Investigation of the Structural Changes in Silk Due to Tin Weighting
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Analytical Techniques
2.2.1. SEM-EDX Analysis
2.2.2. FTIR-ATR Analysis
2.2.3. XRD Analysis
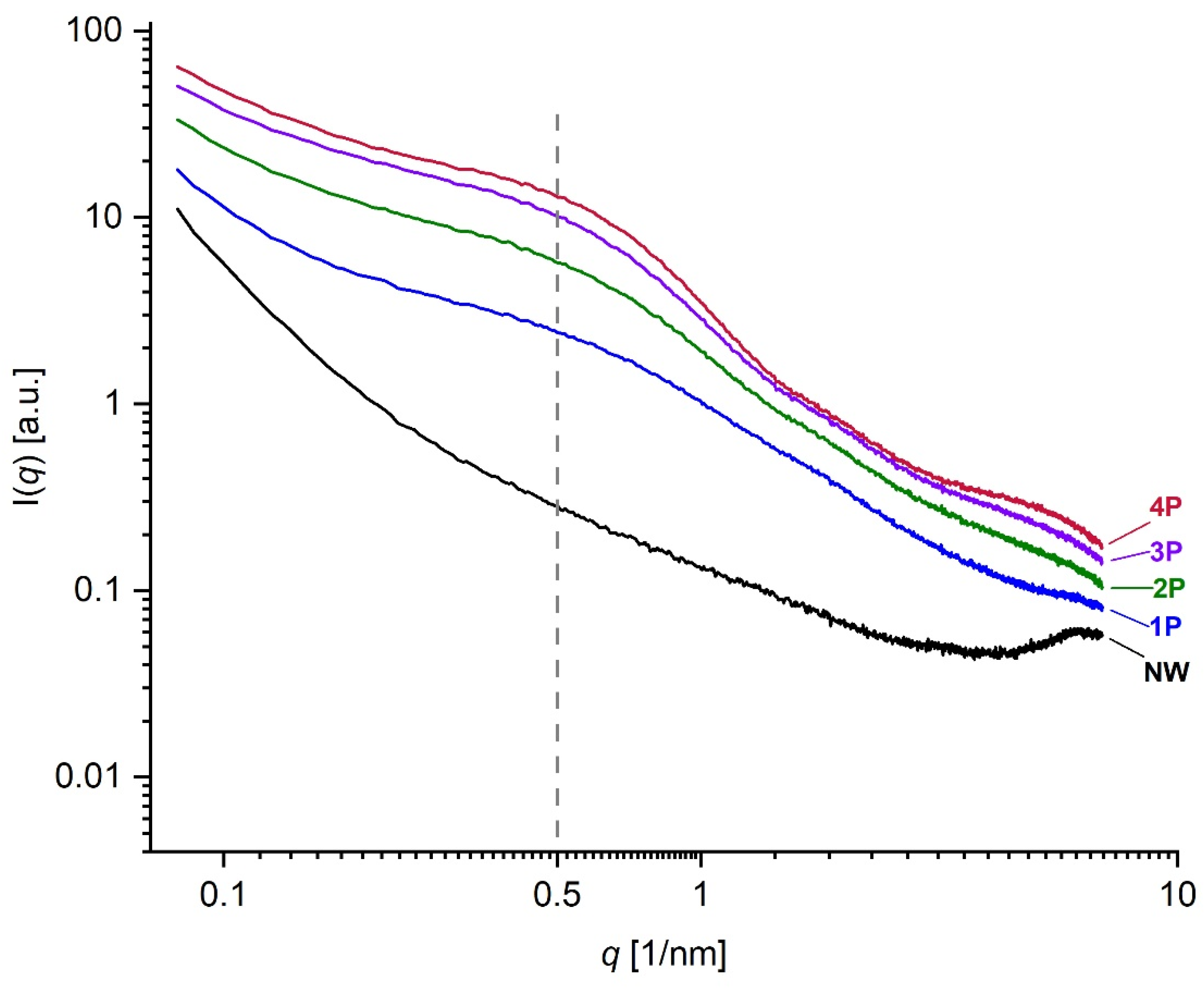
2.2.4. SAXS and SWAXS Analysis
2.2.5. Contact Angle and Hydrophilicity
3. Results
3.1. Weight Gain of Tin-Weighted Silk
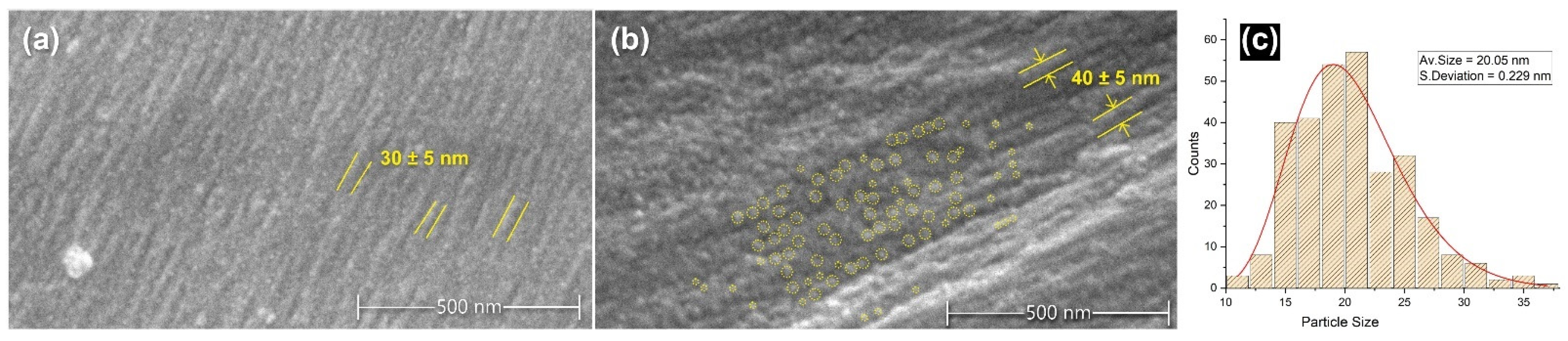
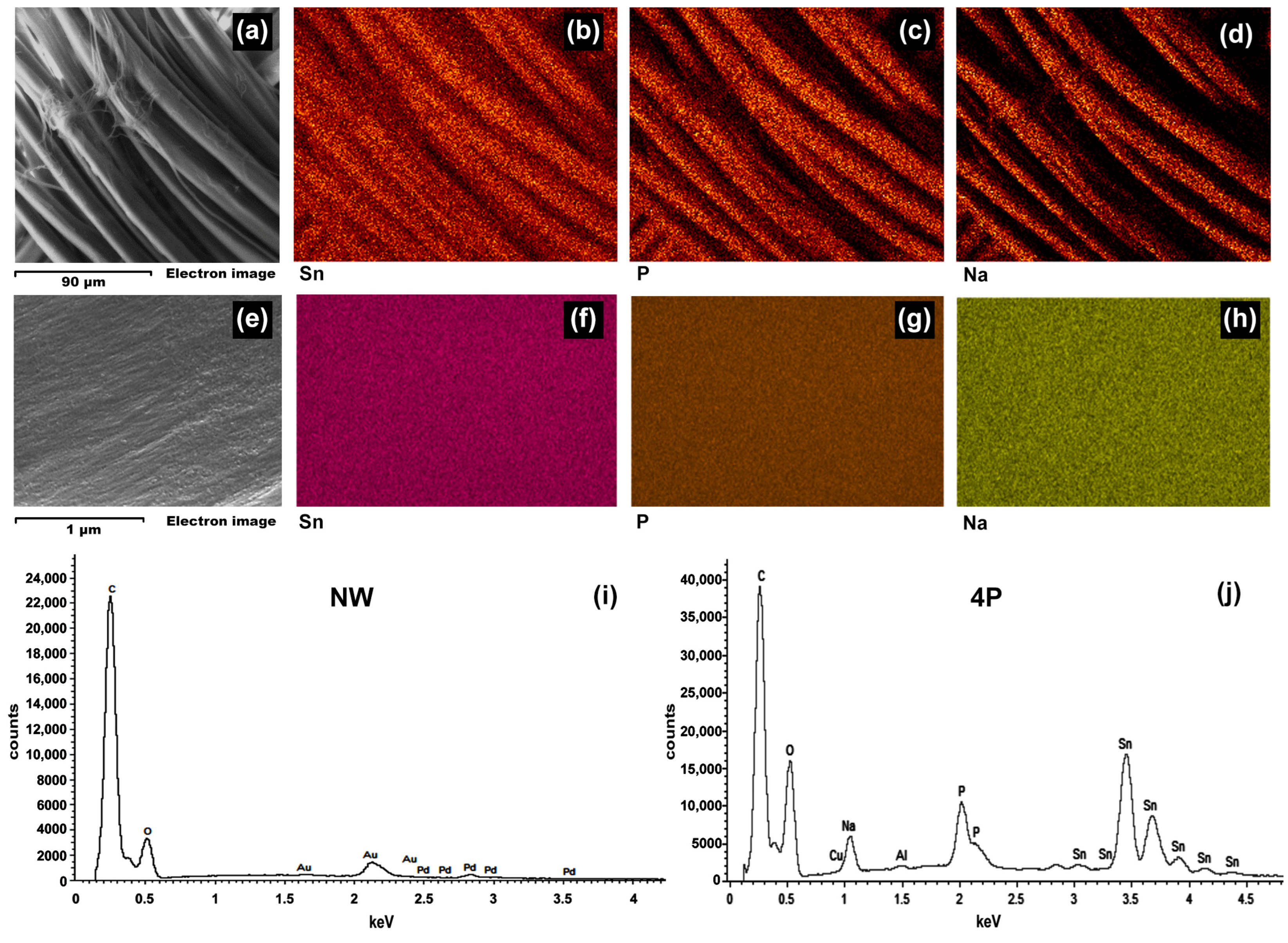
3.2. Morphology and Elemental Mapping
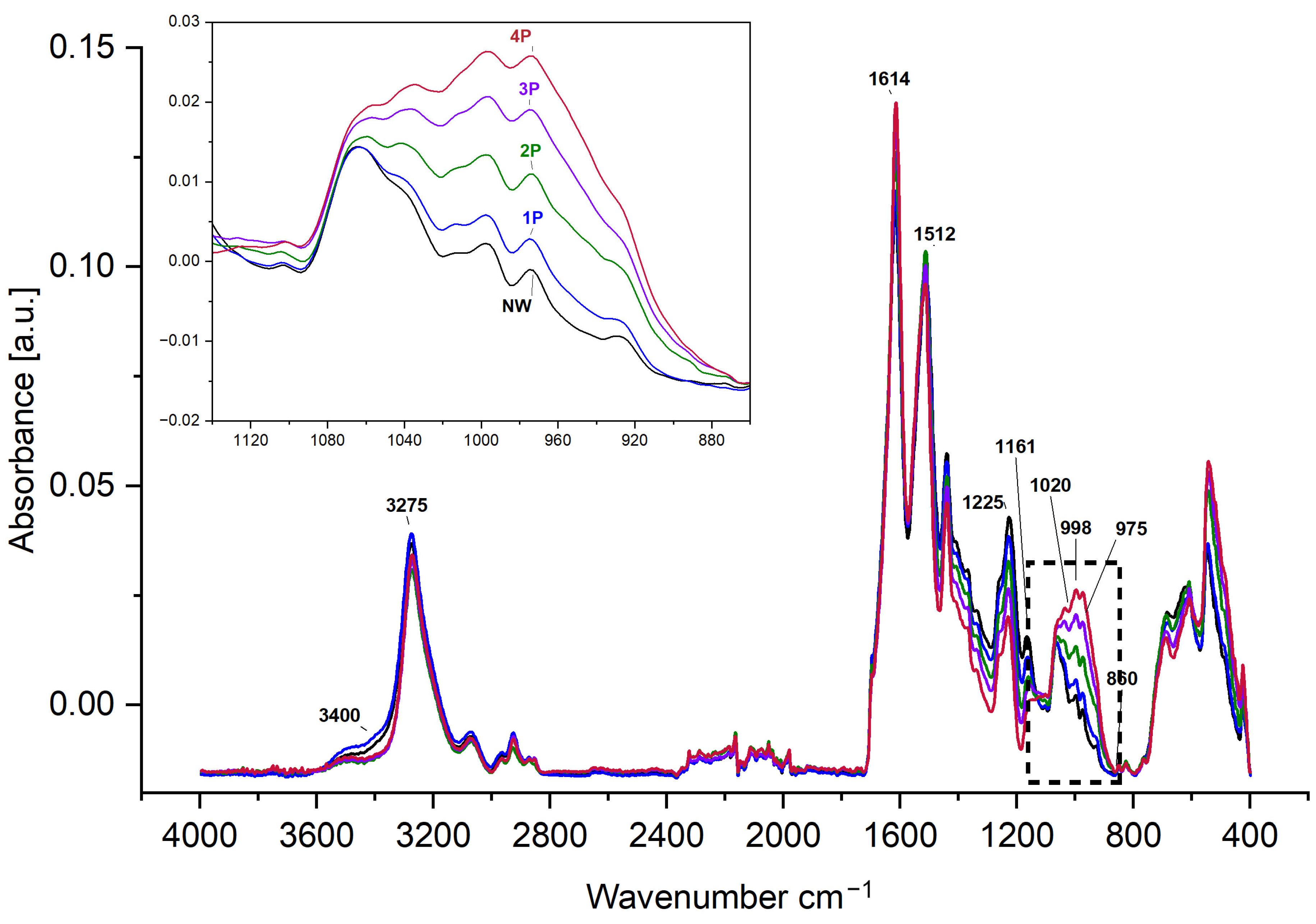
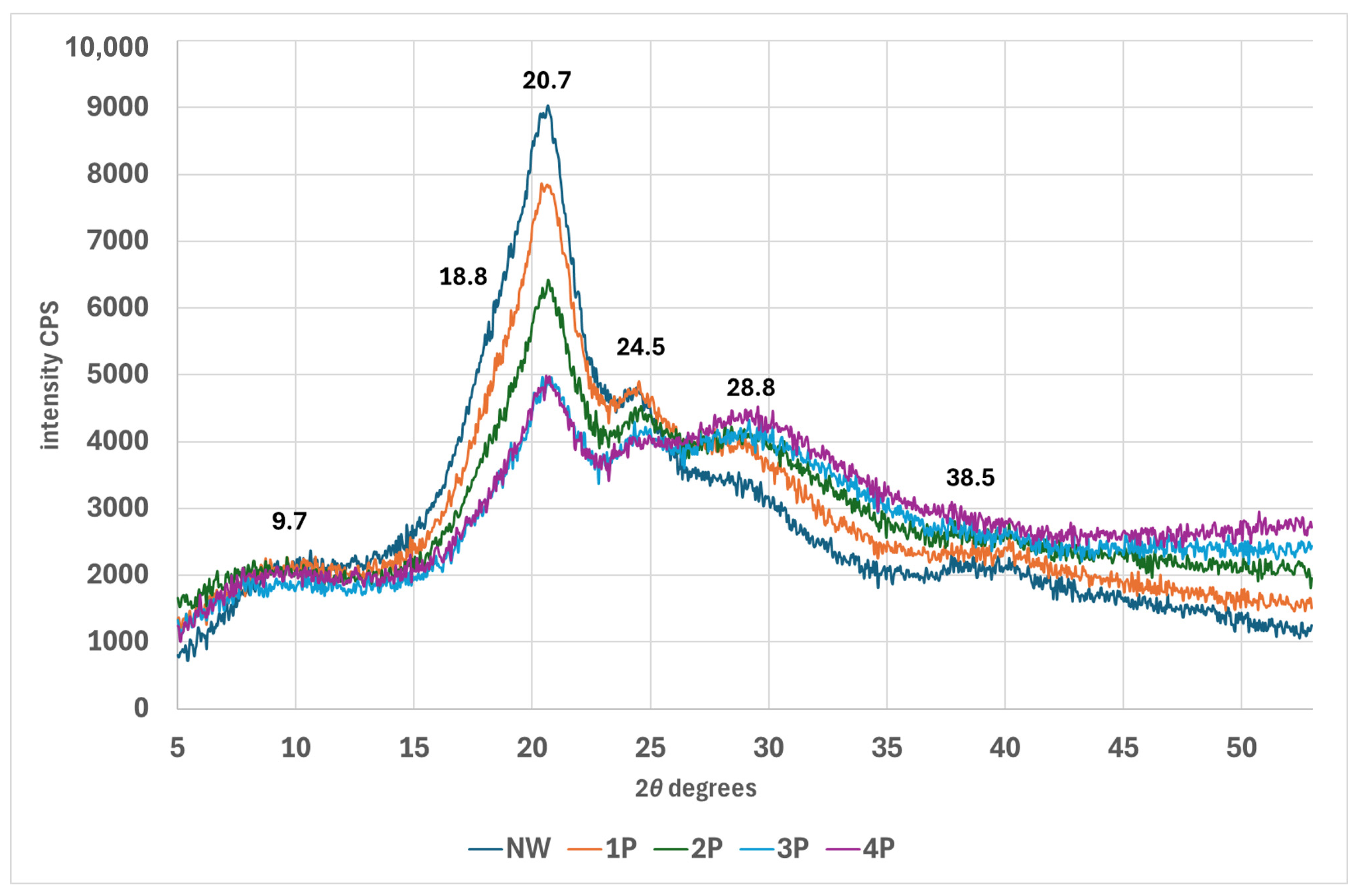
3.3. Structural Investigations
4. Conclusions
- (1)
- SEM-EDX analysis showed the deposition of tin-phosphate nanoparticles on the surface of silk fibres and estimated nanofeatures’ average size to be around 20 nm.
- (2)
- FTIR-ATR spectroscopy indicated alterations in the hydrogen bonding network and water accessibility within the silk fibres, while also suggesting a slight increase in β-sheet content in heavily weighted silk compared to NW silk, pointing to some changes in the protein structure.
- (3)
- Using XRD and SWAXS analyses, significant modifications to the crystalline and amorphous regions of silk fibres could be evidenced, with a notable reverse order in crystallinity with a decrease in long-range order and an increase in short-range ordered or amorphous regions.
- (4)
- SAXS analysis further demonstrated changes in the nanoscale structure of silk fibres, particularly at the 12.5 nm scale, in addition to the effect it has on the structure of the silk fibres close to the atomistic-scale size level.
- (5)
- Contact angle measurements demonstrated a significant increase in silk hydrophilicity after tin-phosphate weighting, transforming the material from moderately hydrophobic to highly hydrophilic.
- (6)
- Overall, tin-phosphate weighting significantly alters the microstructure of silk fibres. SEM-EDS showed the nanoscale modification of the fibres by the weighting process. FTIR analysis indicates an increase in β-sheet content, suggesting an enhanced local order within the previously amorphous regions. This is corroborated by XRD data showing increased short-range order, while a decrease in long-range order suggests the formation of smaller, ordered domains. Importantly, the overall fibre structure remains largely intact, as evidenced by SAXS and SWAXS results. A notable consequence of these structural changes is a significant increase in fibre hydrophilicity.
- (7)
- The structural modifications induced by tin-weighting could have important implications for the physical properties and long-term stability of silk textiles. The incorporation of tin-phosphate nanoparticles appears to restrict the flexibility of polymer chains within the silk fibres, potentially affecting their mechanical properties and degradation behaviour.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babu, K.M. Silk: Processing, properties and applications. In Woodhead Publishing Series in Textiles; No. 149; Woodhead Publication: Oxford, UK, 2013. [Google Scholar]
- Chand, S.; Chand, S.; Raula, B. Usage of Silkworm Materials in Various Ground of Science and Research. J. Nat. Fibers 2023, 20, 2139328. [Google Scholar] [CrossRef]
- Kozłowski, R.M. (Ed.) Handbook of Natural Fibres. Vol. 1: Types, Properties and Factors Affecting Breeding and Cultivation; Woodhead Publishing Series in Textiles; No. 118; Woodhead Publication: Oxford, UK, 2012. [Google Scholar]
- Asakura, T.; Williamson, M.P. A review on the structure of Bombyx mori silk fibroin fiber studied using solid-state NMR: An antipolar lamella with an 8-residue repeat. Int. J. Biol. Macromol. 2023, 245, 125537. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Zuo, B. Effect of sodium carbonate concentrations on the degumming and regeneration process of silk fibroin. J. Text. Inst. 2015, 106, 311–319. [Google Scholar] [CrossRef]
- Sen, K.; Babu, K.M. Macrocharacterization and analysis of amino acid composition. J Appl. Polym. Sci 2004, 92, 1080–1097. [Google Scholar] [CrossRef]
- Reddy, R.; Jiang, Q.; Aramwit, P.; Reddy, N. Litter to Leaf: The Unexplored Potential of Silk Byproducts. Trends Biotechnol. 2021, 39, 706–718. [Google Scholar] [CrossRef]
- The practice and ethics of silk weighting. J. Text. Inst. Proc. 1925, 16, P327–P334. [CrossRef]
- Sakaguchi, I. Cumulative Tin-Weighting of Silk with Stannic Chloride. Text. Res. J. 1969, 39, 1053–1054. [Google Scholar] [CrossRef]
- Roberts, N.M.; Mack, P.B. A Study of the Effects of Light and Air on the Physical Properties of Weighted and Unweighted Silks. J. Home Econ. 1932, 2, 151–165. [Google Scholar]
- Gilcrease, S. The Effect of Humidification on Artificially Aged Tin-Weighted Silks; University of Rhode Island: Kingston, RI, USA, 2018. [Google Scholar]
- Garside, P.; Wyeth, P.; Zhang, X. Understanding the ageing behaviour of nineteenth and twentieth century tin-weighted silks. J. Inst. Conserv. 2010, 33, 179–193. [Google Scholar] [CrossRef]
- Zhang, X.; Berghe, I.V.; Wyeth, P. Heat and moisture promoted deterioration of raw silk estimated by amino acid analysis. J. Cult. Herit. 2011, 12, 408–411. [Google Scholar] [CrossRef]
- Annadurai, V.; Subramanyam, G.; Urs, R.G.; Somashekar, R. Structure-property relation in varieties of silk fibers. J. Appl. Polym. Sci. 2001, 79, 1979–1985. [Google Scholar] [CrossRef]
- Garside, P.; Mills, G.A.; Smith, J.R.; Wyeth, P. An Investigation of Weighted and Degraded Silk by Complementary Microscopy Techniques. 2014. Available online: https://api.semanticscholar.org/CorpusID:135323639 (accessed on 22 August 2024).
- Mahltig, B.; Grethe, T. High-Performance and Functional Fiber Materials—A Review of Properties, Scanning Electron Microscopy SEM and Electron Dispersive Spectroscopy EDS. Textiles 2022, 2, 209–251. [Google Scholar] [CrossRef]
- Sevim, A.M. Investigation of the Degradation Stages of Archaeological and Historical Silk Textiles:An ATR-FTIR Spectroscopic Study. 2019. Available online: https://api.semanticscholar.org/CorpusID:150356886 (accessed on 22 August 2024).
- Koperska, M.A.; Pawcenis, D.; Bagniuk, J.; Zaitz, M.M.; Missori, M.; Łojewski, T.; Łojewska, J. Degradation markers of fibroin in silk through infrared spectroscopy. Polym. Degrad. Stab. 2014, 105, 185–196. [Google Scholar] [CrossRef]
- Badillo-Sanchez, D.; Chelazzi, D.; Giorgi, R.; Cincinelli, A.; Baglioni, P. Understanding the structural degradation of South American historical silk: A Focal Plane Array (FPA) FTIR and multivariate analysis. Sci. Rep. 2019, 9, 17239. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Qi, Z.; Knight, D.P.; Shao, Z.; Chen, X. Synchrotron FTIR Microspectroscopy of Single Natural Silk Fibers. Biomacromolecules 2011, 12, 3344–3349. [Google Scholar] [CrossRef]
- Pawcenis, D.; Koperska, M.A.; Milczarek, J.M.; Łojewski, T.; Łojewska, J. Size exclusion chromatography for analyses of fibroin in silk: Optimization of sampling and separation conditions. Appl. Phys. A 2014, 114, 301–308. [Google Scholar] [CrossRef][Green Version]
- Pawcenis, D.; Smoleń, M.; Aksamit-Koperska, M.A.; Łojewski, T.; Łojewska, J. Evaluating the impact of different exogenous factors on silk textiles deterioration with use of size exclusion chromatography. Appl. Phys. A 2016, 122, 576. [Google Scholar] [CrossRef]
- Tse, S.; Dupont, A.-L. Measuring Silk Deterioration by High-Performance Size-Exclusion Chromatography, Viscometry and Electrophoresis. 2001. Available online: https://api.semanticscholar.org/CorpusID:138624253 (accessed on 22 August 2024).
- Riekel, C.; Burghammer, M.; Rosenthal, M. Nanoscale X-Ray Diffraction of Silk Fibers. Front. Mater. 2019, 6, 315. [Google Scholar] [CrossRef]
- Ryu, M.; Honda, R.; Cernescu, A.; Vailionis, A.; Balčytis, A.; Vongsvivut, J.; Juodkazis, S. Nanoscale optical and structural characterisation of silk. Beilstein J. Nanotechnol. 2019, 10, 922–929. [Google Scholar] [CrossRef]
- Martel, A.; Burghammer, M.; Davies, R.J.; Di Cola, E.; Vendrely, C.; Riekel, C. Silk Fiber Assembly Studied by Synchrotron Radiation SAXS/WAXS and Raman Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17070–17074. [Google Scholar] [CrossRef]
- Garside, P.; Wyeth, P. Crystallinity and degradation of silk: Correlations between analytical signatures and physical condition on ageing. Appl. Phys. A 2007, 89, 871–876. [Google Scholar] [CrossRef]
- Gong, D.; Zhang, X.; Gong, Y. Insight Into the Crystallinity of Chinese Ancient Silk by Synchrotron Radiation-Based and Conventional X-ray Diffraction Methods. J. Conserv. Sci. 2020, 36, 1–14. [Google Scholar] [CrossRef]
- Greiff, S.; Kutzke, H.; Riekel, C.; Wyeth, P.; Lahlil, S. Surveying silk fibre degradation by crystallinity determination: A study on the Tang dynasty silk treasure from Famen Temple, China. In Proceedings of the AHRC Research Centre for Textile Conservation and Textile Studies: First Annual Conference: Scientific Analysis of Ancient and Historic Textiles: Informing Preservation, Display and Interpretation, Winchester, UK, 13–15 July 2004. [Google Scholar]
- Hacke, M. Weighted silk: History, analysis and conservation. Stud. Conserv. 2009, 54, 3–15. [Google Scholar] [CrossRef]
- Carboni, P. Silk: Biology, Chemistry, Technology; Chapman & Hall: London, UK, 1952. [Google Scholar]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature. J. Phys. Chem. Solids 2021, 151, 109928. [Google Scholar] [CrossRef]
- Cerar, J.; Jamnik, A.; Szilágyi, I.; Tomšič, M. Solvation of nonionic poly(ethylene oxide) surfactant Brij 35 in organic and aqueous-organic solvents. J. Colloid Interface Sci. 2021, 594, 150–159. [Google Scholar] [CrossRef]
- Bras, W.; Hammel, M. Scattering Methods and their Application in Colloid and Interface Science. J. Appl. Crystallogr. 2019, 52, 243–244. [Google Scholar] [CrossRef]
- Wang, Q.; Schniepp, H.C. Nanofibrils as Building Blocks of Silk Fibers: Critical Review of the Experimental Evidence. JOM 2019, 71, 1248–1263. [Google Scholar] [CrossRef]
- Wang, Q.; Ling, S.; Yao, Q.; Li, Q.; Hu, D.; Dai, Q.; Zhang, Y. Observations of 3 nm Silk Nanofibrils Exfoliated from Natural Silkworm Silk Fibers. ACS Mater. Lett. 2020, 2, 153–160. [Google Scholar] [CrossRef]
- Greving, I.; Cai, M.; Vollrath, F.; Schniepp, H.C. Shear-Induced Self-Assembly of Native Silk Proteins into Fibrils Studied by Atomic Force Microscopy. Biomacromolecules 2012, 13, 676–682. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Asakura, T.; Kuzuhara, A.; Tabeta, R.; Saito, H. Conformational characterization of Bombyx mori silk fibroin in the solid state by high-frequency carbon-13 cross polarization-magic angle spinning NMR, x-ray diffraction, and infrared spectroscopy. Macromolecules 1985, 18, 1841–1845. [Google Scholar] [CrossRef]
- Koshy, J.; Chandran, A.; Samuel, S.; George, K.C. Optical properties of SnO2 nanoparticles. In Proceedings of the Light and Its Interactions with Matter, NIT, Calicut, India, 19–21 March 2014; pp. 192–196. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, C.; Ma, X.; Chen, C.; Zhu, H. Study on the preparation of collagen-modified silk fibroin films and their properties. Biomed. Mater. 2006, 1, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.K.M.M.; Shubhra, Q.T.H.; Al-Imran, G.; Barai, S.; Islam, M.R.; Rahman, M.M. Preparation and characterization of natural silk fiber-reinforced polypropylene and synthetic E-glass fiber-reinforced polypropylene composites: A comparative study. J. Compos. Mater. 2011, 45, 2301–2308. [Google Scholar] [CrossRef]
- Pham, D.T.; Saelim, N.; Tiyaboonchai, W. Crosslinked fibroin nanoparticles using EDC or PEI for drug delivery: Physicochemical properties, crystallinity and structure. J. Mater. Sci. 2018, 53, 14087–14103. [Google Scholar] [CrossRef]
- Ki, C.S.; Kim, J.W.; Oh, H.J.; Lee, K.H.; Park, Y.H. The effect of residual silk sericin on the structure and mechanical property of regenerated silk filament. Int. J. Biol. Macromol. 2007, 41, 346–353. [Google Scholar] [CrossRef]
- Fang, G.; Huang, Y.; Tang, Y.; Qi, Z.; Yao, J.; Shao, Z.; Chen, X. Insights into Silk Formation Process: Correlation of Mechanical Properties and Structural Evolution during Artificial Spinning of Silk Fibers. ACS Biomater. Sci. Eng. 2016, 2, 1992–2000. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Luo, X.; Zhang, L.; Wu, M.; Zhang, W. Systematic assessment of the silk deterioration behaviors for silk aging prediction. Polym. Degrad. Stab. 2023, 218, 110532. [Google Scholar] [CrossRef]
- Martel, A.; Burghammer, M.; Davies, R.J.; Riekel, C. Thermal Behavior of Bombyx mori Silk: Evolution of Crystalline Parameters, Molecular Structure, and Mechanical Properties. Biomacromolecules 2007, 8, 3548–3556. [Google Scholar] [CrossRef]
- Lu, Y.H.; Lin, H.; Chen, Y.Y.; Wang, C.; Hua, Y.R. Structure and performance ofBombyx mori silk modified with nano-TiO2 and chitosan. Fibers Polym 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Szirtes, L.; Révai, Z.; Megyeri, J.; Kuzmann, E. Investigations of tin (II/IV) phosphates prepared by various methods, Mössbauer study and X-ray diffraction characterization. Radiat. Phys. Chem. 2009, 78, 239–243. [Google Scholar] [CrossRef]
- Parlin, A.F.; Stratton, S.M.; Culley, T.M.; Guerra, P.A. A laboratory-based study examining the properties of silk fabric to evaluate its potential as a protective barrier for personal protective equipment and as a functional material for face coverings during the COVID-19 pandemic. PLoS ONE 2020, 15, e0239531. [Google Scholar] [CrossRef]
- Parlin, A.F.; Yermakov, M.; Stratton, S.M.; Reutman, S.R.; Culley, T.M.; Guerra, P.A.; Grinshpun, S.A. Effect of Double Masking with Silk or Cotton Over-masks on the Source Control Capabilities of a Surgical Mask. Aerosol Air Qual. Res. 2022, 22, 220036. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elrefaey, I.; Mahgoub, H.; Vettorazzo, C.; Marinšek, M.; Meden, A.; Jamnik, A.; Tomšič, M.; Strlič, M. Investigation of the Structural Changes in Silk Due to Tin Weighting. Polymers 2024, 16, 2481. https://doi.org/10.3390/polym16172481
Elrefaey I, Mahgoub H, Vettorazzo C, Marinšek M, Meden A, Jamnik A, Tomšič M, Strlič M. Investigation of the Structural Changes in Silk Due to Tin Weighting. Polymers. 2024; 16(17):2481. https://doi.org/10.3390/polym16172481
Chicago/Turabian StyleElrefaey, Ibrahim, Hend Mahgoub, Chiara Vettorazzo, Marjan Marinšek, Anton Meden, Andrej Jamnik, Matija Tomšič, and Matija Strlič. 2024. "Investigation of the Structural Changes in Silk Due to Tin Weighting" Polymers 16, no. 17: 2481. https://doi.org/10.3390/polym16172481
APA StyleElrefaey, I., Mahgoub, H., Vettorazzo, C., Marinšek, M., Meden, A., Jamnik, A., Tomšič, M., & Strlič, M. (2024). Investigation of the Structural Changes in Silk Due to Tin Weighting. Polymers, 16(17), 2481. https://doi.org/10.3390/polym16172481







