Utilization of Forest Residues for Cellulose Extraction from Timber Species in the High Montane Forest of Chimborazo, Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Sample Collection and Taxonomic Identification
2.3. Herbarium of Dendrological Samples
2.4. Taxonomic Identification
2.5. Cellulose Extraction
2.6. Characterization Techniques
2.6.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.2. Polarized Light Optical Microscopy
2.7. Data Analysis
3. Results
3.1. Mass Fractions of the Components in Each Species
3.2. Amount of Extracted Cellulose
3.2.1. Cellulose Fiber and Pulp Yield
- x = Amount of fiber (g).
- p = Amount of plant material (g).
- C = Amount of pulp (g).
- Z = Cellulose fiber (g).
| Species | Sample Amount (g) | Fiber Amount (g) | Cellulose Fiber (%) | Pulp Amount (g) | Bleached Cellulose Pulp (%) | Total Cellulose Yield (%) |
|---|---|---|---|---|---|---|
| Cedrela montana Moritz ex Turcz | 50 | 32.1 | 64.2% | 20.21 | 62.96% | 40.41% |
| Buddleja incana Ruiz & Pav | 50 | 29.98 | 59.96% | 21.12 | 70.45% | 42.22% |
| Vallea stipularis L. f. | 50 | 33.12 | 66.24% | 26.77 | 80.83% | 53.54% |
| Myrsine andina (Mez) Pipoly | 50 | 30.56 | 61.12% | 19.77 | 64.69% | 39.43% |
3.2.2. ANOVA Results Cellulose Fiber and Pulp
3.3. Characterization of Extracted Cellulose
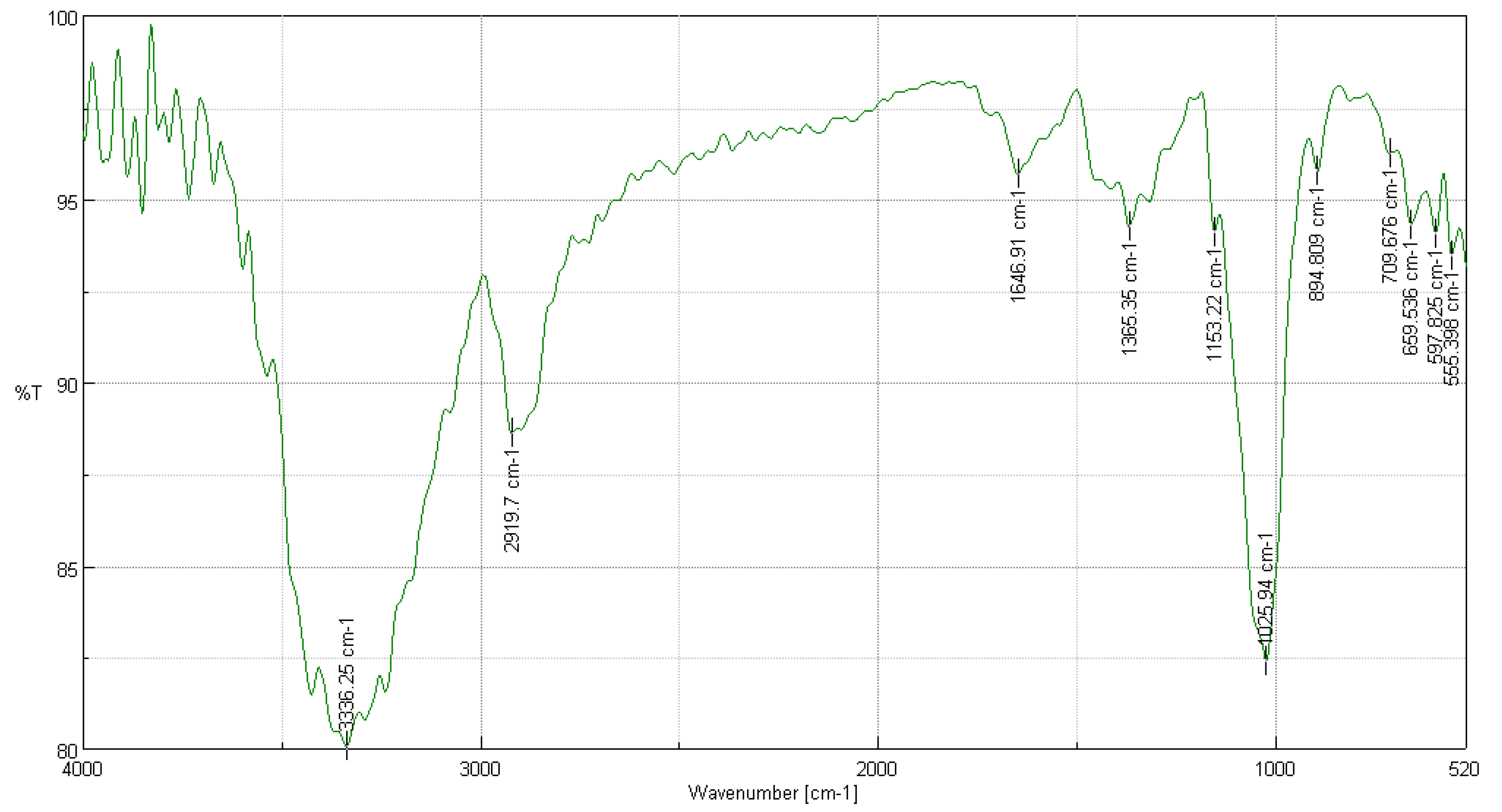
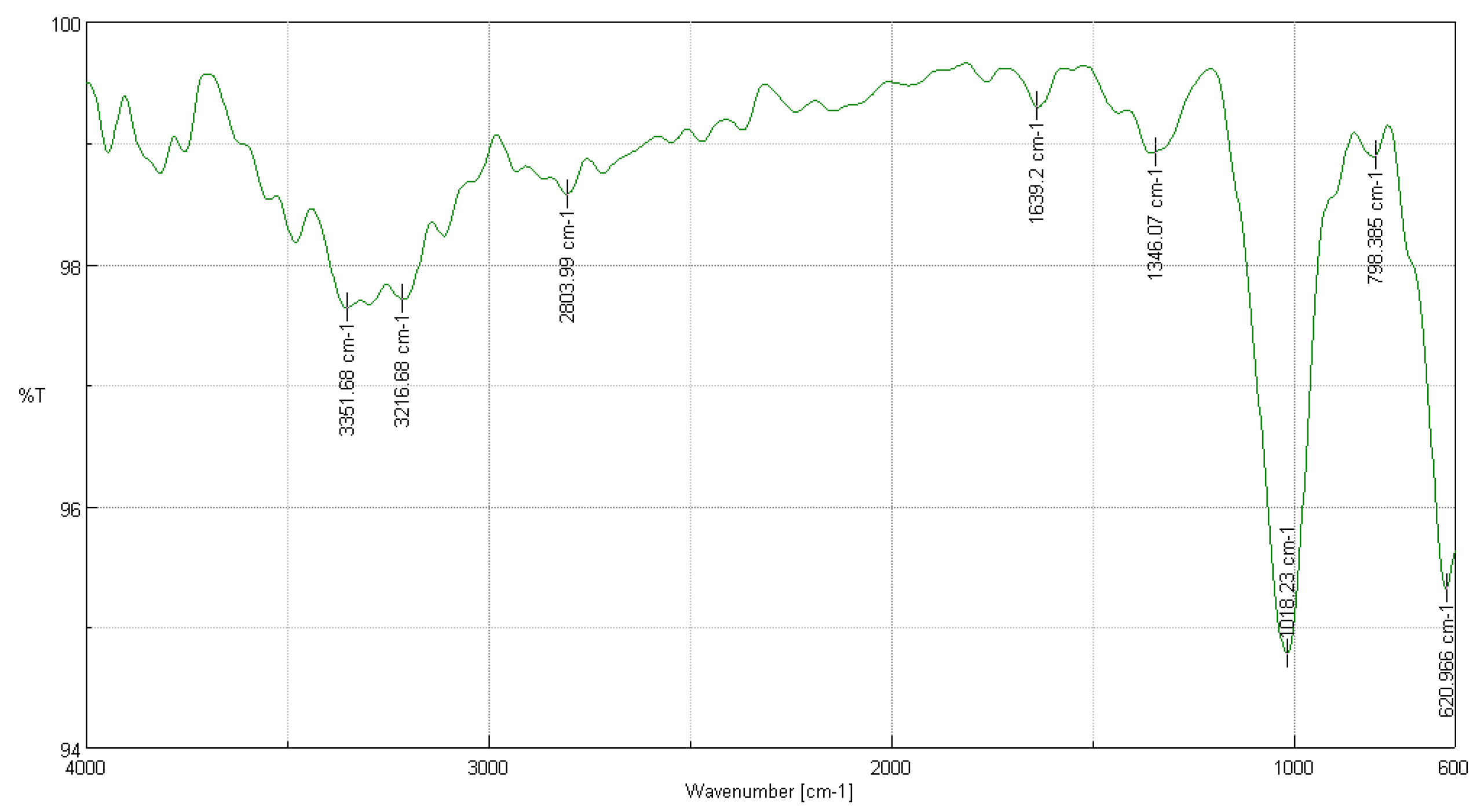
3.3.1. FTIR Analysis of Cedrela montana Moritz ex Turcz
3.3.2. FTIR Analysis of Buddleja incana Ruiz & Pav
3.3.3. FTIR Analysis of Vallea stipularis L. f.
3.3.4. FTIR Analysis of Myrsine andina (Mez) Pipoly
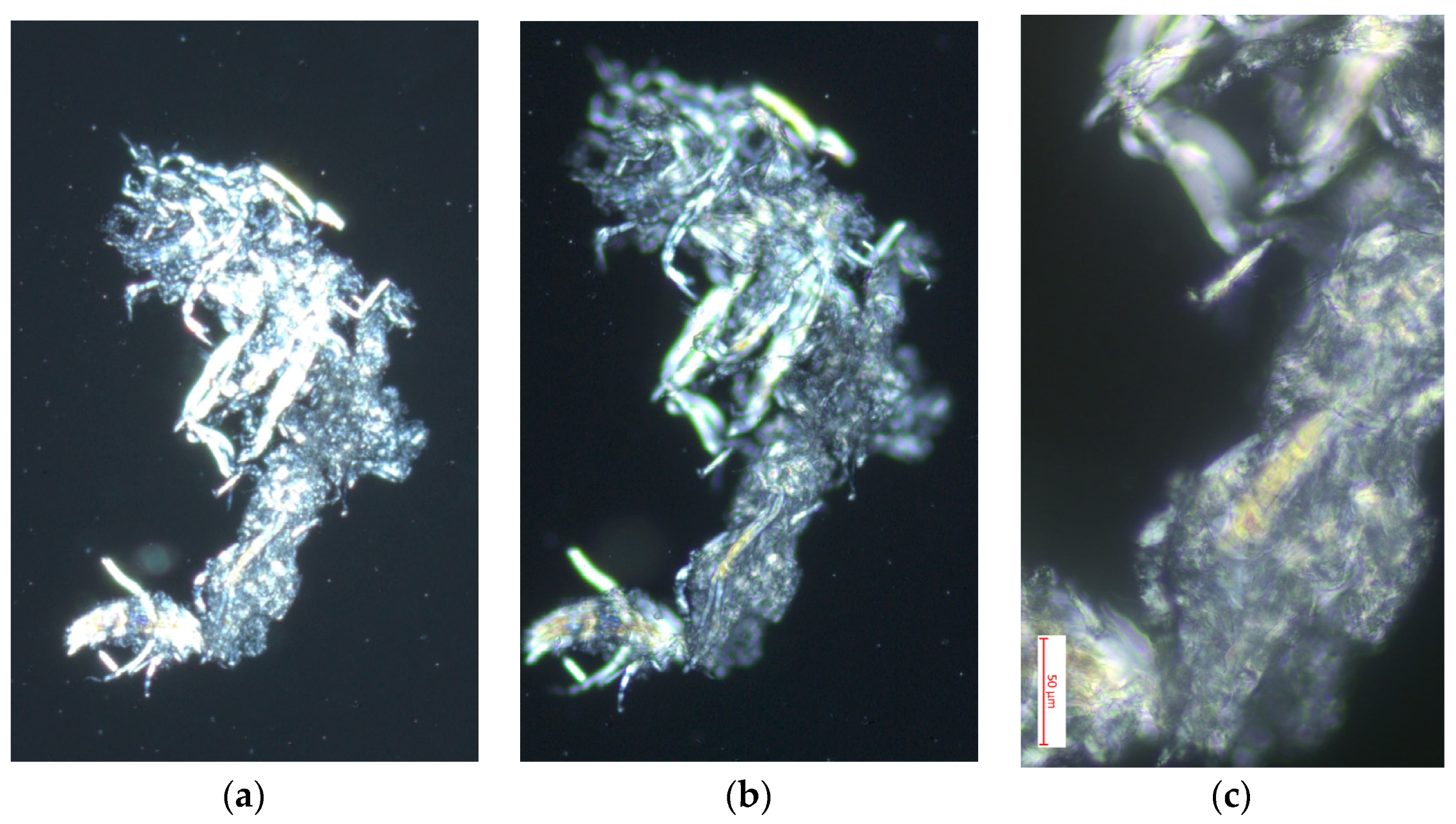
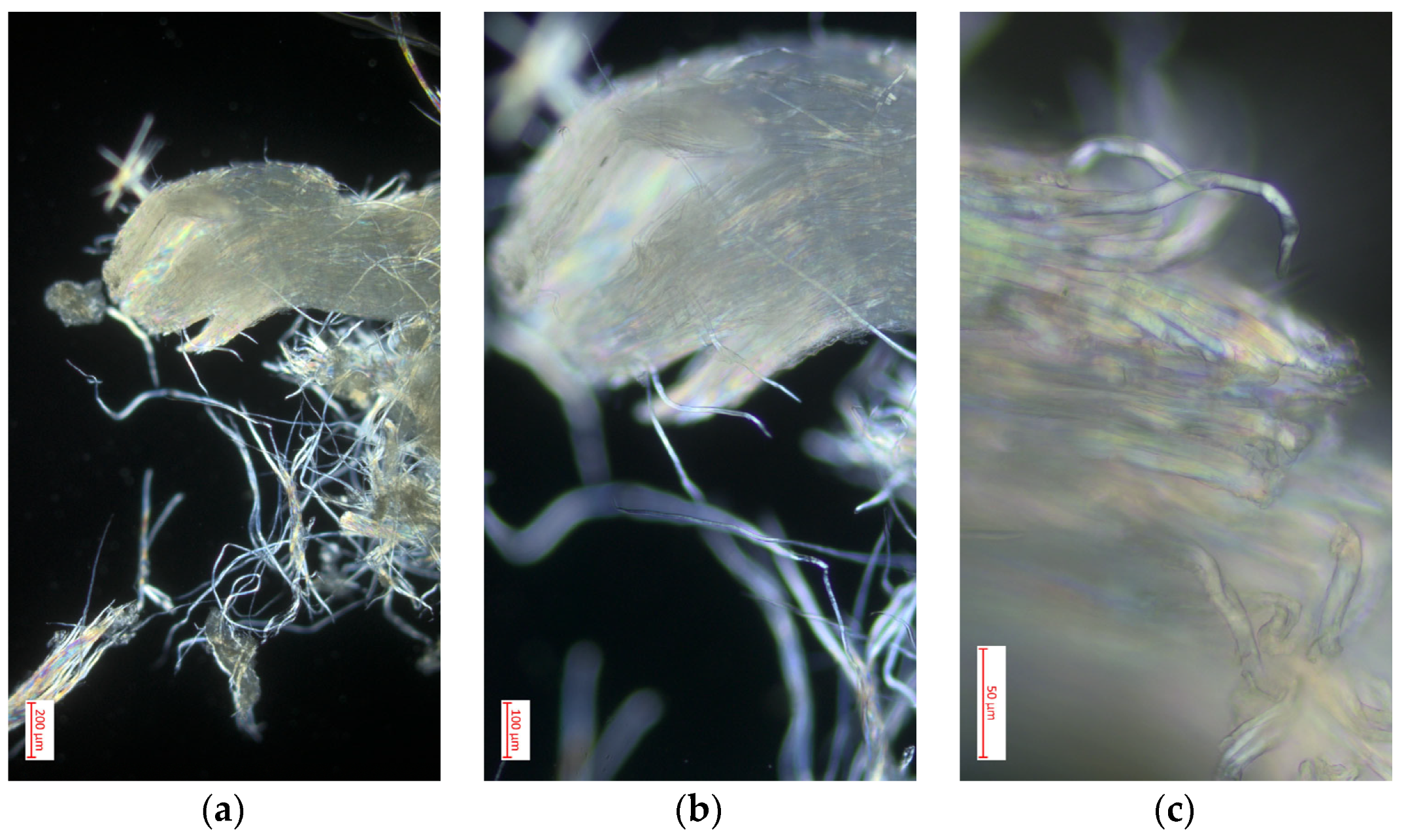
3.4. Optical Microscopy
3.4.1. Polarized Light Optical Microscopy for Cellulose Obtained from the Species Cedrela montana Moritz ex Turcz
3.4.2. Polarized Light Optical Microscopy for Cellulose Obtained from the Species Buddleja incana Ruiz & Pav
3.4.3. Polarized Light Optical Microscopy for Cellulose Obtained from the Species Vallea stipularis L. f.
3.4.4. Polarized Light Optical Microscopy for Cellulose Obtained from the Species Myrsine andina (Mez) Pipoly
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. Design of nano and micro fibrillated cellulose production processes from forest industrial wastes in a multiproduct biorefinery. Chem. Eng. Res. Des. 2021, 167, 1–14. [Google Scholar] [CrossRef]
- Wang, C.; Su, J.; Liu, T.; Ge, S.; Liew, R.K.; Zhang, H.; Naushad, M.; Lam, S.S.; Ng, H.S.; Sonne, C.; et al. A sustainable strategy to transform cotton waste into renewable cellulose fiber self-reinforcing composite paper. J. Clean. Prod. 2023, 429, 139567. [Google Scholar] [CrossRef]
- Liu, A.; Wu, H.; Naeem, A.; Du, Q.; Ni, B.; Liu, H.; Li, Z.; Ming, L. Cellulose nanocrystalline from biomass wastes: An overview of extraction, functionalization and applications in drug delivery. Int. J. Biol. Macromol. 2023, 241, 124557. [Google Scholar] [CrossRef]
- Debnath, B.; Haldar, D.; Purkait, M.K. A critical review on the techniques used for the synthesis and applications of crystalline cellulose derived from agricultural wastes and forest residues. Carbohydr. Polym. 2021, 273, 118537. [Google Scholar] [CrossRef]
- Imani, M.; Carreras, I.M.V.; Dimić-Mišić, K.; Kostić, M.; Barceló, E.; García, M.A.C.; Gane, P. Investigating waste mineral-filled cellulose sourcing in circular economy for regeneration into composite: Matching existing market volumes of oil-based plastics for packaging. Clean. Circ. Bioeconomy 2024, 8, 100089. [Google Scholar] [CrossRef]
- Procel, S.; Núñez, G.; Puebla, R.; Hirata, R.; Manciati, C.; Mendoza, B. Conceptual model of groundwater flow in a volcanic-sedimentary aquifer system of the Andean region of Chimborazo, Ecuador. J. S. Am. Earth Sci. 2023, 131, 104641. [Google Scholar] [CrossRef]
- Orejuela-Escobar, L.M.; Landázuri, A.C.; Goodell, B. Second generation biorefining in Ecuador: Circular bioeconomy, zero waste technology, environment and sustainable development: The nexus. J. Bioresour. Bioprod. 2021, 6, 83–107. [Google Scholar] [CrossRef]
- Pucha, G.; Pérez, M.; Aguay, D.; Chávez, E.; Chávez, N.; Giroletti, E.; Reino, W.; Recalde, C. Soil radioactivity in the highest volcanic region of Northern Andes. J. Environ. Radioact. 2023, 262, 107142. [Google Scholar] [CrossRef]
- Lozano, P.; MAE (Ministerio del Ambiente del Ecuador). Especies Forestales Árboreas y Arbustivas de los Bosques Montanos del Ecuador. 2015. Available online: https://www.flacsoandes.edu.ec/libros/142985-opac (accessed on 21 August 2024).
- Grzybek, P.; Dudek, G.; van der Bruggen, B. Cellulose-based films and membranes: A comprehensive review on preparation and applications. Chem. Eng. J. 2024, 495, 153500. [Google Scholar] [CrossRef]
- Dang, X.; Li, N.; Yu, Z.; Ji, X.; Yang, M.; Wang, X. Advances in the preparation and application of cellulose-based antimicrobial materials: A review. Carbohydr. Polym. 2024, 342, 122385. [Google Scholar] [CrossRef]
- Harini, U.; Harish, S.; Harishankar, A.; Buvaneswaran, M.; Sinija, V.R. Extraction and characterization of microcrystalline cellulose from wine waste. Food Bioprod. Process. 2024, 144, 92–101. [Google Scholar] [CrossRef]
- Garnett, M.T.; Kumar, H.K.S.; Beckingham, B.S.; Alexander, S.L.M. Extraction of cellulose from restaurant food waste. RSC Sustain. 2024, 2, 170–178. [Google Scholar] [CrossRef]
- Vela, D.R.M.; Logroño, F.A.N. Preparation and Characterization of Cellulose Acetate and Cellulose Nitrate Prepared from Cellulose Extracted from Calamagrostis intermedia. Asian J. Chem. 2022, 34, 2099–2104. [Google Scholar] [CrossRef]
- Vela, D.R.M.; Vizuete, R.F.Z.; Flores Mancheno, A.C. Implications of Particle Size in the Extraction of Cellulose from the Cala-magrostis Intermedia Species. Bionatura 2023, 8, 57. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Fractional study of alkali-soluble hemicelluloses obtained by graded ethanol precipitation from sugar cane bagasse. J. Agric. Food Chem. 2010, 58, 1768–1776. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Hua, W.; Li, Y.; Bian, F.; Xiao, T. Extraction of cellulose nanofibrils from pine sawdust by integrated chemical pretreatment. Heliyon 2024, 10, e25355. [Google Scholar] [CrossRef]
- Biswas, S.; Rahaman, T.; Gupta, P.; Mitra, R.; Dutta, S.; Kharlyngdoh, E.; Guha, S.; Ganguly, J.; Pal, A.; Das, M. Cellulose and lignin profiling in seven, economically important bamboo species of India by anatomical, biochemical, FTIR spectroscopy and thermogravimetric analysis. Biomass Bioenergy 2022, 158, 106362. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Miś, A. Characteristics of the chemical processes induced by celluloses in the model and gluten dough studied with application of FTIR spectroscopy. Food Hydrocoll. 2018, 85, 176–184. [Google Scholar] [CrossRef]
- Johansson, S.; Scattarella, F.; Kalbfleisch, S.; Johansson, U.; Ward, C.; Hetherington, C.; Sixta, H.; Hall, S.; Giannini, C.; Olsson, U. Scanning WAXS microscopy of regenerated cellulose fibers at mesoscopic resolution. IUCrJ 2024, 11, 570–577. [Google Scholar] [CrossRef]
- Jacob, N.D.; Hassanzadeh, H.; Oliver, D.R.; Sherif, S.S.; Kordi, B. Classification of degradation in oil-impregnated cellulose insulation using texture analysis of optical microscopy images. Electr. Power Syst. Res. 2016, 133, 104–112. [Google Scholar] [CrossRef]
- Tom, C.; Paineau, E.; Pujala, R.K. Investigating the phase behaviour of binary suspensions of cellulose nanocrystals and montmorillonite with nonlinear rheology, SAXS and polarized optical microscopy. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 132972. [Google Scholar] [CrossRef]
- Zhu, Z.; Fu, S.; Lavoine, N.; Lucia, L.A. Structural reconstruction strategies for the design of cellulose nanomaterials and aligned wood cellulose-based functional materials—A review. Carbohydr. Polym. 2020, 247, 116722. [Google Scholar] [CrossRef]
- Zhang, C.; Mo, J.; Fu, Q.; Liu, Y.; Wang, S.; Nie, S. Wood-cellulose-fiber-based functional materials for triboelectric nanogenerators. Nano Energy 2021, 81, 105637. [Google Scholar] [CrossRef]
- Li, M.; Wang, F.; Ouyang, S.; Liu, Y.; Hu, Z.; Wu, Y.; Qian, J.; Li, Z.; Wang, L.; Ma, S.; et al. A comprehensive review on preparation and functional application of the wood aerogel with natural cellulose framework. Int. J. Biol. Macromol. 2024, 275, 133340. [Google Scholar] [CrossRef]
- TAPPI. Forming Handsheets for Physical Tests of Pulp; Test Method TAPPI/ANSI T 205; Tappi: Atlanta, GA, USA, 2002; pp. 1–9. [Google Scholar]
- Rummukainen, H.; Hörhammer, H.; Kuusela, P.; Kilpi, J.; Sirviö, J.; Mäkelä, M. Traditional or adaptive design of experiments? A pilot-scale comparison on wood delignification. Heliyon 2024, 10, e24484. [Google Scholar] [CrossRef]
- Mendes, I.S.; Prates, A.; Evtuguin, D.V. New insights into kinetics and mechanisms of acid sulphite delignification of Eucalyptus globulus wood. Chem. Eng. J. 2024, 496, 153959. [Google Scholar] [CrossRef]
- Huda, E.; Rahmi; Khairan. Preparation and characterization of cellulose acetate from cotton. IOP Conf. Ser. Earth Environ. Sci. 2019, 364, 012021. [Google Scholar] [CrossRef]
- Kostryukov, S.G.; Matyakubov, H.B.; Masterova, Y.Y.; Kozlov, A.S.; Pryanichnikova, M.K.; Pynenkov, A.A.; Khluchina, N.A. Determination of Lignin, Cellulose, and Hemicellulose in Plant Materials by FTIR Spectroscopy. J. Anal. Chem. 2023, 78, 718–727. [Google Scholar] [CrossRef]
- Femina, K.S.; Asokan, A. Extraction of Cellulose. In Handbook of Biomass; Springer: Singapore, 2024; pp. 485–512. [Google Scholar] [CrossRef]
- Asogan, A.; Sazali, N.; Salleh, W.N.W.; Ibrahim, H.; Krishnan, R.N. FTIR Analysis of Plant-Based Cellulose as Adsorbents for Water Remediation. In Lecture Notes in Mechanical Engineering; Springer: Singapore, 2023; pp. 89–94. [Google Scholar] [CrossRef]
- Fuller, M.E.; Andaya, C.; McClay, K. Evaluation of ATR-FTIR for analysis of bacterial cellulose impurities. J. Microbiol. Methods 2018, 144, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- EL-Mously, H.; Midani, M.; Darwish, E.A. Date Palm Byproducts for Cellulose and Cellulose Derivatives Production. In Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2023; pp. 129–137. [Google Scholar] [CrossRef]
- Kita, Y.; Setiyobudi, T.; Awano, T.; Yoshinaga, A.; Sugiyama, J. Simultaneous cell-by-cell recognition and microfibril angle determination in Japanese hardwoods by polarized optical microscopy combined with semantic segmentation. Cellulose 2023, 30, 8439–8450. [Google Scholar] [CrossRef]
- Gopalan, G.P.; Suku, A.; Anas, S. Nanostructured Cellulose: Extraction and Characterization. In Handbook of Biomass; Springer: Singapore, 2024; pp. 877–917. [Google Scholar] [CrossRef]
- Plappert, S.; Liebner, F. Potential of Anisotropic Cellulose Aerogels. In Springer Handbook of Aerogels; Springer: Cham, Switzerland, 2023; pp. 727–745. [Google Scholar] [CrossRef]
- Villar, L.; Pita, M.; Paez, J.; Sánchez, P.B. Dissolution kinetics of cellulose in ionic solvents by polarized light microscopy. Cellulose 2023, 30, 3027–3039. [Google Scholar] [CrossRef]
- Magalhães, S.; Fernandes, C.; Pedrosa, J.F.S.; Alves, L.; Medronho, B.; Ferreira, P.J.T.; Rasteiro, M.d.G. Eco-Friendly Methods for Extraction and Modification of Cellulose: An Overview. Polymers 2023, 15, 3138. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Ramos, L.P. The chemistry involved in the steam treatment of lignocellulosic materials. Quim. Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.E.; Allen, M.S.; Laser, M.; Lynd, L.R. Protein feeds coproduction in biomass conversion to fuels and chemicals. Biofuels Bioprod. Biorefining 2009, 3, 219–230. [Google Scholar] [CrossRef]
- Alothman, O.Y.; Kian, L.K.; Saba, N.; Jawaid, M.; Khiari, R. Cellulose nanocrystal extracted from date palm fibre: Morphological, structural and thermal properties. Ind. Crops Prod. 2021, 159, 113075. [Google Scholar] [CrossRef]
- Sathishkumar, T.P.; Shah, M.A.; Panchal, H.; Sharma, K.; Gopinath, R.; Sanjay, M.R.; Siengchin, S.; Kumar, L.R.; Rampradheep, G.S. Characterization of new cellulose fiber extracted from second generation Bitter Albizia tree. Sci. Rep. 2024, 14, 1693. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sathishkumar, T.P.; Rajeshkumar, L. Characterization of novel cellulosic fiber extracted from agro-waste of Ricinus communis plant for sustainable green composite materials. Biomass Convers. Biorefin. 2024, 14, 1–17. [Google Scholar] [CrossRef]
- Ichwan, M.; Onyianta, A.; Trask, R.; Etale, A.; Eichhorn, S. Production and characterization of cellulose nanocrystals of different allomorphs from oil palm empty fruit bunches for enhancing composite interlaminar fracture toughness. Carbohydr. Polym. Technol. Appl. 2023, 5, 100272. [Google Scholar] [CrossRef]
- Oshikata, M.S.K.; Blas, N.S.; Silva, B.d.L.; Fukamizu, D.I.; da Silva, D.R.B.; Gauto, L.P.; Cruz, A.J.G.; Morandim-Giannetti, A.d.A.; Pratto, B. Cotton waste upcycling: Biofuel and cellulose derivatives production. Cellulose 2024, 31, 6693–6704. [Google Scholar] [CrossRef]
- Gonultas, O.; Candan, Z. Chemical characterization and ftir spectroscopy of thermally compressed eucalyptus wood panels. Maderas Cienc. Tecnol. 2018, 20, 431–442. [Google Scholar] [CrossRef]
- Rosa, S.M.; Rehman, N.; de Miranda, M.I.G.; Nachtigall, S.M.; Bica, C.I. Chlorine-free extraction of cellulose from rice husk and whisker isolation. Carbohydr. Polym. 2012, 87, 1131–1138. [Google Scholar] [CrossRef]
- Jaiswal, D.; Devnani, G.; Rajeshkumar, G.; Sanjay, M.; Siengchin, S. Review on extraction, characterization, surface treatment and thermal degradation analysis of new cellulosic fibers as sustainable reinforcement in polymer composites. Curr. Res. Green. Sustain. Chem. 2022, 5, 100271. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Reiner, R.S.; Baez, C. Probing crystallinity of never-dried wood cellulose with Raman spectroscopy. Cellulose 2016, 23, 125–144. [Google Scholar] [CrossRef]
- Shokri, S.; Hedjazi, S.; Lê, H.Q.; Abdulkhani, A.; Sixta, H. High-purity cellulose production from birch wood by γ-valerolactone/water fractionation and IONCELL-P process. Carbohydr. Polym. 2022, 288, 119364. [Google Scholar] [CrossRef] [PubMed]
- Emenike, E.C.; Iwuozor, K.O.; Saliu, O.D.; Ramontja, J.; Adeniyi, A.G. Advances in the extraction, classification, modification, emerging and advanced applications of crystalline cellulose: A review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100337. [Google Scholar] [CrossRef]




| Component | Cedrela montana Moritz ex Turcz | Buddleja incana Ruiz & Pav | Vallea stipularis L. f. | Myrsine andina (Mez) Pipoly | Analytical Method |
|---|---|---|---|---|---|
| Hemicellulose (% by weight) | 26.4% | 27.8% | 24.6% | 26.9% | Hydrolysis |
| Lignin (% by weight) | 20.1% | 22.4% | 18.3% | 19.5% | Klason method |
| Ash (% by weight) | 2.1% | 1.7% | 2.5% | 2.0% | Calcination at 550 °C |
| Fat–wax fraction (% by weight) | 2.9% | 2.8% | 4.4% | 3.0% | Soxhlet extraction with hexane |
| Bleached Cellulose Pulp | Cellulose Fiber | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Df | Sum Sq | Mean Sq | F Value | p Value | Df | Sum Sq | Mean Sq | F Value | p Value | |
| Species | 3 | 584.9 | 194.96 | 22.64 | 0.00029 *** | 3 | 74.03 | 24.68 | 1291 | 0.342 |
| Residuals | 8 | 68.9 | 8.61 | 8 | 152.95 | 19.12 | ||||
| Species | Bleached Cellulose Pulp | Groups 95% LSD = 5.52 | Groups 99% LSD = 8.04 |
|---|---|---|---|
| Cedrela montana Moritz ex Turcz | 62.96 | c | b |
| Buddleja incana Ruiz & Pav | 70.45 | b | b |
| Vallea stipularis L. f. | 80.83 | a | a |
| Myrsine andina (Mez) Pipoly | 64.69 | c | b |
| Assumption | Test | Cellulose Fiber (p-Value) | Bleached Cellulose Pulp (p-Value) |
|---|---|---|---|
| Normality | Shapiro–Wilk | 0.1487 | 0.8084 |
| Homoscedasticity | Bartlett | 0.7172 | 0.5174 |
| Independence | Runs | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano Vela, D.R.; Villegas Freire, C.N.; Zabala Vizuete, R.F.; Flores Mancheno, A.C. Utilization of Forest Residues for Cellulose Extraction from Timber Species in the High Montane Forest of Chimborazo, Ecuador. Polymers 2024, 16, 2713. https://doi.org/10.3390/polym16192713
Manzano Vela DR, Villegas Freire CN, Zabala Vizuete RF, Flores Mancheno AC. Utilization of Forest Residues for Cellulose Extraction from Timber Species in the High Montane Forest of Chimborazo, Ecuador. Polymers. 2024; 16(19):2713. https://doi.org/10.3390/polym16192713
Chicago/Turabian StyleManzano Vela, Dennis Renato, Cristina Nataly Villegas Freire, Rolando Fabian Zabala Vizuete, and Ana Carola Flores Mancheno. 2024. "Utilization of Forest Residues for Cellulose Extraction from Timber Species in the High Montane Forest of Chimborazo, Ecuador" Polymers 16, no. 19: 2713. https://doi.org/10.3390/polym16192713
APA StyleManzano Vela, D. R., Villegas Freire, C. N., Zabala Vizuete, R. F., & Flores Mancheno, A. C. (2024). Utilization of Forest Residues for Cellulose Extraction from Timber Species in the High Montane Forest of Chimborazo, Ecuador. Polymers, 16(19), 2713. https://doi.org/10.3390/polym16192713







