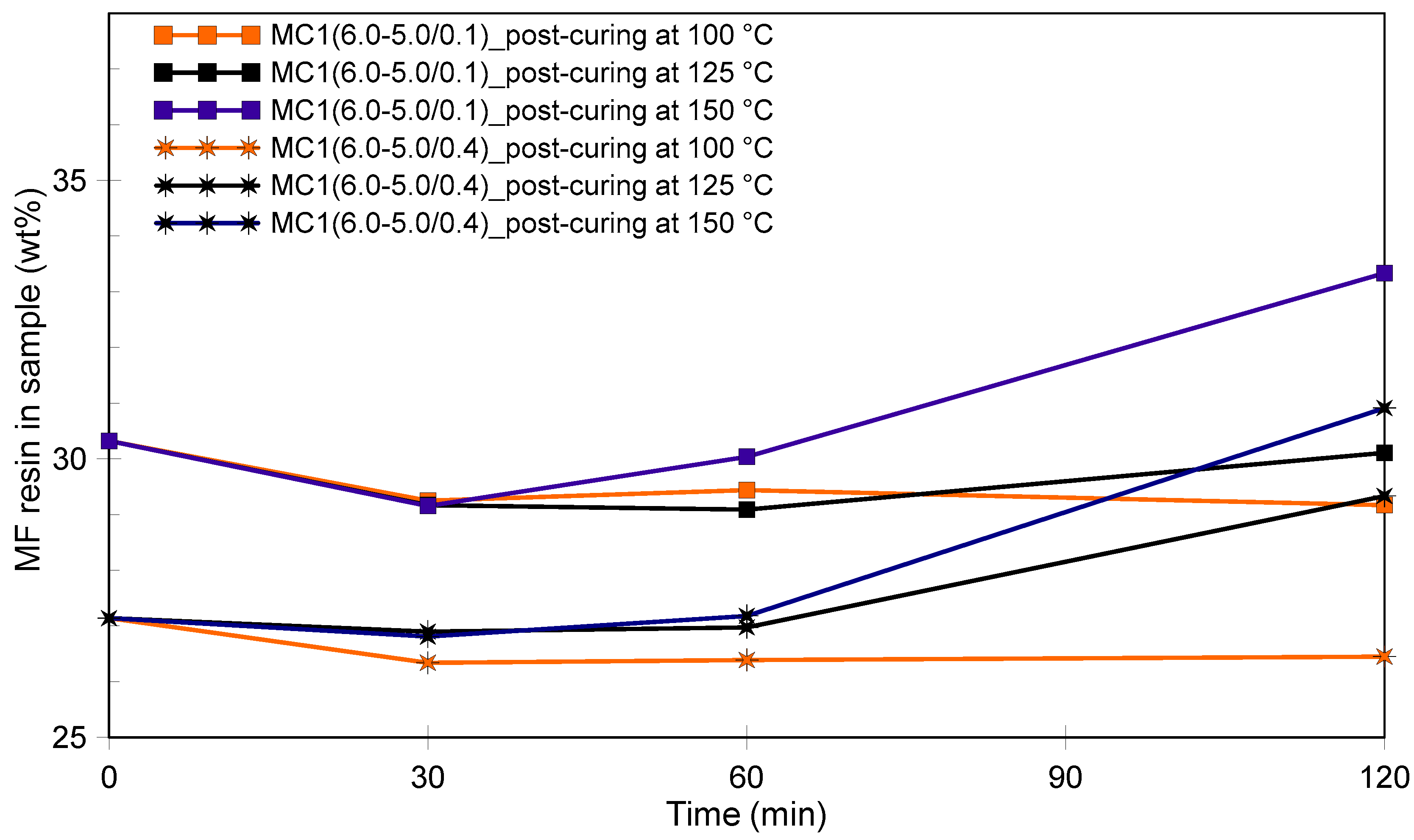
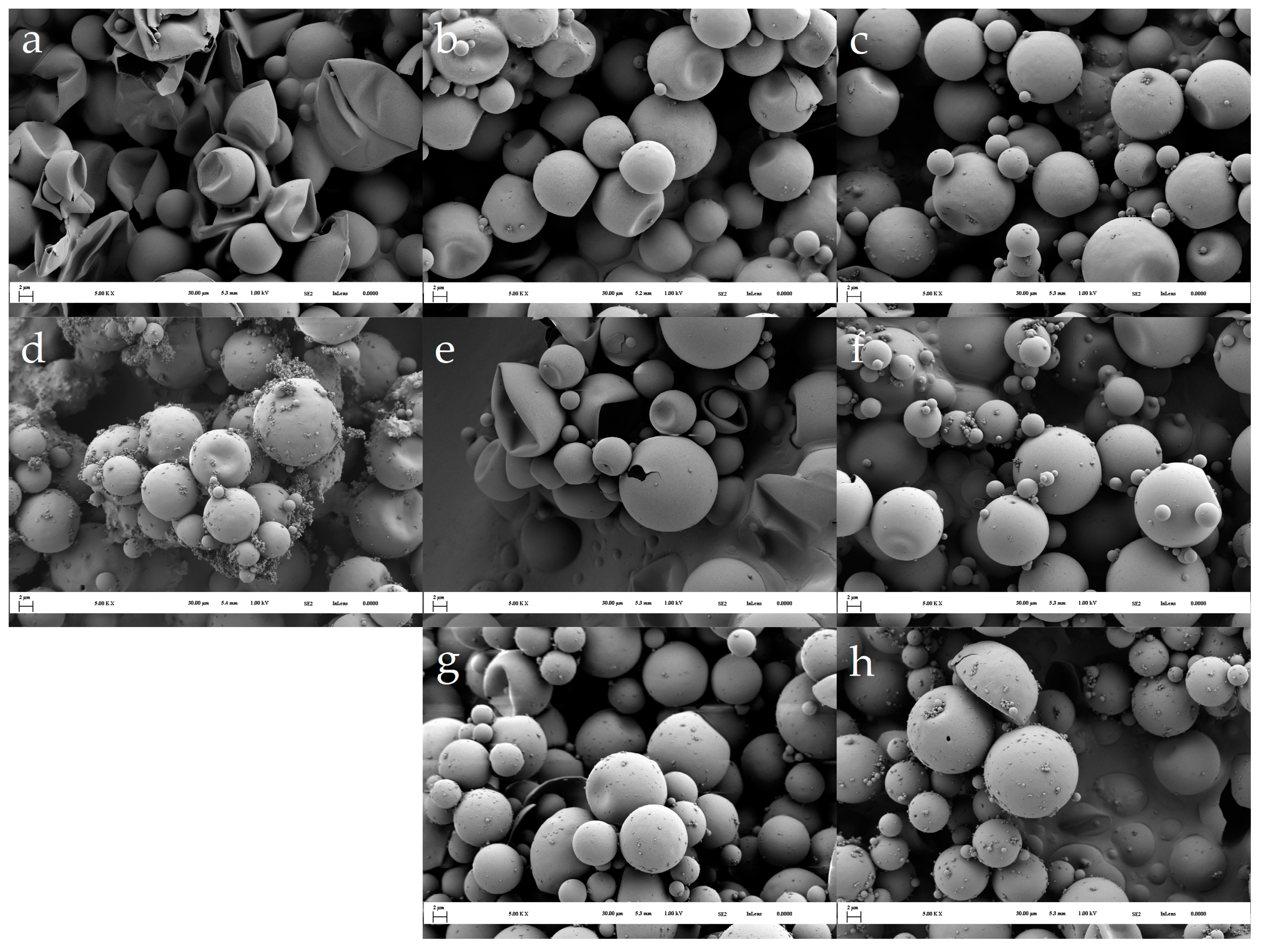
3.1. Morphology of the Microcapsules during the Encapsulation Process and Post-Curing
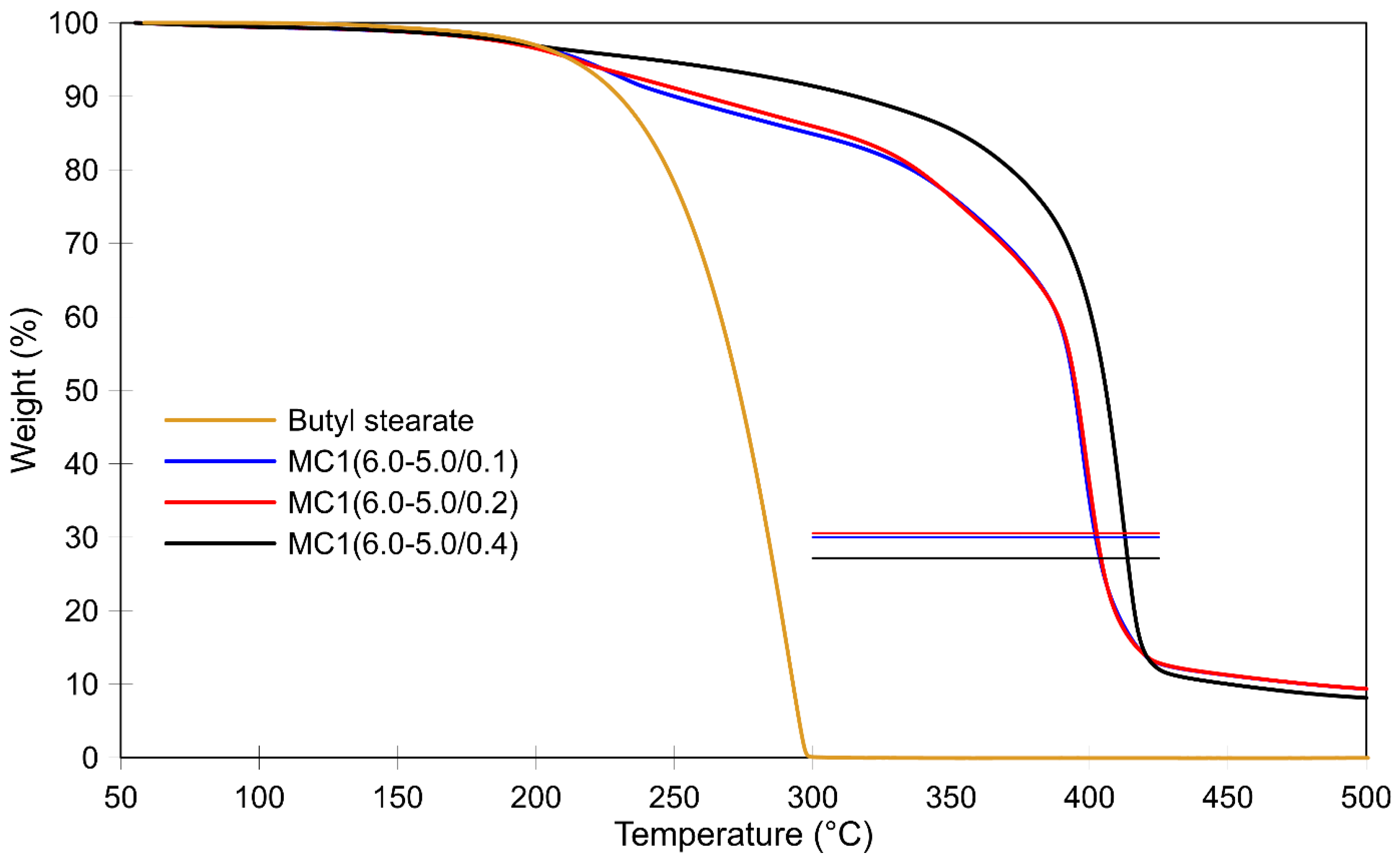
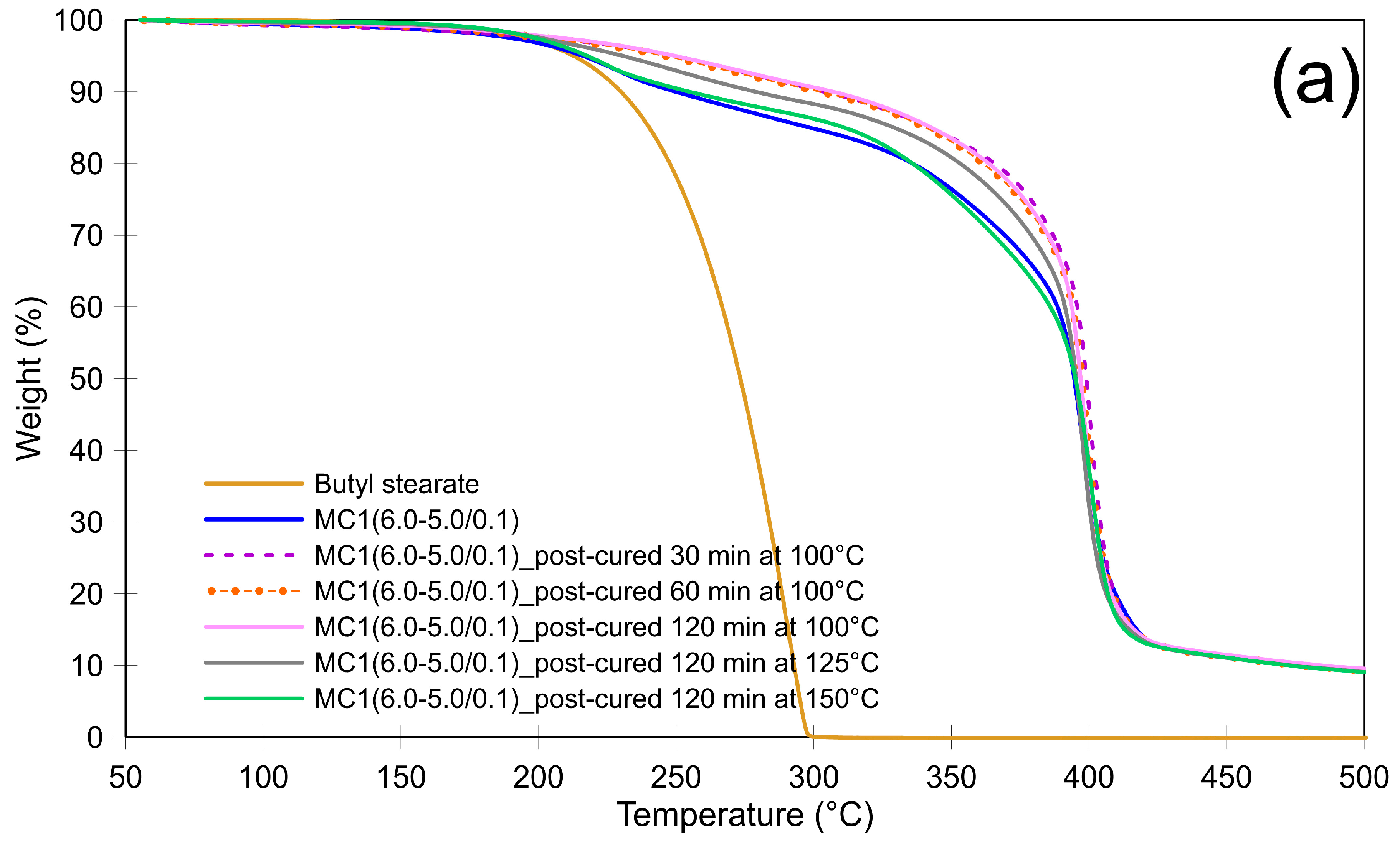
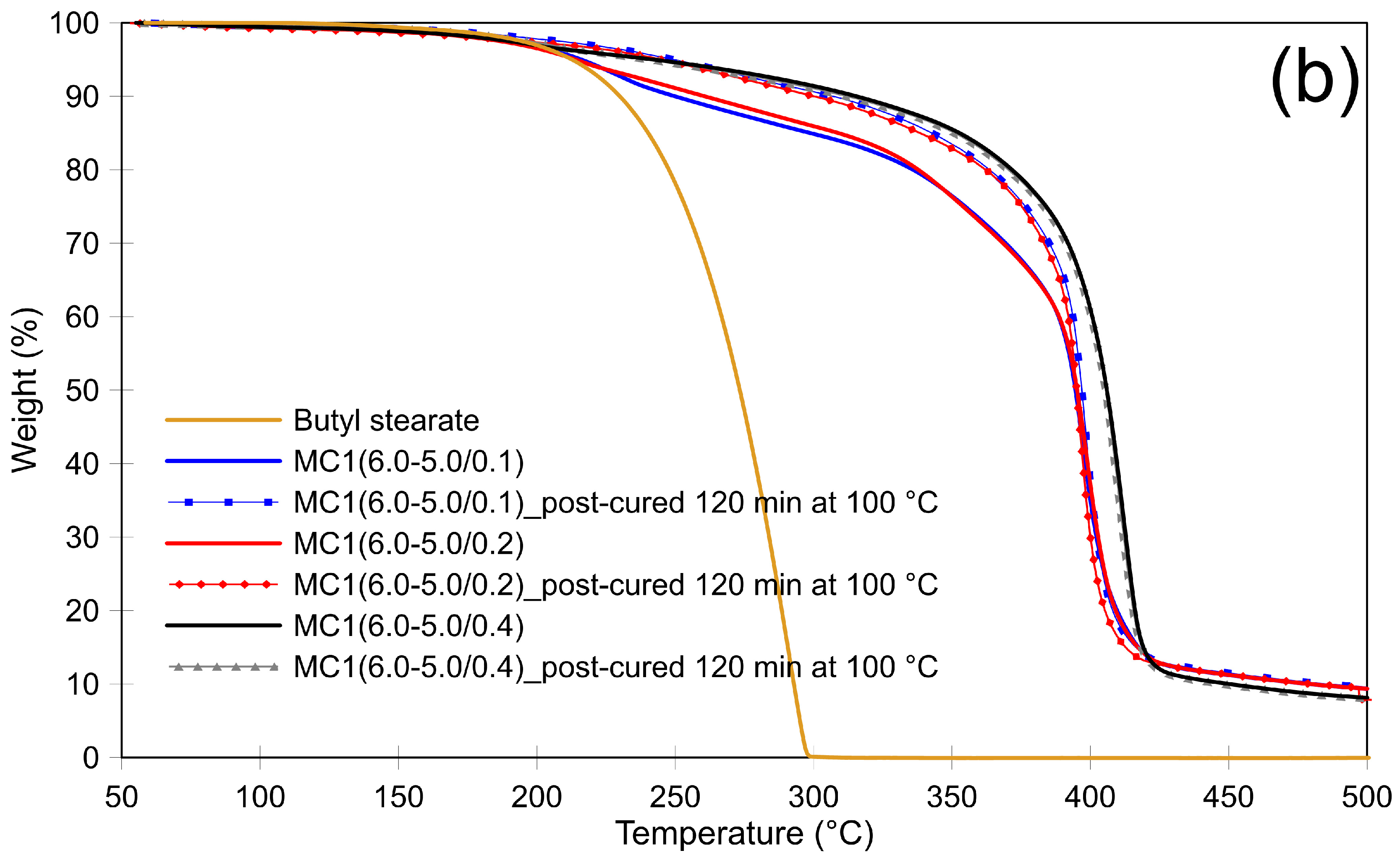
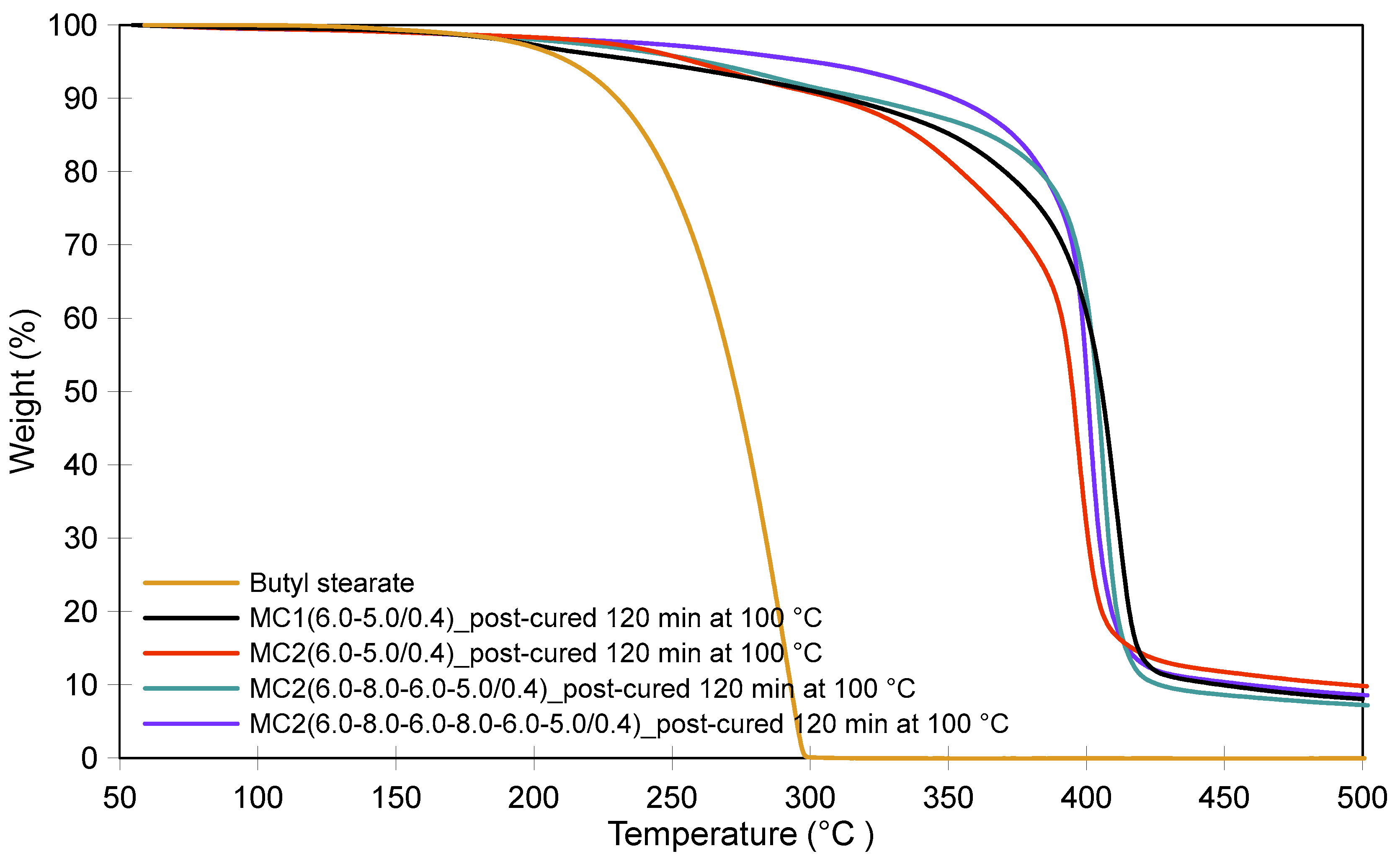
3.2. Thermal Stability of Microcapsules and the Effect of Post-Curing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kurdi, A.; Almoatham, N.; Mirza, M.; Ballweg, T.; Alkahlan, B. Potential phase change materials in building wall construction—A review. Materials 2021, 14, 5328. [Google Scholar] [CrossRef]
- Mehling, H.; Brütting, M.; Haussmann, T. PCM products and their fields of application—An overview of the state in 2020/2021. J. Energy Storage 2022, 51, 10254. [Google Scholar] [CrossRef]
- Ismail, K.A.R.; Lino, F.A.M.; Teggar, M.; Arici, M.; Machado, P.L.O.; Alves, T.A.; De Paula, A.C.O.; Benhorma, A. A comprehensive review on phase change materials and applications in buildings and components. ASME Open J. Eng. 2022, 1, 011049. [Google Scholar]
- Wang, Y.; Liu, Z.; Niu, X.; Ling, X. Preparation, characterization, and thermal properties of microencapsulated phase change material for low-temperature thermal energy storage. Energy Fuels 2019, 33, 1631–1636. [Google Scholar] [CrossRef]
- Saraç, E.G.; Öner, E.; Kahraman, M.V. Developing a thermo-regulative system for nonwoven textiles using microencapsulated organic coconut oil. J. Ind. Text. 2022, 51, 1952S–1963S. [Google Scholar] [CrossRef]
- Moreira, A.C.G.; Manrique, Y.A.; Martins, I.M.; Fernandes, I.P.; Rodrigues, A.E.; Lopes, J.C.B.; Dias, M.M. Continuous production of melamine-formaldehyde microcapsules using a mesostructured reactor. Ind. Eng. Chem. Res. 2020, 59, 18510–18519. [Google Scholar] [CrossRef]
- Cunha, S.; Sarcinella, A.; Aguiar, J.; Frigione, M. Perspective on the development of energy storage technology using phase change materials in the construction industry: A review. Energies 2023, 16, 4806. [Google Scholar] [CrossRef]
- Togun, H.; Sultan, H.S.; Mohammed, H.I.; Sadeq, A.M.; Biswas, N.; Hasan, H.A.; Homod, R.Z.; Abdulkadhim, A.H.; Yaseen, Z.M.; Talebizadehsardari, P. A critical review on phase change materials (PCM) based heat exchanger: Different hybrid techniques for the enhancement. J. Energy Storage 2024, 79, 109840. [Google Scholar] [CrossRef]
- Reddy, V.J.; Ghazali, M.F.; Kumarasamy, S. Advancements in phase change materials for energy-efficient building construction: A comprehensive review. J. Energy Storage 2024, 81, 110494. [Google Scholar] [CrossRef]
- McLaggan, M.S.; Hadden, R.M.; Gillie, M. Flammability assessment of phase change material wall lining and insulation materials with different weight fractions. Energy Build. 2017, 153, 439–447. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Innovative design of microencapsulated phase change materials for thermal energy storage and versatile applications: A review. Sustain. Energy Fuels 2019, 3, 1091–1149. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Yang, D.; Tu, S.; Chen, J.; Zhang, H.; Chen, W.; Hu, D.; Lin, J. Phase change composite microcapsules with low-dimensional thermally conductive nanofillers: Preparation, performance and applications. Polymers 2022, 15, 1562. [Google Scholar] [CrossRef] [PubMed]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium, Renew. Sust. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Weng, K.; Xu, X.; Chen, Y.; Li, X.; Qing, C.; Zou, D. Development and applications of multifunctional microencapsulated PCMs: A comprehensive review. Nano Energy 2024, 122, 109308. [Google Scholar] [CrossRef]
- Trivedi, G.V.N.; Parameshwaran, R. Microencapsulated phase change material suspensions for cool thermal energy storage. Mater. Chem. Phys. 2020, 242, 122519. [Google Scholar] [CrossRef]
- Naikwadi, A.T.; Samui, A.B.; Mahanwar, P.A. Melamine-formaldehyde microencapsulated n-tetracosane phase change material for solar thermal energy storage in coating. Sol. Energy Mater. Sol. Cells. 2020, 215, 110676. [Google Scholar] [CrossRef]
- Zhang, X.X.; Tao, X.M.; Yick, K.L.; Wang, X.C. Structure and thermal stability of microencapsulated phase-change materials. Colloid. Polym. Sci. 2004, 282, 330–336. [Google Scholar] [CrossRef]
- Kumar, G.N.; Al-Aifan, B.; Parameshwaran, R.; Ram, V.V. Facile synthesis of microencapsulated 1-dodecanol/melamine-formaldehyde phase change material using in-situ polymerization for thermal energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125698. [Google Scholar] [CrossRef]
- Lu, X.; Qian, R.; Xu, X.; Liu, M.; Liu, Y.; Zou, D. Modifications of microencapsulated phase change materials: Supercooling suppression, thermal conductivity en-hancement and stability improvement. Nano Energy 2024, 124, 109520. [Google Scholar] [CrossRef]
- Cellat, K.; Tezcan, F.; Kardaş, G.; Paksoy, H. Comprehensive investigation of butyl stearate as a multifunctional smart concrete additive for energy-efficient buildings. Int. J. Energy Res. 2019, 43, 7146–7158. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Appukuttan, S. Thermo-physical investigation of butyl stearate as potential phase change material for thermal energy storage in cooling application. Energy Storage 2020, 2, e160. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Hawes, D.; Ghanbari, E. Obtaining an energy storing building material by direct incorporation of an organic phase change material in gypsum wallboard. Sol. Energy Mater. 1991, 22, 231–242. [Google Scholar] [CrossRef]
- Hashempour, S.; Vakili, M.H. Preparation and characterisation of nano enhanced phase change material by adding carbon nano tubes to butyl stearate. J. Exp. Nanosci. 2018, 13, 188–198. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, C. Thermal Performance Analysis of Aluminum Alloy Phase Change Panels for Regions with Hot Summers and Warm Winters. Appl. Sci. 2024, 14, 6936. [Google Scholar] [CrossRef]
- Duquesne, M.; Mailhé, C.; Ruiz-Onofre, K.; Achchaq, F. Biosourced organic materials for latent heat storage: An economic and eco-friendly alternative. Energy 2019, 188, 116067. [Google Scholar] [CrossRef]
- Mehrizi, A.A.; Karimi-Maleh, H.; Naddafi, M.; Karimi, F. Application of bio-based phase change materials for effective heat management. J. Energy Storage 2023, 61, 106859. [Google Scholar] [CrossRef]
- Chen, W.; Fu, X.; Ge, W.; Xu, J.; Jiang, M. Microencapsulation of bisneopentyl glycol dithiopyrophosphate and its flame retardant effect on polyvinyl alcohol. Polym. Degrad. Stab. 2014, 102, 81–87. [Google Scholar] [CrossRef]
- Lkü, G.; Köken, N.; Akar, A.; Kizilcan, N.; Yaman, D. Tris (1-chloro-2-propyl) phosphate (TCPP) microcapsules for the preparation of flame-retardant rigid polyurethane foam. Polym. Plastics Tech. Mat. 2021, 60, 562–570. [Google Scholar] [CrossRef]
- Han, S.; Li, J.; Ding, Q.; Zang, J.; Lu, Y.; Zhang, L.; Hu, L. Effects of Processing Conditions on the Properties of Monoammonium Phosphate Microcapsules with Melamine-Formaldehyde Resin Shell. Polymers 2023, 15, 2991. [Google Scholar] [CrossRef]
- Su, J.F.; Wang, L.X.; Ren, L.; Huang, Z. Mechanical properties and thermal stability of double-shell thermal-energy-storage microcapsules. J. Appl. Polym. Sci. 2007, 103, 1295–1302. [Google Scholar] [CrossRef]
- Su, J.F.; Huang, Z.; Ren, L. High compact melamine-formaldehyde microPCMs containing n-octadecane fabricated by a two-step coacervation method. Colloid. Polym. Sci. 2007, 285, 1581–1591. [Google Scholar] [CrossRef]
- Su, J.F.; Wang, L.; Ren, L. Fabrication and thermal properties of microPCMs: Used melamine-formaldehyde resin as shell material. J. Appl. Polym. Sci. 2006, 101, 1522–1528. [Google Scholar] [CrossRef]
- Geng, X.; Huang, R.; Zhang, X.; Li, W. Research on long-chain alkanol etherified melamine-formaldehyde resin microPCMs for energy storage. Energy 2021, 214, 119029. [Google Scholar] [CrossRef]
- Erzin, F.; Konuklu, Y. Facile synthesis of resorcinol-melamine-formaldehyde microcapsules containing hexadecane for thermal energy storage. J. Energy Storage 2021, 44, 103285. [Google Scholar] [CrossRef]
- Chai, Y.; Zhao, T.; Gao, X.; Zhang, J. Low cracking ratio of paraffin microcapsules shelled by hydroxyl terminated polydimethylsiloxane modified melamine-formaldehyde resin. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 86–93. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Wang, K.; Huang, Y.; Chen, Z. Highly efficient photothermal conversion capric acid phase change microcapsule: Silicon carbide modified melamine urea formaldehyde. J. Colloid. Interface Sci. 2021, 582, 30–40. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yu, F. Enhanced thermal conductivity of microencapsulated phase change materials based on graphene oxide and carbon nanotube hybrid filler. Sol. Energy Mater. Sol. Cells 2019, 192, 72–80. [Google Scholar] [CrossRef]
- Geng, X.; He, Y.; Han, N.; Zhang, X.; Li, W. Synthesis and characterization of hydrophobic reversible thermochromic microPCMs with amino resins shell for thermal energy storage. Energy Build. 2021, 230, 110528. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Zhao, T. Fabrication and characterization of poly(melamine-formaldehyde)/silicon carbide hybrid microencapsulated phase change materials with enhanced thermal conductivity and light-heat performance. Sol. Energy Mater. Sol. Cells 2018, 183, 82–91. [Google Scholar] [CrossRef]
- Zhao, Q.; He, F.; Zhang, Q.; Fan, J.; He, R.; Zhang, K.; Yan, H.; Yang, W. Microencapsulated phase change materials based on graphene Pickering emulsion for light-to-thermal energy conversion and management. Sol. Energy Mater. Sol. Cells 2019, 203, 110204. [Google Scholar] [CrossRef]
- Zhang, B.; Li, S.; Fei, X.; Zhao, H.; Lou, X. Enhanced mechanical properties and thermal conductivity of paraffin microcapsules shelled by hydrophobic-silicon carbide modified melamine-formaldehyde resin. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125219. [Google Scholar] [CrossRef]
- Dong, B.; Li, S.; Zhang, X.; Wang, J.; Peng, H. Synthesis and characterization of nanoalumina and CNTs-reinforced microcapsules with n-dodecane as a phase change material for cold energy storage. Energy Fuels 2020, 34, 7700–7708. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly(melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Aiswarya, V.; Das, S.; Watmode, P.D.; Gajghate, S.S. Development of hybrid MgO/GO modified microencapsulated phase change material for thermal energy management: An experimental approach. J. Clean. Prod. 2024, 434, 140399. [Google Scholar] [CrossRef]
- Salaün, F.; Devaux, E.; Bourbigot, S.; Rumeau, P. Influence of process parameters on microcapsules loaded with n-hexadecane prepared by in situ polymerization. Chem. Eng. J. 2009, 155, 457–465. [Google Scholar] [CrossRef]
- Fei, X.; Zhao, H.; Zhang, B.; Cao, L.; Yu, M.; Zhou, J.; Yu, L. Microencapsulation mechanism and size control of fragrance microcapsules with melamine resin shell. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 300–306. [Google Scholar] [CrossRef]
- Ollier, R.P.; Alvarez, V.A. Synthesis of epoxy-loaded poly(melamine-formaldehyde) microcapsules: Effect of pH regulation method and emulsifier selection. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 872–882. [Google Scholar] [CrossRef]
- Han, S.; Chen, Y.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. Effects of processing conditions on the properties of paraffin/melamine-urea-formaldehyde microcapsules prepared by in situ polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124046. [Google Scholar] [CrossRef]
- Ambrosi, M.; Fratini, E.; Baglioni, P.; Vannucci, C.; Bartolini, A.; Pintens, A.; Smets, J. Microcapsules for confining fluids: Prediction of shell stability from advanced SAXS investigations. J. Phys. Chem. C 2016, 120, 13514–13522. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, S.J.; Cheong, I.W.; Kim, J.H. Microencapsulation of fragrant oil via in situ polymerization: Effects of pH and melamine-formaldehyde molar ratio. J. Microencapsul. 2002, 19, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, T. Effects of parameters of the shell formation process on the performance of microencapsulated phase change materials based on melamine-formaldehyde. Text. Res. J. 2017, 87, 1848–1859. [Google Scholar] [CrossRef]
- Khakzad, F.; Alinejad, Z.; Shirin-Abadi, A.R.; Ghasemi, M.; Mahdavian, A.R. Optimization of parameters in preparation of PCM microcapsules based on melamine formaldehyde through dispersion polymerization. Colloid Polym. Sci. 2014, 292, 355–368. [Google Scholar] [CrossRef]
- Zheng, T.; Pilla, S. Encapsulation of hydrophilic payload by PU-PMF capsule: Effect of melamine-formaldehyde pre-polymer content, pH and temperature on capsule morphology. Colloids Surf. A Physicochem. Eng. Asp. 2018, 542, 59–67. [Google Scholar] [CrossRef]
- Huang, R.; Li, W.; Wang, J.; Zhang, X. Effects of oil-soluble etherified melamine-formaldehyde prepolymers on in situ microencapsulation and macroencapsulation of n-dodecanol. New J. Chem. 2017, 41, 9424–9437. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Huang, R.; Wang, N.; Wang, J.; Zhang, X. Microstructure regulation of microencapsulated bio-based n-dodecanol as phase change materials via in situ polymerization. New J. Chem. 2017, 41, 14696–14707. [Google Scholar] [CrossRef]
- Pavlyuchenko, V.N.; Ivanchev, S.S.; Rätzsch, M.; Bucka, H.; Primachenko, O.N.; Leitner, P.; Khaikin, S.Y. Transetherification of melamine–formaldehyde resin methyl ethers and competing reaction of self-condensation. J. Appl. Polym. Sci. 2006, 101, 2977–2985. [Google Scholar] [CrossRef]
- Escobar, C.F.; Roldo, L.; Rocha, T.L.; Kindlein, W., Jr. Nanoencapsulation mechanism of fragrant oil: Effect of formaldehyde—Melamine molar ratio. Polym. Int. 2018, 67, 220–226. [Google Scholar] [CrossRef]
- Merline, D.; Vukusic, S.; Abdala, A.A. Melamine formaldehyde: Curing studies and reaction mechanism. Polym. J. 2013, 45, 413–419. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.H.; Zeng, X.R. Preparation, characterization, and thermal properties of microPCMs containing n-dodecanol by using different types of styrene-maleic anhydride as emulsifier. Colloid. Polym. Sci. 2009, 287, 549–560. [Google Scholar] [CrossRef]
- Li, S.; Dong, B.; Wang, J.; Li, J.; Shen, T.; Peng, H.; Ling, X. Synthesis and characterization of mixed alkanes microcapsules with phase change temperature below ice point for cryogenic thermal energy storage. Energy 2019, 187, 115898. [Google Scholar] [CrossRef]
- Fu, D.; Su, Y.; Xie, B.; Liu, G.; Li, Z.; Jiang, K.; Wang, D. Pore decoration on microcapsule surface using nonionic surfactant micelles as template: Temperature effect and encapsulation mechanism investigation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 219–227. [Google Scholar] [CrossRef]
- Alič, B.; Šebenik, U.; Krajnc, M. Differential scanning calorimetric examination of melamine–formaldehyde microcapsules containing decane. J. Appl. Polym. Sci. 2011, 119, 3687–3695. [Google Scholar] [CrossRef]
- Alič, B.; Šebenik, U.; Krajnc, M. Microencapsulation of butyl stearate with melamine- formaldehyde resin: Effect of decreasing the pH value on the composition and thermal stability of microcapsules. Express Polym. Lett. 2012, 6, 826–836. [Google Scholar] [CrossRef]
- Hill, L.W.; Lee, S.B. Effect of melamine-formaldehyde structure on cure response of thermoset coatings. J. Coat. Technol. 1999, 71, 127–133. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Wang, X.; Wu, S.; Zhang, X. Effects of ammonium chloride and heat treatment on residual formaldehyde contents of melamine-formaldehyde microcapsules. Colloid. Polym. Sci. 2007, 285, 1691–1697. [Google Scholar] [CrossRef]
- Butyl Stearate—Safety Data Sheet. Available online: https://www.sigmaaldrich.com/SI/en/product/aldrich/85720 (accessed on 15 November 2023).
- Elder, R.L. Final report on the safety assessment of butyl stearate, cetyl stearate, isobutyl stearate, isopropyl stearate, myristyl stearate, and octyl stearate. J. Am. Coll. Toxicol. 1985, 4, 107–146. [Google Scholar] [CrossRef]
- Manley, T.R.; Higgs, D.A. Thermal stability of melamine-formaldehyde resins. J. Polym. Sci. Polym. Symp. 1973, 42, 1377–1382. [Google Scholar] [CrossRef]
- Devallencourt, C.; Saiter, J.M.; Fafet, A.; Ubrich, E. Thermogravimetry/Fourier transform infrared coupling investigations to study the thermal stability of melamine formaldehyde resin. Thermochim. Acta 1995, 259, 143–151. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alič, B.; Šebenik, U.; Krajnc, M. Applying pH Modulation to Improve the Thermal Stability of Melamine–Formaldehyde Microcapsules Containing Butyl Stearate as a Phase-Change Material. Polymers 2024, 16, 2463. https://doi.org/10.3390/polym16172463
Alič B, Šebenik U, Krajnc M. Applying pH Modulation to Improve the Thermal Stability of Melamine–Formaldehyde Microcapsules Containing Butyl Stearate as a Phase-Change Material. Polymers. 2024; 16(17):2463. https://doi.org/10.3390/polym16172463
Chicago/Turabian StyleAlič, Branko, Urška Šebenik, and Matjaž Krajnc. 2024. "Applying pH Modulation to Improve the Thermal Stability of Melamine–Formaldehyde Microcapsules Containing Butyl Stearate as a Phase-Change Material" Polymers 16, no. 17: 2463. https://doi.org/10.3390/polym16172463
APA StyleAlič, B., Šebenik, U., & Krajnc, M. (2024). Applying pH Modulation to Improve the Thermal Stability of Melamine–Formaldehyde Microcapsules Containing Butyl Stearate as a Phase-Change Material. Polymers, 16(17), 2463. https://doi.org/10.3390/polym16172463






