Efficient Removal of Mercury from Wastewater Solutions by a Nitrogen-Doped Hyper-Crosslinked Polyamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
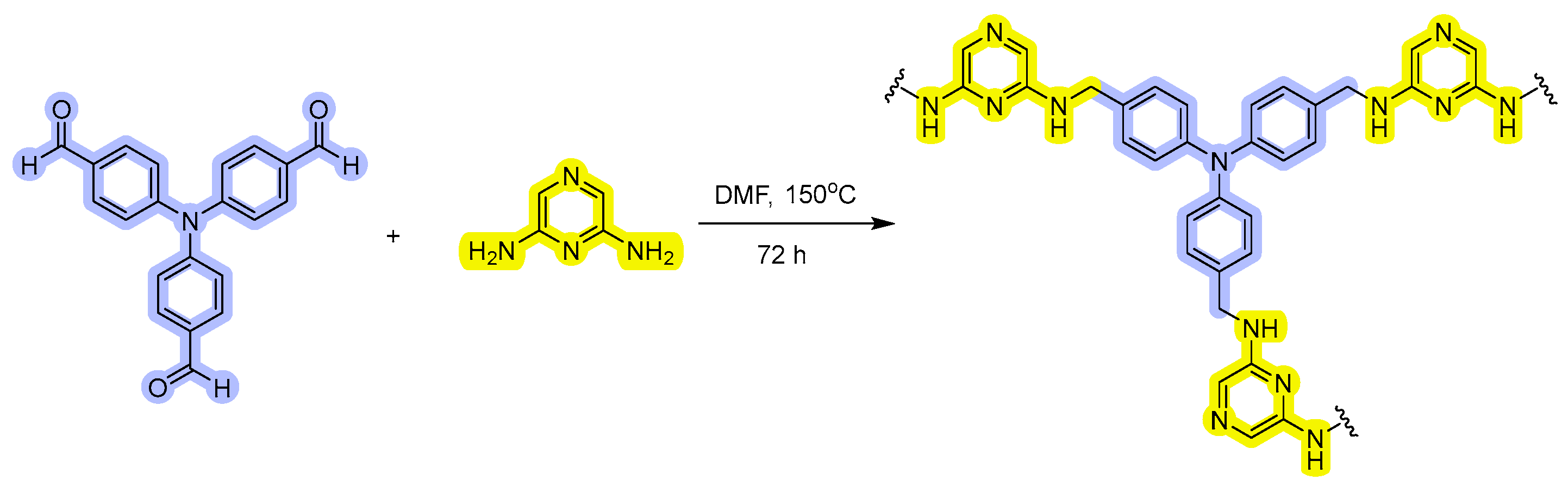
2.2.1. Synthesis of HCPA
2.2.2. Equipment
2.2.3. Adsorption Experiments
2.2.4. Reuse and Regeneration of HCPA Polymer for the Adsorption of Hg (II) Ions
3. Results
3.1. Structural Performance of HCPA Polymer
3.2. Effect of pH
3.3. Effect of Initial Concentration on the Adsorption Capacity of HCPA Polymer
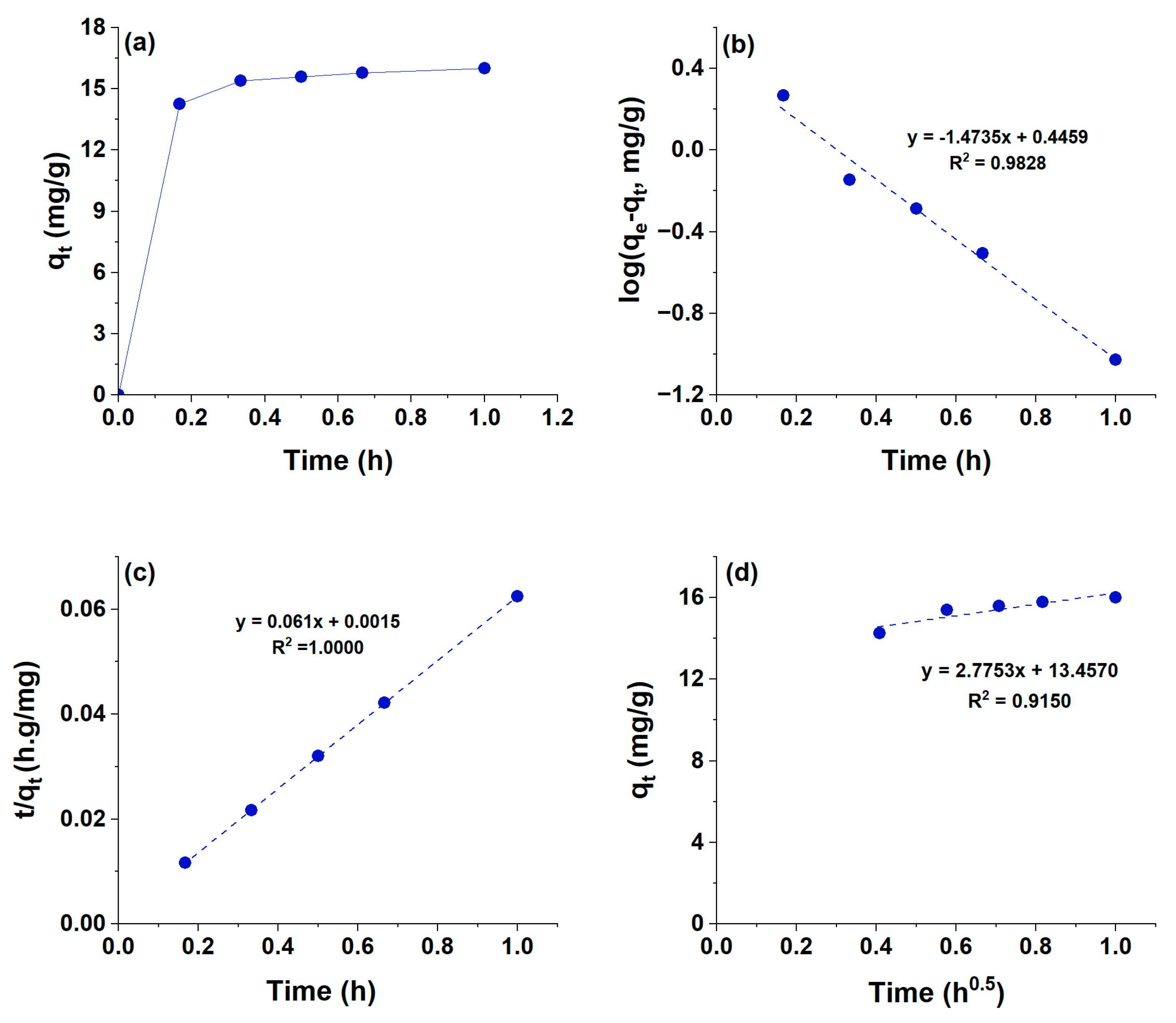
3.4. Effect of Time on the Adsorption Capacity of HCPA Polymer
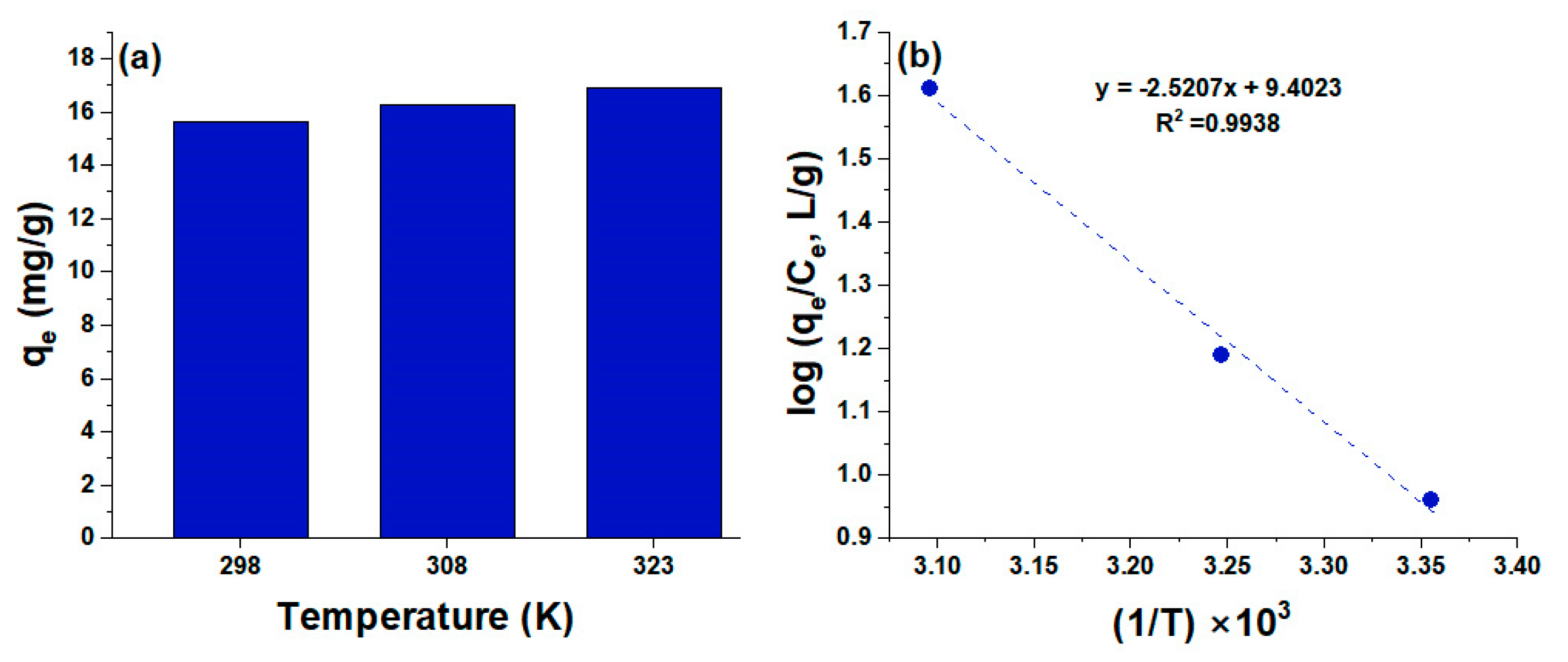
3.5. Effect of Temperature on the Adsorption Capacity of HCPA Polymer
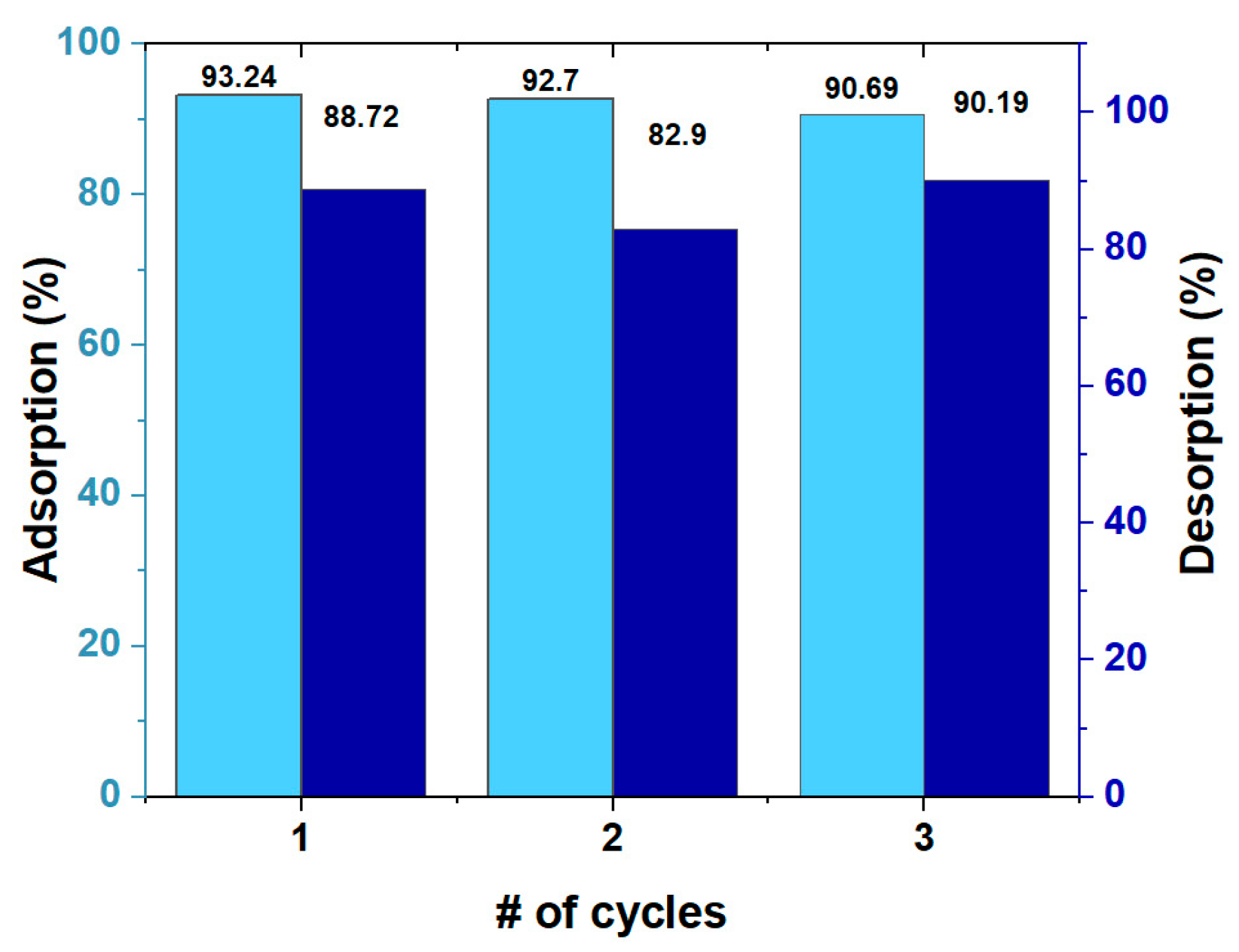
3.6. Reuse and Regeneration of HCPA Polymer
3.7. Wastewater Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, R.; Liu, J.; Sun, C.; Zhang, Y.; Ji, C.; Yin, P. Removal and separation of Hg (II) ions from aqueous solutions by macroporous polystyrene-co-divinylbenzene-supported polyamine chelating resins. J. Chem. Eng. Data 2010, 55, 4650–4659. [Google Scholar] [CrossRef]
- Akintola, O.S.; Saleh, T.A.; Khaled, M.M.; Al Hamouz, O.C.S. Removal of mercury (II) via a novel series of cross-linked polydithiocarbamates. J. Taiwan Inst. Chem. Eng. 2016, 60, 602–616. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Bloom, N. Mercury in petroleum. Fuel Process. Technol. 2000, 63, 1–27. [Google Scholar] [CrossRef]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Wang, Q.; Kim, D.; Dionysiou, D.D.; Sorial, G.A.; Timberlake, D. Sources and remediation for mercury contamination in aquatic systems--a literature review. Environ. Pollut. 2004, 131, 323–336. [Google Scholar] [CrossRef] [PubMed]
- AlRashidi, R.B.; Al Hamouz, O.C.S. Synthesis of Cross-Linked Pyrazine-Based Polymers for Selective Removal of Mercury (II) Ions from Wastewater Solutions. Arab. J. Sci. Eng. 2022, 47, 7207–7218. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Lou, Z.; Yang, K.; Lv, D.; Zhou, J.; Baig, S.A.; Xu, X. Enhanced performance for Hg (II) removal using biomaterial (CMC/gelatin/starch) stabilized FeS nanoparticles: Stabilization effects and removal mechanism. Chem. Eng. J. 2018, 344, 616–624. [Google Scholar] [CrossRef]
- Liang, W.; Li, M.; Zhang, Z.; Jiang, Y.; Awasthi, M.K.; Jiang, S.; Li, R. Decontamination of Hg(II) from aqueous solution using polyamine-co-thiourea inarched chitosan gel derivatives. Int. J. Biol. Macromol. 2018, 113, 106–115. [Google Scholar] [CrossRef]
- Shen, W.; Fang, Y.; Azeem, M.; Gao, Y.; Li, X.; Zhao, P.; Ali, A.; Li, M.; Li, R. Chitosan crosslinked with polyamine-co-melamine for adsorption of Hg(2+): Application in purification of polluted water. Int. J. Biol. Macromol. 2021, 181, 778–785. [Google Scholar] [CrossRef]
- Adly, M.S.; El-Dafrawy, S.M.; Ibrahim, A.A.; El-Hakam, S.A.; El-Shall, M.S. Efficient removal of heavy metals from polluted water with high selectivity for Hg(ii) and Pb(ii) by a 2-imino-4-thiobiuret chemically modified MIL-125 metal-organic framework. RSC Adv. 2021, 11, 13940–13950. [Google Scholar] [CrossRef]
- Štandeker, S.; Veronovski, A.; Novak, Z.; Knez, Ž. Silica aerogels modified with mercapto functional groups used for Cu (II) and Hg (II) removal from aqueous solutions. Desalination 2011, 269, 223–230. [Google Scholar] [CrossRef]
- Bibby, A.; Mercier, L. Mercury (II) ion adsorption behavior in thiol-functionalized mesoporous silica microspheres. J. Chem. Mater. 2002, 14, 1591–1597. [Google Scholar] [CrossRef]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, preparation, and applications (with emphasis on aqueous media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Choudhary, P.; Das, S.K. Biomaterial functionalized graphene-magnetite nanocomposite: A novel approach for simultaneous removal of anionic dyes and heavy-metal ions. ACS Sustain. Chem. Eng. 2018, 6, 6328–6341. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, L.; Zhang, D.; Wang, F.; Sharma, V.K.; Wang, C. Amido-functionalized carboxymethyl chitosan/montmorillonite composite for highly efficient and cost-effective mercury removal from aqueous solution. J. Colloid Interface Sci. 2019, 554, 479–487. [Google Scholar] [CrossRef]
- Albakri, M.A.; Saleh, T.A.; Mankour, Y.; Garrison, T.F.; Al Hamouz, O.C.S. Synthesis of a new thiophenol-thiophene polymer for the removal of mercury from wastewater and liquid hydrocarbons. J. Colloid Interface Sci. 2021, 582, 428–438. [Google Scholar] [CrossRef]
- Jal, P.K.; Patel, S.; Mishra, B.K. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 2004, 62, 1005–1028. [Google Scholar] [CrossRef]
- Liang, W.; Li, M.; Jiang, S.; Ali, A.; Zhang, Z.; Li, R. Polyamine-co-2, 6-diaminopyridine covalently bonded on chitosan for the adsorptive removal of Hg(II) ions from aqueous solution. Int. J. Biol. Macromol. 2019, 130, 853–862. [Google Scholar] [CrossRef]
- Yang, G.; Han, H.; Du, C.; Luo, Z.; Wang, Y. Facile synthesis of melamine-based porous polymer networks and their application for removal of aqueous mercury ions. Polymer 2010, 51, 6193–6202. [Google Scholar] [CrossRef]
- Quesada, H.B.; de Araújo, T.P.; Vareschini, D.T.; de Barros, M.A.S.D.; Gomes, R.G.; Bergamasco, R. Chitosan, alginate and other macromolecules as activated carbon immobilizing agents: A review on composite adsorbents for the removal of water contaminants. Int. J. Biol. Macromol. 2020, 164, 2535–2549. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, Z.; Guo, Y.; Zhang, Y.; Zhao, W.; Yang, J.; Zhang, S.; Zhang, W. Facile synthesis of Melamine-Modified porous organic polymer for mercury (II) removal. Sep. Purif. Technol. 2021, 274, 119097. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, S.; Xi, C.; Jiang, B.; Zhang, F. Adsorption and Removal of Mercury(II) by a Crosslinked Hyperbranched Polymer Modified via Sulfhydryl. ACS Omega 2022, 7, 12231–12241. [Google Scholar] [CrossRef] [PubMed]
- Da'na, E.; Sayari, A. Adsorption of heavy metals on amine-functionalized SBA-15 prepared by co-condensation: Applications to real water samples. Desalination 2012, 285, 62–67. [Google Scholar] [CrossRef]
- Kotnik, T.; Žerjav, G.; Pintar, A.; Žagar, E.; Kovačič, S. Azine- and imine-linked conjugated polyHIPEs through Schiff-base condensation reaction. Polym. Chem. 2022, 13, 474–478. [Google Scholar] [CrossRef]
- Hamdy, L.B.; Goel, C.; Rudd, J.A.; Barron, A.R.; Andreoli, E. The application of amine-based materials for carbon capture and utilisation: An overarching view. Mater. Adv. 2021, 2, 5843–5880. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; AlJarrah, M.; Mayyas, M.; Alrebaki, M. High-stability polyamine/amide-functionalized magnetic nanoparticles for enhanced extraction of uranium from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2018, 86, 148–157. [Google Scholar] [CrossRef]
- Cegłowski, M.; Gierczyk, B.; Frankowski, M.; Popenda, Ł. A new low-cost polymeric adsorbents with polyamine chelating groups for efficient removal of heavy metal ions from water solutions. React. Funct. Polym. 2018, 131, 64–74. [Google Scholar] [CrossRef]
- Abdelnaby, M.M.; Cordova, K.E.; Abdulazeez, I.; Alloush, A.M.; Al-Maythalony, B.A.; Mankour, Y.; Alhooshani, K.; Saleh, T.A.; Al Hamouz, O.C.S. Novel Porous Organic Polymer for the Concurrent and Selective Removal of Hydrogen Sulfide and Carbon Dioxide from Natural Gas Streams. ACS Appl. Mater. Interfaces 2020, 12, 47984–47992. [Google Scholar] [CrossRef]
- Englert, C.; Tauhardt, L.; Hartlieb, M.; Kempe, K.; Gottschaldt, M.; Schubert, U.S. Linear poly(ethylene imine)-based hydrogels for effective binding and release of DNA. Biomacromolecules 2014, 15, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, H.; Gupta, R.; Dash, S. Efficient synthesis of branched polyamine based thermally stable heterogeneous catalyst for Knoevenagel condensation at room temperature. Catal. Lett. 2018, 148, 1703–1713. [Google Scholar] [CrossRef]
- Al Hamouz, O.C.S. New phenol–glycol cross-linked polymers for efficient removal of mercury from aqueous solutions. Arab. J. Sci. Eng. 2018, 43, 211–219. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Manzar, M.S.; Haladu, S.A.; Zubair, M.; Mu'azu, N.D.; Qureshi, A.; Blaisi, N.I.; Garrison, T.F.; Al Hamouz, O.C.S. Synthesis and characterization of a series of cross-linked polyamines for removal of Erichrome Black T from aqueous solution. Chin. J. Chem. Eng. 2021, 32, 341–352. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Gai, L.; Gong, Y.; Guan, H.; He, R.; Lyu, H. Different approaches for preparing a novel thiol-functionalized graphene oxide/Fe-Mn and its application for aqueous methylmercury removal. Chem. Eng. J. 2017, 319, 229–239. [Google Scholar] [CrossRef]
- Al Hamouz, O.C.S.; Ali, S.A. Novel Cross-Linked Polyphosphonate for the Removal of Pb2+ and Cu2+ from Aqueous Solution. Ind. Eng. Chem. Res. 2012, 51, 14178–14187. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O'Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Xu, T.; Shao, G. Novel negatively charged hybrids. 1. copolymers: Preparation and adsorption properties. Sep. Purif. Technol. 2009, 66, 135–142. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Wu, X.; Zhang, J.; Feng, J.; Li, M.; Zong, S.; Yan, W. Nitrogen-Based conjugated microporous polymers for efficient Hg(Ⅱ) removal from Water: Performance and mechanism. Chem. Eng. J. 2023, 471, 144659. [Google Scholar] [CrossRef]
- Saman, N.; Johari, K.; Tien, S.S.; Mat, H. Removal of Hg(II) and CH3Hg(I) Using Rasped Pith Sago Residue Biosorbent. CLEAN Soil Air Water 2014, 42, 1541–1548. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Ozcan, A.; Ozcan, A.S.; Gok, O. Adsorption kinetics and isotherms of anionic dye of Reactive Blue 19 from aqueous solutions onto DTMA-sepiolite. In Hazardous Materials and Wastewater—Treatment, Removal and Analysis; Nova Science Publishers: New York, NY, USA, 2007; pp. 225–249. [Google Scholar]
- Merrifield, J.D.; Davids, W.G.; MacRae, J.D.; Amirbahman, A. Uptake of mercury by thiol-grafted chitosan gel beads. Water Res. 2004, 38, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Oliveros, H.B.; Flores-Espinosa, R.M.; Ordoñez-Regil, E.; Fernández-Valverde, S.M. Synthesis of α-Ti(HPO4)2·H2O and sorption of Eu (III). Chem. Eng. J. 2014, 236, 398–405. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.; Sudhakar, P.P. Adsorption of mercury(II), methyl mercury(II) and phenyl mercury(II) on chitosan cross-linked with a barbital derivative. Carbohydr. Polym. 2011, 86, 1055–1062. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, N.; Liu, S.; Zheng, C.; Wang, X.; Huang, T.; Guo, Y.; Bai, R. Thiol functionalization of short channel SBA-15 through a safe, mild and facile method and application for the removal of mercury (II). J. Environ. Chem. Eng. 2018, 6, 5420–5433. [Google Scholar] [CrossRef]
- Xia, Z.; Baird, L.; Zimmerman, N.; Yeager, M. Heavy metal ion removal by thiol functionalized aluminum oxide hydroxide nanowhiskers. Appl. Surf. Sci. 2017, 416, 565–573. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Zhou, X.; Bai, R. Removal of mercury (II) from aqueous solution with three commercial raw activated carbons. Res. Chem. Intermed. 2017, 43, 2273–2297. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.; Makhlouf, A.S.H.; Chong, K.F.; Alharbi, N.S.; Agarwal, S.; Gupta, V.K. MWCNTs-Fe3O4 nanocomposite for Hg (II) high adsorption efficiency. J. Mol. Liq. 2018, 258, 345–353. [Google Scholar] [CrossRef]
- Caicedo Salcedo, O.D.; Vargas, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Study of mercury [Hg (II)] adsorption from aqueous solution on functionalized activated carbon. ACS Omega 2021, 6, 11849–11856. [Google Scholar] [CrossRef]
- Labidi, S.N.; Mechati, B. Quartz Mineral as new Sorbent for Hg (II) Removal from Aqueous Solution: Adsorption Kinetics and Isotherm. Pollution 2023, 9, 445–458. [Google Scholar]


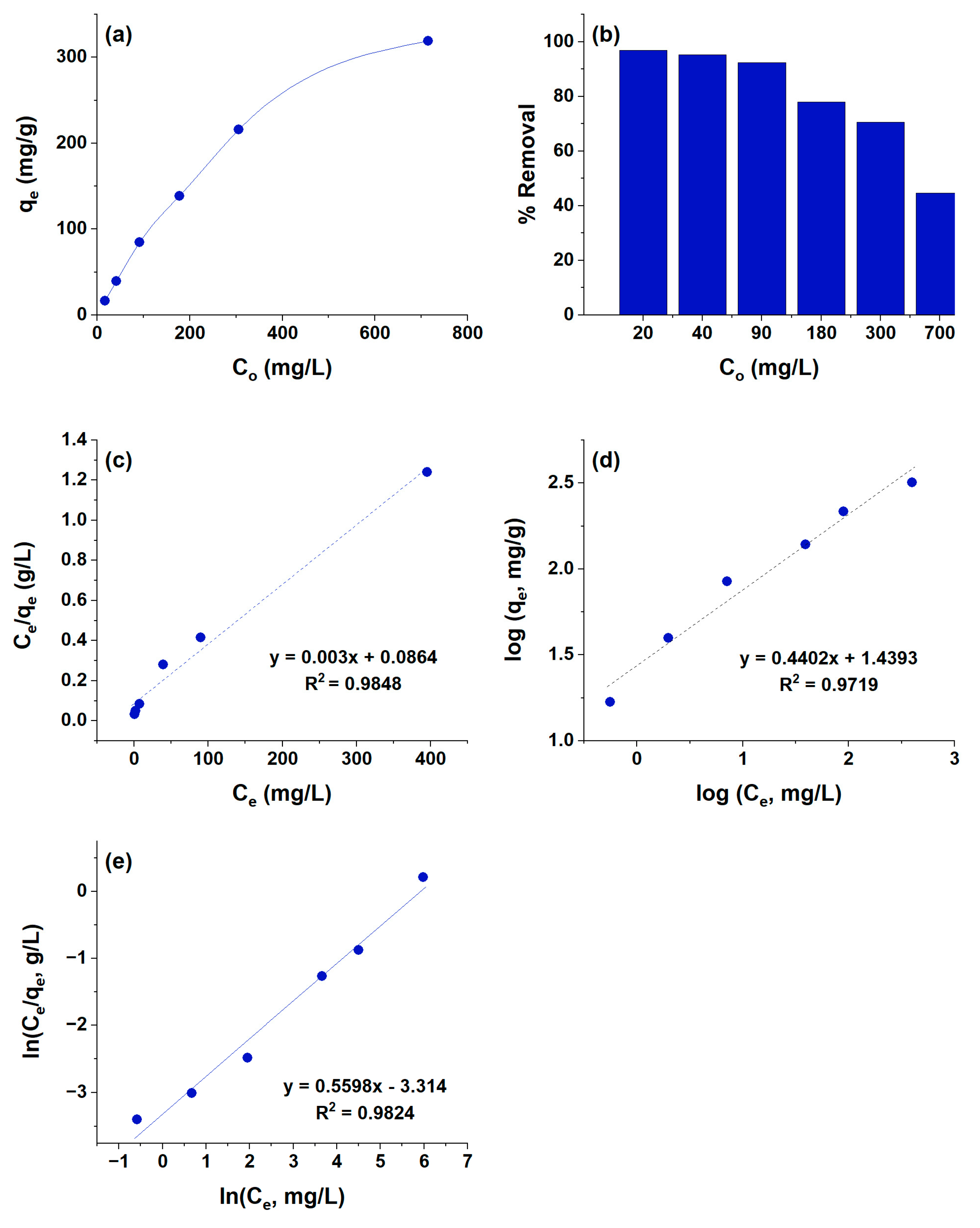
| Langmuir Isotherm Model | |||
|---|---|---|---|
| HCPA | qm (mg/g) | Kl (L/mg) | R2 |
| 333.3 | 0.0347 | 0.9848 | |
| Freundlich Isotherm Model | |||
| Kf (mg(1−1/n)L1/ng−1) | 1/n | R2 | |
| 27.50 | 0.4402 | 0.9719 | |
| Redlich–Peterson Isotherm Model | |||
| β | Kr (L/g) | R2 | |
| 0.5598 | 27.4948 | 0.9824 | |
| Pseudo-First-Order Model | |||
| qe(exp) (mg/g) | qe(calc.) (mg/g) | k1 (min−1) | R2 |
| 16.6 | 2.791 | 3.246 | 0.9828 |
| Pseudo-Second-Order Model | |||
| qe(exp) (mg/g) | qe(calc.) (mg/g) | k2 (min−1) | R2 |
| 16.6 | 16.39 | 2.481 | 1.0000 |
| Intraparticle Diffusion | |||
| kp (mg/g.h) | C | R2 | |
| 2.7753 | 13.4570 | 0.9150 | |
| Polymer | T (K) | |||
|---|---|---|---|---|
| HCPA | 48.26 | 180.0 | −4.511 | 293.15 |
| −7.211 | 308.15 | |||
| −9.912 | 323.15 |
| pH: 8.124 | |||
|---|---|---|---|
| Metal Ion | Before Adsorption (mg/L) | After Adsorption (mg/L) | Removal % |
| Hg | 22.34 | 0.484 | 97.8 |
| Fe | 32.10 | 32.10 | 0.0 |
| B | 5.10 | 3.0 | 41.2 |
| Mg | 195 | 182.4 | 6.5 |
| Ca | 422.4 | 270 | 36.1 |
| Ag | 2.40 | 2.40 | 0.0 |
| Cr | 2.10 | 0.60 | 71.4 |
| Pb | 3.00 | 2.10 | 30.0 |
| K | 121.2 | 121.2 | 0.0 |
| Cu | 1.80 | 0.30 | 83.3 |
| Zn | 8.10 | 2.70 | 66.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Ghamdi, K.; Ahmad, A.; Falca, G.; Alrefaeia, M.N.; Al-Hamouz, O.C.S. Efficient Removal of Mercury from Wastewater Solutions by a Nitrogen-Doped Hyper-Crosslinked Polyamine. Polymers 2024, 16, 2495. https://doi.org/10.3390/polym16172495
Al Ghamdi K, Ahmad A, Falca G, Alrefaeia MN, Al-Hamouz OCS. Efficient Removal of Mercury from Wastewater Solutions by a Nitrogen-Doped Hyper-Crosslinked Polyamine. Polymers. 2024; 16(17):2495. https://doi.org/10.3390/polym16172495
Chicago/Turabian StyleAl Ghamdi, Khalid, Aqeel Ahmad, Gheorghe Falca, Meshal Nawaf Alrefaeia, and Othman Charles S. Al-Hamouz. 2024. "Efficient Removal of Mercury from Wastewater Solutions by a Nitrogen-Doped Hyper-Crosslinked Polyamine" Polymers 16, no. 17: 2495. https://doi.org/10.3390/polym16172495






