Hazardous Materials from Threats to Safety: Molecularly Imprinted Polymers as Versatile Safeguarding Platforms
Abstract
:1. Introduction
1.1. Types of Hazardous Compounds
- Hazardous materials, in a broad sense, refer to any substance or material, regardless of its form or quantity, that presents an unreasonable risk to safety, health, and property [3]. Hazardous materials are defined either as matter (solid, liquid, or gas) or energy form, which, when released, have the potential to cause harm to individuals, the surrounding environment, and property, including weapons of mass destruction (WMD), as well as any other criminal use of hazardous materials, such as illicit laboratories, environmental crimes, or industrial sabotage [3];
- Hazardous substances—any substance posing a threat to waterways and the environment when released [3];
- Common hazardous materials include nitrogen oxides, sulfur oxides, carbon oxides, hydrogen sulfide, volatile organic compounds (VOCs), nitrogen-containing compounds (NCCs), sulfur-containing compounds (SCCs), dyes, pharmaceuticals, and personal care products (PPCPs), etc. [2];
- Volatile organic compounds (VOCs)—chemicals with high vapor pressure, often emitted from solvents, resins, paints, adhesives, and similar substances. Hazardous VOCs include benzene, naphthalene, toluene, phenolics, xylenes, and similar compounds, posing risks to both the environment and human health [2];
- Hazardous chemicals—any chemical that would be a risk to employees if exposed in the workplace [3];
- Dangerous goods—in international transportation, hazardous materials are commonly referred to as “dangerous goods” [3];
- Hazardous drugs—medication used to treat illnesses such as cancer, arthritis, multiple sclerosis, and viral diseases possessing one/more of the following properties: carcinogenicity, reproductive toxicity, teratogenicity, genotoxicity, organ toxicity at low doses [3];
- Illicit drugs—legally produced drugs that are abused and drugs produced for no reason other than abuse are called abused drugs, drugs of abuse, or illicit drugs. In addition to legally produced pharmaceutical drugs, there are also substances that have no legitimate, recognized medicinal purpose but are produced and ingested entirely for their psychoactive effects [4];
- Extremely Hazardous Substances (EHS)—extremely hazardous to a community during a spill or release due to their toxicities and physical/chemical properties [3];
- Hazardous wastes—discarded materials regulated by the authorities due to public health and safety concerns [3];
- Weapons of Mass Destruction (WMD)—(1) any destructive device, such as any explosive, incendiary, or poison gas bomb, grenade, rocket having a propellant charge of more than four ounces, missile having an explosive or incendiary charge of more than one-quarter ounce (7 g), mine, or device similar to the above; (2) any weapon involving toxic or poisonous chemicals; (3) any weapon involving a disease organism; or (4) any weapon that is designed to release radiation or radioactivity at a level dangerous to human life [3];
1.2. Hazardous Compounds Assessment Strategies and Designed Polymeric Platforms
1.3. Green Aspects of MIPs
| Imprinting Strategy | Characteristics | Refs. |
|---|---|---|
| Dummy-template/Segment imprinting | Replacing hazardous or very expensive targets with a dummy template that emulates the size, conformation, and functional groups of the target except for its undesirable characteristics, avoiding all types of risks and hazardous waste; using green dummy templates with high solubility to avoid poor solubility in porogen media; reducing danger to personnel; offering availability of using other analytes; segment/fragment imprinting (the case of biomacromolecules or hazardous templates) replacing dummy imprinting when using a partial target as a pseudotemplate for cost-effectiveness, regenerability, and non-toxicity. | [26,34] |
| Dual/multi-template imprinting | Versatility by the use of two or more target templates to generate multiple types of active sites in a single polymer material; comprising the use of self-synthetic and dual/multiple functional monomers and dual/multiple template ions; rarely reported for bio-macromolecules, affecting thus the heterogeneity of binding sites and poor selectivity; highly desirable for sustainability by simultaneously recognizing multi-templates; several different templates can be extracted, separated, and detected. | [29,35] |
| Stimuli-responsive (SR)imprinting | Smart polymers offer a plethora of alternatives for producing specific, powerful responses to a wide range of stimuli, i.e., changes in pH, gas, temperature, solvent, radiation, and biological or chemical agents; two main synthesizing methods: grafting/incorporating the SR into MIPs and the integration of SR elements within the MIP network; the use of safe biomaterials as SR will replace hazardous byproducts like ozone. | [26,36] |
| Click chemistryimprinting | Highly reliable one-pot synthesis tool first proposed in 2001 by Sharpless; biocompatible small-molecule reactions, generally used in bioconjugation with moderate reaction temperatures, leading to inoffensive byproducts; relies on new compounds and combinatorial libraries through heteroatom links (C–X–C). | [26,29] |
| Microwave-assisted heatingimprinting | Widely applied to almost all types of polymerization based on their heating speed, selectivity, and efficiency properties; rapid energy transfer and high energy efficiency of microwave irradiation. | [28] |
2. MIPs Designed for CWAs and Other Hazardous-Related Compounds
2.1. Detection/Sensing of CWAs and Related Compounds
2.2. Decontamination of CWAs and Related Compounds
3. MIPs Designed for Explosives Assessment
3.1. MIPs for Sensing/Detection of Explosives by Optical Techniques (e.g., Colorimetry, Fluorescence, Surface-Enhanced RAMAN Scattering (SERS))
3.2. MIPs for Sensing/Detection of Explosives by Electrochemical Methods
4. MIPs Designed for Illicit Drugs Assessment
4.1. Ultra-Potent Synthetic Opioids
4.2. MIPs for Sensing/Detection of Illicit Drugs by Optical Techniques
| Sensor Type/Detection Method | MIP Polymerization Method | Sensor Modification | Target | LoD 1 (M) | Linear Range | Real Samples | Refs. |
|---|---|---|---|---|---|---|---|
| Colorimetric/Non-enzymatic | Precipitation | MIP and CS2-Cu(II) complex | Ephedrine | 0.6 μM | 1–100 μM | Urine | [153] |
| Colorimetric/Non-enzymatic | Precipitation | MIP and ninhydrin | Methamphetamine | 1.44 μM | 5–100 μM | Urine | [154] |
| Colorimetric/UV Spectroscopy | Bulk | MIP-Based Dye DC | Amphetamine | 57 μM | 0.01–0.20 mg mL−1 | Urine | [155] |
| Fibre Optic- long period grating (LPG) | SPE | nanoMIPs/PG array via EDC/ NHS coupling | Carboxyl-fentanyl | 0.13μM | 0–1000 ng mL−1 | Blood, human serum | [155] |
| Fluorescence | Free radical | AuZnFeSeSQDs@MIP core/shell nanocomposite | Levamisole | 0.05 μM | 0.5–100 μM | Mixed drug containing cocaine | [157] |
| Fluorescence | Free radical | AuZnCeSeS QDs-MIP nanocomposite | Methamphetamine | 0.02 nM | 0.05–50.000 nM | Urine | [158] |
| Photoluminescent/Fluorescence | photoluminescence | GQDs-MIP | Methamphetamine | 12 nM | 5–50 μM | NA 2 | [159] |
4.3. MIPs for Sensing/Detection of Illicit Drugs by Electrochemical Methods
| Electrode Type/Detection Method | MIP Polymerization Method | Electrode Modification | Target | LoD 1 (M) | Linear Range | Real Samples | Recovery Rate (%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| GCE/CV, DPV | Electro polymerization | Zn/La3+/MOF/MIP | Buprenorphine BUP | 0.0021 μM | 0.0079–0.0992 μM | Blood | 99.1–100.2 | [161] |
| SPCE/CV, HPLC | Precipitation | graphene-UiO-66 composites/MMIP | Cannabidiol CBD | 0.05 μM | 5–100 μM | CBD product | 99.5–99.8 | [162] |
| SPCE/DPV | Electro-polymerization | MIP/MWCNTs | THC | 0.54 nM | 0–3150 nM | Human blood plasma | 99.75 | [163] |
| SPEISE/Potentiometric | Precipitation | MIP/MWCNTs | Pholcodine PHO | 0.25 µM | 5.5 µM–0.01 M | Serum | 91–95.5 | [164] |
| ITO/DPV | Sol–gel and electropolymerization | pyrrole@sol-gelMIP/fMWCNT | Naloxone | 0.02 µM | 0–12 µM | Urine | >88 | [165] |
| SPCE/UPLC-MS/MS | SPE/UV radiation | Chitosan/RGO/Electroactive nanoMIPs | MDMA | 1.6 nM | 1–200 nM | Street probes | 92–99 | [166] |
| SPPE/DPV | SPE/UPLC-MS | nanoMIPs/graphene by 3D printing andnanoMIP/silane by drop-casting | Amphetamine | 68 and 37.6 nM | 75–220 nM and 25–220 nM | Human plasma and street | NA 2 | [167] |
| GCE/SWV | Electropolymerization | MIM-ErGO | Fentanyl | 1.28 nM | 0.0038–1.72 μM | Human serum | 97.0–110 | [168] |
| Pt/DPV | Electropolymerization | polyacrylate-based MIP | Aminoacids/indazole-based cannabinoids | 0.01 mM | Up to 0.8 mM | Simulated pills and smoking mixtures | 70–115 | [169] |
| Au-E/DPV | Electro-polymerization | polydopamine-based MIP | Homopiperonylamine (MDPEA) | 54 nM | 0.1–7.5 μM | Urine | 99.27–108 | [170] |
| GPE/SWV | Electro-polymerization | o-phenylenediamine-based MIP | Oxycodone | 1.8 ± 0.239 nM | 0.4–5.0 nM | Wastewater | 96.0–102.5 | [171] |
| Au/EIS | SPE | nanoMIPs | Cocaine | 0.70 nM | 0.30–147 nM | Diluted cocaine | NA 2 | [172] |
| CPE/SWV | Precipitation | Magnetic Fe3O4 nanoMIPs | Sufentanil | 0.02 μM | 0.001–0.06 μM | Urine and plasma | 96.0–102.5 | [173] |
| SPPE/DPV | SPE/UV radiation | nanoMIPs | Fentanyl | 0.28 nM | 5–60 nM | Human plasma | NA 2 | [174] |
| Au transducers/Capacitance | Photoinitiatedemulsion | MIP/flow-injection | 4-methyl-5-phenylpyrimidine (4M5PP) | 80 μM | 100–3000 μM | Wastewater | 95–101 | [175] |
| Au electrodes/Capacitance | Bulk polymerization | immobilized MIPs | benzyl methyl ketone (BMK) | 1 μM | 50 to 1000 μM | Spiked tap water and real water | NA 2 | [176] |
| Au/Multiplex capacitive, CV, and optical microscopy | Electropolymerization | MIP, Poly-tyramine/ | Amphetamine | 50 μM | NA2 | Sewage and tap water | NA 2 | [177] |
5. MIPs Designed for Biological Agents Assessment
5.1. MIP Versatile Tools for Detecting Biological Agents
| Sensor Type | Polymerization Method | Substrate/Sensitive Material | Target | Detection | Performances | Refs. |
|---|---|---|---|---|---|---|
| Optical | Free radical polymerization by thermal initiation | E. coli—stamp imprinted Poly(styrene-co-divinylbenzene) | E. coli and B. cereus | confocal Raman Microscopy and AFM |
| [238] |
| Electrochemical | Electro-copolymerization of aniline and p-aminophenyl boronic acid | Binding sites of boronic acid group | E. coli K-12 | MIP film reversibly binds glycan on E.coli cell surface |
| [239] |
| Electrochemical sandwich sensor | One-step electro-copolymerization of 3-thiopheneethanol (TE) monomer and S. aureus template | Sandwich-type electrode: Gold nanoparticles modified with aptamers and electroactive 6- (Ferrocenyl)hexanethiol (Fc) /bacteria-imprinted polymer film/Glass carbon electrode | S. aureus | Dual recognition by the bacteria-imprinted polymer film (BIF) and Aptamer |
| [240] |
| Optical/ Surface imprinting | Free radical polymerization method by thermal initiation | Carboxylic-terminated polystyrene (CPS) microparticles/monomers: acrylamide, methacrylic acid, methyl methacrylate, N-vinylpyrrolidone Dihydroxyethylenebisacrylamide | E. coli OP50 | Selective entrapment of E.coli OP50 |
| [241] |
| Electrochemical EIS- | One-step electro-polymerization | Conductive poly(3-thiopheneacetic acid) deposited on gold electrode | S. aureus, L. monocytogenes, E. coli and S. paratyphi | Selective detection of S. aureus from artificially contaminated milk |
| [242] |
| Electrochemical EIS- | Electro-copolymerization of the template and TE monomer on a GCE | Bacteria-imprinted polythiophene (3-thiopheneethanol—based film) | S. aureus | Identifying S. aureus from multi-bacterial strain mixtures. |
| [243] |
| Electrochemical EIS- | Electro-polymerization | Electrochemically fabricated poly(3-aminophenyl boronic acid)—based MIP deposited on gold disk electrodes | S. epidermidis | Label-free detection - |
| [244] |
| Electrochemical EIS- | Electro-polymerization | Poly(o-phenylenediamine) on glass carbon electrode | E. coli O157:H7 and S. aureus | Dual bacteria-imprinted polymer (DBIP) - |
| [245] |
| Electrochemical EIS- | One-step electro-polymerization | Bacteria-imprinted polypyrrole (BIP) film on -GCE- surface | E. coli O157:H7 | Noncavity imprinted sites situated at the surface of the PPy matrix |
| [246] |
| Electro-chemi-luminescence (ECL) | Electro-polymerization | Polydopamine (PDA) surface imprinted polymer (SIP) film - and nitrogen-doped graphene quantum dots | E. coli O157:H7 | E.coli detection and quantitative measurements |
| [247] |
| Fluorescence-colorimetric dual-mode | Ionic polymerization | Fe3O4 coated with carbon quantum dots; Phenolphthalein was coated with ZIF-8 and then surface-modified with EV71 aptamers to specifically bind to the target | virus EV71 | Aptamers introduced into the imprinting layer to enhance the recognition of the target virus |
| [248] |
5.2. Integrating Bacterial Sensing and Intrinsic/Stimulus-Driven Decontamination in MIPs
6. Conclusions, Emerging Trends, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-230 | Methyl-(bis(diethylamino)methylene)phosphonamidofluoridate |
| A-232 | Methyl (1-(diethylamino)ethylidene)phosphoramidofluoridate |
| A-234 | Ethyl (1-(diethylamino)ethylidene)phosphoramidofluoridate |
| A-242 | O-n-Decyl N-(1-(di-n-decylamino)-n-decylidene)phosphoramidofluoridate |
| A-262 | Guanidine-bearing phosphoramidofluoridate structure |
| ABA | 4-Aminobenzoic acid |
| AchE | Acetylcholinesterase |
| AH-7921 | [3,4-dichloro-N-[[1(dimethylamino) cyclohexyl]methyl] benzamide] |
| ANFO | Ammonium nitrate/fuel oil |
| ATR-FTIR | Total reflectance Fourier transform-infrared |
| NIR | Near-infrared spectroscopy |
| Au-E | Gold disk electrode |
| AuNCs | Gold nanoclusters |
| AuNPs | Gold nanoparticles |
| B. anthracis, B. subtilis | Bacillus species |
| B. cereus | Bacillus cereus |
| BPAEC | Bovine pulmonary artery endothelial cells |
| B. suis, B. melitensis, B. abortus | Brucella species |
| BUP | Buprenorphine |
| BWAs | Biological warfare agents |
| BWC | Biological Weapons Convention |
| BZ3 | Quinuclidinyl benzilate or 1-azabicyclo[2.2.2]octan-3-yl hydroxy(diphenyl)acetate |
| C. botulinum | Clostridium botulinum toxin |
| C. psittaci | Chlamydia psittaci |
| CBD | Cannabidiol |
| CG | Phosgene or Carbonyl dichloride |
| Cl | Chlorine |
| CN | ω-Chloroacetophenone |
| CPE | Carbon paste electrode |
| CPS | Carboxylic-terminated polystyrene |
| CR | Dibenz[b,f][1,4]oxazepin |
| CS | α-chlorbenzylidenemalonitrile |
| CV | Cyclic voltammetry |
| CWAs | Chemical warfare agents |
| DC | Diphenylarsinous cyanide |
| DEET | N,N-diethyl-meta-toluamide |
| DFP | Diisopropylfluorophosphate |
| DMMP | Dimethyl Methylphosphonate |
| DNB | 1,3-Dinitrobenzene |
| DNP | 2,4-Dinitrophenol |
| DNT | 2,4-Dinitrotoluene |
| DPV | Differential pulse voltammetry |
| ECL | Electro-chemiluminescence |
| EDOT | 3, 4-Ethylenedioxythiophene |
| EGDMA | Ethylene glycol dimethacrylate |
| EIS | Electrochemical Impedance Spectroscopy |
| ELISA | Enzyme-linked immunosorbent assays |
| EMPA | Ethyl methyl phosphonic acid |
| ErGO | Reduced graphene oxide |
| Escherichia coli species | E. coli |
| EV71 | Enterovirus 71 |
| FRET | Förster resonance energy transfer |
| FXR | Farnesoid X receptor |
| GA | Tabun O-Ethyl N,N-dimethylphosphoramidocyanidate |
| GB | Sarin or O-Isopropyl methylphosphonofluoridate |
| GC | Gas chromatography |
| GCE | Glassy carbon electrode |
| GC-MS | Gas chromatography-mass spectrometry |
| GD | Soman or O-Pinacolyl methylphosphonofluoridate |
| GO | Graphene oxide |
| GQDs | Graphene quantum dots |
| GWI | Gulf War Illness |
| HCN | Hydrogen cyanide |
| HD | Sulfur mustard or 2-Chloroethylchloromethylsulfide |
| HHC | Hexahydrocannabinol |
| HMX | Octogen |
| HPLC | High-pressure liquid chromatography |
| HPLC | MS/MS High-performance liquid chromatography-tandem mass spectrometry |
| HRMS | High-resolution mass spectrometry |
| IC | MS/MS Ion chromatography-tandem mass spectrometry |
| IF | Imprinting factor |
| IFAT | Indirect fluorescence antibody test |
| IMS | Ion mobility spectroscopy |
| IPD-IC | Indirect photometric detection ion chromatography |
| ISE | Ion-selective electrode |
| ITO | Indium tin oxide |
| L | Lewisite 1 or 2-Chlorovinyldichloroarsine |
| LC | MS/MS Liquid chromatography-tandem mass spectrometry |
| LFA | Lateral flow assay |
| LSD | Lysergic acid diethylamide |
| LSV | Linear sweep voltammetry |
| MAA | Methacrylic acid |
| MABs | Monoclonal Antibodies |
| MBA | N,N′-Methylenebisacrylamide |
| MDMA | Ecstasy or 3,4-methylenedioxymethamphetamine |
| MDPEA | 3,4-Methylenedioxyphenethylamine |
| MESNA | Sodium 2-mercaptoethane sulfonate |
| METH | Methamphetamine |
| MI | Molecular imprinting |
| MIM | Molecularly imprinted membrane |
| MIPs | Molecular imprinted polymers |
| MMIP | Magnetic molecularly imprinted polymers |
| MOF | Metal–organic frameworks |
| MT-45 | [1-cyclohexyl-4-(1,2-diphenylethyl)piperazine] |
| MTs | Metallothioneins |
| MWCNTs | Multi-wall carbon nanotubes |
| nanoMIPs | Nano-sized molecularly imprinted polymers |
| NC | Membrane nitrocellulose membrane |
| NCHS | National Center for Health Statistics |
| NG | Nitroglycerine |
| NLX | Naloxone |
| NM | Nitrogen mustard or 2-Chloro-N,N-bis(2-chloroethyl)ethanamine |
| NTO | 3-nitro-1,2,4-triazol-5-one |
| OC | Oleoresin of capsicum or pepper spray |
| OP | Organophosphate |
| OPCW | Organization for Prohibition of Chemical Weapons |
| PSI-MS | Paper spray ionization mass spectrometry |
| PB | Pyridostigmine bromide |
| PDA | Polydopamine |
| PEI | Polyethyleneimine |
| PER | Permethrin |
| PETN | pentaerythritol tetranitrate |
| PHO | Pholcodine (3-(2-morpholinoethyl)morphine) |
| POC | Point-of-care |
| Ps. Aeruginosa | Pseudomonas aeruginosa |
| PSIMS | Paper spray ionization mass spectrometry |
| PANI | Polyaniline |
| PPy | Polypyrole |
| PVA | Poly(vinylalcohol) |
| QCM | Quartz crystal microbalance |
| QDs | Quantum dots |
| RDX | Hexogen |
| RGO | Reduced graphene oxide |
| RS | Raman spectroscopy/scattering |
| SAW | Surface acoustic wave |
| SDC | Substrate displacement colorimetry |
| SF | Cyclosarin or cyclohexyl methylphosphonofluoridate |
| SIP | Surface imprinted polymer |
| SM | Mustard gas or Bis(2-chloroethyl)sulfide |
| SPCE | Screen-Printed Carbon Electrode |
| SPE | Solid phase extraction |
| SPEs | Screen-Printed Electrodes |
| SPME | Solid phase microextraction |
| SPPE | Screen-printed platinum electrodes |
| S. Paratyphi | Salmonella Paratyphi |
| S. aureus, S. epidermidis | Staphylococcus species |
| S. pneumoniae | Streptococcus pneumoniae |
| STX | Saxitoxin |
| SUF | Sufentanil |
| SWV | Square Wave Voltammetry |
| SWV | Square wave voltammetry |
| TDG | Thiodiglycol |
| TEOS | Tetraethyl orthosilicate |
| THC | Trans-Δ9-tetrahydrocannabinol |
| THCP | Tetrahydrocannabiphorol |
| TNB | 1,3,5-Trinitrobenzene |
| TNP | 2,4,6-Trinitrophenol |
| TNT | 2,4,6-Trinitrotoluene |
| U-47700 | [3,4-dichloro-N-[(1R,2R)-2-(dimethylamino)yclohexyl]-N-methylbenzamide] |
| U-48800 | [trans-2-(2,4-dichlorophenyl)-N-2-(dimethylamino)cyclohexyl)-N-methylacetamide,monohydrochloride] |
| U-49900 | [3,4-dichloro-N-(2-(diethylamino)cyclohexyl)-N-methylbenzamide] |
| U-50488 | [trans-3,4-dichloro-N-methyl-N-[ 2-(1-pyrrolidinyl) cyclohexyl]-benzeneacetamide] |
| V. cholera | Vibrio cholerae |
| VX | O-Ethyl S-2-diisopropylaminoethyl methylphosphonothiolate |
| Y. pestis | Yersinia pestis |
| ZnO | Zinc oxide |
References
- Guo, J.; Luo, C. Risk assessment of hazardous materials transportation: A review of research progress in the last thirty years. J. Traffic Transp. Eng. (Engl. Ed.) 2022, 9, 571–590. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Noll, G.G.; Hildebrand, M.S.; Schnepp, R.; Rudner, G.D. Hazardous Materials: Managing the Incident; Jones & Bartlett Learning: Burlington, MA, USA, 2014. [Google Scholar]
- Houck, M.M.; Siegel, J.A. Chapter 13—Illicit Drugs. In Fundamentals of Forensic Science, 2nd ed.; Houck, M.M., Siegel, J.A., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 305–340. [Google Scholar]
- Üzümcü, A. Working Together for a World Free of Chemical Weapons, and Beyond. Nobel Peace Prize Lecture OPCW. 2013. Available online: www.nobelprize.org (accessed on 10 December 2023).
- Das, S.; Kataria, V.K. Bioterrorism: A Public Health Perspective. Med. J. Armed Forces India 2010, 66, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Richardt, A.; Hülseweh, B.; Niemeyer, B.; Sabath, F. CBRN Protection: Managing the Threat of Chemical, Biological, Radioactive and Nuclear Weapons; Wiley: Hoboken, HJ, USA, 2012. [Google Scholar]
- Mohamed, R.R.; Elshiekh, A.O.; Mohamed, A.M.; Abdul, M.M.; Hamid; Kamal, H.A.; Heikal, A.M. Smart Polymers and Their Different Applications. In Sustainable Nanomaterials: Synthesis and Environmental Applications; Uddin, I., Ed.; Springer Nature: Singapore, 2024; pp. 271–300. [Google Scholar]
- Polyakov, M. Adsorption properties and structure of silica gel. Zhur Fiz Khim 1931, 2, 799–805. [Google Scholar]
- Wulff, G.; Sarhan, A. Über die Anwendung von enzymanalog gebauten Polymeren zur Racemattrennung. Angew. Chem. 1972, 84, 364. [Google Scholar] [CrossRef]
- Komiyama, M. Chapter 8—Molecular Imprinting as Key Technology for Smart Nanoarchitectonics. In Materials Nanoarchitectonics; Ariga, K., Azzaroni, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 161–174. [Google Scholar]
- Gavrila, A.-M.; Zaharia, A.; Paruch, L.; Perrin, F.X.; Sarbu, A.; Olaru, A.G.; Paruch, A.M.; Iordache, T.-V. Molecularly imprinted films and quaternary ammonium-functionalized microparticles working in tandem against pathogenic bacteria in wastewaters. J. Hazard. Mater. 2020, 399, 123026. [Google Scholar] [CrossRef]
- Gavrilă, A.-M.; Stoica, E.-B.; Iordache, T.-V.; Sârbu, A. Modern and Dedicated Methods for Producing Molecularly Imprinted Polymer Layers in Sensing Applications. Appl. Sci. 2022, 12, 3080. [Google Scholar] [CrossRef]
- Sengar, M.S.; Kumari, P.; Sengar, N.; Singh, S.K. Molecularly Imprinted Polymer Technology for the Advancement of Its Health Surveillances and Environmental Monitoring. ACS Appl. Polym. Mater. 2024, 6, 1086–1105. [Google Scholar] [CrossRef]
- Shevchenko, K.G.; Garkushina, I.S.; Canfarotta, F.; Piletsky, S.A.; Barlev, N.A. Nano-molecularly imprinted polymers (nanoMIPs) as a novel approach to targeted drug delivery in nanomedicine. RSC Adv. 2022, 12, 3957–3968. [Google Scholar] [CrossRef]
- Rahman, S.; Bozal-Palabiyik, B.; Unal, D.N.; Erkmen, C.; Siddiq, M.; Shah, A.; Uslu, B. Molecularly imprinted polymers (MIPs) combined with nanomaterials as electrochemical sensing applications for environmental pollutants. Trends Environ. Anal. Chem. 2022, 36, e00176. [Google Scholar] [CrossRef]
- Udaya Rajesh, R.; Mathew, T.; Kumar, H.; Singhal, A.; Thomas, L. Metal-organic frameworks: Recent advances in synthesis strategies and applications. Inorg. Chem. Commun. 2024, 162, 112223. [Google Scholar] [CrossRef]
- Geng, L.; Huang, J.; Fang, M.; Wang, H.; Liu, J.; Wang, G.; Hu, M.; Sun, J.; Guo, Y.; Sun, X. Recent progress of the research of metal-organic frameworks-molecularly imprinted polymers (MOFs-MIPs) in food safety detection field. Food Chem. 2024, 458, 140330. [Google Scholar] [CrossRef]
- Saylan, Y.; Kılıç, S.; Denizli, A. Biosensing Applications of Molecularly Imprinted-Polymer-Based Nanomaterials. Processes 2024, 12, 177. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; Li, Y.; Wang, Q.; Wang, M.; Li, Y.; Davenport, A.; Li, B. Recent advances in molecularly imprinted polymer-based electrochemical sensors. Biosens. Bioelectron. 2024, 249, 116018. [Google Scholar] [CrossRef]
- Su, M.; Song, Y. Printable Smart Materials and Devices: Strategies and Applications. Chem. Rev. 2022, 122, 5144–5164. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Adesanmi, B.O.; McLamore, E.S.; Wei, J.; Obare, S.O. Molecularly Imprinted Plasmonic Sensors as Nano-Transducers: An Effective Approach for Environmental Monitoring Applications. Chemosensors 2023, 11, 203. [Google Scholar] [CrossRef]
- Donato, L.; Nasser, I.I.; Majdoub, M.; Drioli, E. Green Chemistry and Molecularly Imprinted Membranes. Membranes 2022, 12, 472. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Arabi, M.; Ostovan, A.; Li, J.; Wang, X.; Zhang, Z.; Choo, J.; Chen, L. Molecular Imprinting: Green Perspectives and Strategies. Adv. Mater. 2021, 33, 2100543. [Google Scholar] [CrossRef]
- Martín-Esteban, A. Green molecularly imprinted polymers for sustainable sample preparation. J. Sep. Sci. 2022, 45, 233–245. [Google Scholar] [CrossRef]
- Martins, R.O.; Bernardo, R.A.; Machado, L.S.; Batista Junior, A.C.; Maciel, L.Í.L.; Aguiar, D.V.A.d.; Sanches Neto, F.O.; Oliveira, J.V.A.; Simas, R.C.; Chaves, A.R. Greener molecularly imprinted polymers: Strategies and applications in separation and mass spectrometry methods. TrAC Trends Anal. Chem. 2023, 168, 117285. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Tavengwa, N.T.; Tutu, H.; Chimuka, L. Green aspects in molecular imprinting technology: From design to environmental applications. Trends Environ. Anal. Chem. 2018, 17, 14–22. [Google Scholar] [CrossRef]
- Del Sole, R.; Mele, G.; Bloise, E.; Mergola, L. Green Aspects in Molecularly Imprinted Polymers by Biomass Waste Utilization. Polymers 2021, 13, 2430. [Google Scholar] [CrossRef]
- Eissa, M.S.; Imam, M.S.; AbdElrahman, M.; Ghoneim, M.M.; Abdullah, M.; Bayram, R.; Ali, H.M.; Abdelwahab, N.S.; Gamal, M. Magnetic molecularly imprinted polymers and carbon dots molecularly imprinted polymers for green micro-extraction and analysis of pharmaceuticals in a variety of matrices. Microchem. J. 2024, 205, 111235. [Google Scholar] [CrossRef]
- Marć, M.; Jatkowska, N.; Płotka-Wasylka, J.; Gallart Mateu, D.; Esteve Turrillas, F.A.; de la Guardia, M. Molecularly imprinted polymers based on deep eutectic solvents as a greenest materials for selective extraction of emerging contaminants from complex samples. TrAC Trends Anal. Chem. 2024, 178, 117837. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Arabi, M.; Ghaedi, M.; Ostovan, A.; Wang, X.; Li, J.; Chen, L. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 2019, 195, 390–400. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zeng, J.; Hu, X.; Wang, X.; Yu, L.; Wang, D.; Cheng, L.; Ahmed, R.; Romanovski, V.; et al. Multi-templates molecularly imprinted polymers for simultaneous recognition of multiple targets: From academy to application. TrAC Trends Anal. Chem. 2023, 166, 117173. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhao, X.; Ma, Y.; Zhang, H.; Pan, G. Molecularly Imprinted Nanomaterials with Stimuli Responsiveness for Applications in Biomedicine. Molecules 2023, 28, 918. [Google Scholar] [CrossRef]
- Marć, M.; Kupka, T.; Wieczorek, P.P.; Namieśnik, J. Computational modeling of molecularly imprinted polymers as a green approach to the development of novel analytical sorbents. TrAC Trends Anal. Chem. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Yıldız, Ü.Y.; Hussain, C.G.; Keçili, R.; Hussain, C.M. Chapter 3—Green approaches for the preparation of molecularly imprinted polymers. In Green Imprinted Materials; Hussain, C.M., Keçili, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 69–94. [Google Scholar]
- Aoulad El Hadj Ali, Y.; Hejji, L.; Ait Lahcen, A.; Pérez-Villarejo, L.; Azzouz, A.; Kim, K.-H. Progress and prospects in the green synthesis of molecularly imprinted polymers for sorptive extraction and sensing applications toward emerging contaminants in various sample matrices. TrAC Trends Anal. Chem. 2024, 170, 117466. [Google Scholar] [CrossRef]
- Keçili, R.; Hussain, C.G.; Hussain, C.M. Fluorescent nanosensors based on green carbon dots (CDs) and molecularly imprinted polymers (MIPs) for environmental pollutants: Emerging trends and future prospects. Trends Environ. Anal. Chem. 2023, 40, e00213. [Google Scholar] [CrossRef]
- Marć, M.; Wojnowski, W.; Pena-Pereira, F.; Tobiszewski, M.; Martín-Esteban, A. AGREEMIP: The Analytical Greenness Assessment Tool for Molecularly Imprinted Polymers Synthesis. ACS Sustain. Chem. Eng. 2024, 12, 12516–12524. [Google Scholar] [CrossRef]
- Reddy, D.S. Progress and Challenges in Developing Medical Countermeasures for Chemical, Biological, Radiological, and Nuclear Threat Agents. J. Pharmacol. Exp. Ther. 2024, 388, 260–267. [Google Scholar] [CrossRef]
- Steindl, D.; Boehmerle, W.; Körner, R.; Praeger, D.; Haug, M.; Nee, J.; Schreiber, A.; Scheibe, F.; Demin, K.; Jacoby, P.; et al. Novichok nerve agent poisoning. Lancet 2021, 397, 249–252. [Google Scholar] [CrossRef]
- DeFalco, A.P. Chemical warfare agents and delivery systems. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2024; pp. 761–768. [Google Scholar]
- OPCW. Schedule 1 List. Available online: https://www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/schedule-1 (accessed on 20 August 2024).
- Opravil, J.; Pejchal, J.; Finger, V.; Korabecny, J.; Rozsypal, T.; Hrabinova, M.; Muckova, L.; Hepnarova, V.; Konecny, J.; Soukup, O.; et al. A-agents, misleadingly known as “Novichoks”: A narrative review. Arch. Toxicol. 2023, 97, 2587–2607. [Google Scholar] [CrossRef]
- Smolkin, B.; Nahum, V.; Bloch-Shilderman, E.; Nili, U.; Fridkin, G.; Ashkenazi, N. Acetohydroxamic acid salts: Mild, simple and effective degradation reagents to counter Novichok nerve agents. RSC Adv. 2024, 14, 14904–14909. [Google Scholar] [CrossRef]
- de Koning, M.C.; Vieira Soares, C.; van Grol, M.; Bross, R.P.T.; Maurin, G. Effective Degradation of Novichok Nerve Agents by the Zirconium Metal–Organic Framework MOF-808. ACS Appl. Mater. Interfaces 2022, 14, 9222–9230. [Google Scholar] [CrossRef]
- Harvey, S.P.; McMahon, L.R.; Berg, F.J. Hydrolysis and enzymatic degradation of Novichok nerve agents. Heliyon 2020, 6, e03153. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Obare, S.O.; Wei, J. Molecularly imprinted polymers as chemosensors for organophosphate pesticide detection and environmental applications. TrAC Trends Anal. Chem. 2023, 167, 117231. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, N.; Liu, S.; Wei, K.; Ma, C.; Pan, J.; Feng, S. Direct and specific detection of methyl-paraoxon using a highly sensitive fluorescence strategy combined with phosphatase-like nanozyme and molecularly imprinted polymer. Talanta 2024, 277, 126434. [Google Scholar] [CrossRef]
- Suwannapattana, P.; Kongkaew, M.; Thongchai, W.; Sirasunthorn, N. The Selective Detection of Cyantraniliprole Insecticides Using Molecularly Imprinted Polymers Coupled with an Acetylcholinesterase Inhibition-Based Biosensor. Chem. Biodivers. 2023, 20, e202300171. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Ahmadian, F. A chemiluminescence biosensor based on the peroxidase-like property of molybdenum disulfide/zirconium metal-organic framework nanocomposite for diazinon monitoring. Anal. Chim. Acta 2023, 1253, 341055. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Liu, J.; Wang, J.; Liu, J.; Gao, Y.; Ma, N. Chemiluminescence sensors based on molecularly imprinted polymers for the determination of organophosphorus in milk. J. Dairy Sci. 2022, 105, 3019–3031. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wu, Q.; Yuan, Y.; Liu, W.; Han, C.; Wang, X. Detection of Dimethyl Methyl Phosphonate by Silica Molecularly Imprinted Materials. Nanomaterials 2023, 13, 2871. [Google Scholar] [CrossRef]
- Radi, A.-E.; Oreba, R.; Elshafey, R. Molecularly Imprinted Electrochemical Sensor for the Detection of Organophosphorus Pesticide Profenofos. Electroanalysis 2021, 33, 1945–1951. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Xu, Y.; Pan, H.; Guo, K.; Zhang, Y.; Chen, Y.; Liu, D.; Zhang, Y.; Yao, C.; et al. A novel molecularly imprinted polymer composite based on polyaniline nanoparticles as sensitive sensors for parathion detection in the field. Food Control 2022, 133, 108638. [Google Scholar] [CrossRef]
- Yola, B.B.; Kotan, G.; Akyıldırım, O.; Atar, N.; Yola, M.L. Electrochemical determination of fenitrothion pesticide based on ultrathin manganese oxide nanowires/molybdenum titanium carbide MXene ionic nanocomposite and molecularly imprinting polymer. Microchim. Acta 2024, 191, 230. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Athira, V.S.; Nair, S.S. Detection of chlorpyrifos based on molecular imprinting with a conducting polythiophene copolymer loaded on multi-walled carbon nanotubes. Food Chem. 2022, 381, 132010. [Google Scholar] [CrossRef]
- Aghoutane, Y.; Bari, N.E.; Laghrari, Z.; Bouchikhi, B. Electrochemical Detection of Fenthion Insecticide in Olive Oils by a Sensitive Non-Enzymatic Biomimetic Sensor Enhanced with Metal Nanoparticles. Chem. Proc. 2021, 5, 64. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, M.; Ding, L.; Xie, S.; Liu, P.; Xie, D.; Wang, S.; Cheng, F. Molecularly imprinted polymer photoelectrochemical sensor for the detection of triazophos in water based on carbon quantum dot-modified titanium dioxide. Microchim. Acta 2024, 191, 277. [Google Scholar] [CrossRef] [PubMed]
- Daizy, M.; Ali, M.R.; Bacchu, M.S.; Aly, M.A.S.; Khan, M.Z.H. ZnO hollow spheres arrayed molecularly-printed-polymer based selective electrochemical sensor for methyl-parathion pesticide detection. Environ. Technol. Innov. 2021, 24, 101847. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.; Zhang, A.; Peng, Y.; Zhou, H.; Wang, B.; Xie, L.; Guo, Y. A molecularly imprinted electrochemical sensor MIP/Cu-MOF/rGO/AuNPs/GCE for highly sensitive detection of electroneutral organophosphorus pesticide residues. Microchim. Acta 2024, 191, 338. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Yola, M.L.; Atar, N.; Orooji, Y.; Karimi, F.; Senthil Kumar, P.; Rouhi, J.; Baghayeri, M. A novel detection method for organophosphorus insecticide fenamiphos: Molecularly imprinted electrochemical sensor based on core-shell Co3O4@MOF-74 nanocomposite. J. Colloid Interface Sci. 2021, 592, 174–185. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Y.; Zang, X.; Chen, Y.; Wang, X.; Wang, N.; Meng, X.; Cui, B. Gold-copper-doped lanthanide luminescent metal-organic backbone induced self-enhanced molecularly imprinted ECL sensors for ultra-sensitive detection of chlorpyrifos. Food Chem. 2024, 443, 138533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, R.; Wang, X.; Zhang, J.; Wang, N.; Fang, Y.; Cui, B. Molecularly imprinted electrochemiluminescence sensor based on flake-like Au@Cu:ZIF-8 nanocomposites for ultrasensitive detection of malathion. Sens. Actuators B Chem. 2024, 399, 134837. [Google Scholar] [CrossRef]
- Sezigen, S.; Kaya, S.I.; Bakirhan, N.K.; Ozkan, S.A. Development of a molecularly imprinted polymer-based electrochemical sensor for the selective detection of nerve agent VX metabolite ethyl methylphosphonic acid in human plasma and urine samples. Anal. Bioanal. Chem. 2024, 416, 1505–1515. [Google Scholar] [CrossRef]
- Yağmuroğlu, O. Molecularly Imprinted Polymer Based Potentiometric Sensor for the Selective and Sensitive Detection of Nerve Agent Simulant Parathion. Def. Sci. J. 2022, 72, 343–352. [Google Scholar] [CrossRef]
- Luo, Y.; Ye, Q.; Xie, T.; Xie, J.; Mao, K.; Zou, H.; Li, Y.; Huang, C.; Zhen, S. A Novel Molecular Imprinted Polymers-Based Lateral Flow Strip for Sensitive Detection of Thiodiglycol. J. Anal. Test. 2023, 7, 110–117. [Google Scholar] [CrossRef]
- Luo, Y.J.; Ye, Q.C.; Xie, T.J.; Tian, L.L.; Yan, Y.; Lei, Z.; Wang, D.M.; Huang, C.Z.; Li, Y.F.; Zhen, S.J. Electrostatic assemblies of molecularly imprinted polymers on the surface of electrospun nanofiber membranes for the point-of-care detection of thiodiglycol, a sulfur mustard poisoning metabolic marker. Anal. Methods 2023, 15, 1500–1505. [Google Scholar] [CrossRef]
- Ye, Q.; Men, C.; Tian, L.; Liu, Y.; Zhan, L.; Li, Y.F.; Huang, C.Z.; Zhen, S.J. Preparation of a molecularly imprinted test strip for point-of-care detection of thiodiglycol, a sulfur mustard poisoning metabolic marker. Talanta 2021, 234, 122701. [Google Scholar] [CrossRef] [PubMed]
- Diltemiz, S.E. Chapter 13—Optical sensors based on green molecularly imprinted polymers. In Green Imprinted Materials; Hussain, C.M., Keçili, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 419–433. [Google Scholar]
- Fang, L.; Jia, M.; Zhao, H.; Kang, L.; Shi, L.; Zhou, L.; Kong, W. Molecularly imprinted polymer-based optical sensors for pesticides in foods: Recent advances and future trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-Imprinting-Based Surface-Enhanced Raman Scattering Sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, X.; Zhang, P.; Zhou, M.; Liu, C.; Shi, X.; Ma, J. Surface-Enhanced Raman Spectroscopy Detection for Fenthion Pesticides Based on Gold Molecularly Imprinted Polymer Solid-State Substrates. Appl. Spectrosc. 2024, 78, 00037028241253860. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Su, L.; Teng, Y.; Hao, J.; Bi, Y. Fluorescent nanomaterials combined with molecular imprinting polymer: Synthesis, analytical applications, and challenges. Microchim. Acta 2020, 187, 399. [Google Scholar] [CrossRef]
- Chen, M.-j.; Yang, H.-l.; Si, Y.-m.; Tang, Q.; Chow, C.-f.; Gong, C.-b. Photoresponsive Surface Molecularly Imprinted Polymers for the Detection of Profenofos in Tomato and Mangosteen. Front. Chem. 2020, 8, 583036. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, P.; Niu, Y.-L.; Cao, J.-P.; Guo, T.-Y. Fabrication of Molecularly Imprinted Electrospun Nanofibers with Mono-amidoxime Functional Ligand for Efficient Decontamination of Toxic Organophosphates. Chin. J. Polym. Sci. 2024, 42, 446–456. [Google Scholar] [CrossRef]
- Cowen, T.; Bedwell, T.S.; Piletska, E.V.; Rice, H.; Piletsky, S.A. Nanoparticle-induced enhancement of cholinesterase activity in the presence of malathion: A potential nerve agent therapeutic. Int. J. Pharm. 2022, 629, 122406. [Google Scholar] [CrossRef] [PubMed]
- Disley, J.; Gil-Ramírez, G.; Gonzalez-Rodriguez, J. Chitosan-Based Molecularly Imprinted Polymers for Effective Trapping of the Nerve Agent Simulant Dimethyl Methylphosphonate. ACS Appl. Polym. Mater. 2023, 5, 935–942. [Google Scholar] [CrossRef]
- Jiang, P.; Niu, Y.; Cao, J.; Xie, D.; Li, J.; Guo, T. A MOF-doped molecularly imprinted polymer/MOF hybrid gel incorporating with pH-buffering sodium acrylate for practical detoxification of organophosphorus nerve agents. Chem. Eng. J. 2024, 481, 148377. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, P.; Liu, W.; Li, J.; Chen, Z.; Guo, T. Molecularly imprinted self-buffering double network hydrogel containing bi-amidoxime functional groups for the rapid hydrolysis of organophosphates. J. Hazard. Mater. 2023, 444, 130332. [Google Scholar] [CrossRef] [PubMed]
- Gros, C.; Brandès, S.; Yang, J.; Monot, C.; Sabat, D.; Pacquelet, S.; Desbois, N.; André, L.; Estour, F.; Baati, R. Corroles As Precursors of Porous Organic Polymers (POPs) and Molecularly Imprinted Polymers (MIPs)—Application to the Detection of CO and the Decontamination of Chemical Nerve Agents. ECS Meet. Abstr. 2022, MA2022-01, 940. [Google Scholar] [CrossRef]
- Carbonelli, M.; Quaranta, R.; Malizia, A.; Gaudio, P.; Giovanni, D.D. An Analysis of Terrorist Attacks on Soft and Hard Targets in the Period 2000–2019. Int. J. Saf. Secur. Eng. 2024, 14, 865–873. [Google Scholar] [CrossRef]
- To, K.C.; Ben-Jaber, S.; Parkin, I.P. Recent Developments in the Field of Explosive Trace Detection. ACS Nano 2020, 14, 10804–10833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, Y.; Shi, A.; Bao, L.; Shen, Y.; Shen, R.; Ye, Y. Recent Developments in Spectroscopic Techniques for the Detection of Explosives. Materials 2018, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.; Roy Choudhury, D.; Ghosh, D.; Saha, C. Advancements in optical sensors for explosive materials Identification: A comprehensive review. Results Chem. 2024, 8, 101602. [Google Scholar] [CrossRef]
- Khan, S.; Valiyaneerilakkal, U.; Kumar, S.; Singh, A.; Ahmed, A.; Perera, H.C.S.; Mahadeva, R.; Alawatugoda, J.; Arya, S. Nanosensors in hazardous explosives trace detection—Challenges and Future directions. Microchem. J. 2024, 200, 110474. [Google Scholar] [CrossRef]
- De Iacovo, A.; Mitri, F.; De Santis, S.; Giansante, C.; Colace, L. Colloidal Quantum Dots for Explosive Detection: Trends and Perspectives. ACS Sens. 2024, 9, 555–576. [Google Scholar] [CrossRef]
- Fortes, F.J.; Moros, J.; Lucena, P.; Cabalín, L.M.; Laserna, J.J. Laser-Induced Breakdown Spectroscopy. Anal. Chem. 2013, 85, 640–669. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Liu, Z.; Chen, J.; Shi, L.; Huang, L.; Liu, Y.; Cui, S.; He, X. Utilizing an Automated SERS-Digital Microfluidic System for High-Throughput Detection of Explosives. ACS Sens. 2023, 8, 1733–1741. [Google Scholar] [CrossRef]
- Caygill, J.S.; Davis, F.; Higson, S.P.J. Current trends in explosive detection techniques. Talanta 2012, 88, 14–29. [Google Scholar] [CrossRef]
- Virumbrales, C.; Hernández-Ruiz, R.; Trigo-López, M.; Vallejos, S.; García, J.M. Sensory Polymers: Trends, Challenges, and Prospects Ahead. Sensors 2024, 24, 3852. [Google Scholar] [CrossRef] [PubMed]
- Giannoukos, S.; Brkić, B.; Taylor, S.; Marshall, A.; Verbeck, G.F. Chemical Sniffing Instrumentation for Security Applications. Chem. Rev. 2016, 116, 8146–8172. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Bagheri, A.R.; Bhatt, P.; Chen, S. Environmental occurrence, toxicity concerns, and remediation of recalcitrant nitroaromatic compounds. J. Environ. Manag. 2021, 291, 112685. [Google Scholar] [CrossRef]
- Anniyappan, M.; Talawar, M.B.; Sinha, R.K.; Murthy, K.P.S. Review on Advanced Energetic Materials for Insensitive Munition Formulations. Combust. Explos. Shock Waves 2020, 56, 495–519. [Google Scholar] [CrossRef]
- Mahbub, P.; Hasan, C.K.; Rudd, D.; Voelcker, N.H.; Orbell, J.; Cole, I.; Macka, M. Rapid and selective screening of organic peroxide explosives using acid-hydrolysis induced chemiluminescence. Anal. Chim. Acta 2023, 1255, 341156. [Google Scholar] [CrossRef] [PubMed]
- Forbes, T.P.; Krauss, S.T.; Gillen, G. Trace detection and chemical analysis of homemade fuel-oxidizer mixture explosives: Emerging challenges and perspectives. TrAC Trends Anal. Chem. 2020, 131, 116023. [Google Scholar] [CrossRef]
- Kyriakidis, S. Aliphatic Nitro, Nitrate, and Nitrite Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Karadurmus, L.; Bilge, S.; Sınağ, A.; Ozkan, S.A. Molecularly imprinted polymer (MIP)-Based sensing for detection of explosives: Current perspectives and future applications. TrAC Trends Anal. Chem. 2022, 155, 116694. [Google Scholar] [CrossRef]
- Singh, S. Sensors—An effective approach for the detection of explosives. J. Hazard. Mater. 2007, 144, 15–28. [Google Scholar] [CrossRef]
- Ewing, R.G.; Waltman, M.J.; Atkinson, D.A.; Grate, J.W.; Hotchkiss, P.J. The vapor pressures of explosives. TrAC Trends Anal. Chem. 2013, 42, 35–48. [Google Scholar] [CrossRef]
- Aznar-Gadea, E.; Sanchez-Alarcon, I.; Soosaimanickam, A.; Rodriguez-Canto, P.J.; Perez-Pla, F.; Martínez-Pastor, J.P.; Abargues, R. Molecularly imprinted nanocomposites of CsPbBr3 nanocrystals: An approach towards fast and selective gas sensing of explosive taggants. J. Mater. Chem. C 2022, 10, 1754–1766. [Google Scholar] [CrossRef]
- Yang, L.; Hu, W.; Pei, F.; Du, B.; Tong, Z.; Mu, X.; Xia, M.; Wang, F.; Liu, B. Novel dual-emission fluorescence imprinted sensor based on Mg, N-CDs and metal-organic frameworks for rapid and smart detection of 2, 4, 6-trinitrophenol. Talanta 2024, 266, 125115. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, Ş.; Üzer, A.; Apak, R. Direct Determination of Peroxide Explosives on Polycarbazole/Gold Nanoparticle-Modified Glassy Carbon Sensor Electrodes Imprinted for Molecular Recognition of TATP and HMTD. Anal. Chem. 2022, 94, 17662–17669. [Google Scholar] [CrossRef] [PubMed]
- Głosz, K.; Fabin, M.; Janasik, P.; Kołodziej, W.; Stolarczyk, A.; Jarosz, T. The Failure of Molecular Imprinting in Conducting Polymers: A Case Study of Imprinting Picric Acid on Polycarbazole. Sensors 2024, 24, 424. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ling, Y.; Chen, J.; Yuan, X.; Li, S.; Zhang, Z. Design of a versatile and selective electrochemical sensor based on dummy molecularly imprinted PEDOT/laser-induced graphene for nitroaromatic explosives detection. Environ. Res. 2023, 236, 116769. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Wojnarowicz, A.; Kelm, A.; Woznicki, P.; Borowicz, P.; Majka, A.; D’Souza, F.; Kutner, W. Chemosensor for Selective Determination of 2,4,6-Trinitrophenol Using a Custom Designed Imprinted Polymer Recognition Unit Cross-Linked to a Fluorophore Transducer. ACS Sens. 2016, 1, 636–639. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Hu, B.; Meng, M.; Yan, Y. Molecularly imprinted polydopamine coated CdTe@SiO2 as a ratiometric fluorescent probe for ultrafast and visual p-nitrophenol monitoring. Microchem. J. 2022, 172, 106899. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Huang, F.; Ji, X.; Dai, H.; Wu, W. Surface enhanced Raman scattering substrate for the detection of explosives: Construction strategy and dimensional effect. J. Hazard. Mater. 2020, 387, 121714. [Google Scholar] [CrossRef]
- Aznar-Gadea, E.; Rodríguez-Canto, P.J.; Martínez-Pastor, J.P.; Lopatynskyi, A.; Chegel, V.; Abargues, R. Molecularly Imprinted Silver Nanocomposites for Explosive Taggant Sensing. ACS Appl. Polym. Mater. 2021, 3, 2960–2970. [Google Scholar] [CrossRef]
- Lazau, C.; Iordache, T.-V.; Florea, A.-M.; Orha, C.; Bandas, C.; Radu, A.-L.; Sarbu, A.; Rotariu, T. Towards developing an efficient sensitive element for trinitrotoluene detection: TiO2 thin films functionalized with molecularly imprinted copolymer films. Appl. Surf. Sci. 2016, 384, 449–458. [Google Scholar] [CrossRef]
- Gavrila, A.M.; Iordache, T.V.; Lazau, C.; Rotariu, T.; Cernica, I.; Stroescu, H.; Stoica, M.; Orha, C.; Bandas, C.E.; Sarbu, A. Biomimetic Sensitive Elements for 2,4,6-Trinitrotoluene Tested on Multi-Layered Sensors. Coatings 2020, 10, 273. [Google Scholar] [CrossRef]
- Apak, R.; Üzer, A.; Sağlam, Ş.; Arman, A. Selective Electrochemical Detection of Explosives with Nanomaterial Based Electrodes. Electroanalysis 2023, 35, e202200175. [Google Scholar] [CrossRef]
- Kamel, A.H.; Abd-Rabboh, H.S.M.; Hefnawy, A. Molecularly imprinted polymer-based electrochemical sensors for monitoring the persistent organic pollutants chlorophenols. RSC Adv. 2024, 14, 20163–20181. [Google Scholar] [CrossRef]
- Tancharoen, C.; Sukjee, W.; Yenchitsomanus, P.-t.; Panya, A.; Lieberzeit, P.A.; Sangma, C. Selectivity enhancement of MIP-composite sensor for explosive detection using DNT-dengue virus template: A co-imprinting approach. Mater. Lett. 2021, 285, 129201. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Sosnowska, M.; Sobczak, J.W.; Kc, C.B.; Nesterov, V.N.; D’Souza, F.; Kutner, W. Simultaneous Chronoamperometry and Piezoelectric Microgravimetry Determination of Nitroaromatic Explosives Using Molecularly Imprinted Thiophene Polymers. Anal. Chem. 2013, 85, 8361–8368. [Google Scholar] [CrossRef]
- Peltzer, K.; Ramlagan, S.; Johnson, B.D.; Phaswana-Mafuya, N. Illicit Drug Use and Treatment in South Africa: A Review. Substance Use & Misuse 2010, 45, 2221–2243. [Google Scholar] [CrossRef]
- Janik, P.; Kosticova, M.; Pecenak Prof, J.; Turcek, M. Categorization of psychoactive substances into “hard drugs” and “soft drugs”: A critical review of terminology used in current scientific literature. Am. J. Drug Alcohol Abus. 2017, 43, 636–646. [Google Scholar] [CrossRef]
- Nations, U. PRESS RELEASE—UNODC World Drug Report 2024: Harms of World Drug Problem Continue to Mount Amid Expansions in Drug Use and Markets. Available online: https://www.unodc.org/unodc/en/press/releases/2024/June/unodc-world-drug-report-2024_-harms-of-world-drug-problem-continue-to-mount-amid-expansions-in-drug-use-and-markets.html (accessed on 20 August 2024).
- EUDA. European Drug Report 2024: Trends and Developments. Available online: https://www.euda.europa.eu/publications/european-drug-report/2024_en (accessed on 20 August 2024).
- Alzu’bi, A.; Almahasneh, F.; Khasawneh, R.; Abu-El-Rub, E.; Baker, W.B.; Al-Zoubi, R.M. The synthetic cannabinoids menace: A review of health risks and toxicity. Eur. J. Med. Res. 2024, 29, 49. [Google Scholar] [CrossRef]
- Pires, B.; Rosendo, L.M.; Brinca, A.T.; Simão, A.Y.; Barroso, M.; Rosado, T.; Gallardo, E. The Therapeutic Potential of Amphetamine-like Psychostimulants. Life 2023, 13, 2180. [Google Scholar] [CrossRef]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. Synthetic opioids: A review and clinical update. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221139616. [Google Scholar] [CrossRef] [PubMed]
- Krausz, R.M.; Westenberg, J.N.; Meyer, M.; Choi, F. The upcoming synthetic ultrapotent opioid wave as a foreseeable disaster. Lancet Psychiatry 2022, 9, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.R.; Garnett, M.; Miniño, A.M. Drug Overdose Deaths in the United States, 2002–2022. NCHS Data Brief 2024, 491, 1941–4935. [Google Scholar] [CrossRef]
- U.S. Department of Justice, D.E.A. Carfentanil: A Dangerous New Factor in the U.S. Opioid Crisis. Available online: https://www.justice.gov/usao-edky/file/898991/dl (accessed on 20 August 2024).
- Edinoff, A.N.; Martinez Garza, D.; Vining, S.P.; Vasterling, M.E.; Jackson, E.D.; Murnane, K.S.; Kaye, A.M.; Fair, R.N.; Torres, Y.J.L.; Badr, A.E.; et al. New Synthetic Opioids: Clinical Considerations and Dangers. Pain Ther. 2023, 12, 399–421. [Google Scholar] [CrossRef]
- Schedules of Controlled Substances: Permanently Placement of Etodesnitazene, N-Pyrrolidino Etonitazene, and Protonitazene in Schedule I, 89 FR 25514 (11 April 2024); Temporary Placement of Butonitazene, Flunitazene, Metodesnitazene, Metonitazene in Schedule I, 87 FR 21556 (12 April 2022). Available online: https://www.govinfo.gov/content/pkg/FR-2024-04-11/pdf/2024-07684.pdf (accessed on 20 August 2024).
- Guidance for Local Areas on Planning to Deal with Potent Synthetic Opioids. Available online: https://www.gov.uk/government/publications/fentanyl-preparing-for-a-future-threat/guidance-for-local-areas-on-planning-to-deal-with-fentanyl-or-another-potent-opioid (accessed on 20 August 2024).
- Moe, J.; Godwin, J.; Purssell, R.; O’Sullivan, F.; Hau, J.P.; Purssell, E.; Curran, J.; Doyle-Waters, M.M.; Brasher, P.M.A.; Buxton, J.A.; et al. Naloxone dosing in the era of ultra-potent opioid overdoses: A systematic review. CJEM 2020, 22, 178–186. [Google Scholar] [CrossRef]
- Bremer, P.T.; Burke, E.L.; Barrett, A.C.; Desai, R.I. Investigation of monoclonal antibody CSX-1004 for fentanyl overdose. Nat. Commun. 2023, 14, 7700. [Google Scholar] [CrossRef]
- Żubrycka, A.; Kwaśnica, A.; Haczkiewicz, M.; Sipa, K.; Rudnicki, K.; Skrzypek, S.; Poltorak, L. Illicit drugs street samples and their cutting agents. The result of the GC-MS based profiling define the guidelines for sensors development. Talanta 2022, 237, 122904. [Google Scholar] [CrossRef]
- Schram, J.; Parrilla, M.; Sleegers, N.; Van Durme, F.; van den Berg, J.; van Nuijs, A.L.N.; De Wael, K. Electrochemical profiling and liquid chromatography–mass spectrometry characterization of synthetic cathinones: From methodology to detection in forensic samples. Drug Test. Anal. 2021, 13, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, X.; Lyu, L.; Duan, H.; Chen, Y.; Bian, J.; Xu, Z.; Liu, L.; Zhang, Y. Determination of Fentanyl, Alpha-Methylfentanyl, Beta-Hydroxyfentanyl and the Metabolite Norfentanyl in Rat Urine by LC–MS-MS. J. Anal. Toxicol. 2021, 46, 421–431. [Google Scholar] [CrossRef]
- Hadi, E.A.; Al-Bayati, Y.K. Preparation and characterized study of new molecularly imprinted polymers for determination Cocaine by GC-Mass based on different Functional Monomers. Egypt. J. Chem. 2022, 65, 107–116. [Google Scholar] [CrossRef]
- Kardani, F.; Khezeli, T.; Shariati, S.; Hashemi, M.; Mahdavinia, M.; Jelyani, A.Z.; Rashedinia, M.; Noori, S.M.A.; Karimvand, M.N.; Ramezankhani, R. Application of novel metal organic framework-deep eutectic solvent/molecularly imprinted polymer multiple monolithic fiber for solid phase microextraction of amphetamines and modafinil in unauthorized medicinal supplements with GC-MS. J. Pharm. Biomed. Anal. 2024, 242, 116005. [Google Scholar] [CrossRef]
- Ferreira, J.B.; Santos, N.A.d.; Borges, K.B.; Conceição, N.S.; Baptista, C.S.D.; França, H.S.; Romão, W. Synthesis and Characterization of Molecularly Imprinted Polymers for the Determination of Cocaine in Urine Using Microextraction in Packed Sorvent. J. Braz. Chem. Soc. 2023, 34, 1677–1690. [Google Scholar] [CrossRef]
- Fu, Y.; Pessagno, F.; Manesiotis, P.; Borrull, F.; Fontanals, N.; Maria Marcé, R. Preparation and evaluation of molecularly imprinted polymers as selective SPE sorbents for the determination of cathinones in river water. Microchem. J. 2022, 175, 107100. [Google Scholar] [CrossRef]
- Han, C.; Tan, D.; Wang, Y.; Yu, Z.; Sun, X.; Wang, D. Selective extraction of synthetic cathinones new psychoactive substances from wastewater, urine and cocktail using dummy molecularly imprinted polymers. J. Pharm. Biomed. Anal. 2022, 215, 114765. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Liang, Y.; Guo, T.; Wang, Y.; Li, Y.; Sun, X.; Wang, D. Dummy molecularly imprinted polymers-agarose gel mixed matrix membrane for extraction of amphetamine-type stimulants in wastewater and urine. J. Chromatogr. A 2023, 1708, 464368. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.P.d.; Aguiar, D.V.A.d.; Pereira, I.; Vaz, B.G. Determining Methamphetamine in Urine by Molecularly Imprinted Polymer Assisted Paper Spray Ionization Mass Spectrometry. J. Braz. Chem. Soc. 2021, 32, 269–276. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Brady, P.N.; Grant, C.; Fernand Narcisse, V.; Rosado Flores, P.; Lisse, C.H.; Bwambok, D.K. Electrochemical Sensors, Biosensors, and Optical Sensors for the Detection of Opioids and Their Analogs: Pharmaceutical, Clinical, and Forensic Applications. Chemosensors 2024, 12, 58. [Google Scholar] [CrossRef]
- Ott, C.E.; Burns, A.; Sisco, E.; Arroyo, L.E. Targeted fentanyl screening utilizing electrochemical surface-enhanced Raman spectroscopy (EC-SERS) applied to authentic seized drug casework samples. Forensic Chem. 2023, 34, 100492. [Google Scholar] [CrossRef]
- McKeown, H.E.; Rook, T.J.; Pearson, J.R.; Jones, O.A.H. Classification of fentanyl precursors by multivariate analysis of low-field nuclear magnetic resonance spectroscopy data. Forensic Chem. 2020, 21, 100285. [Google Scholar] [CrossRef]
- Ferguson, K.; Tupik, S.L.; Haddad, H.; Perr, J.; Gilbert, M.; Newman, R.; Almirall, J. Utility of gas chromatography infrared spectroscopy (GC-IR) for the differentiation of positional isomers of fentanyl related substances. Forensic Chem. 2022, 29, 100425. [Google Scholar] [CrossRef]
- Kranenburg, R.F.; Verduin, J.; Weesepoel, Y.; Alewijn, M.; Heerschop, M.; Koomen, G.; Keizers, P.; Bakker, F.; Wallace, F.; van Esch, A.; et al. Rapid and robust on-scene detection of cocaine in street samples using a handheld near-infrared spectrometer and machine learning algorithms. Drug Test. Anal. 2020, 12, 1404–1418. [Google Scholar] [CrossRef]
- Darie, I.-F.; Anton, S.R.; Praisler, M. Machine Learning Systems Detecting Illicit Drugs Based on Their ATR-FTIR Spectra. Inventions 2023, 8, 56. [Google Scholar] [CrossRef]
- Yeganegi, A.; Fardindoost, S.; Tasnim, N.; Hoorfar, M. Molecularly imprinted polymers (MIP) combined with Raman spectroscopy for selective detection of Δ⁹-tetrahydrocannabinol (THC). Talanta 2024, 267, 125271. [Google Scholar] [CrossRef] [PubMed]
- Azhdary, P.; Janfaza, S.; Fardindoost, S.; Tasnim, N.; Hoorfar, M. Highly selective molecularly imprinted polymer nanoparticles (MIP NPs)-based microfluidic gas sensor for tetrahydrocannabinol (THC) detection. Anal. Chim. Acta 2023, 1278, 341749. [Google Scholar] [CrossRef]
- Akhoundian, M.; Alizadeh, T. An ultra-selective and non-enzymatic colorimetric sensor based on imprinted polymer for ephedrine assay in urine samples. Mater. Today Commun. 2024, 39, 109193. [Google Scholar] [CrossRef]
- Akhoundian, M.; Alizadeh, T. Enzyme-free colorimetric sensor based on molecularly imprinted polymer and ninhydrin for methamphetamine detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121866. [Google Scholar] [CrossRef] [PubMed]
- Lowdon, J.W.; Eersels, K.; Arreguin-Campos, R.; Caldara, M.; Heidt, B.; Rogosic, R.; Jimenez-Monroy, K.L.; Cleij, T.J.; Diliën, H.; van Grinsven, B. A Molecularly Imprinted Polymer-based Dye Displacement Assay for the Rapid Visual Detection of Amphetamine in Urine. Molecules 2020, 25, 5222. [Google Scholar] [CrossRef]
- Liu, L.; Grillo, F.; Canfarotta, F.; Whitcombe, M.; Morgan, S.P.; Piletsky, S.; Correia, R.; He, C.; Norris, A.; Korposh, S. Carboxyl-fentanyl detection using optical fibre grating-based sensors functionalised with molecularly imprinted nanoparticles. Biosens. Bioelectron. 2021, 177, 113002. [Google Scholar] [CrossRef]
- Adegoke, O.; Zolotovskaya, S.; Abdolvand, A.; Daeid, N.N. Fabrication of a near-infrared fluorescence-emitting SiO2-AuZnFeSeS quantum dots-molecularly imprinted polymer nanocomposite for the ultrasensitive fluorescence detection of levamisole. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 129013. [Google Scholar] [CrossRef]
- Adegoke, O.; Nsuamani, M.L.; Nic Daeid, N. Cadmium-free silica-encapsulated molecularly imprinted AuZnCeSeS quantum dots nanocomposite as an ultrasensitive fluorescence nanosensor for methamphetamine detection. Mater. Sci. Semicond. Process. 2023, 159, 107387. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Mashhadi-Ramezani, S.; Mosleh, N. Molecularly imprinted polymer containing fluorescent graphene quantum dots as a new fluorescent nanosensor for detection of methamphetamine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 118021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kan, Y.; Chen, Z.; Li, H.; Zhang, W. MOFs-Modified Electrochemical Sensors and the Application in the Detection of Opioids. Biosensors 2023, 13, 284. [Google Scholar] [CrossRef]
- Yahyapour, M.; Ranjbar, M.; Mohadesi, A.; Rejaeinegad, M. Determination of Buprenorphine (BUP) with Molecularly Imprinted Polymer Zn/La3+ Metal Organic Framework on Modified Glassy Carbon Electrode (GCE). Electroanalysis 2022, 34, 1012–1020. [Google Scholar] [CrossRef]
- Tang, X.; Gu, Y.; Tang, P.; Liu, L. Electrochemical Sensor Based on Magnetic Molecularly Imprinted Polymer and Graphene-UiO-66 Composite Modified Screen-printed Electrode for Cannabidiol Detection. Int. J. Electrochem. Sci. 2022, 17, 220562. [Google Scholar] [CrossRef]
- Zhao, Y.; Moon, Y.; Savari, R. Molecularly Imprinted Electrochemical Sensor for Determination of Tetrahydrocannabinol in Human Blood Plasma. Int. J. Electrochem. Sci. 2022, 17, 221185. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Amr, A.E.-G.E.; Almehizia, A.A.; Kamel, A.H. All-Solid-State Potentiometric Ion-Sensors Based on Tailored Imprinted Polymers for Pholcodine Determination. Polymers 2021, 13, 1192. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, N.; Chan, N.W.C.; Jemere, A.B. A Molecularly Imprinted Sol-Gel Electrochemical Sensor for Naloxone Determination. Nanomaterials 2021, 11, 631. [Google Scholar] [CrossRef]
- Truta, F.M.; Cruz, A.G.; Dragan, A.-M.; Tertis, M.; Cowen, T.; Stefan, M.-G.; Topala, T.; Slosse, A.; Piletska, E.; Van Durme, F.; et al. Design of smart nanoparticles for the electrochemical detection of 3,4-methylenedioxymethamphetamine to allow in field screening by law enforcement officers. Drug Test. Anal. 2024, 16, 865–878. [Google Scholar] [CrossRef]
- Almabadi, M.H.; Truta, F.M.; Adamu, G.; Cowen, T.; Tertis, M.; Drăgan, A.-M.; Alanazi, K.D.M.; Ștefan, M.-G.; Piletska, E.; Kiss, B.; et al. Integration of smart nanomaterials for highly selective disposable sensors and their forensic applications in amphetamine determination. Electrochim. Acta 2023, 446, 142009. [Google Scholar] [CrossRef]
- Li, M.; Chen, H.; Xu, A.; Duan, S.; Liu, Q.; Zhang, R.; Wang, S.; Bai, H. High-performance fentanyl molecularly imprinted electrochemical sensing platform designed through molecular simulations. Anal. Chim. Acta 2024, 1312, 342686. [Google Scholar] [CrossRef]
- Merli, D.; Lio, E.; Protti, S.; Coccia, R.; Profumo, A.; Alberti, G. Molecularly Imprinted Polymer-based voltammetric sensor for amino acids/indazole derivatives synthetic cannabinoids detection. Anal. Chim. Acta 2024, 1288, 342151. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, D.; Wu, Z.; Liang, Z.; Han, F.; Chen, K.; Fu, W.; Han, D.; Wang, Y.; Niu, L. Polydopamine-based molecularly imprinted electrochemical sensor for the highly selective determination of ecstasy components. Analyst 2022, 147, 3291–3297. [Google Scholar] [CrossRef]
- Charkravarthula, P.; Mugweru, A. Molecularly Imprinted Electrochemical Sensor Based on Poly (O-Phenylenediamine) for Sensitive Detection of Oxycodone in Water. Electrochem 2023, 4, 435–446. [Google Scholar] [CrossRef]
- D’Aurelio, R.; Chianella, I.; Goode, J.A.; Tothill, I.E. Molecularly Imprinted Nanoparticles Based Sensor for Cocaine Detection. Biosensors 2020, 10, 22. [Google Scholar] [CrossRef]
- Abedi, H.; Roostaie, A. Magnetic molecularly imprinted polymer for trace pharmaceutical analysis: Preconcentration and the electrochemical determination of sufentanil in biological fluids. Vietnam J. Chem. 2023, 61, 383–396. [Google Scholar] [CrossRef]
- Almabadi, M. Electrochemical Method Based On Molecularly Imprinted Polymers for Drug Detection. Ph.D. Thesis, University of Leicester, Leicester, UK, 2024. [Google Scholar]
- El-Akaad, S.; De Saeger, S.; Beloglazova, N. Molecularly imprinted polymer based capacitive sensing of a specific Leuckart marker 4-methyl-5-phenylpyrimidine in wastewater. Sens. Actuators B Chem. 2021, 343, 130116. [Google Scholar] [CrossRef]
- De Rycke, E.; Trynda, A.; Jaworowicz, M.; Dubruel, P.; De Saeger, S.; Beloglazova, N. Capacitive sensing of an amphetamine drug precursor in aqueous samples: Application of novel molecularly imprinted polymers for benzyl methyl ketone detection. Biosens. Bioelectron. 2021, 172, 112773. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, E.; Leman, O.; Dubruel, P.; Hedström, M.; Völker, M.; Beloglazova, N.; De Saeger, S. Novel multiplex capacitive sensor based on molecularly imprinted polymers: A promising tool for tracing specific amphetamine synthesis markers in sewage water. Biosens. Bioelectron. 2021, 178, 113006. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S. Chapter 1—Biological warfare agents: History and modern-day relevance. In Handbook on Biological Warfare Preparedness; Flora, S.J.S., Pachauri, V., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–11. [Google Scholar]
- Nikolelis, D.P. Portable Chemical Sensors: Weapons Against Bioterrorism; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Bruce-Tagoe, T.A.; Bhaskar, S.; Kavle, R.R.; Jeevanandam, J.; Acquah, C.; Ohemeng-Boahen, G.; Agyei, D.; Danquah, M.K. Advances in aptamer-based biosensors for monitoring foodborne pathogens. J. Food Sci. Technol. 2024, 61, 1252–1271. [Google Scholar] [CrossRef]
- Mi, F.; Hu, C.; Wang, Y.; Wang, L.; Peng, F.; Geng, P.; Guan, M. Recent advancements in microfluidic chip biosensor detection of foodborne pathogenic bacteria: A review. Anal. Bioanal. Chem. 2022, 414, 2883–2902. [Google Scholar] [CrossRef]
- Upadhyay, P.; Surar, F.; Kim, G.; Reddy, J.; Shakir, S.; Alexander, B.D.; Hanson, K.; Singh, V. 2223. A comparative analysis of the detection of UTI pathogens via culture method and the Open Array-nanofluidic real time PCR method. Open Forum Infect. Dis. 2022, 9, S907–S908. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.-n.; Han, B.; Cheng, L.; Li, D.; Zheng, W.; Zhao, Y. Biochemical sensor based on functional material assisted optical fiber surface plasmon resonance: A review. Measurement 2023, 207, 112353. [Google Scholar] [CrossRef]
- Roy, P. Chapter 6—Development of optical biosensors for the diagnosis of pathogens. In Biosensors for Emerging and Re-Emerging Infectious Diseases; Das, J., Dave, S., Radhakrishnan, S., Mohanty, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 137–168. [Google Scholar]
- Anand, U.; Chandel, A.K.S.; Oleksak, P.; Mishra, A.; Krejcar, O.; Raval, I.H.; Dey, A.; Kuca, K. Recent advances in the potential applications of luminescence-based, SPR-based, and carbon-based biosensors. Appl. Microbiol. Biotechnol. 2022, 106, 2827–2853. [Google Scholar] [CrossRef] [PubMed]
- Frigoli, M.; Lowdon, J.W.; Caldara, M.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Emerging Biomimetic Sensor Technologies for the Detection of Pathogenic Bacteria: A Commercial Viability Study. ACS Omega 2024, 9, 23155–23171. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, A.; Youssef, K.; Akhtarian, S.; Kraft, G.; Rezai, P. Fluorescent bacteria detection in water using cell imprinted polymer (CIP) coated microparticles in a magnetophoretic microfluidic device. Talanta 2024, 268, 125290. [Google Scholar] [CrossRef]
- Cassedy, A.; Parle-McDermott, A.; O’Kennedy, R. Virus Detection: A Review of the Current and Emerging Molecular and Immunological Methods. Front. Mol. Biosci. 2021, 8, 637559. [Google Scholar] [CrossRef]
- Schöler, L.; Le-Trilling, V.T.K.; Eilbrecht, M.; Mennerich, D.; Anastasiou, O.E.; Krawczyk, A.; Herrmann, A.; Dittmer, U.; Trilling, M. A Novel In-Cell ELISA Assay Allows Rapid and Automated Quantification of SARS-CoV-2 to Analyze Neutralizing Antibodies and Antiviral Compounds. Front. Immunol. 2020, 11, 573526. [Google Scholar] [CrossRef]
- Walper, S.A.; Lasarte Aragonés, G.; Sapsford, K.E.; Brown, C.W., III; Rowland, C.E.; Breger, J.C.; Medintz, I.L. Detecting Biothreat Agents: From Current Diagnostics to Developing Sensor Technologies. ACS Sens. 2018, 3, 1894–2024. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Lisse, C.; Medawala, W.; Brady, P.N.; Bwambok, D.K.; Anum, D.; Alonge, T.; Taylor, M.E.; Baker, G.A.; Mehari, T.F.; et al. Fluorescent chemical sensors: Applications in analytical, environmental, forensic, pharmaceutical, biological, and biomedical sample measurement, and clinical diagnosis. Appl. Spectrosc. Rev. 2024, 59, 1–89. [Google Scholar] [CrossRef]
- Eskandari, V.; Sahbafar, H.; Zeinalizad, L.; Hadi, A. A review of applications of surface-enhanced raman spectroscopy laser for detection of biomaterials and a quick glance into its advances for COVID-19 investigations. ISSS J. Micro Smart Syst. 2022, 11, 363–382. [Google Scholar] [CrossRef]
- Nazim, T.; Lusina, A.; Cegłowski, M. Recent Developments in the Detection of Organic Contaminants Using Molecularly Imprinted Polymers Combined with Various Analytical Techniques. Polymers 2023, 15, 3868. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, K.; Coşansu, S.; Orhan-Yanıkan, E.; Gülseren, G. Advance methods for the qualitative and quantitative determination of microorganisms. Microchem. J. 2021, 166, 106188. [Google Scholar] [CrossRef]
- Rajpal, S.; Mizaikoff, B.; Mishra, P. Rational design of MIPs for the detection of Myxovirus resistance protein A (MxA), a biomarker for viral infection. Int. J. Biol. Macromol. 2024, 266, 131101. [Google Scholar] [CrossRef] [PubMed]
- Duracova, M.; Klimentova, J.; Fucikova, A.; Dresler, J. Proteomic Methods of Detection and Quantification of Protein Toxins. Toxins 2018, 10, 99. [Google Scholar] [CrossRef]
- Sharma, H.; Mutharasan, R. Review of biosensors for foodborne pathogens and toxins. Sens. Actuators B Chem. 2013, 183, 535–549. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lin, Y.; Chu, W.; Luo, Z.; Zhao, M.; Hu, J.; Miao, X.; He, F. A catalytic hairpin assembly–based Förster resonance energy transfer sensor for ratiometric detection of ochratoxin A in food samples. Anal. Bioanal. Chem. 2023, 415, 867–874. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Ebdali, H.; Soltani, M.; Abdolahi-Fard, P.; Heydari, M.; Assadpour, E.; Azizi-Lalabadi, M.; Zhang, F.; Jafari, S.M. Nanomaterial-based sensors for the detection of pathogens and microbial toxins in the food industry; a review on recent progress. Coord. Chem. Rev. 2024, 500, 215545. [Google Scholar] [CrossRef]
- Drinkard, K.K.; Barr, J.R.; Kalb, S.R. Mass Spectrometric Detection and Differentiation of Enzymatically Active Abrin and Ricin Combined with a Novel Affinity Enrichment Technique. Chem. Res. Toxicol. 2024, 37, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ge, K.; Qadir, M.F.; Wang, X.; Gu, Y.; Yang, Y. MIPs-Based Sensors and Biosensors for Environmental Monitoring. In Molecularly Imprinted Polymers as Artificial Antibodies for the Environmental Health: A Step Towards Achieving the Sustainable Development Goals; Patra, S., Sillanpaa, M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 167–200. [Google Scholar]
- Toaleb, N.I.; Shaapan, R.M. Zoonotic Protozoan Parasites Infecting Camels, Diagnosis and Control—A Review. Egypt. J. Vet. Sci. 2024, 55, 1131–1142. [Google Scholar] [CrossRef]
- Johnson, M.; Tetzlaff, S.; Katz, A.; Sperry, J. Comparison of qPCR and metabarcoding for environmental DNA surveillance of a freshwater parasite. Ecol. Evol. 2024, 14, e11382. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, S.; Gorki, V. Chapter 4—Molecular diagnostic tools in detection of mixed parasite infections: Current scenario and challenges. In Falciparum Malaria; Qidwai, T., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 59–76. [Google Scholar]
- Upadhyay, A.; Pal, D.; Kumar, A. Future Development of Automated Technique for Clinical Microbiology. In Automated Diagnostic Techniques in Medical Microbiology; Kumar, S., Kumar, A., Eds.; Springer Nature: Singapore, 2024; pp. 191–204. [Google Scholar]
- Sachdeva, P.; Nath, G.; Jain, U. Phage based biosensors: Enhancing early detection of emerging pathogens in diagnostics. Talanta Open 2024, 10, 100345. [Google Scholar] [CrossRef]
- Venkataraman, R.; Yadav, U.; Shivalingegowda, R.K.; Shrestha, Y. Vaccination strategies to combat nosocomial infections. Vacunas (Engl. Ed.) 2023, 24, 60–67. [Google Scholar] [CrossRef]
- Hjort, R.G.; Pola, C.C.; Soares, R.R.A.; Oliveira, D.A.; Stromberg, L.; Claussen, J.C.; Gomes, C.L. Advances in Biosensors for Detection of Foodborne Microorganisms, Toxins, and Chemical Contaminants. In Encyclopedia of Food Safety, 2nd ed.; Smithers, G.W., Ed.; Academic Press: Oxford, UK, 2024; pp. 372–384. [Google Scholar]
- Blasi, F.; Mantero, M.; Santus, P.; Tarsia, P. Understanding the burden of pneumococcal disease in adults. Clin. Microbiol. Infect. 2012, 18, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rahim, G.R.; Gupta, N.; Maheshwari, P.; Singh, M.P. Monomicrobial Klebsiella pneumoniae necrotizing fasciitis: An emerging life-threatening entity. Clin. Microbiol. Infect. 2019, 25, 316–323. [Google Scholar] [CrossRef]
- Planet, P.J. 155—Pseudomonas aeruginosa. In Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Long, S.S., Prober, C.G., Fischer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 866–870.e861. [Google Scholar]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Logue, C.M.; Barbieri, N.L.; Nielsen, D.W. Chapter Eight—Pathogens of Food Animals: Sources, Characteristics, Human Risk, and Methods of Detection. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 277–365. [Google Scholar]
- Harris, A. Clostridium botulinum. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 141–145. [Google Scholar]
- Kumar, A.; Flora, S.J.S. Chapter 13—Genome information of BW agents and their application in biodefence. In Handbook on Biological Warfare Preparedness; Flora, S.J.S., Pachauri, V., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 257–271. [Google Scholar]
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40. [Google Scholar] [CrossRef]
- Warawa, J.; Shannon, J.G.; Bosio, C.M. Immunology of Bacterial Biodefense Agents: Francisella tularensis, Burkholderia mallei, and Yersinia pestis. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 66–74. [Google Scholar]
- Reece, R.; Smit, M.A.; Flanigan, T.P. Ebola Virus. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 355–362. [Google Scholar]
- Ascenzi, P.; Bocedi, A.; Heptonstall, J.; Capobianchi, M.R.; Di Caro, A.; Mastrangelo, E.; Bolognesi, M.; Ippolito, G. Ebolavirus and Marburgvirus: Insight the Filoviridae family. Mol. Asp. Med. 2008, 29, 151–185. [Google Scholar] [CrossRef]
- Lupi, O.; Tyring, S.K.; Cosenza, P.P.; Motta, R.N.; Kouri, G.; Guzman, M.G.; De Aguiar, F.C.; Correa, A.R.; de Almeida Ferry, F.R.; Boleira, M.; et al. 12—Hemorrhagic Fever and Arboviruses. In Tropical Dermatology, 2nd ed.; Tyring, S.K., Lupi, O., Hengge, U.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 127–151. [Google Scholar]
- Prakash, P.O.; Rayasam, K.; Chaitanya, K.V.; Peddireddy, V. Chapter 10—Biofilms: Cities of microorganisms. In Bacterial Survival in the Hostile Environment; Kumar, A., Tenguria, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 131–148. [Google Scholar]
- Crump, J.A.; Wain, J. Salmonella. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Academic Press: Oxford, UK, 2017; pp. 425–433. [Google Scholar]
- Woldehiwet, Z. Q fever (coxiellosis): Epidemiology and pathogenesis. Res. Vet. Sci. 2004, 77, 93–100. [Google Scholar] [CrossRef]
- Bradberry, S. Ricin and abrin. Medicine 2016, 44, 109–110. [Google Scholar] [CrossRef]
- Eremeeva, M.E.; Dasch, G.A. 179—Other Rickettsia Species. In Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Long, S.S., Prober, C.G., Fischer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 957–966.e954. [Google Scholar]
- Idil, N.; Aslıyüce, S.; Perçin, I.; Mattiasson, B. Recent Advances in Optical Sensing for the Detection of Microbial Contaminants. Micromachines 2023, 14, 1668. [Google Scholar] [CrossRef]
- Ebralidze, I.I.; Laschuk, N.O.; Poisson, J.; Zenkina, O.V. Chapter 1—Colorimetric Sensors and Sensor Arrays. In Nanomaterials Design for Sensing Applications; Zenkina, O.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–39. [Google Scholar]
- Yang, Q.; Li, J.; Wang, X.; Peng, H.; Xiong, H.; Chen, L. Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens. Bioelectron. 2018, 112, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Petrou, P.; Kakabakos, S. Advances in interferometric sensors for the detection of food contaminants. TrAC Trends Anal. Chem. 2024, 175, 117714. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar, S.; M, Y.; S, R.; C A, M.; Thirunavookarasu S, N.; C K, S. Recent advances in Surface Plasmon Resonance (SPR) biosensors for food analysis: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1055–1077. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Y.; Wang, L.; Lu, Y.; Li, J.; Lu, D.; Zhou, T.; Huang, Z.; Huang, J.; Huang, H.; et al. Interference-free and high precision biosensor based on surface enhanced Raman spectroscopy integrated with surface molecularly imprinted polymer technology for tumor biomarker detection in human blood. Biosens. Bioelectron. 2019, 143, 111599. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, C.; Huang, J.; Wang, M.; Qi, W.; Wang, H.; He, Z. Specific and quantitative detection of bacteria based on surface cell imprinted SERS mapping platform. Biosens. Bioelectron. 2022, 215, 114524. [Google Scholar] [CrossRef]
- Monzó, J.; Insua, I.; Fernandez-Trillo, F.; Rodriguez, P. Fundamentals, achievements and challenges in the electrochemical sensing of pathogens. Analyst 2015, 140, 7116–7128. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Lu, X. Molecular imprinting technology for sensing foodborne pathogenic bacteria. Anal. Bioanal. Chem. 2021, 413, 4581–4598. [Google Scholar] [CrossRef]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef]
- El-Schich, Z.; Zhang, Y.; Feith, M.; Beyer, S.; Sternbæk, L.; Ohlsson, L.; Stollenwerk, M.; Wingren, A.G. Molecularly Imprinted Polymers in Biological Applications. BioTechniques 2020, 69, 407–420. [Google Scholar] [CrossRef]
- Norouzi, S.; Dashtian, K.; Amourizi, F.; Zare-Dorabei, R. Red-emissive carbon nanostructure-anchored molecularly imprinted Er-BTC MOF: A biosensor for visual anthrax monitoring. Analyst 2023, 148, 3379–3391. [Google Scholar] [CrossRef]
- Bräuer, B.; Thier, F.; Bittermann, M.; Baurecht, D.; Lieberzeit, P.A. Raman Studies on Surface-Imprinted Polymers to Distinguish the Polymer Surface, Imprints, and Different Bacteria. ACS Appl. Bio Mater. 2022, 5, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, N.; Etienne, M.; Sharma, P.S.; El-Kirat-Chatel, S.; Helú, M.B.; Kutner, W. Molecularly imprinted polymer as a synthetic receptor mimic for capacitive impedimetric selective recognition of Escherichia coli K-12. Anal. Chim. Acta 2021, 1188, 339177. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, P.P.; Yan, J.; Luan, D.; Sun, T.; Bian, X. Dual Synthetic Receptor-Based Sandwich Electrochemical Sensor for Highly Selective and Ultrasensitive Detection of Pathogenic Bacteria at the Single-Cell Level. Anal. Chem. 2023, 95, 5561–5567. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Youssef, K.; Akhtarian, S.; Tabesh, E.; Kraft, G.; Brar, S.K.; Rezai, P. Molecularly imprinted polymer (MIP) based core-shell microspheres for bacteria isolation. Polymer 2022, 251, 124917. [Google Scholar] [CrossRef]
- Hasseb, A.A.; Abdel Ghani, N.d.T.; Shehab, O.R.; El Nashar, R.M. Application of molecularly imprinted polymers for electrochemical detection of some important biomedical markers and pathogens. Curr. Opin. Electrochem. 2022, 31, 100848. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Liu, T.; Zhang, Z.; Kong, J.; Yu, H.; Yan, J.; Luan, D.; Zhao, Y.; Bian, X. Reusable and universal impedimetric sensing platform for the rapid and sensitive detection of pathogenic bacteria based on bacteria-imprinted polythiophene film. Analyst 2022, 147, 4433–4441. [Google Scholar] [CrossRef]
- Golabi, M.; Kuralay, F.; Jager, E.W.H.; Beni, V.; Turner, A.P.F. Electrochemical bacterial detection using poly(3-aminophenylboronic acid)-based imprinted polymer. Biosens. Bioelectron. 2017, 93, 87–93. [Google Scholar] [CrossRef]
- Xu, X.; Lin, X.; Wang, L.; Ma, Y.; Sun, T.; Bian, X. A Novel Dual Bacteria-Imprinted Polymer Sensor for Highly Selective and Rapid Detection of Pathogenic Bacteria. Biosensors 2023, 13, 868. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Lu, Y.; Jia, M.; Yan, J.; Bian, X. Facile Preparation of a Bacteria Imprinted Artificial Receptor for Highly Selective Bacterial Recognition and Label-Free Impedimetric Detection. Anal. Chem. 2019, 91, 1027–1033. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Zhang, L.; Gao, J.; Ma, Q. Electrochemiluminescence Detection of Escherichia coli O157:H7 Based on a Novel Polydopamine Surface Imprinted Polymer Biosensor. ACS Appl. Mater. Interfaces 2017, 9, 5430–5436. [Google Scholar] [CrossRef]
- Tang, L.; Liang, K.; Wang, L.; Chen, C.; Cai, C.; Gong, H. Construction of an Ultrasensitive Molecularly Imprinted Virus Sensor Based on an “Explosive” Secondary Amplification Strategy for the Visual Detection of Viruses. Anal. Chem. 2022, 94, 13879–13888. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, M.; Babaeipour, V.; Jabbari, F.; Nouralishahi, A. Optimization of Sensor Based on Molecularly Imprinted Polymeric Nanoparticles for Detection of Dipicolinic Acid as a Biomarker in Bacterial Spores. Iran. J. Chem. Chem. Eng. 2024, 43, 556–568. [Google Scholar] [CrossRef]
- Nasirahmadi, S.; Akbari-Adergani, B.; Shoeibi, S. Construction of eco-biosensor and its potential application for highly selective, sensitive and fast detection of viscumin. Anal. Chim. Acta 2020, 1107, 213–224. [Google Scholar] [CrossRef]
- Pradhan, S.; Boopathi, M.; Kumar, O.; Baghel, A.; Pandey, P.; Mahato, T.H.; Singh, B.; Vijayaraghavan, R. Molecularly imprinted nanopatterns for the recognition of biological warfare agent ricin. Biosens. Bioelectron. 2009, 25, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Tsurugi, K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987, 262, 8128–8130. [Google Scholar] [CrossRef]
- Hua, Y.; Ahmadi, Y.; Sonne, C.; Kim, K.-H. Progress and challenges in sensing of mycotoxins using molecularly imprinted polymers. Environ. Pollut. 2022, 305, 119218. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, M.; Huang, Z.; Sun, Y.; Ye, L. Molecularly Imprinted Polymers for Targeting Lipopolysaccharides and Photothermal Inactivation of Pseudomonas aeruginosa. ACS Appl. Polym. Mater. 2023, 5, 3055–3064. [Google Scholar] [CrossRef]
- Shao, S.; Gao, S.; Li, Y.; Lv, Y. Rapid Screening and Synthesis of Abiotic Synthetic Receptors for Selective Bacterial Recognition. ACS Appl. Mater. Interfaces 2023, 15, 16408–16419. [Google Scholar] [CrossRef]
- Yang, L.; Luo, Y.; Zhou, Y.; Huang, C.; Shen, X. Specific nanoantibiotics for selective removal of antibiotic-resistant bacteria: New insights in bacterial imprinting based on interfacial biomimetic mineralization. J. Hazard. Mater. 2023, 443, 130254. [Google Scholar] [CrossRef]
- Mustafa, G.; Lieberzeit, P.A. MIP Sensors on the Way to Real-World Applications. In Designing Receptors for the Next Generation of Biosensors; Piletsky, S.A., Whitcombe, M.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 167–187. [Google Scholar]
- Srivastava, A.; Singh, M. Limitations and Challenges in the Practical Implementation of MIPs. In Molecularly Imprinted Polymers as Artificial Antibodies for the Environmental Health: A Step Towards Achieving the Sustainable Development Goals; Patra, S., Sillanpaa, M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 389–412. [Google Scholar]
- Ayankojo, A.G.; Reut, J.; Syritski, V. Electrochemically Synthesized MIP Sensors: Applications in Healthcare Diagnostics. Biosensors 2024, 14, 71. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Mishra, P.; Bhakta, S. Chapter 16—MIP-based commercial materials: Molecularly-imprinted polymers for commercial application: Potentials and barriers. In Molecularly Imprinted Polymers (MIPs); Singh, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 391–415. [Google Scholar]
- Lu, Z.; Du, X.; Sun, M.; Zhang, Y.; Li, Y.; Wang, X.; Wang, Y.; Du, H.; Yin, H.; Rao, H. Novel dual-template molecular imprinted electrochemical sensor for simultaneous detection of CA and TPH based on peanut twin-like NiFe2O4/CoFe2O4/NCDs nanospheres: Fabrication, application and DFT theoretical study. Biosens. Bioelectron. 2021, 190, 113408. [Google Scholar] [CrossRef] [PubMed]
- Rawool, C.R.; Srivastava, A.K. A dual template imprinted polymer modified electrochemical sensor based on Cu metal organic framework/mesoporous carbon for highly sensitive and selective recognition of rifampicin and isoniazid. Sens. Actuators B Chem. 2019, 288, 493–506. [Google Scholar] [CrossRef]
- Cortés Rodríguez, F.; Dal Peraro, M.; Abriata, L.A. Online tools to easily build virtual molecular models for display in augmented and virtual reality on the web. J. Mol. Graph. Model. 2022, 114, 108164. [Google Scholar] [CrossRef]
- Xu, J.; Merlier, F.; Avalle, B.; Vieillard, V.; Debré, P.; Haupt, K.; Tse Sum Bui, B. Molecularly Imprinted Polymer Nanoparticles as Potential Synthetic Antibodies for Immunoprotection against HIV. ACS Appl. Mater. Interfaces 2019, 11, 9824–9831. [Google Scholar] [CrossRef]
- Batista, A.D.; Rajpal, S.; Keitel, B.; Dietl, S.; Fresco-Cala, B.; Dinc, M.; Groß, R.; Sobek, H.; Münch, J.; Mizaikoff, B. Plastic Antibodies Mimicking the ACE2 Receptor for Selective Binding of SARS-CoV-2 Spike. Adv. Mater. Interfaces 2022, 9, 2101925. [Google Scholar] [CrossRef]
- Yao, Z.; Diao, Y.; Gao, J.; Pan, G. Emerging theragnostic molecularly imprinted nano-antibodies. Colloid Interface Sci. Commun. 2023, 57, 100753. [Google Scholar] [CrossRef]
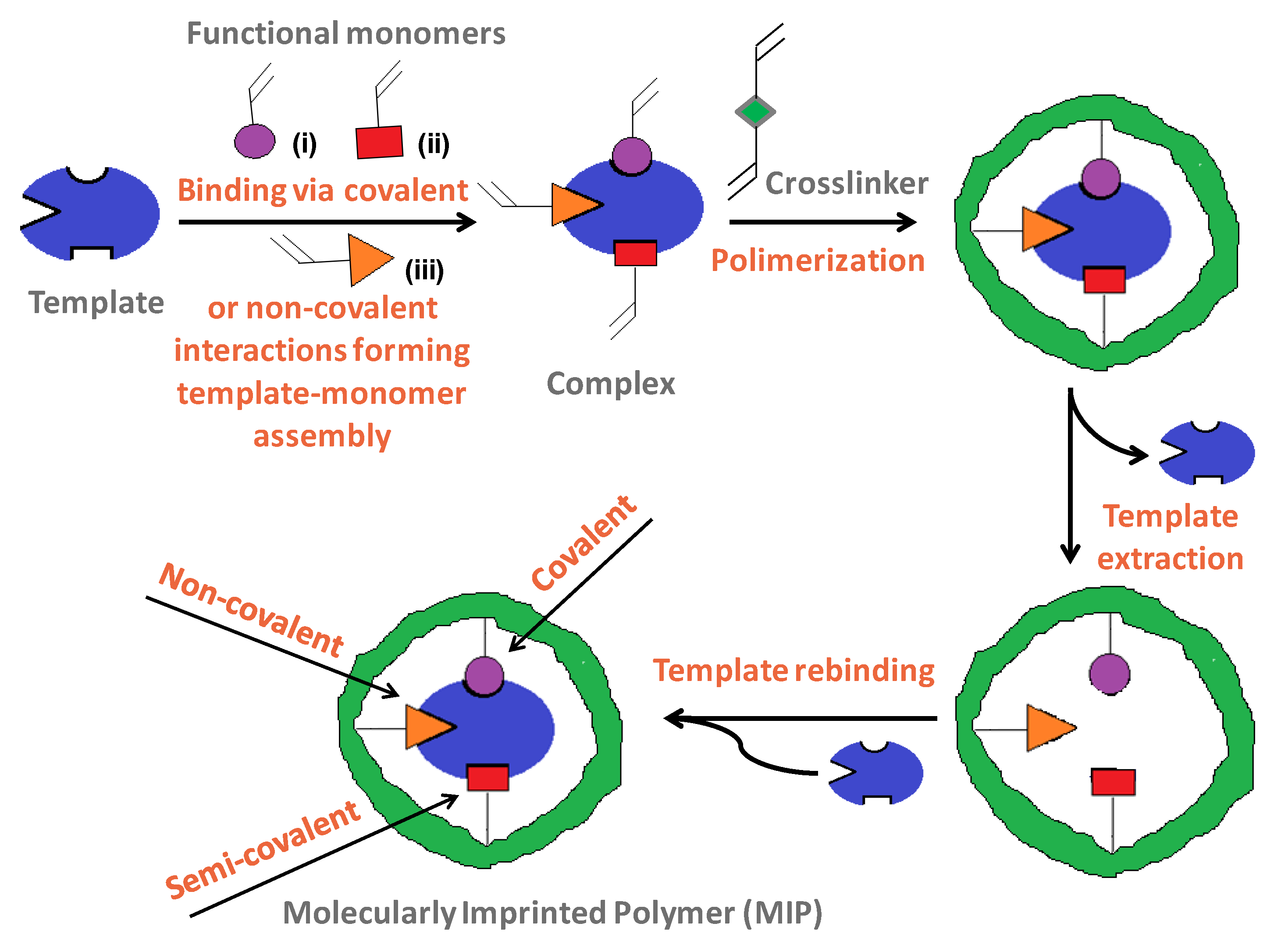


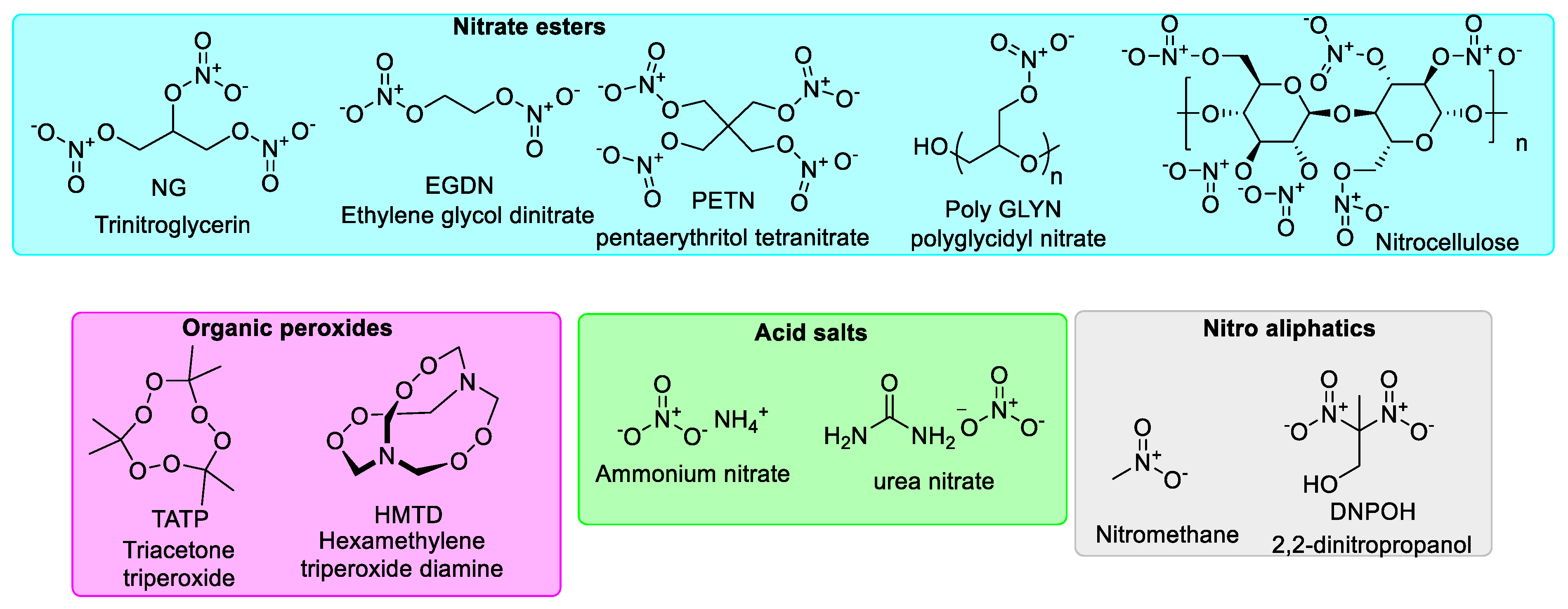
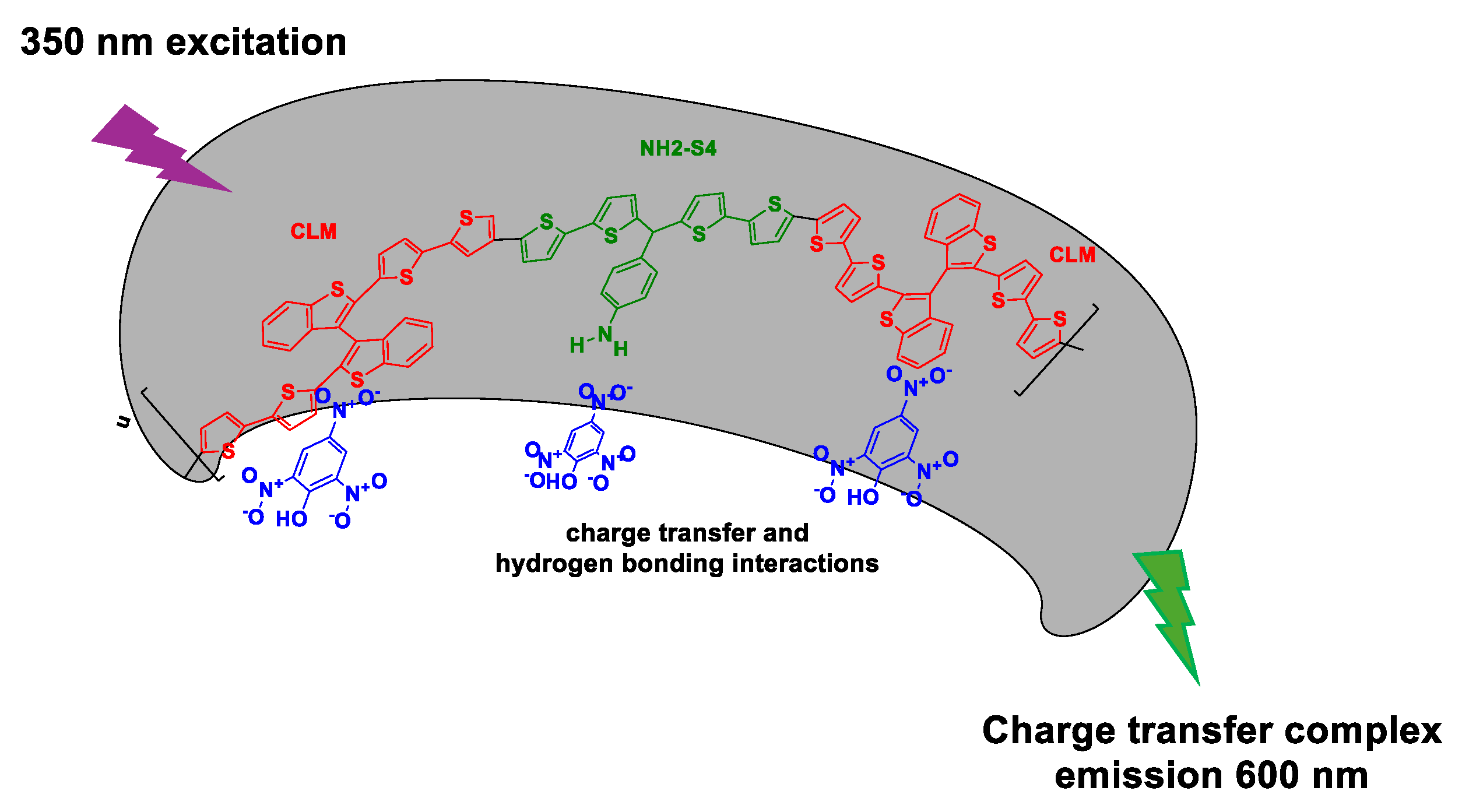
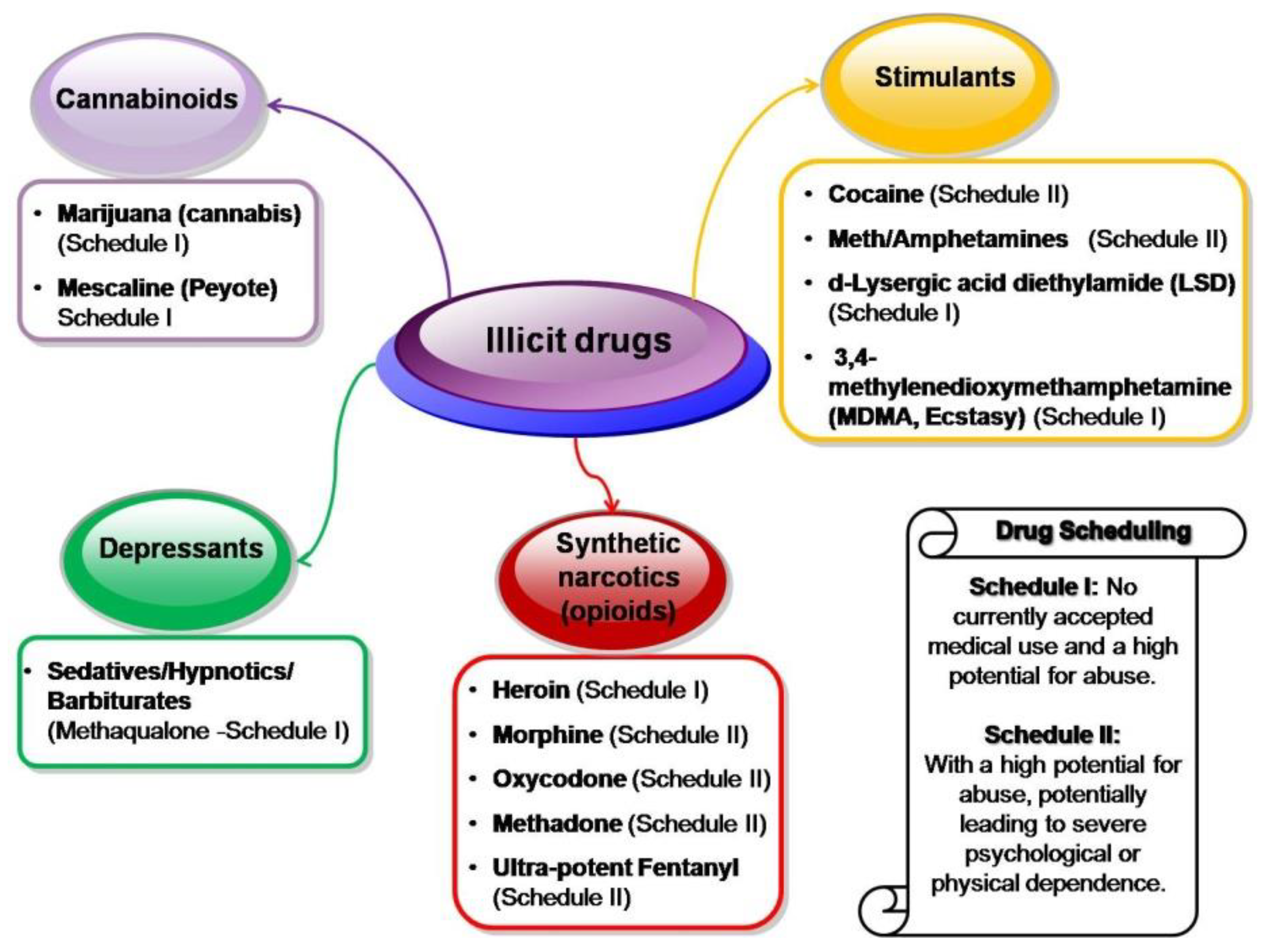
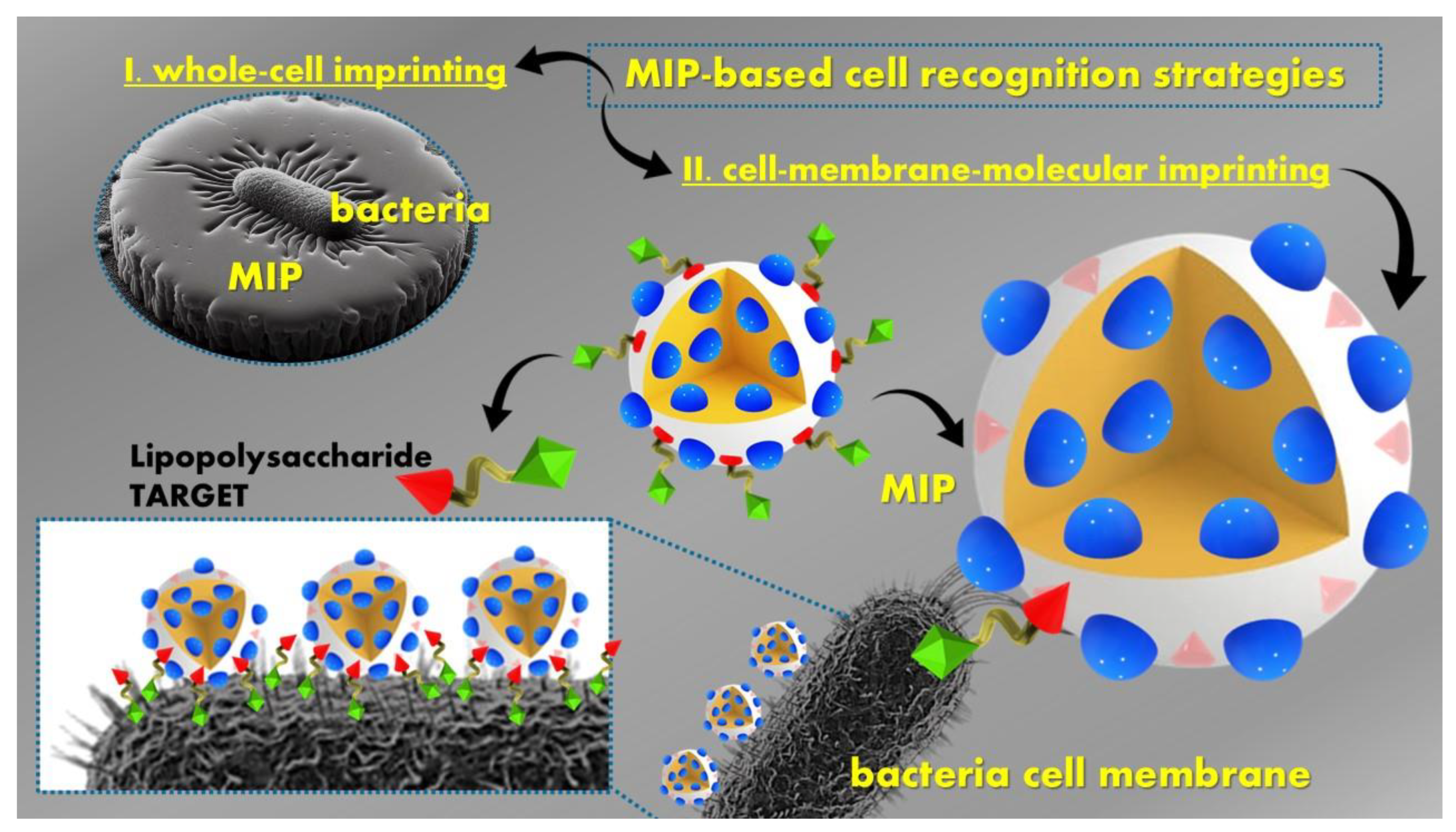

| CWAs | Main Properties/Toxicology | Treatment/Therapy | Refs. | |
|---|---|---|---|---|
| Blister or vesicants | Sulfur mustard HD or Mustard gas SM, Nitrogen mustard HN1, lewisite L1, O-mustard | Damage occurs in the tissues; affects the lungs, eyes and produces skin blistering/ are relatively persistent | Anti-inflammatory agents (Dexamethasone), antioxidants, intracellular proteins like Dynasore, farnesoid receptor activation, Mesna for SM exposure, immunomodulators, British anti-Lewisite antidotes, and wound/tissue repair agents | [42,44] |
| Nerve agents | G-type: Sarin GB, Soman GD, Cyclosarin GF, Tabun GA; V-type O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate VX, VG, VM, VE; A-type: A 230, A232, A234, A-242, A-262 | Highly fatal due to their neurotoxicity; reacts irreversibly with cholinesterase; this results in acetylcholine accumulation, breakdown of the nervous system, convulsive status epilepticus, and death within minutes/ range in persistency | Antidotes like antimuscarinic agent atropine; benzodiazepines midazolam and ketamine; combined therapy for GD: allopregnanolone and ganaxolone; Pralidoxime; hydrophilic neurosteroids as anticonvulsants; parasympatholytic and neuroprotective agents for Novichok | [42,43,44,45] |
| Incapacitating agents | 3-Quinuclidinyl benzilate BZ; | Prevents normal activity by producing mental or physiological effects | Antidotes like 7-MEOTA, atropine, and tacrine THA | [5,42,44] |
| Irritants/riot control agents | Tear aerosols CR, CS, CN, and OC pepper spray | Causes tearing and irritation of the skin, lungs, and eyes | TRPA1 with HC-030031 or A-967079 inhibitors for CS-induced skin injuries; Proliferating BPAECs; Bronchodilators like beta-2 agonists | [5,42,44] |
| Choking agents | Chlorine Cl, CG phosgene | Affects the respiratory tract and lungs | Limited or nonexistent therapeutic interventions (FXR activation on lung injury), oxidative stress, and fibrosis | [5,42,44] |
| Blood or vomiting agents | Cyanides DC and HCN, Adamsite, Arsine | Causes acute pain, nausea, and vomiting; prevents the transfer of oxygen to the body’s tissues | Hydroxocobalamin, cobalt (II/III) complex CoN4 [14] | [5,42,44] |
| Other agents: Gulf War | Pyridostigmine bromide PB, N,N-diethyl-m-toluamide DEET, permethrinPER and chlorpyrifos | GWI causing chronic pain, fatigue, sleep disturbances, cognitive problems | Ketamine for treating GWI-associated neuropsychiatric disorders | [5,42,44] |
| Sensor Type/Detection Method | Polymerization Method | Electrode Modification | Target Molecule | LOD | Linear Range | Real Samples | Recovery Rate (%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Fluorescence combined with phosphatase-like nanozyme | One-pot reverse microemulsion | Gold nanoclusters (AuNCs) with MIPs polydopamine (PDA) and hollow CeO2 nanospheres CeO2@PDA@AuNCs-MIPs | Methyl-paraoxon | 0.15 nM | 0.45–125 nM | River and tap water | 93.06–102.22 | [51] |
| Colorimetric | Photo-polymerization | MIP-AchE inhibition | Cyantraniliprole Insecticides | 4.1 ppm | 15–50 ppm | Spiked melon | 86.00–105.55 | [52] |
| Colorimetric/fluorometric enzymatic | Chemiluminescence | Molybdenum disulfide/zirconium-based MOF MoS2@MIP-202(Zr) nanocomposite (NC) | Diazinon | 0.12 nmol L−1 | 0.5–300.0 nmol L−1 | Real water samples, river | 95–102.5 | [53] |
| Chemiluminescence (CL) | Bulk polymerization | MIP-based microtitration CL | Coumaphos, fenthion, chlorpyrifos, parathion, diazinon, fenchlorphos, and fenitrothion | 1–3 pg mL−1 | 1–20 ng mL−1 | Milk samples | Intraday86.1–86.5 and interday83.6–94.2 | [54] |
| SAW gas/adsorption | Sol-gel polymerization | Piezoelectric/mesoporous SiO2MIP | Dimethyl methylphosphonateDMMP | 80 ppb | 80 ppb | NA * | NA * | [55] |
| Electrochemical/CV, DPV, EIS | Electropolymerization | MIP(O-PPy)/GCE | Profenofos | 1 nM | 1.0 × 10−9–5.0 × 10−6 M | Sweet pepper samples | 108 | [56] |
| Electrochemical/ CV, DPV | Surface and Electropolymerization | polyaniline nanomaterials (PANIs)-MIPs/GC | Parathion | 0.011 μM | 0.034–18.67 μM | Vegetables pakchoi, radish, lettuce, brassica chinensis, spinach, cabbage | 98.2–100.1 | [57] |
| Electrochemical/CV, EIS | Electropolymerization | Manganese oxide nanowires/two-dimensional molybdenum titanium carbide MXene (MnO2NWs@Mo2TiC2MXene)/GCE | Fenitrothion | 3.0 × 10−10 mol L−1 | 1.0 × 10−9–2.0 × 10−8 mol L−1 | White flour samples | Close to 100 | [58] |
| Electrochemical/CV, EIS | Surface and Electropolymerization | Polythiophene copolymer loaded on—MWCNTs | Chlorpyrifos | 4.0 pM | 0.02–1000 nM | Vegetable samples | NA * | [59] |
| Non-Enzymatic Biomimetic Electrochemical/CV, DPV, EIS | Electropolymerization | 2-aminothiophenol complex mixed with AuNPs/Au-SPE | Fenthion | 0.05 mgkg−1 | 0.01–17.3 µg-mL−1 | Olive oil samples | NA * | [60] |
| Photo electrochemical/CV | Electropolymerization | Carbon QD-modified titanium dioxide (MIP/C/TiO2NTs) | Triazophos | 0.03 nM | 0.1–1000 nM | Dongjiang River, drinking water and tap water | 102–107 | [61] |
| Electrochemical /DPV | Electropolymerization | -ZnO-hollow spheres (ZnOHS)—MIP/GCE | Methyl-parathion | 0.5 × 10−9 mol L−1 | 5 × 10−9–0.1 × 10−4 mol L−1 | Green beans, strawberry, tomato, and cabbage | 90.4–91.9 and 96.3 | [62] |
| Electrochemical /DPV | Electropolymerization | MIP/Cu-MOF/rGO/AuNPs/GCE | Phosmet | 7.2 × 10−15 M | 1.00 × 10−14–5.00 × 10−7 M | Apples, Cucumbers | 94.2–106.5 | [63] |
| Electrochemical /CV, EIS | Electropolymerization | MIP/Co3O4 nanowire and core-shell Co3O4@MOF-74 nanocomposite | Fenamiphos | 3.0 × 10−12 M | 10−11–10−9 M | Orange juice | 99.65–100.48 | [64] |
| Electrochemiluminescence (ECL) | Electropolymerization | MIP gold copper doped Tb-MOFs (Au@Cu:Tb-MOFs)/GCE | Chlorpyrifos | 0.083 pM | 0.285–0.285 × 106 pM | Apples and cabbage samples | 97.83–103.62 | [65] |
| Electrochemiluminescence (ECL) | Electropolymerization | Flake-like nanocomposites Au@Cu:ZIF-8 | Malathion | 0.18 pgmL−1 | 10 pgmL−1 to 0.1 μgmL−1 | Food and agricultural products | 91.94–104.50 | [66] |
| Electrochemical/ CV, EIS | Thermal polymerization | MIP based on4-ABA and TEOS/GCE | Ethyl methylphosphonic acid | 2.75 × 10−11 M (standard), 2.11 × 10−11 M (urine), and 2.36 × 10−11 M (plasma) | 1.0 × 10–10– 2.5 × 10–9 (standard), 1.0 × 10–10–2.5 × 10–9 (urine); 1.0 × 10–10–1.0 × 10–9 (plasma) | Human plasma and urine | 99.86–101.30 in urine; 100.62–101.08 in plasma | [67] |
| Electrochemical/CV, EIS | Emulsion polymerization | MIP based on 4-ABA and TEOS/GCE- | Parathion | 1.86 × 10−8 mol L−1 | 10−8–10−4 mol L−1 | Tap and lake water | 97–99 in tap water; 94–96 in lake water | [68] |
| Biomarkers POCT—Test strip/(MIPs)-based LFA strategy | Bulk | Sample pad, conjugate, absorption and backing pad/AuNPs, MIPs, and MTs | Thiodiglycol | 0.41 pgmL−1 | 10.0 pgmL−1–10,000.0 ng mL−1 | Urine samples | 96.2–105.4 | [69] |
| Biomarkers POCT—test strip/(MIPs)-based LFA strategy | Electrostatic assemblies of MIPs on the surface of PEI/PVA NFs membranes. | MIPs- PEI-PVA electrospun nanofiber membranes and AuNPs | Thiodiglycol | 38 pgmL−1 | 0.1 ng mL−1–1.0 µg mL−1 | Urine samples | 105–111.6 | [70] |
| Biomarkers POCT—test strip/(MIPs)-based LFA strategy | TDG was combined with the MAA through hydrogen bonding. | Coating MIPs supported on an NC membrane to obtain MIM and AuNPs | Thiodiglycol | 1.0 ng mL−1–100.0 μg mL−1 | 0.23 ng mL−1 | Urine samples | 97.9–102 | [71] |
| Sensor Type/ Detection Method | Preparation Method | Substrate/ Sensitive Material | Target | LoD | Linearity Range | Refs. |
|---|---|---|---|---|---|---|
| Photoluminescence | Deposited by spin-coating | CsPbBr3 nanocrystals (NCs) embedded in a polycaprolactone (PCL) matrix | Vapors of 3-nitrotoluene (3-NT) and nitromethane (NM) | 0.218 mg mL−1 | 10−9–10−3 M 3-NT- | [103] |
| Fluorescence | Sol–gel process | Core-shell structure (MOF, Mg, N-CDs,r-CdTe), 3-amino-propyrtriethoxysilane (functional monomer) and tetraethyl orthosilicate (cross-linker) | Picric acid(2,4,6-trinitrophenol) | 0.56 μM | 1–100 μM | [104] |
| Electrochemical/CV | Electro polymerization | polycarbazole (PCz) films decorated with gold nanoparticles | Triacetone triperoxide (TATP) and hexamethylene triperoxide diamine (HMTD) | 15 μgL−1 | 0.1−1.0 mg L−1 | [105] |
| Electrochemical/DPV | Electrochemical polymerization | polycarbazole (PCz) deposited on Pt and -GCE- | Picric acid(2, 4, 6-trinitrophenol) | 0.26 mM (MIP PCz/Pt) 0.57 mM (MIP PCz/GCE) | 0.1–0.9 mM picric acid | [106] |
| Electrochemical | Electrochemical polymerization | trimesic acid and 3,4-ethylene-dioxythiophene were copolymerized on a tailormade laser-induced graphene electrode | 2,4,6-Trinitrotolueen (TNT), 2,4,6-trinitrophenol (TNP), 2,4-dinitrotoluene (DNT), 1,3,5-trinitrobenzene (TNB), 2,4-dinitrophenol (DNP), and 1,3-dinitrobenzene (DNB) | TNT—1.95 ppbTNP—3.06 ppbDNT—2.49 ppbTNB—1.67 ppbDNP -1.94 ppbDNB—4.56 ppb | 10 ppb–1000 ppb and1000 ppb–5000 ppb | [107] |
| Biological Agents | Detection Methods | References |
|---|---|---|
| Pathogens | Culture and colony counting, Immunology-based methods (PCR, lateral flow, ELISA, biosensors, fluorescence immunoassay, chemiluminescence assay, electrochemical immunoassay, SPR, fiber optic sensor, microfluidic biochip), MIPs, MOFs, etc. | [18,180,181,182,183,184,185,186,187] |
| Viruses | Cell culturing, PCR, ELISA, Flow cytometry, Biosensors Fluorescence, Raman and mass spectroscopy, NMR, SPR, Electrochemistry, HPLC, GC–MS, Electrogenerated ECL, ELISA, MIPs | [188,189,190,191,192,193,194,195] |
| Toxins | Radioimmunoassay, ELISA, Lateral flow, ECL, Biosensors, Fluorescence, Förster resonance energy transfer (FRET) | [194,196,197,198,199,200] |
| Parasites | Fluorescent microscopy, Flow cytometry, Automated blood cell analyzers, Serology antibody detection, Molecular methods, Laser desorption mass spectrometry, ELISA, Indirect fluorescence antibody test (IFAT) | [201,202,203,204,205,206] |
| Pathogen | Main Properties/Toxicology | Treatment /Therapy | Refs. |
|---|---|---|---|
| Bacillus anthracis BWA—cat. A | Gram-positive, rod-shaped bacteria that is an obligate, endospore-forming pathogen; spore form or vegetative form; skin (lesions) represents the main route to enter the organism; responsible for cutaneous, pulmonary, and gastrointestinal anthrax; symptoms: necrotic lesions, fever, nausea, vomiting, respiratory distress. | Anthrax vaccines and β-lactam antibiotics (e.g., penicillin), ciprofloxacin, doxycycline | [213] |
| Clostridium Botulinum BWA—cat. A | Anaerobic spore-forming gram-positive bacillus, rod-shaped neurotoxin; spores are highly resistant to heat, light, and drying; it blocks acetylcholine release across nerve synapses, leading to muscular paralysis and potentially death. | Antitoxins and antibiotics | [214] |
| Yersinia pestis BWA—cat. A | A nonmotile, gram-negative, facultative anaerobic, non-spore-forming, rod-shaped coccobacillus bacteria; transmission: flea bites, respiratory droplets; it is the causative agent of bubonic plague; bubonic, septicemic, or pneumonic forms. | Antibiotics (streptomycin, gentamicin) | [215] |
| Variola virus BWA—cat. A | Brick-shaped enveloped virus; the causative agent of smallpox; most frequently transmitted by droplet infection; symptoms: fever, rash, vomiting, skin blisters leaving scars. | Smallpox vaccines (eradicated globally) | [216] |
| Francisella tularensis BWA—cat. A | Intracellular, cytosolic, gram-negative, bacterial pathogen, often leads to a fatal disease called tularemia; symptoms include fever, headache, muscle aches, swollen lymph nodes, mouth ulcers, etc. | Antibiotics (streptomycin, gentamicin, doxycycline, ciprofloxacin) | [217] |
| Ebola Virus BWA—cat. A | Single-stranded RNA virus belonging to the Filoviridae family along with Marburg virus; filoviruses are enveloped, non-segmented RNA viruses with filamentous particles; Ebola virus causes a highly lethal hemorrhagic fever (the virus invades and kills the endothelial cells that line small blood vessels); symptoms: fever, fatigue, abdominal pain, severe headache, vomiting, diarrhea, myalgia, arthralgia, weakness, and hemorrhages. | Antiviral, monoclonal antibody treatments | [218] |
| Marburg virus BWA—cat. A | Hemorrhagic fever virus belonging to the family Filoviridae along with Ebola and has a single-stranded, negative-sense RNA genome; these viruses produce hemorrhagic shock syndrome and visceral organ necrosis. | Monoclonal antibodies | [219] |
| Arenaviridae (Lassa, Machupo) BWA—cat. B | Enveloped RNA viruses with bi-segmented genome with ambisense coding strategy; arenavirus infections could result in severe illness and death: Lassa fever (Sub-Saharan Africa), Machupo (South America). | Supportive care, ribavirin (Lassa) | [220] |
| Brucella BWA—cat. B | Gram-negative, coccobacilli-shaped bacterium; symptoms of brucellosis: severe fever (Malta fever), sweating, fatigue, headache, joint pain, endocarditis, and neurological complications. | Rifampin, doxycycline, streptomycin, no human vaccines available | [213] |
| Vibrio cholerae BWA—cat. B | Comma-shaped gram-negative bacterium; Vibrio cholerae is the aetiologic agent of cholera, a profound secretory diarrheal illness associated with the rapid onset of dehydration and hypovolemia. | Electrolytes, furazolidone, ampicillin, erythromycin, and fluoroquinolones | [221] |
| Salmonellae BWA—cat.B | Gram-negative rod-shaped bacteria; salmonella infections cause gastroenteritis, abdominal pain, fever, vomiting, nausea, diarrhea | Electrolytes, loperamide, cephalosporins | [222] |
| Coxiella burnetii BWA—cat. B | Pleomorphic, gram-negative intracellular bacterium generating Q fever (coxiellosis); symptoms: high fever, myalgia, malaise, nonproductive cough | Doxycycline, hydroxychloroquine | [223] |
| Ricin toxin BWA—cat. B | A type 2 ribosome-inactivating protein (heterodimer glycoprotein) isolated from the beans of the castor oil plant (Ricinus communis) leads to fluid and protein leakage and tissue edema, causing so-called ‘vascular leak syndrome’; inhalational exposure is the primary concern in terms of its use as a potential bioterrorism agent. | Vaccination (prophylaxis) and antitoxin (therapeutic) approaches | [224] |
| Rickettsia prowazekii BWA—cat. B | A small, gram-negative, obligately intracellular, rod-shaped bacterium leading to epidemic typhus fever; it remains highly infectious after drying in media with high osmolarity and has been weaponized so that it could be used as an agent of bioterrorism; symptoms include fever, headache, prostration, small and pink macules, and hemorrhagic rash. | Doxycycline, chloramphenicol | [225] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrila, A.-M.; Diacon, A.; Iordache, T.-V.; Rotariu, T.; Ionita, M.; Toader, G. Hazardous Materials from Threats to Safety: Molecularly Imprinted Polymers as Versatile Safeguarding Platforms. Polymers 2024, 16, 2699. https://doi.org/10.3390/polym16192699
Gavrila A-M, Diacon A, Iordache T-V, Rotariu T, Ionita M, Toader G. Hazardous Materials from Threats to Safety: Molecularly Imprinted Polymers as Versatile Safeguarding Platforms. Polymers. 2024; 16(19):2699. https://doi.org/10.3390/polym16192699
Chicago/Turabian StyleGavrila, Ana-Mihaela, Aurel Diacon, Tanta-Verona Iordache, Traian Rotariu, Mariana Ionita, and Gabriela Toader. 2024. "Hazardous Materials from Threats to Safety: Molecularly Imprinted Polymers as Versatile Safeguarding Platforms" Polymers 16, no. 19: 2699. https://doi.org/10.3390/polym16192699
APA StyleGavrila, A.-M., Diacon, A., Iordache, T.-V., Rotariu, T., Ionita, M., & Toader, G. (2024). Hazardous Materials from Threats to Safety: Molecularly Imprinted Polymers as Versatile Safeguarding Platforms. Polymers, 16(19), 2699. https://doi.org/10.3390/polym16192699












