Optimization of Polylactide-Co-Glycolide-Rifampicin Nanoparticle Synthesis, In Vitro Study of Mucoadhesion and Drug Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rifampicin Loaded PLGA Nanoparticles
2.3. Experimental Design of Central Composite Design
2.4. Measurement of Particle Size, Polydispersity and Zeta Potential
2.5. Determination of Drug Loading Efficiency and Nanoparticle Yield
2.6. In Vitro Release of Drug from Polymer Nanoparticles
2.7. Thermogravimetric Analysis and Differential Scanning Calorimetry
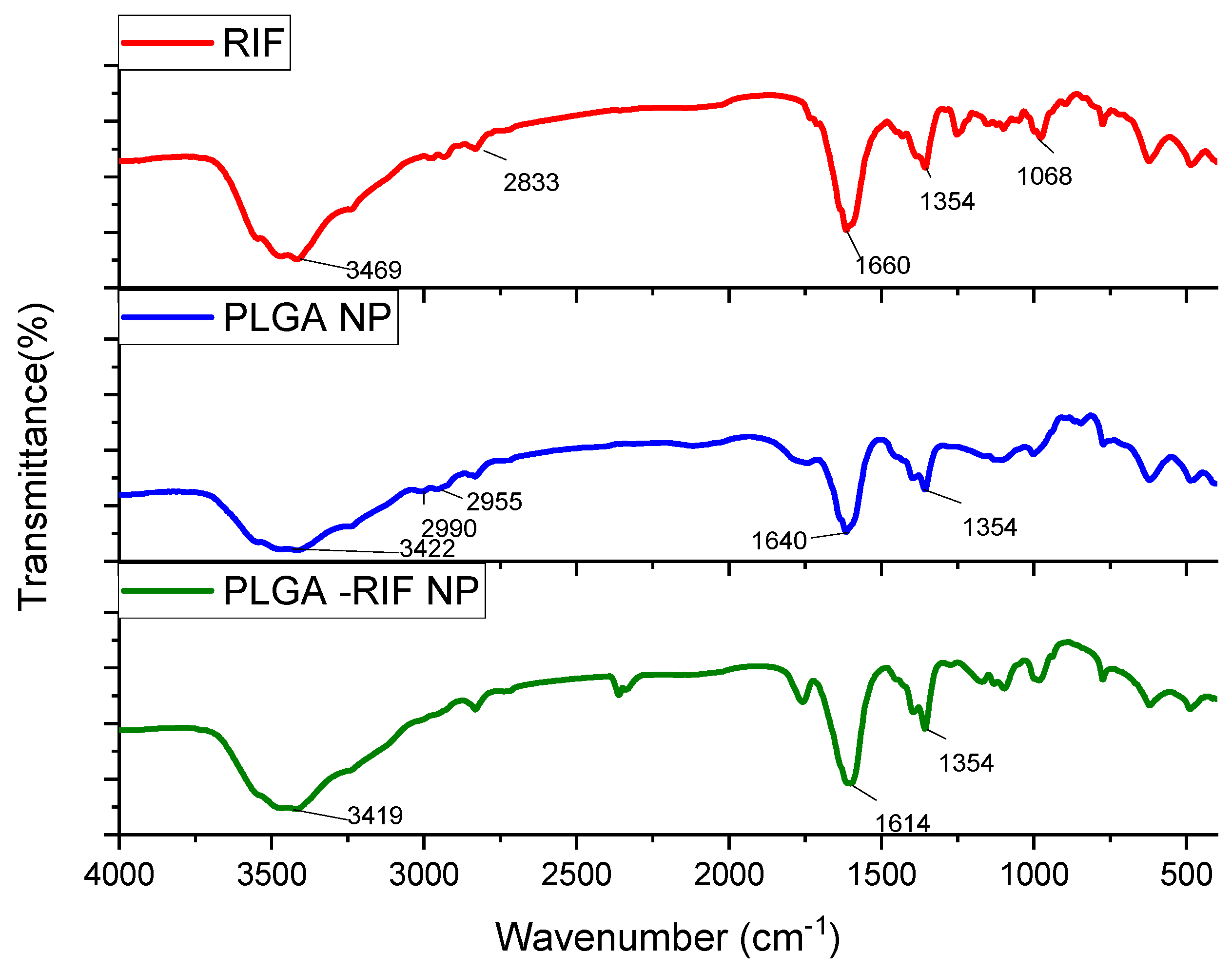
2.8. Study of Prepared Nanoparticles by Infrared Spectroscopy
2.9. In Vitro Study of Nanoparticle Mucoadhesion
2.10. Statistical Processing of the Produced Data
3. Results and Discussion
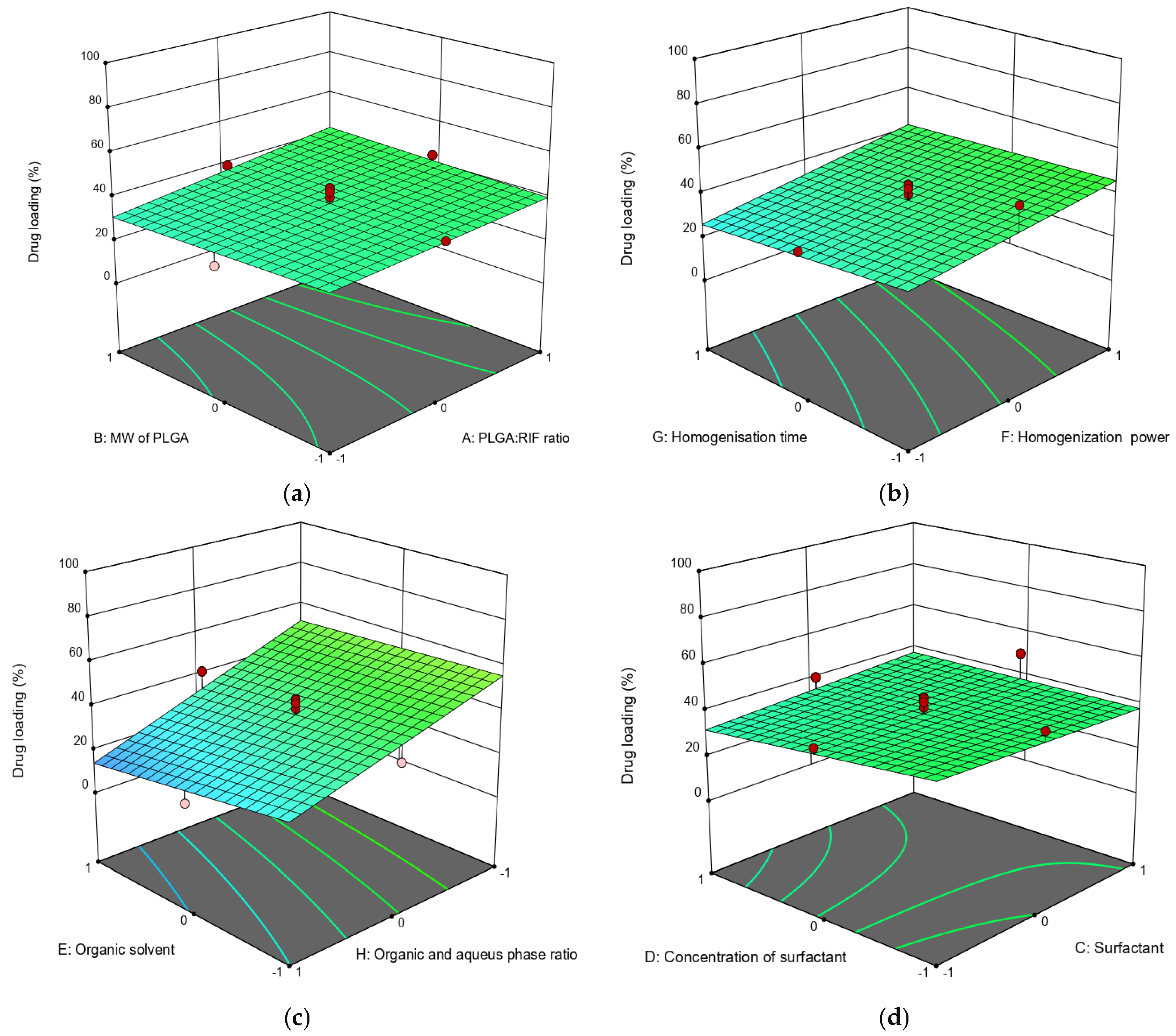
3.1. Synthesis and Optimization of Conditions for Producing PLGA-RIF NPs
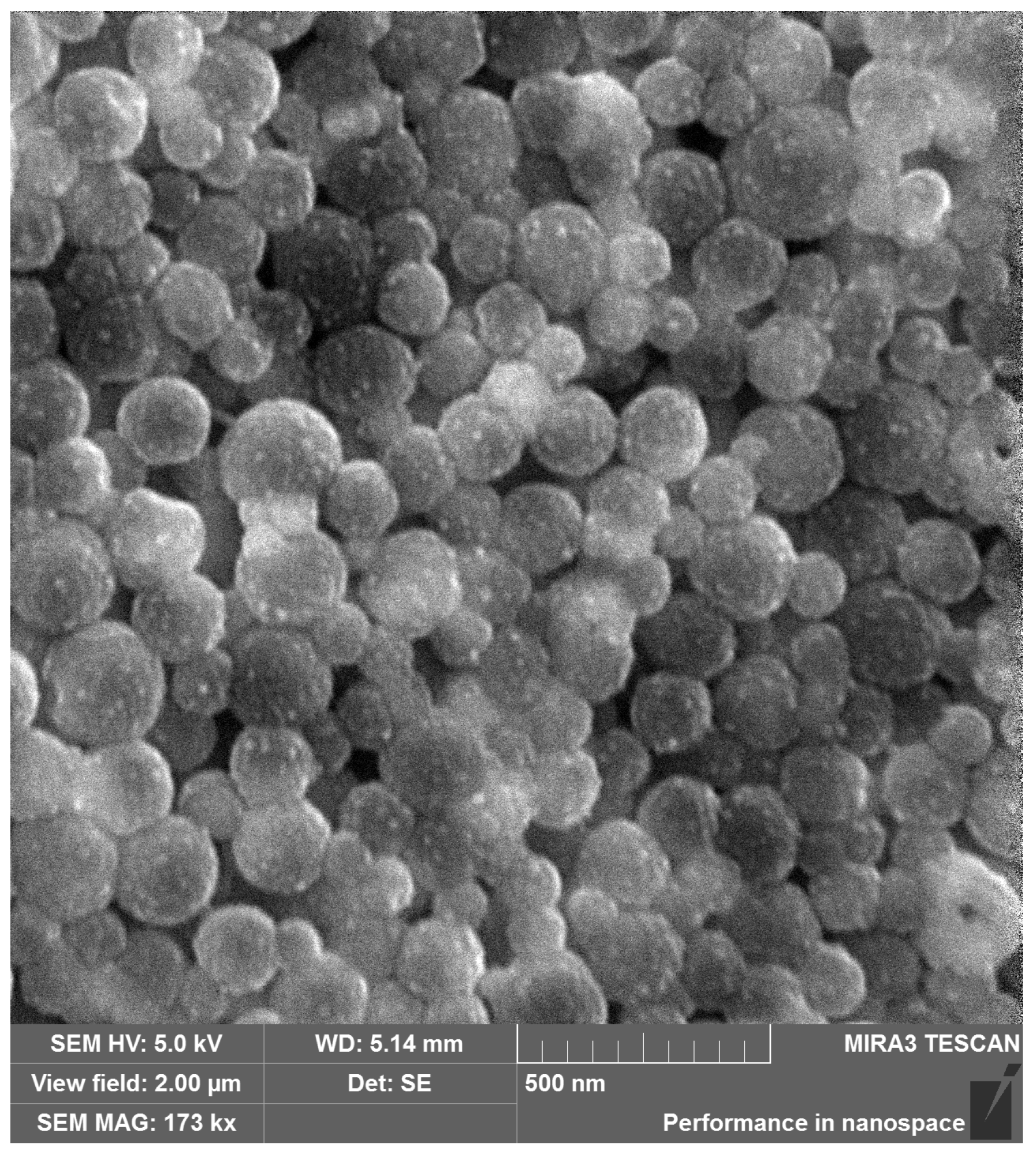
3.2. Physicochemical Characterization of PLGA-RIF Nanoparticles
3.3. In Vitro Release Profile of PLGA-RIF NPs
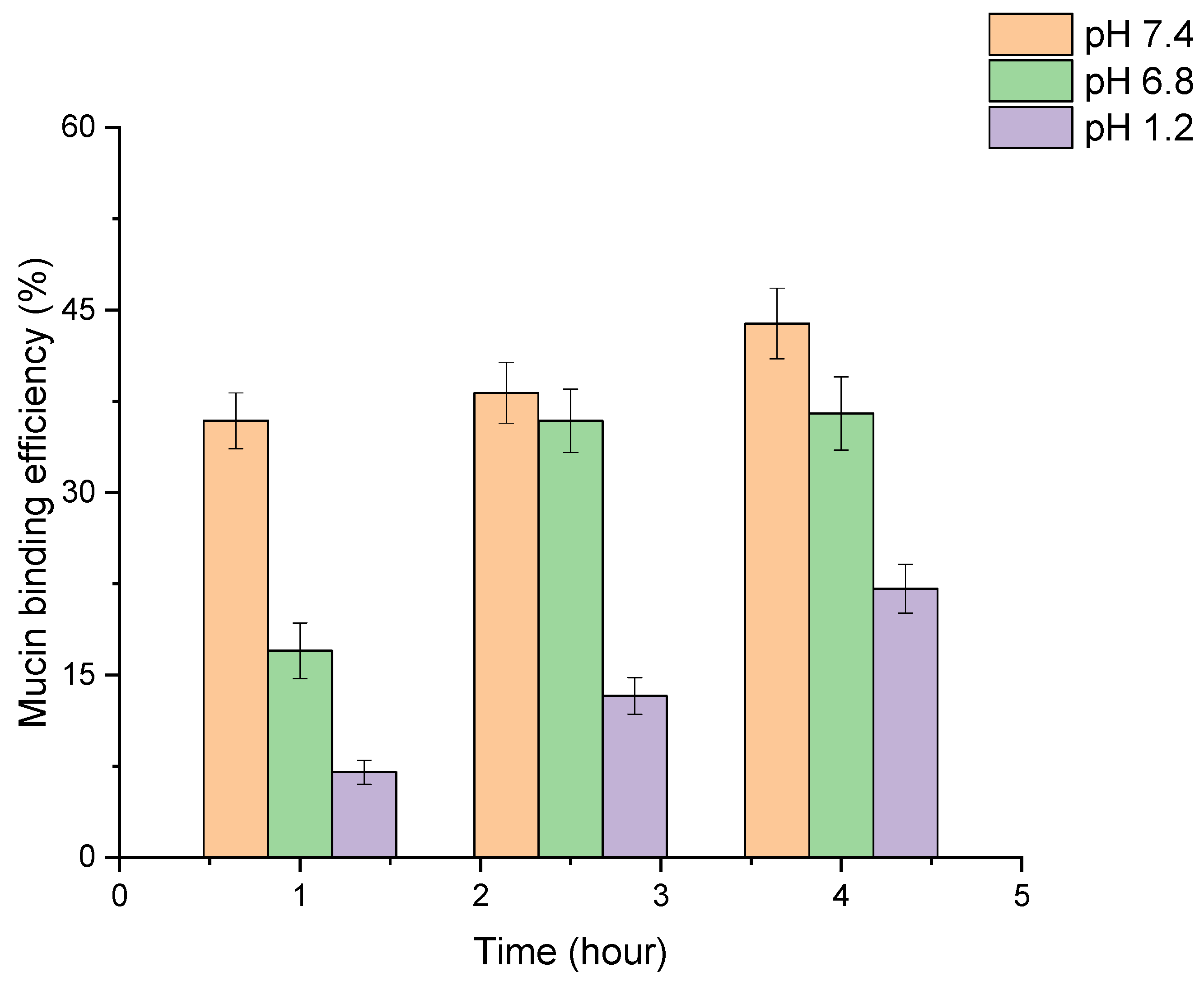
3.4. In Vitro Mucoadhesion of PLGA-RIF NPs
3.5. In Vitro Efficacy of PLGA-RIF NPs against Strain H37Rv
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up polymeric-based nanoparticles drug delivery systems: Development and challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Keum, C.G.; Noh, Y.W.; Baek, J.S.; Lim, J.H.; Hwang, C.J.; Na, Y.G.; Shin, S.C.; Cho, C.W. Practical preparation procedures for docetaxel-loaded nanoparticles using polylactic acid-co-glycolic acid. Int. J. Nanomed. 2011, 6, 2225–2234. [Google Scholar] [CrossRef]
- Turk, T.S.; Bayindir, Z.S.; Badilli, U. Preparation of polymeric nanoparticles using different stabilizing agents. J. Fac. Pharm. Ank. Univ. 2009, 38, 257–268. [Google Scholar] [CrossRef]
- Galiyeva, A.R.; Tazhbayev, Y.M.; Zhumagaliyeva, T.S.; Sadyrbekov, D.T.; Kaikenov, D.A.; Karimova, B.N.; Shokenova, S.S. Polylactide-co-glycolide nanoparticles immobilized with isoniazid: Optimization using the experimental Taguchi method. Bull. Karaganda Univ. Chem. Ser. 2022, 105, 69–77. [Google Scholar] [CrossRef]
- Motiei, M.; Pleno de Gouveia, L.; Šopík, T.; Vícha, R.; Škoda, D.; Císař, J.; Khalili, R.; DomincováBergerová, E.; Münster, L.; Fei, H.; et al. Nanoparticle-Based Rifampicin Delivery System Development. Molecules 2021, 26, 2067. [Google Scholar] [CrossRef]
- Hakkimane, S.S.; Shenoy, V.P.; Gaonkar, S.L.; Bairy, I.; Guru, B.R. Antimycobacterial susceptibility evaluation of rifampicin and isoniazid benz-hydrazone in biodegradable polymeric nanoparticles against Mycobacterium tuberculosis H37Rv strain. Int. J. Nanomed. 2018, 13, 4303–4318. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Mariappan, T.T.; Singh, S. Positioning of Rifampicin in the Biopharmaceutics Classification System (BCS). Clin. Res. Regul. Aff. 2006, 23, 1–10. [Google Scholar] [CrossRef]
- Iacobino, A.; Fattorini, L.; Giannoni, F. Drug-Resistant Tuberculosis 2020: Where We Stand. Appl. Sci. 2020, 10, 2153. [Google Scholar] [CrossRef]
- Jang, J.G.; Chung, J.H. Diagnosis and treatment of multidrug-resistant tuberculosis. Yeungnam Univ. J. Med. 2020, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Pinheiro, M.; Magalhães, J.; Ribeiro, R.; Seabra, V.; Reis, S.; Sarmento, B. The formulation of nanomedicines for treating tuberculosis. Adv. Drug Deliv. Rev. 2016, 102, 102–115. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Al Shaal, L.; Mitri, K. Present status of nanoparticle research for treatment of Tuberculosis. J. Pharm. Pharm. Sci. 2011, 14, 100. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, S.; Khuller, G. Oral poly(lactide-co-glycolide) nanoparticle based antituberculosis drug delivery: Toxicological and chemotherapeutic implications. Indian J. Exp. Biol. 2006, 44, 459–467. [Google Scholar] [PubMed]
- Rather, I.; Shafiq, N.; Shukla, J.; Kaur, G.; Pandey, S.; Bhandari, R.K.; Pandey, A.K.; Mittal, B.R.; Khuller, G.K.; Sharma, N.; et al. Bio-evaluation of poly(lactic-co-glycolic) acid nanoparticles loaded with radiolabelled rifampicin. Br. J. Clin. Pharmacol. 2023, 89, 3702–3714. [Google Scholar] [CrossRef]
- Hernández-Giottonini, K.Y.; Arellano-Reynoso, B.; Rodríguez-Córdova, R.J.; de la Vega-Olivas, J.; Díaz-Aparicio, E.; Lucero-Acuña, A. Enhancing Therapeutic Efficacy against Brucella canis Infection in a Murine Model Using Rifampicin-Loaded PLGA Nanoparticles. ACS Omega 2023, 8, 49362–49371. [Google Scholar] [CrossRef]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Lamberti, F.; Mazzariol, C.; Spolaore, F.; Ceccato, R.; Salmaso, L.; Gross, S. Design of Experiment: A Rational and Still Unexplored Approach to Inorganic Materials’ Synthesis. Sustain. Chem. 2022, 3, 114–130. [Google Scholar] [CrossRef]
- Rather, M.A.; Amin, S.; Maqbool, M.; Bhat, Z.S.; Gupta, P.N.; Ahmad, Z. Preparation and in vitro characterization of albumin nanoparticles encapsulating an anti-tuberculosis drug-levofloxacin. Adv. Sci. Eng. Med. 2016, 8, 912–917. [Google Scholar] [CrossRef]
- Krishna, V.; Reddy, M.S. Isocratic High Performance Liquid Chromatographic (HPLC) Determination of Rifampicin in Presence of Isoniazid. Res. J. Pharm. Technol. 2014, 7, 328–331. [Google Scholar]
- Gordeeva, D.S.; Sitenkova, A.V.; Moustafine, R.I. New Carriers for Bioadhesive Gastroretentive Drug Delivery Systems Based on Eudragit® EPO/Eudragit® L100 Interpolyelectrolyte Complexes. Sci. Pharm. 2024, 92, 14. [Google Scholar] [CrossRef]
- Timergalieva, V.R.; Gennari, C.G.M.; Cilurzo, F.; Selmin, F.; Moustafine, R.I. Comparative Evaluation of Metformin and Metronidazole Release from Oral Lyophilisates with Different Methods. Sci. Pharm. 2023, 91, 23. [Google Scholar] [CrossRef]
- Samprasit, W.; Opanasopit, P.; Chamsai, B. Alpha-mangostin and resveratrol, dual-drugs-loaded mucoadhesive thiolated chitosan-based nanoparticles for synergistic activity against colon cancer cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 110, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Guang, W.; Baraldo, M.; Furlanut, M. Calculating percentage prediction error: A user’s note. Pharmacol. Res. 1995, 32, 241–248. [Google Scholar] [CrossRef]
- Doan, T.V.P.; Olivier, J.C. Preparation of rifampicin-loaded PLGA microspheres for lung delivery as aerosol by premix membrane homogenization. Int. J. Pharm. 2009, 382, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Snejdrova, E.; Loskot, J.; Martiska, J.; Soukup, T.; Prokes, L.; Frolov, V.; Kucera, T. Rifampicin-loaded PLGA nanoparticles for local treatment of musculoskeletal infections: Formulation and characterization. J. Drug Deliv. Sci. Technol. 2022, 73, 103435. [Google Scholar] [CrossRef]
- Tazhbayev, Y.M.; Galiyeva, A.R.; Zhumagaliyeva, T.S.; Burkeyev, M.Z.; Kazhmuratova, A.T.; Zhakupbekova, E.Z.; Zhaparova, L.Z.; Bakibayev, A.A. Synthesis and characterization of isoniazid immobilized polylactide-co-glycolide nanoparticles. Bull. Karaganda Univ. Chem. Ser. 2021, 101, 61–70. [Google Scholar] [CrossRef]
- Youshia, J.; Ali, M.E.; Lamprecht, A. Artificial neural network based particle size prediction of polymeric nanoparticles. Eur. J. Pharm. Biopharm. 2017, 119, 333–342. [Google Scholar] [CrossRef]
- Galiyeva, A.; Daribay, A.; Zhumagaliyeva, T.; Zhaparova, L.; Sadyrbekov, D.; Tazhbayev, Y. Human Serum Albumin Nanoparticles: Synthesis, Optimization and Immobilization with Antituberculosis Drugs. Polymers 2023, 15, 2774. [Google Scholar] [CrossRef]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro, M.D.L. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. Hematol. E Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef]
- Agrawal, S.; Ashokraj, Y.; Bharatam, P.V.; Pillai, O.; Panchagnula, R. Solid-state characterization of rifampicin samples and its biopharmaceutic relevance. Eur. J. Pharm. Sci. 2004, 22, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Reis, T.V.D.S.; Silva, L.C.C.D.; Storpírtis, S.; Mercuri, L.P.; Matos, J.D.R. Thermal behavior and decomposition kinetics of rifampicin polymorphs under isothermal and non-isothermal conditions. Braz. J. Pharm. Sci. 2010, 46, 343–351. [Google Scholar] [CrossRef]
- Qi, J.; Feng, S.; Liu, X.; Xing, L.; Chen, D.; Xiong, C. Morphology, thermal properties, mechanical property and degradation of PLGA/PTMC composites. J. Polym. Res. 2020, 27, 387. [Google Scholar] [CrossRef]
- Silva, A.T.C.R.; Cardoso, B.C.O.; Silva, M.E.S.R.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer In Vitro Degradation. J. Biomater. Nanobiotechnol. 2015, 6, 8–19. [Google Scholar] [CrossRef]
- Yessentayeva, N.A.; Galiyeva, A.R.; Daribay, A.T.; Sadyrbekov, D.T.; Zhumagalieva, T.S.; Marsel, D.T. Synthesis and Optimization of Bovine Serum Albumin Nanoparticles Immobilized with Antituberculosis Drugs. Eurasian J. Chem. 2024, 29, 33–42. [Google Scholar] [CrossRef]
- Sharma, A.; Puri, V.; Kumar, P.; Singh, I.; Huanbutta, K. Development and Evaluation of Rifampicin Loaded Alginate–Gelatin Biocomposite Microfibers. Polymers 2021, 13, 1514. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, O.; Tomila, T.; Ulyanchich, N.; Yarmola, T.; Uvarova, I. Fourier-Transform Infrared Spectroscopy of Antibiotic Loaded Ag-Free and Ag-Doped Hydroxyapatites. Adv. Sci. Eng. Med. 2014, 6, 193–202. [Google Scholar] [CrossRef]
- Portaccio, M.; Menale, C.; Diano, N.; Serri, C.; Mita, D.G.; Lepore, M. Monitoring production process of cisplatin-loaded PLGA nanoparticles by FT-IR microspectroscopy and univariate data analysis. J. Appl. Polym. Sci. 2014, 132, 41305. [Google Scholar] [CrossRef]
- Anwer, M.D.K.; Al-Mansoor, M.A.; Jamil, S.; Al-Shdefat, R.; Ansari, M.N.; Shakeel, F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int. J. Biol. Macromol. 2016, 92, 213–219. [Google Scholar] [CrossRef]
- Devi, M.G.; Dutta, S.; Al Hinai, A.T.; Feroz, S. Studies on encapsulation of Rifampicin and its release from chitosan-dextran sulfate capsules. Korean J. Chem. Eng. 2014, 32, 118–124. [Google Scholar] [CrossRef]
- Ahmad, N. Rasagiline-encapsulated chitosan-coated PLGA nanoparticles targeted to the brain in the treatment of parkinson’s disease. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 677–690. [Google Scholar] [CrossRef]
- Galiyeva, A.R.; Tazhbayev, Y.M.; Yessentayeva, N.A.; Daribay, A.T.; Marsel, D.T.; Sadyrbekov, D.T.; Zhaparova, L.Z.; Arystanova, Z.T. PEGylation of Albumin Nanoparticles Immobilized with the Anti-Tuberculosis Drug “Isoniazid”. Eurasian J. Chem. 2023, 110, 42–50. [Google Scholar] [CrossRef]
- Bourguignon, T.; Godinez-Leon, J.A.; Gref, R. Nanosized Drug Delivery Systems to Fight Tuberculosis. Pharmaceutics 2023, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Martínez, N.; Guillen, D.; Moreno-Mendieta, S.A.; Sanchez, S.; Rodríguez-Sanoja, R. The Role of Mucoadhesion and Mucopenetration in the Immune Response Induced by Polymer-Based Mucosal Adjuvants. Polymers 2023, 15, 1615. [Google Scholar] [CrossRef]
- Donnelly, R.; Shaikh, R.; Raj Singh, T.; Garland, M.; Woolfson, D. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, R.P.; Khutoryanskiy, V.V. Progress and Current Trends in the Synthesis of Novel Polymers with Enhanced Mucoadhesive Properties. Macromol. Biosci. 2019, 19, 1900194. [Google Scholar] [CrossRef] [PubMed]
- Kaldybekov, D.B.; Tonglairoum, P.; Opanasopit, P.; Khutoryanskiy, V.V. Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder. Eur. J. Pharm. Sci. 2018, 111, 83–90. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2010, 11, 748–764. [Google Scholar] [CrossRef]





| Independent Variable | Variable Level | ||
|---|---|---|---|
| Low –1 | Center 0 | High 1 | |
| PLGA:RIF | 1:1 | 2:1 | 3:1 |
| MW of PLGA | 7000–17,000 | 24,000–38,000 | 30,000–60,000 |
| Surfactant | PVA | Tween 80 | Pluronic F127 |
| Concentration of surfactant | 0.5% | 1% | 2% |
| Organic solvent | DCM | DMSO | EA |
| Organic phase: aqueous phase | 1:1 | 1:5 | 1:10 |
| Homogenization power | 15 W | 35 W | 70 W |
| Homogenization time | 5 min | 10 min | 15 min |
| NPs | PLGA:RIF Ratio | MW PLGA | Surfactant | Surfactant Concentration, % | Organic Solvent | Organic Phase:Aqueous Phase | Homogenization Power, W | Homogenization Time, min | Average Size, nm | PDI | Zeta Potential, mV | LE, % | NPs Yield, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| NP1 | 3:1 | PLGA30 | PVA | 0.5 | EA | 1:1 | 15 | 5 | 189 ± 2 | 0.046 ± 0.003 | −30.9 ± 3.4 | 58 | 75 |
| NP2 | 3:1 | PLGA30 | PVA | 2 | DCM | 1:1 | 15 | 5 | 422 ± 2 | 0.280 ± 0.013 | −13.1 ± 1.8 | 64 | 59 |
| NP3 | 3:1 | PLGA7 | Pluronic F127 | 2 | EA | 1:10 | 15 | 5 | 316 ± 2 | 0.349 ± 0.051 | −28.2 ± 3.3 | 24 | 22 |
| NP4 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 220 ± 2 | 0.177 ± 0.023 | −20.1 ± 0.6 | 45 | 33 |
| NP5 | 3:1 | PLGA7 | Pluronic F127 | 2 | EA | 1:1 | 70 | 5 | 94 ± 1 | 0.285 ± 0.067 | −22.8 ± 2.3 | 88 | 26 |
| NP6 | 1:1 | PLGA7 | PVA | 0.5 | EA | 1:1 | 70 | 15 | 219 ± 2 | 0.489 ± 0.035 | −0.61 ± 2.1 | 78 | 41 |
| NP7 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 15 | 175 ± 2 | 0.191 ± 0.046 | −15.6 ± 3.7 | 29 | 67 |
| NP8 | 3:1 | PLGA30 | PVA | 2 | EA | 1:10 | 15 | 15 | 452 ± 3 | 0.499 ± 0.086 | −15.8 ± 2.9 | 31 | 66 |
| NP9 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 170 ± 2 | 0.358 ± 0.008 | −23.2 ± 4.2 | 40 | 34 |
| NP10 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 172 ± 2 | 0.178 ± 0.008 | −21.6 ± 2.8 | 37 | 37 |
| NP11 | 3:1 | PLGA7 | PVA | 0.5 | DCM | 1:1 | 15 | 5 | 443 ± 3 | 0.261 ± 0.034 | −14.5 ± 3.4 | 52 | 69 |
| NP12 | 1:1 | PLGA30 | Pluronic F127 | 0.5 | DCM | 1:10 | 70 | 5 | 260 ± 2 | 0.262 ± 0.045 | −24.3 ± 2.2 | 30 | 33 |
| NP13 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 184 ± 2 | 0.186 ± 0.080 | −23.2 ± 2.1 | 33 | 31 |
| NP14 | 3:1 | PLGA7 | Pluronic F127 | 2 | DCM | 1:10 | 70 | 5 | 219 ± 2 | 0.248 ± 0.065 | −20.3 ± 3.1 | 23 | 36 |
| NP15 | 1:1 | PLGA7 | PVA | 2 | EA | 1:1 | 15 | 5 | 119 ± 2 | 0.217 ± 0.013 | −11.2 ± 2.7 | 44 | 41 |
| NP16 | 1:1 | PLGA7 | Pluronic F127 | 2 | EA | 1:1 | 15 | 15 | 166 ± 3 | 0.556 ± 0.039 | −20.1 ± 1.4 | 16 | 3 |
| NP17 | 3:1 | PLGA7 | PVA | 0.5 | EA | 1:10 | 70 | 5 | 356 ± 3 | 0.474 ± 0.023 | −12.8 ± 1.3 | 48 | 68 |
| NP18 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:1 | 35 | 10 | 291 ± 2 | 0.373 ± 0.034 | −15.4 ± 0.3 | 37 | 55 |
| NP19 | 2:1 | PLGA24 | PVA | 1 | DMSO | 1:5 | 35 | 10 | 206 ± 3 | 0.135 ± 0.023 | −13.2 ± 1.3 | 39 | 33 |
| NP20 | 1:1 | PLGA30 | Pluronic F127 | 2 | DCM | 1:10 | 15 | 15 | 271 ± 1 | 0.508 ± 0.056 | −9.5 ± 1.5 | 27 | 31 |
| NP21 | 1:1 | PLGA30 | PVA | 0.5 | DCM | 1:1 | 70 | 5 | 282 ± 2 | 0.145 ± 0.034 | −16.4 ± 2.3 | 65 | 61 |
| NP22 | 2:1 | PLGA24 | Tween 80 | 2 | DMSO | 1:5 | 35 | 10 | 193 ± 4 | 0.224 ± 0.006 | −18.5 ± 3.3 | 40 | 28 |
| NP23 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 70 | 10 | 167 ± 3 | 0.276 ± 0.013 | −15.6 ± 1.3 | 41 | 17 |
| NP24 | 1:1 | PLGA7 | Pluronic F127 | 0.5 | EA | 1:1 | 15 | 5 | 94 ± 2 | 0.206 ± 0.043 | −13.9 ± 1.6 | 48 | 32 |
| NP25 | 1:1 | PLGA30 | Pluronic F127 | 0.5 | EA | 1:1 | 70 | 15 | 95 ± 3 | 0.238 ± 0.023 | −20.7 ± 3.1 | 55 | 30 |
| NP26 | 3:1 | PLGA7 | Pluronic F127 | 0.5 | DCM | 1:1 | 70 | 15 | 271 ± 4 | 0.116 ± 0.032 | −17.5 ± 0.7 | 53 | 65 |
| NP27 | 3:1 | PLGA7 | Pluronic F127 | 2 | EA | 1:10 | 70 | 15 | 377 ± 4 | 0.393 ± 0.051 | −23.9 ± 1.9 | 31 | 35 |
| NP28 | 2:1 | PLGA24 | Tween 80 | 1 | EA | 1:5 | 35 | 10 | 225 ± 2 | 0.269 ± 0.042 | −17.8 ± 1.7 | 41 | 49 |
| NP29 | 2:1 | PLGA7 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 182 ± 3 | 0.157 ± 0.023 | −15.7 ± 2.3 | 38 | 30 |
| NP30 | 1:1 | PLGA7 | PVA | 2 | DCM | 1:10 | 15 | 15 | 165 ± 4 | 0.263 ± 0.015 | −12.5 ± 0.4 | 4 | 6 |
| NP31 | 1:1 | PLGA7 | Pluronic F127 | 2 | EA | 1:10 | 70 | 5 | 345 ± 3 | 0.393 ± 0.045 | −12.1 ± 1.1 | 13 | 28 |
| NP32 | 3:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 202 ± 2 | 0.179 ± 0.035 | −22.6 ± 3.9 | 44 | 37 |
| NP33 | 1:1 | PLGA7 | Pluronic F127 | 0.5 | DCM | 1:10 | 15 | 15 | 232 ± 2 | 0.198 ± 0.024 | −23.5 ± 1.2 | 14 | 26 |
| NP34 | 3:1 | PLGA30 | PVA | 0.5 | EA | 1:1 | 70 | 15 | 173 ± 3 | 0.118 ± 0.021 | −9.2 ± 1.1 | 36 | 57 |
| NP35 | 1:1 | PLGA30 | Pluronic F127 | 2 | EA | 1:1 | 15 | 5 | 93 ± 2 | 0.275 ± 0.053 | −7.9 ± 1.4 | 24 | 17 |
| NP36 | 1:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 152 ± 3 | 0.235 ± 0.014 | −16.7 ± 1.2 | 27 | 9 |
| NP37 | 3:1 | PLGA7 | PVA | 0.5 | EA | 1:10 | 15 | 15 | 239 ± 2 | 0.164 ± 0.032 | −9.1 ± 0.3 | 11 | 65 |
| NP38 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:10 | 35 | 10 | 178 ± 2 | 0.226 ± 0.035 | −13.3 ± 2.5 | 15 | 29 |
| NP39 | 1:1 | PLGA30 | PVA | 2 | DCM | 1:10 | 70 | 5 | 221 ± 2 | 0.164 ± 0.003 | −13.5 ± 1.9 | 8 | 29 |
| NP40 | 1:1 | PLGA30 | PVA | 2 | EA | 1:1 | 70 | 15 | 118 ± 3 | 0.187 ± 0.013 | −8.1 ± 0.9 | 35 | 25 |
| NP41 | 3:1 | PLGA7 | Pluronic F127 | 2 | DCM | 1:10 | 15 | 15 | 418 ± 5 | 0.468 ± 0.022 | −11.4 ± 4.1 | 32 | 38 |
| NP42 | 3:1 | PLGA30 | Pluronic F127 | 0.5 | DCM | 1:1 | 15 | 5 | 337 ± 4 | 0.144 ± 0.006 | −21.3 ± 1.1 | 37 | 55 |
| NP43 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 242 ± 2 | 0.238 ± 0.011 | −20.1 ± 0.9 | 43 | 44 |
| NP44 | 3:1 | PLGA30 | Pluronic F127 | 2 | EA | 1:10 | 70 | 5 | 315 ± 3 | 0.473 ± 0.018 | −16.4 ± 0.4 | 35 | 36 |
| NP45 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 5 | 175 ± 2 | 0.184 ± 0.023 | −20.2 ± 0.6 | 52 | 23 |
| NP46 | 3:1 | PLGA30 | PVA | 0.5 | DCM | 1:10 | 70 | 15 | 216 ± 3 | 0.155 ± 0.046 | −9.7 ± 1.1 | 36 | 52 |
| NP47 | 3:1 | PLGA30 | Pluronic F127 | 2 | DCM | 1:1 | 70 | 15 | 327 ± 2 | 0.271 ± 0.007 | −3.4 ± 1.4 | 66 | 35 |
| NP48 | 1:1 | PLGA7 | PVA | 0.5 | DCM | 1:10 | 70 | 5 | 162 ± 2 | 0.288 ± 0.016 | −16.7 ± 5.6 | 30 | 8 |
| NP49 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 15 | 10 | 225 ± 3 | 0.205 ± 0.018 | −15.7 ± 1.6 | 32 | 27 |
| NP50 | 2:1 | PLGA24 | Tween 80 | 1 | DCM | 1:5 | 35 | 10 | 354 ± 4 | 0.416 ± 0.023 | −19.9 ± 1.5 | 34 | 43 |
| NP51 | 2:1 | PLGA30 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 220 ± 2 | 0.175 ± 0.018 | −24.5 ± 1.1 | 39 | 37 |
| NP52 | 1:1 | PLGA30 | PVA | 0.5 | DCM | 1:10 | 15 | 5 | 209 ± 3 | 0.212 ± 0.024 | −16.9 ± 0.6 | 25 | 49 |
| NP53 | 2:1 | PLGA24 | Tween 80 | 0.5 | DMSO | 1:5 | 35 | 10 | 323 ± 4 | 0.248 ± 0.017 | −16.1 ± 4.7 | 45 | 45 |
| NP54 | 3:1 | PLGA7 | PVA | 2 | DCM | 1:1 | 70 | 15 | 302 ± 5 | 0.166 ± 0.050 | −15.0 ± 2.1 | 34 | 59 |
| NP55 | 1:1 | PLGA30 | PVA | 0.5 | EA | 1:10 | 70 | 15 | 163 ± 3 | 0.234 ± 0.080 | −17.1 ± 1.1 | 16 | 22 |
| NP56 | 1:1 | PLGA7 | Pluronic F127 | 2 | DCM | 1:1 | 15 | 5 | 257 ± 2 | 0.176 ± 0.020 | −17.8 ± 2.1 | 50 | 21 |
| NP57 | 2:1 | PLGA24 | Pluronic F127 | 1 | DMSO | 1:5 | 35 | 10 | 197 ± 4 | 0.212 ± 0.030 | −15.6 ± 4.6 | 49 | 55 |
| NP58 | 3:1 | PLGA30 | Pluronic F127 | 0.5 | EA | 1:10 | 15 | 15 | 373 ± 3 | 0.479 ± 0.050 | −24.5 ± 2.3 | 6 | 20 |
| NP59 | 2:1 | PLGA24 | Tween 80 | 1 | DMSO | 1:5 | 35 | 10 | 225 ± 3 | 0.207 ± 0.030 | −22.4 ± 2.9 | 44 | 30 |
| NP60 | 1:1 | PLGA30 | PVA | 0.5 | DCM | 1:1 | 15 | 15 | 311 ± 3 | 0.150 ± 0.050 | −18.2 ± 1.6 | 63 | 67 |
| Response | Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Size | Model | 4.293 × 105 | 44 | 9757.75 | 3.33 | 0.0071 | significant |
| Pure error | 4664.39 | 5 | 932.88 | ||||
| Residual | 43,955.54 | 15 | 2930.37 | ||||
| Lack of fit | 39,291.15 | 10 | 3929.11 | 4.21 | 0.0630 | ||
| Cor total | 4.733 × 105 | 59 | |||||
| Loading efficiency | Model | 15,793.89 | 36 | 438.72 | 8.98 | <0.0001 | significant |
| PureError | 100.33 | 5 | 20.07 | ||||
| Residual | 1124.08 | 23 | 48.87 | ||||
| LackofFit | 1023.75 | 18 | 56.88 | 2.83 | 0.1263 | ||
| CorTotal | 16,917.98 | 59 |
| Name | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| PLGA:RIF ratio | Is in range | 1:1 | 1:3 |
| MW of PLGA | Is in range | 7000–17,000 | 30,000–60,000 |
| Surfactant | Is in range | PVA | Tween80 |
| Organic solvent | Is equal to DCM | DCM | EA |
| Homogenization power | Is equal to 70 W | 15 W | 70 W |
| Homogenization time | Is in range | 5 min | 15 min |
| Organic and aqueous phase ratio | Is equal to 1:1 | 1:1 | 1:10 |
| Size | Minimize | 93.4 | 451.8 |
| Loading efficiency | Maximize | 4 | 88.2 |
| Size, nm | PDI | Zeta Potential, mV | Encapsulation Efficiency, % | Loading Efficiency, % | Yield, % | |
|---|---|---|---|---|---|---|
| Predicted | 228 | 0.120 | −24 | 93 | 70 | 45 |
| Experimental | 223 ± 2 | 0.110 ± 0.01 | −26 ± 2 | 91 ± 2 | 67 ± 1 | 47 ± 2 |
| Error (%) | 2.2 | 9.1 | 7.7 | 2.2 | 4.5 | 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yessentayeva, N.A.; Galiyeva, A.R.; Daribay, A.T.; Sadyrbekov, D.T.; Moustafine, R.I.; Tazhbayev, Y.M. Optimization of Polylactide-Co-Glycolide-Rifampicin Nanoparticle Synthesis, In Vitro Study of Mucoadhesion and Drug Release. Polymers 2024, 16, 2466. https://doi.org/10.3390/polym16172466
Yessentayeva NA, Galiyeva AR, Daribay AT, Sadyrbekov DT, Moustafine RI, Tazhbayev YM. Optimization of Polylactide-Co-Glycolide-Rifampicin Nanoparticle Synthesis, In Vitro Study of Mucoadhesion and Drug Release. Polymers. 2024; 16(17):2466. https://doi.org/10.3390/polym16172466
Chicago/Turabian StyleYessentayeva, Nazgul A., Aldana R. Galiyeva, Arailym T. Daribay, Daniyar T. Sadyrbekov, Rouslan I. Moustafine, and Yerkeblan M. Tazhbayev. 2024. "Optimization of Polylactide-Co-Glycolide-Rifampicin Nanoparticle Synthesis, In Vitro Study of Mucoadhesion and Drug Release" Polymers 16, no. 17: 2466. https://doi.org/10.3390/polym16172466
APA StyleYessentayeva, N. A., Galiyeva, A. R., Daribay, A. T., Sadyrbekov, D. T., Moustafine, R. I., & Tazhbayev, Y. M. (2024). Optimization of Polylactide-Co-Glycolide-Rifampicin Nanoparticle Synthesis, In Vitro Study of Mucoadhesion and Drug Release. Polymers, 16(17), 2466. https://doi.org/10.3390/polym16172466






