Research on the Thermal Aging Mechanism of Polyvinyl Alcohol Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. High-Temperature Accelerated Aging Test
2.3. Characterization
3. Results
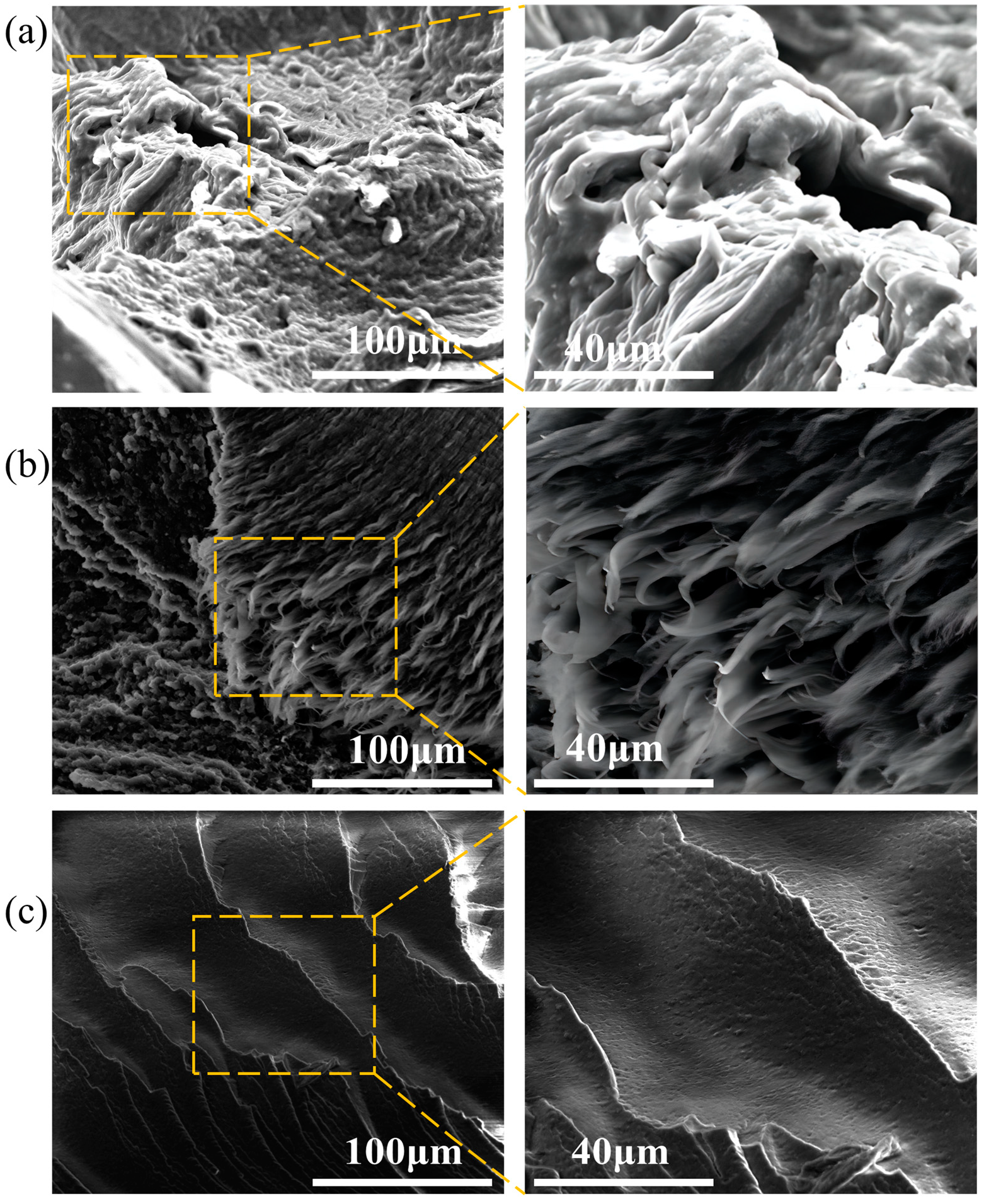
3.1. Macro Performance Analysis
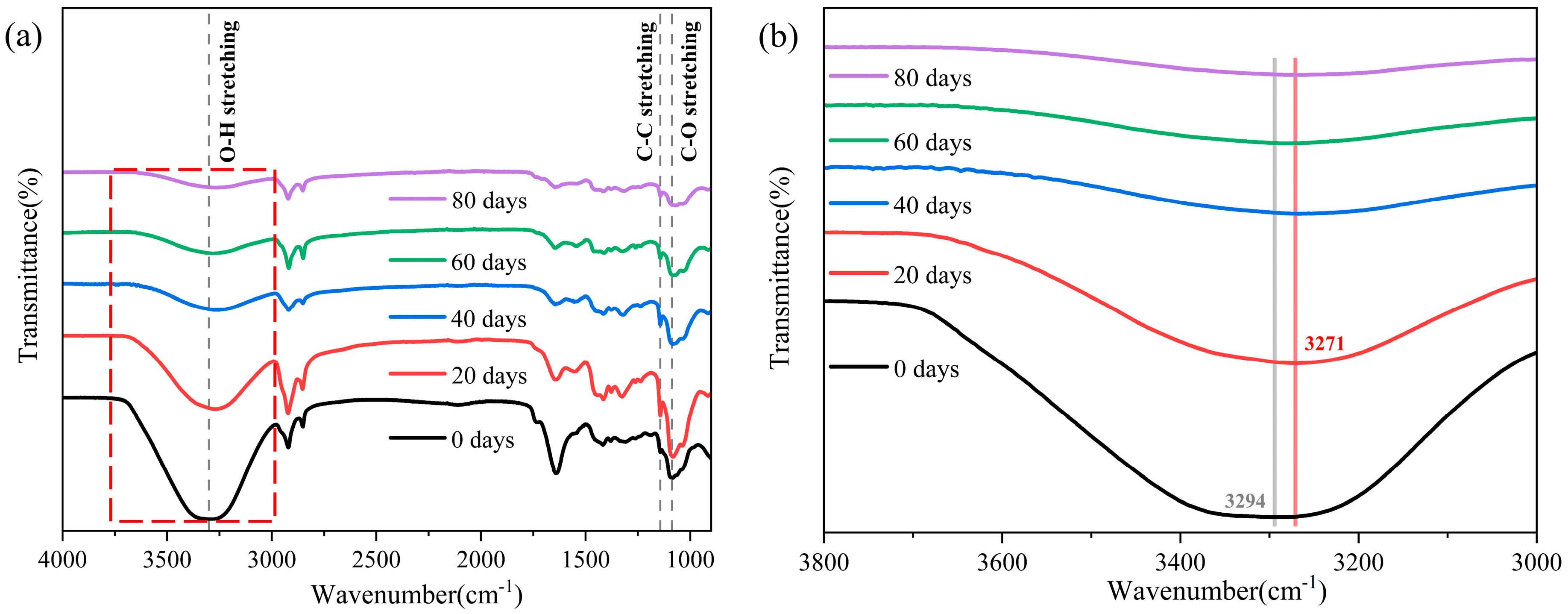
3.2. ATR-FTIR Results
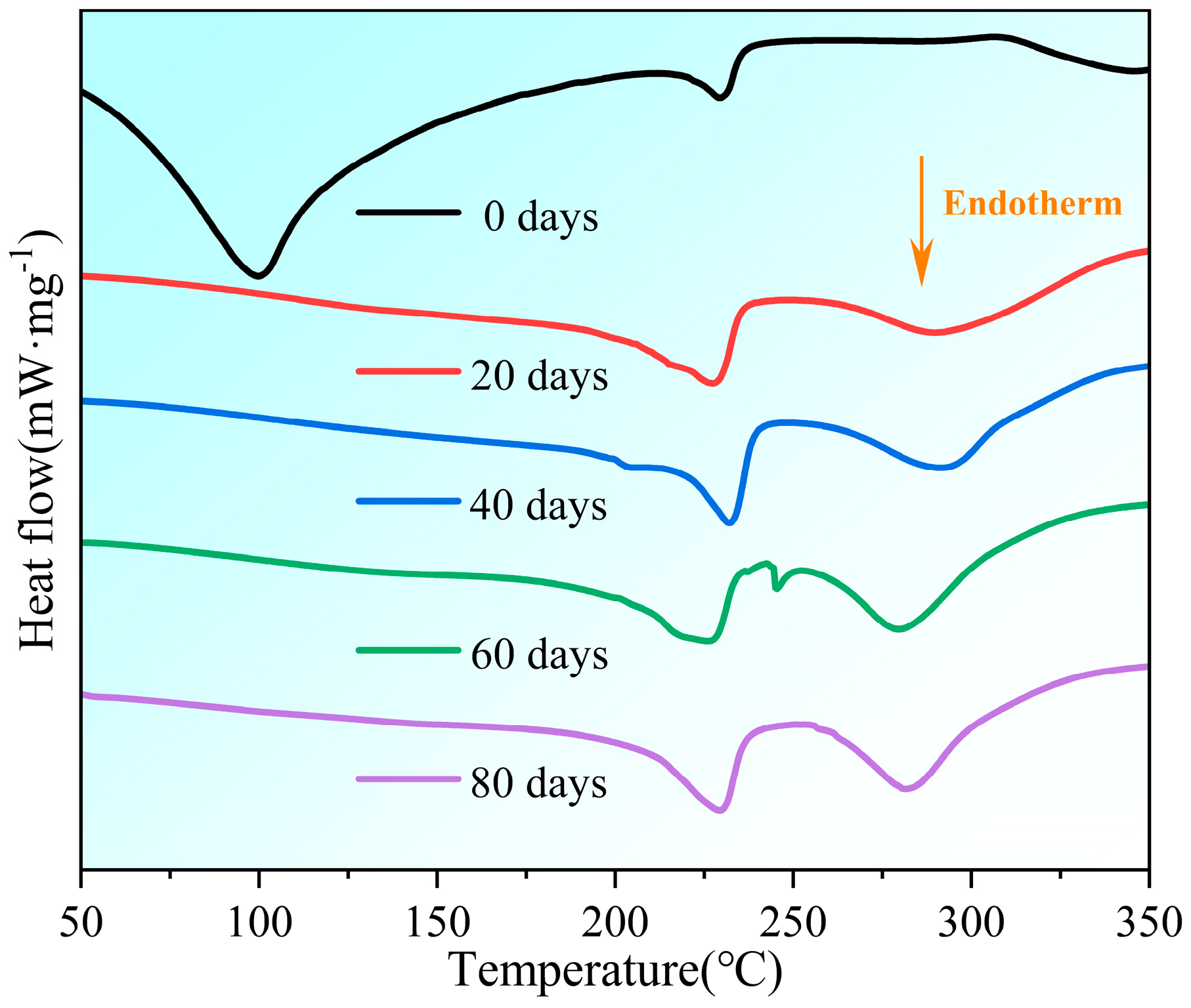
3.3. Thermal Analysis Results
3.4. XRD Results
3.5. Mechanical Properties
4. Discussion
5. Conclusions
- (1)
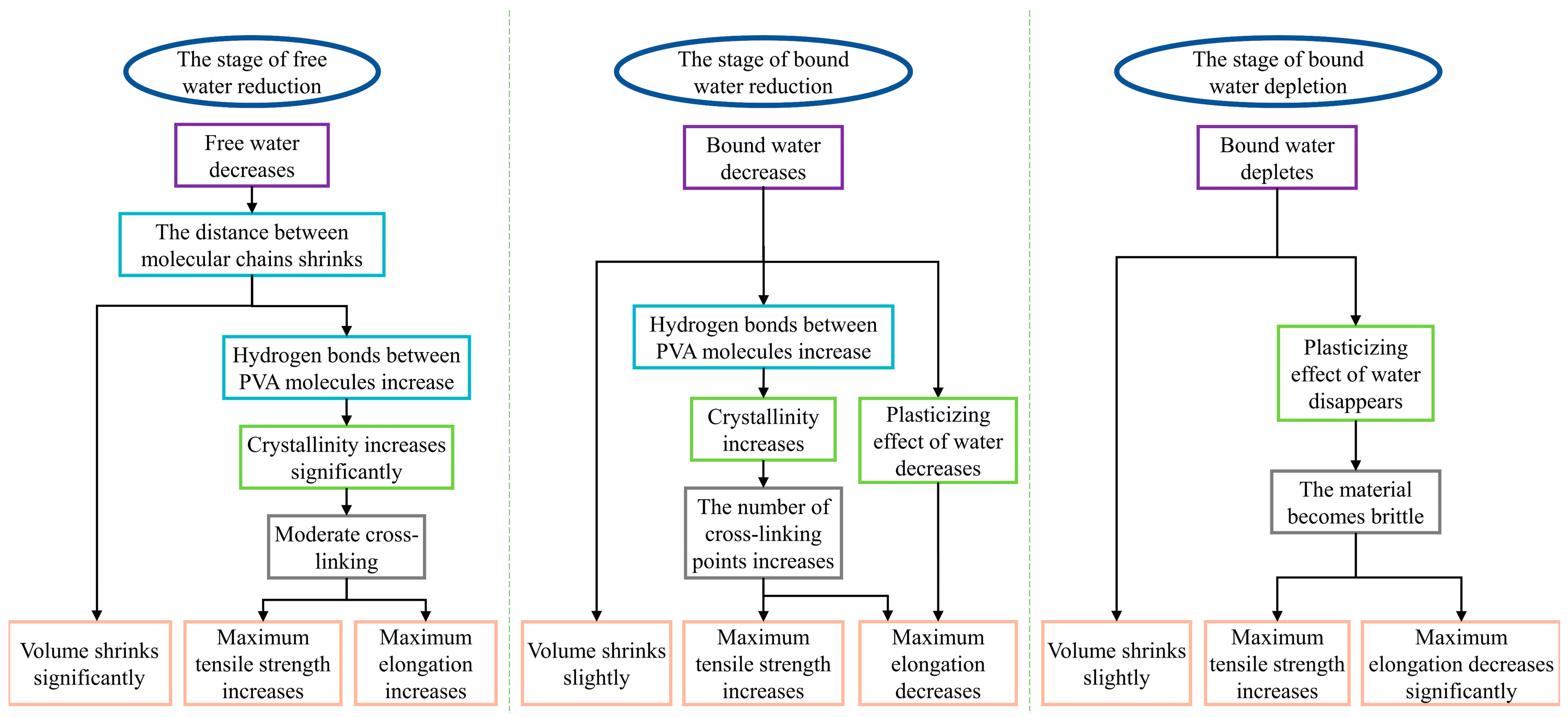
- According to the moisture loss characteristics, the aging of PVA hydrogels occurred in three stages: reduction of free water, reduction in bound water, and depletion of bound water.
- (2)
- Notably, the reduction in bound water significantly influences the lifespan of PVA hydrogels, with the maximum elongation serving as the aging characterization parameter of mechanical properties. In terms of time, the reduction in the bound water stage lasts the longest, and its degree of decrease determines the service life of PVA hydrogels.
- (3)
- Microscopically, the aging process of PVA hydrogels is controlled by molecular chain spacing, hydrogen bonds between PVA molecules, and the plasticizing effect of water. During the reduction in the free water stage, molecular chain spacing is the dominant mechanism; during the reduction in the bound water stage, hydrogen bonds between PVA molecules and the plasticizing effect of water are the dominant mechanisms; and during the depletion of the bound water stage, the plasticizing effect of water is the dominant mechanism.
- (4)
- The loss of bound water emerged as the primary factor determining the lifespan of PVA hydrogel structural components. To prolong the service life of PVA hydrogels, future research should focus on developing strategies to impede the loss of bound water, such as environmental humidity control and hydrophobic coating.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Murphy, W.L. Moving from Static to Dynamic Complexity in Hydrogel Design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhou, Y.; Hao, K.; Liu, C.; Yu, R.; Huang, A.; Wu, C.; Yang, Y. 3D Printed All-Natural Hydrogels: Flame-Retardant Materials Toward Attaining Green Sustainability. Adv. Sci. 2024, 11, 2306360. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Gu, Y.; Xie, Y.; Hu, W.; Huang, M.; Liao, G.; Yang, J.; Fan, Z.; Tan, R. Eco-Friendly Preparation of Carbon-Bonded Carbon Fiber Based on Glucose-Polyacrylamide Hydrogel Derived Carbon as Binder. Nanomaterials 2023, 13, 1045. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, T.; Xie, X.; Song, G.; Ma, X.; Mu, Q.; Huang, Z.; Liu, X.; Sun, C.; Xu, W. Advances and Challenges in Conductive Hydrogels: From Properties to Applications. Eur. Polym. J. 2022, 177, 111454. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically Conductive Hydrogels for Flexible Energy Storage Systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Singh, R.; Veer, B. Hydrogels: Promising Energy Storage Materials. ChemistrySelect 2018, 3, 1309–1320. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent Advances of Injectable Hydrogels for Drug Delivery and Tissue Engineering Applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Chen, H.; Cheng, D. Environmentally Friendly Hydrogel: A Review of Classification, Preparation and Application in Agriculture. Sci. Total Environ. 2022, 846, 157303. [Google Scholar] [CrossRef]
- Liu, D.; Cao, Y.; Jiang, P.; Wang, Y.; Lu, Y.; Ji, Z.; Wang, X.; Liu, W. Tough, Transparent, and Slippery PVA Hydrogel Led by Syneresis. Small 2023, 19, 2206819. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/Thawed Polyvinyl Alcohol Hydrogels: Present, Past and Future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-Based Hydrogels for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Ergul, N.M.; Unal, S.; Kartal, I.; Kalkandelen, C.; Ekren, N.; Kilic, O.; Chi-Chang, L.; Gunduz, O. 3D Printing of Chitosan/Poly(Vinyl Alcohol) Hydrogel Containing Synthesized Hydroxyapatite Scaffolds for Hard-Tissue Engineering. Polym. Test. 2019, 79, 106006. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, M.; Wang, L.; Zhang, X.; Liu, L. Anti-Bacterial, Anti-Freezing Starch/Ionic Liquid/PVA Ion-Conductive Hydrogel with High Performance for Multi-Stimulation Sensitive Responsive Sensors. Chem. Eng. J. 2023, 477, 147065. [Google Scholar] [CrossRef]
- Xue, S.; Wu, Y.; Liu, G.; Guo, M.; Liu, Y.; Zhang, T.; Wang, Z. Hierarchically Reversible Crosslinking Polymeric Hydrogels with Highly Efficient Self-Healing, Robust Mechanical Properties, and Double-Driven Shape Memory Behavior. J. Mater. Chem. A 2021, 9, 5730–5739. [Google Scholar] [CrossRef]
- Chae, A.; Murali, G.; Lee, S.; Gwak, J.; Kim, S.J.; Jeong, Y.J.; Kang, H.; Park, S.; Lee, A.S.; Koh, D.; et al. Highly Oxidation-Resistant and Self-Healable MXene-Based Hydrogels for Wearable Strain Sensor. Adv. Funct. Mater. 2023, 33, 2213382. [Google Scholar] [CrossRef]
- Tayefi, M.; Eesaee, M.; Hassanipour, M.; Elkoun, S.; David, E.; Nguyen-Tri, P. Recent Progress in the Accelerated Aging and Lifetime Prediction of Elastomers: A Review. Polym. Degrad. Stab. 2023, 214, 110379. [Google Scholar] [CrossRef]
- Qi-heng, T.; Run-li, N. Thermal Aging Behavior of High Performance Poly(Vinyl Alcohol) Hydrogel. J. Beijing Inst. Technol. 2012, 21, 558–563. [Google Scholar]
- Cao, J.; Zhao, X.; Ye, L. Facile Method to Fabricate Superstrong and Tough Poly(Vinyl Alcohol) Hydrogels with High Energy Dissipation. Ind. Eng. Chem. Res. 2020, 59, 10705–10715. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.; Shi, D.; Zhou, T.; Kaneko, D.; Chen, M. High Strength and Toughness of Double Physically Cross-linked Hydrogels Composed of Polyvinyl Alcohol and Calcium Alginate. J. Appl. Polym. Sci. 2021, 138, 49987. [Google Scholar] [CrossRef]
- Xiong, C.; Wei, F.; Li, W.; Liu, P.; Wu, Y.; Dai, M.; Chen, J. Mechanism of Polyacrylamide Hydrogel Instability on High-Temperature Conditions. ACS Omega 2018, 3, 10716–10724. [Google Scholar] [CrossRef]
- Law, A.; Simon, L.; Lee-Sullivan, P. Effects of Thermal Aging on Isotactic Polypropylene Crystallinity. Polym. Eng. Sci. 2008, 48, 627–633. [Google Scholar] [CrossRef]
- Sližová, M.; Stašek, M.; Raab, M. Polypropylene after Thirty Years of Storage: Mechanical Proof of Heterogeneous Aging. Polym. J. 2020, 52, 775–781. [Google Scholar] [CrossRef]
- Hodge, R.M.; Bastow, T.J.; Edward, G.H.; Simon, G.P.; Hill, A.J. Free Volume and the Mechanism of Plasticization in Water-Swollen Poly(Vinyl Alcohol). Macromolecules 1996, 29, 8137–8143. [Google Scholar] [CrossRef]
- Briscoe, B.; Luckham, P.; Zhu, S. The Effects of Hydrogen Bonding upon the Viscosity of Aqueous Poly(Vinyl Alcohol) Solutions. Polymer 2000, 41, 3851–3860. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Xu, W.; Zhang, X. Hydrogen Bonding Governs the Elastic Properties of Poly(Vinyl Alcohol) in Water: Single-Molecule Force Spectroscopic Studies of PVA by AFM. Macromolecules 2000, 33, 465–469. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Wang, Y.; Ashammakhi, N.; Alem, H.; Erdem, A.; Chang, Q.; Xu, K.; Liu, Y.; Luo, G.; et al. An Alkaline Based Method for Generating Crystalline, Strong, and Shape Memory Polyvinyl Alcohol Biomaterials. Adv. Sci. 2020, 7, 1902740. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Liu, L.; Song, P.; Cao, Q.; Xu, Z.; Fang, Z.; Wang, H. Water Governs the Mechanical Properties of Poly(Vinyl Alcohol). Polymer 2021, 213, 123330. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, J.; Liu, X.; Li, M.; Chen, C.; Wang, N.; Hou, X. Correlation between the Micro-Structure and Macroscopic Mechanical Properties of GAP-Based Propellant during Aging. Polym. Degrad. Stab. 2023, 214, 110411. [Google Scholar] [CrossRef]
- Liu, J.; Tong, X.; Luo, X.; Chen, X.; Wang, T.; Xu, J. The Relaxation Behavior of Composite Double-Base Propellants with Various Stabilizer Content under Thermal Aging. Mech. Time-Depend. Mater. 2023, 27, 651–663. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Q.; Xiao, S.; Feng, J.; Zhang, X.; Wang, T. Giant Negative Thermopower of Ionic Hydrogel by Synergistic Coordination and Hydration Interactions. Sci. Adv. 2021, 7, eabi7233. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of Poly(Vinyl Alcohol)/Poly(Ethylene Glycol) Hydrogels and PVA-Derived Hybrids by Small-Angle X-Ray Scattering and FTIR Spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Scatena, L.F.; Brown, M.G.; Richmond, G.L. Water at Hydrophobic Surfaces: Weak Hydrogen Bonding and Strong Orientation Effects. Science 2001, 292, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Dasmahapatra, A.K. Effect of Aging on the Microstructure and Physical Properties of Poly(Vinyl Alcohol) Hydrogel. J. Polym. Res. 2021, 28, 269. [Google Scholar] [CrossRef]
- Shi, L.; Han, Q. Molecular Dynamics Study of Deformation Mechanisms of Poly(Vinyl Alcohol) Hydrogel. Mol. Simul. 2018, 44, 1363–1370. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy; Brooks/Cole Cengage Learning: Bellingham, WA, USA, 2008. [Google Scholar]
- Kudo, K.; Ishida, J.; Syuu, G.; Sekine, Y.; Ikeda-Fukazawa, T. Structural Changes of Water in Poly(Vinyl Alcohol) Hydrogel during Dehydration. J. Chem. Phys. 2014, 140, 044909. [Google Scholar] [CrossRef]
- Bercea, M.; Bibire, E.-L.; Morariu, S.; Teodorescu, M.; Carja, G. pH Influence on Rheological and Structural Properties of Chitosan/Poly(Vinyl Alcohol)/Layered Double Hydroxide Composites. Eur. Polym. J. 2015, 70, 147–156. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Differential Scanning Calorimetry of Crystallized PVA Hydrogels. J. Appl. Polym. Sci. 1976, 20, 1457–1465. [Google Scholar] [CrossRef]
- Lee, J.; Jin Lee, K.; Jang, J. Effect of Silica Nanofillers on Isothermal Crystallization of Poly(Vinyl Alcohol): In-Situ ATR-FTIR Study. Polym. Test. 2008, 27, 360–367. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The Thermal Degradation of Poly(Vinyl Alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Sarma, S.; Datta, P. Characteristics of Poly(Vinyl Alcohol)/Lead Sulphide Quantum Dot Device. Nanosci. Nanotechnol. Lett. 2010, 2, 261–265. [Google Scholar] [CrossRef]
- Chandrakala, H.N.; Ramaraj, B.; Shivakumaraiah; Siddaramaiah. Optical Properties and Structural Characteristics of Zinc Oxidecerium Oxide Doped Polyvinyl Alcohol Films. J. Alloys Compd. 2014, 586, 333–342. [Google Scholar] [CrossRef]
- Peng, M.; Xiao, G.; Tang, X.; Zhou, Y. Hydrogen-Bonding Assembly of Rigid-Rod Poly(p-Sulfophenylene Terephthalamide) and Flexible-Chain Poly(Vinyl Alcohol) for Transparent, Strong, and Tough Molecular Composites. Macromolecules 2014, 47, 8411–8419. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, Fatigue, and Friction of Polymers in Which Entanglements Greatly Outnumber Cross-Links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Liu, Z.; Sharma, P. Concurrent Stiffening and Softening in Hydrogels under Dehydration. Sci. Adv. 2023, 9, eade3240. [Google Scholar] [CrossRef]
- Assender, H.E.; Windle, A.H. Crystallinity in Poly(Vinyl Alcohol). 1. An X-ray Diffraction Study of Atactic PVOH. Polymer 1998, 39, 4295–4302. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and Properties of Poly(Vinyl Alcohol) Hydrogels with High Strength and Toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Sekine, Y.; Ikeda-Fukazawa, T. Structural Changes of Water in a Hydrogel during Dehydration. J. Chem. Phys. 2009, 130, 034501. [Google Scholar] [CrossRef] [PubMed]
- Naohara, R.; Narita, K.; Ikeda-Fukazawa, T. Change in Hydrogen Bonding Structures of a Hydrogel with Dehydration. Chem. Phys. Lett. 2017, 670, 84–88. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, C.; Wang, M.; Zhu, C.; Zhao, N.; Xu, J. Skin-Inspired Double-Hydrophobic-Coating Encapsulated Hydrogels with Enhanced Water Retention Capacity. Adv. Funct. Mater. 2021, 31, 2102433. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Q.; Qu, D.-H. Emerging Hydrogen-Bond Design for High-Performance Dynamic Polymeric Materials. ACS Mater. Lett. 2023, 5, 480–490. [Google Scholar] [CrossRef]




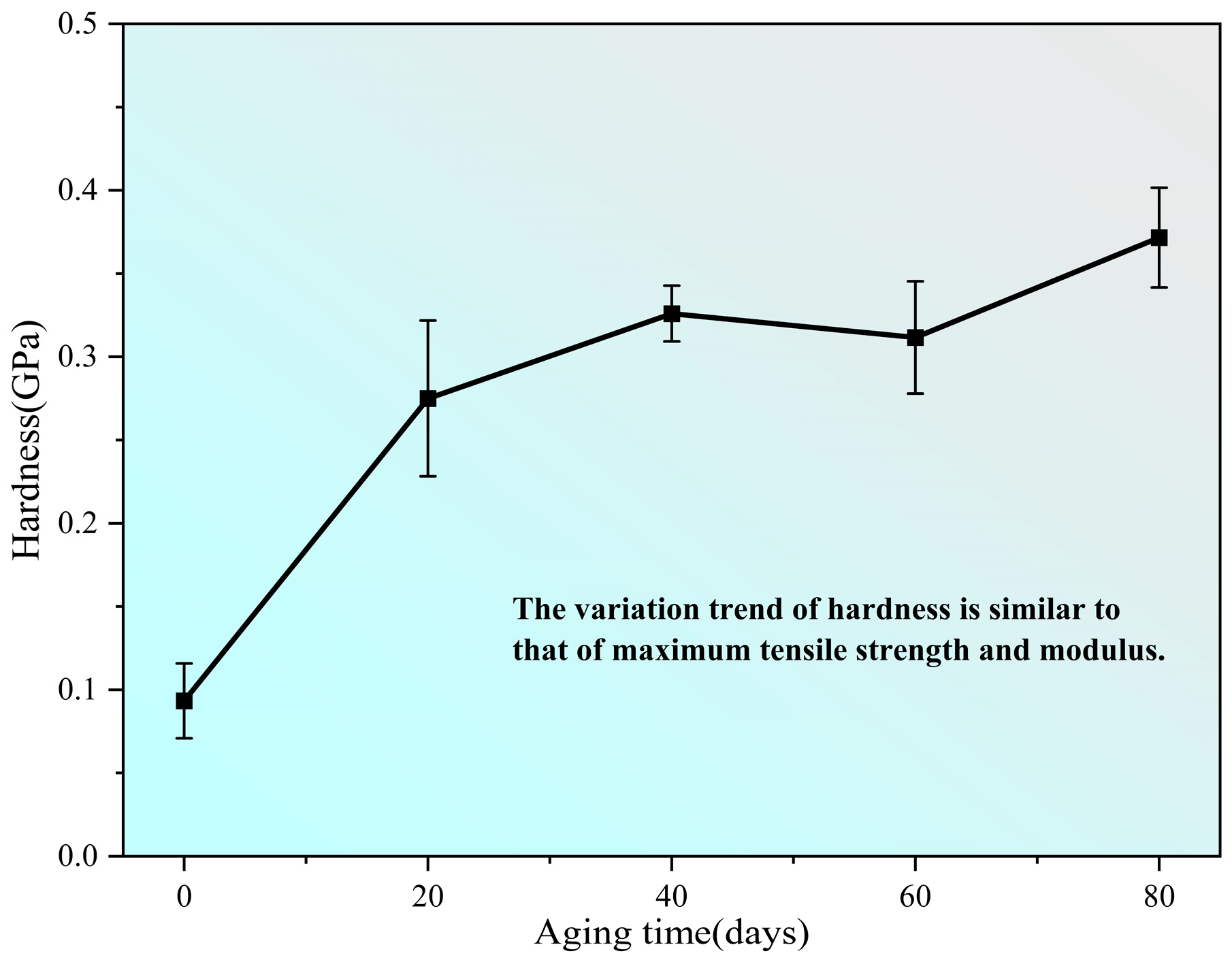
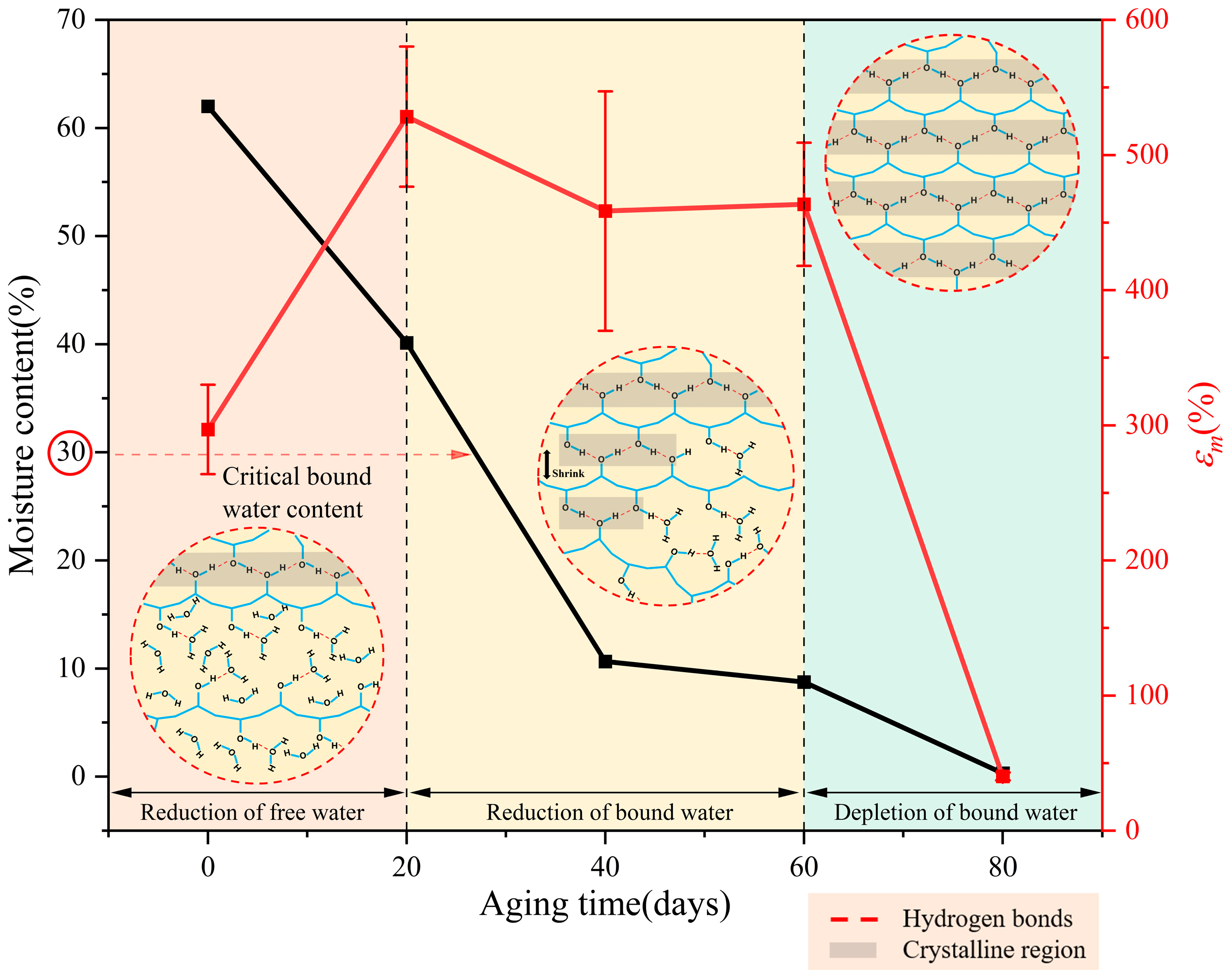
| Aging Time (d) | Volume (mm3) | Volume Shrinkage Ratio (%) | Moisture Content (%) |
|---|---|---|---|
| 0 | 3628 | / | 62 |
| 20 | 2163 | 40.4 | 40.13 |
| 40 | 1817 | 49.9 | 10.65 |
| 60 | 1607 | 55.7 | 8.75 |
| 80 | 1121 | 69.1 | 0.31 |
| Aging Time (d) | Crystallinity (%) |
|---|---|
| 0 | 14.6 |
| 20 | 62.4 |
| 40 | 70.5 |
| 60 | 60.0 |
| 80 | 66.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liu, X.; Wang, J.; Guo, H.; Chen, Y.; Wang, N. Research on the Thermal Aging Mechanism of Polyvinyl Alcohol Hydrogel. Polymers 2024, 16, 2486. https://doi.org/10.3390/polym16172486
Chen C, Liu X, Wang J, Guo H, Chen Y, Wang N. Research on the Thermal Aging Mechanism of Polyvinyl Alcohol Hydrogel. Polymers. 2024; 16(17):2486. https://doi.org/10.3390/polym16172486
Chicago/Turabian StyleChen, Chunkun, Xiangyang Liu, Jiangtao Wang, Haoran Guo, Yingjun Chen, and Ningfei Wang. 2024. "Research on the Thermal Aging Mechanism of Polyvinyl Alcohol Hydrogel" Polymers 16, no. 17: 2486. https://doi.org/10.3390/polym16172486




