Performance Enhancement of Polyurethane Acrylate Resin by Urushiol: Rheological and Kinetic Studies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Urushiol Cross-Linking-Modified PUA
2.3. Preparation of the Urushiol Cross-Linking-Modified PUA Coating Films
2.4. Measurements
2.5. Mechanical Properties and Chemical Resistance of the Coating
2.6. Rheological Analysis
3. Results and Discussion
3.1. Performance Analysis of the Urushiol-Modified PUA
3.1.1. FTIR Characterization of the PUA and Urushiol-Modified PUA
3.1.2. Mechanical Properties and Resistance of the Chemical Medium
3.1.3. Thermal Stability Analysis
3.1.4. Microscopic Morphology Analysis
3.2. Analysis of Rheological Properties of Urushiol-Modified PUA
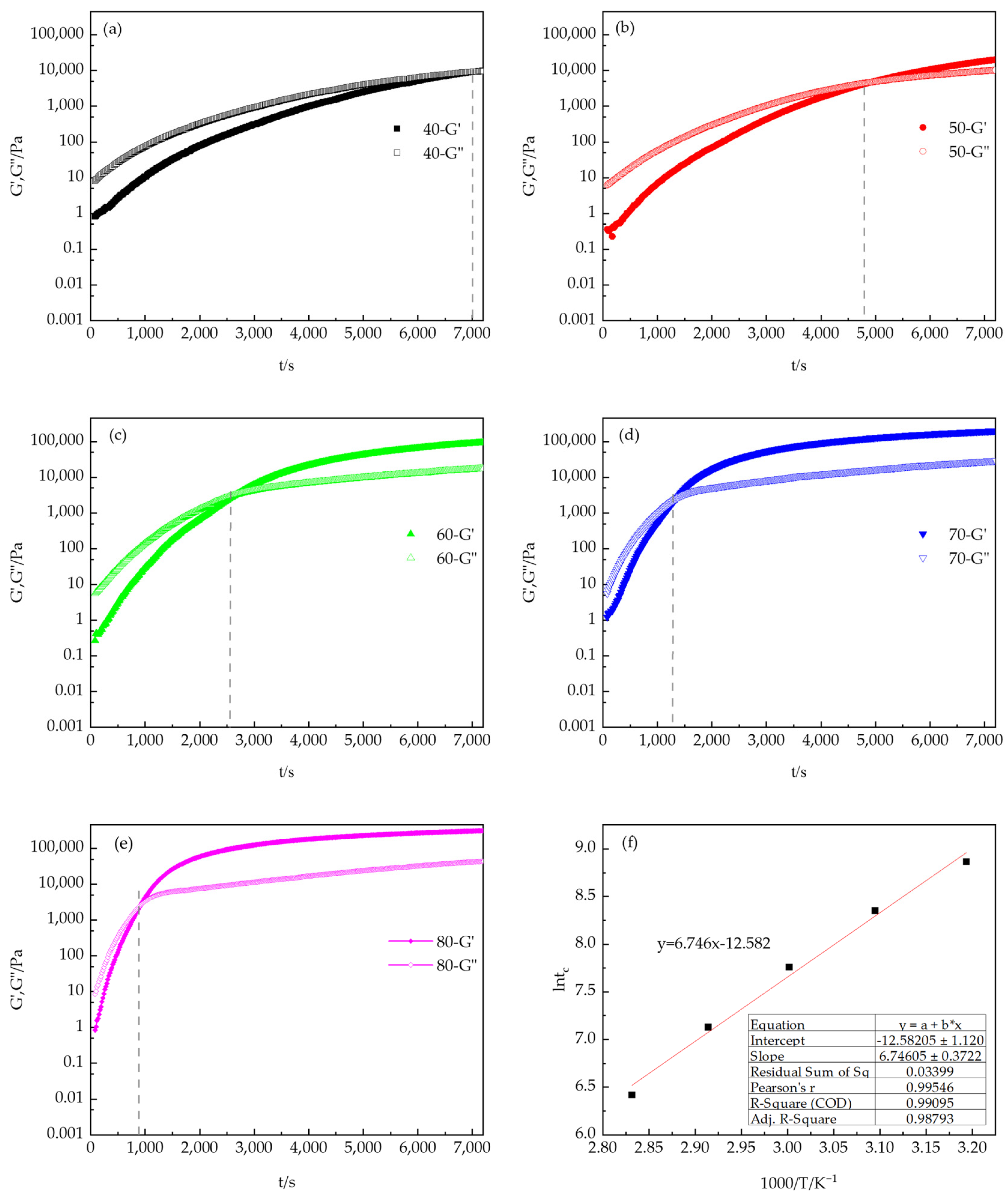
3.2.1. Rheological Characterization: Isothermal Curing
3.2.2. Viscosity Profile
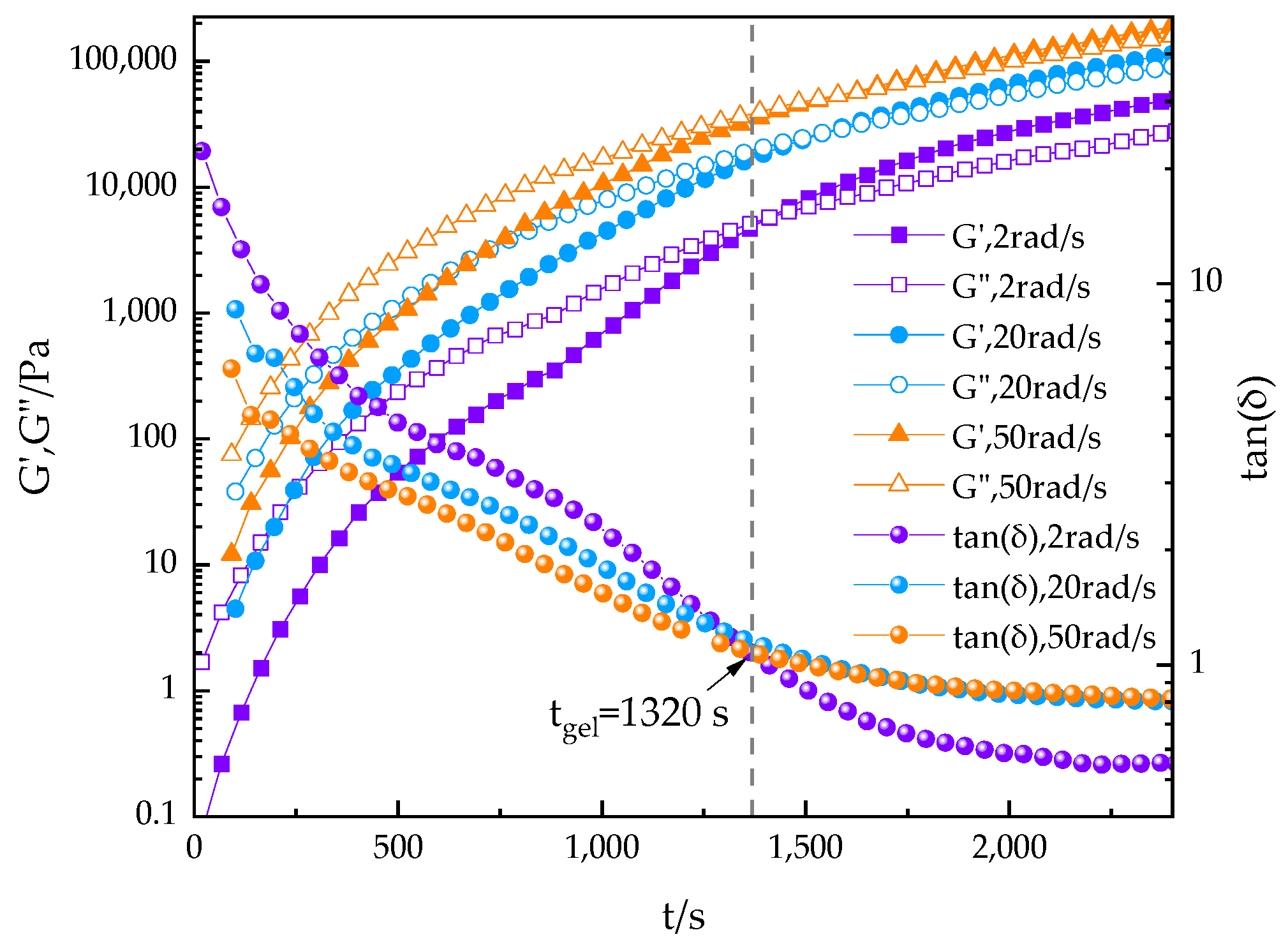
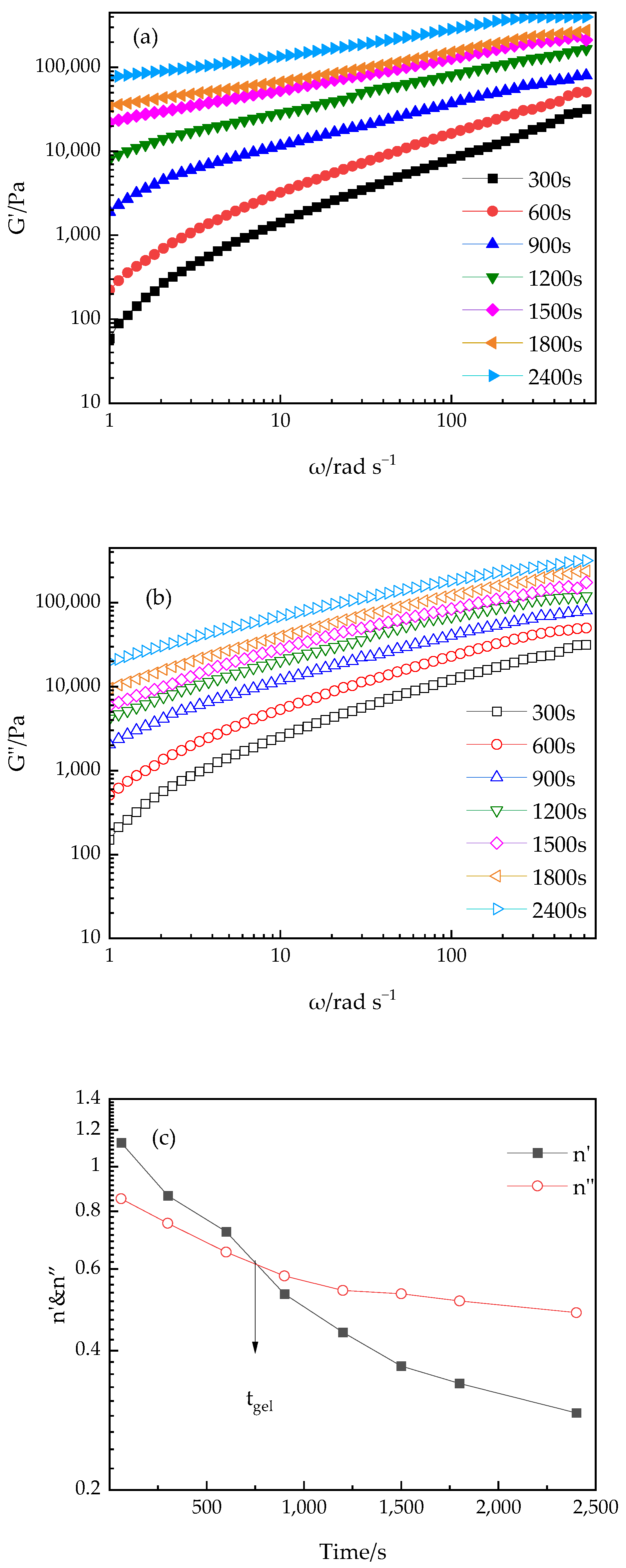
3.2.3. Effect of Shear Frequency
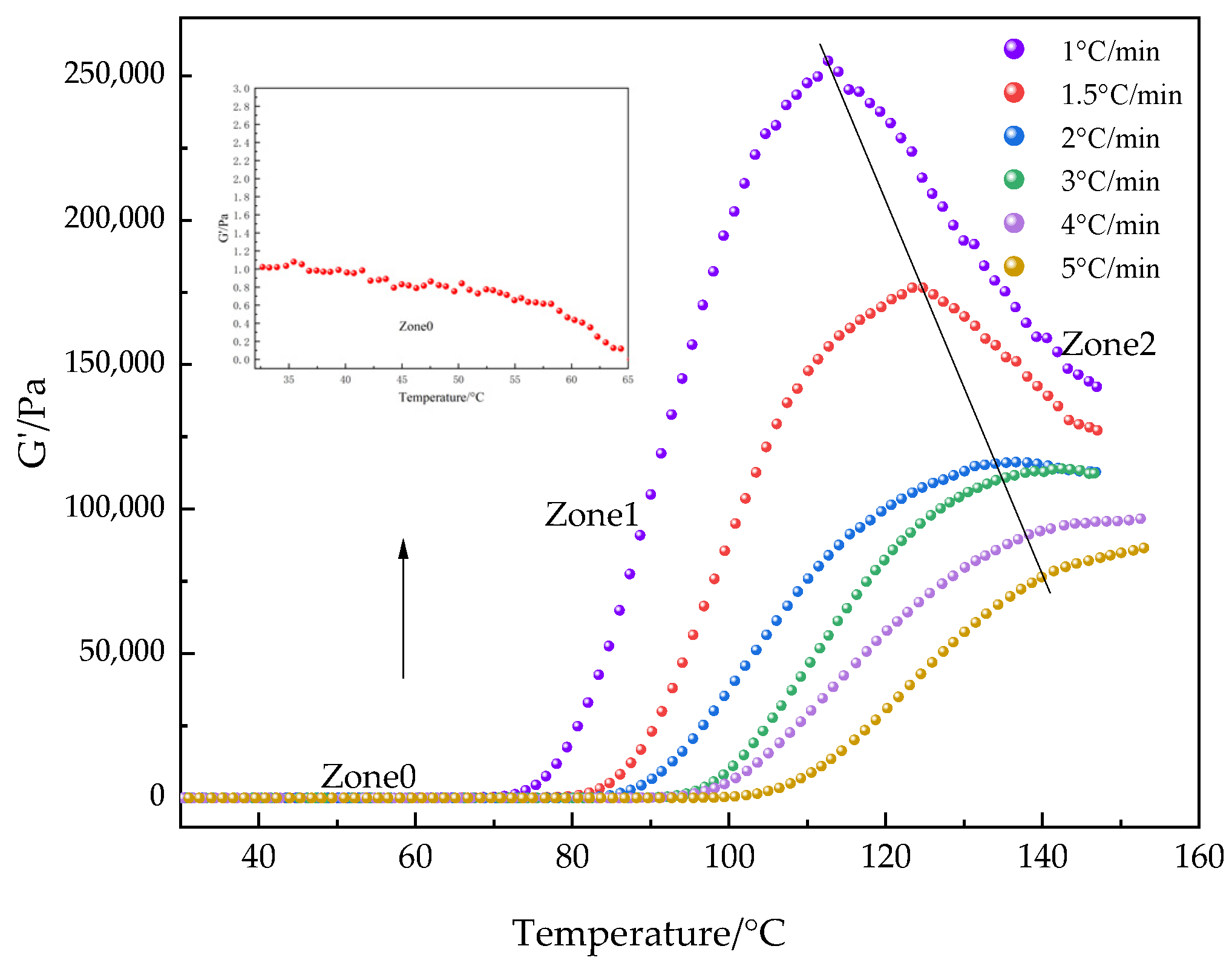
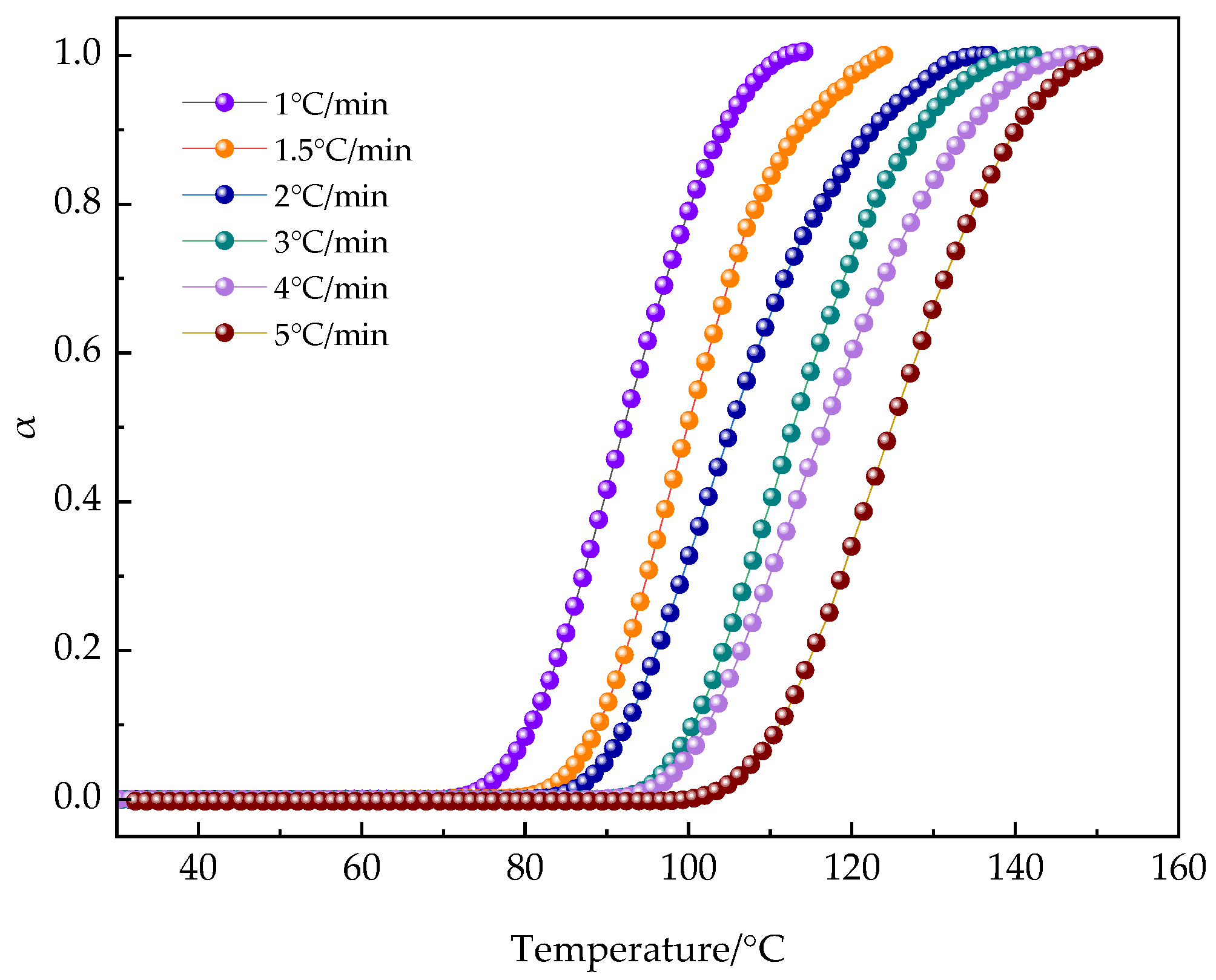
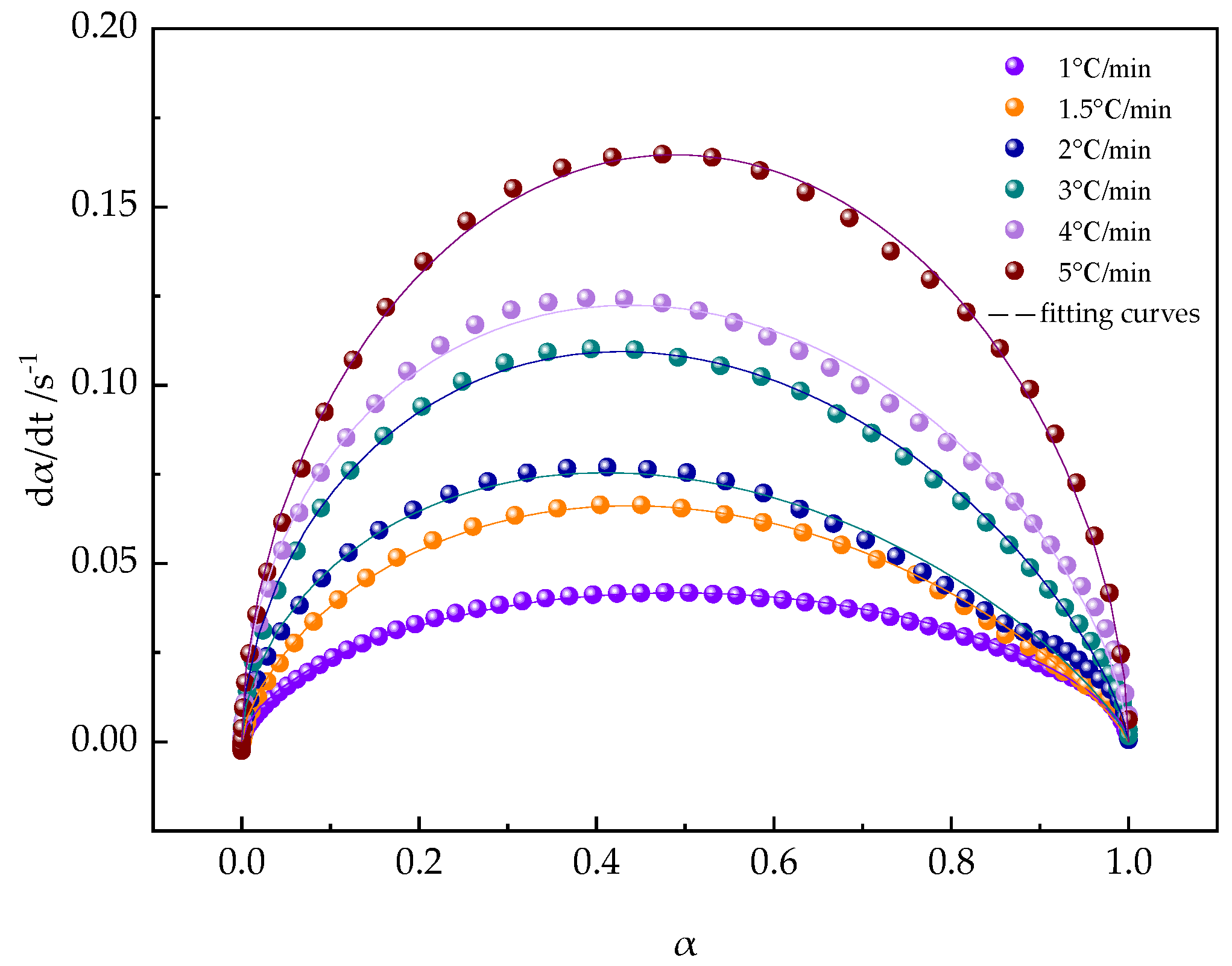
3.2.4. Reaction Kinetics by Rheometry: Non-Isothermal Curing
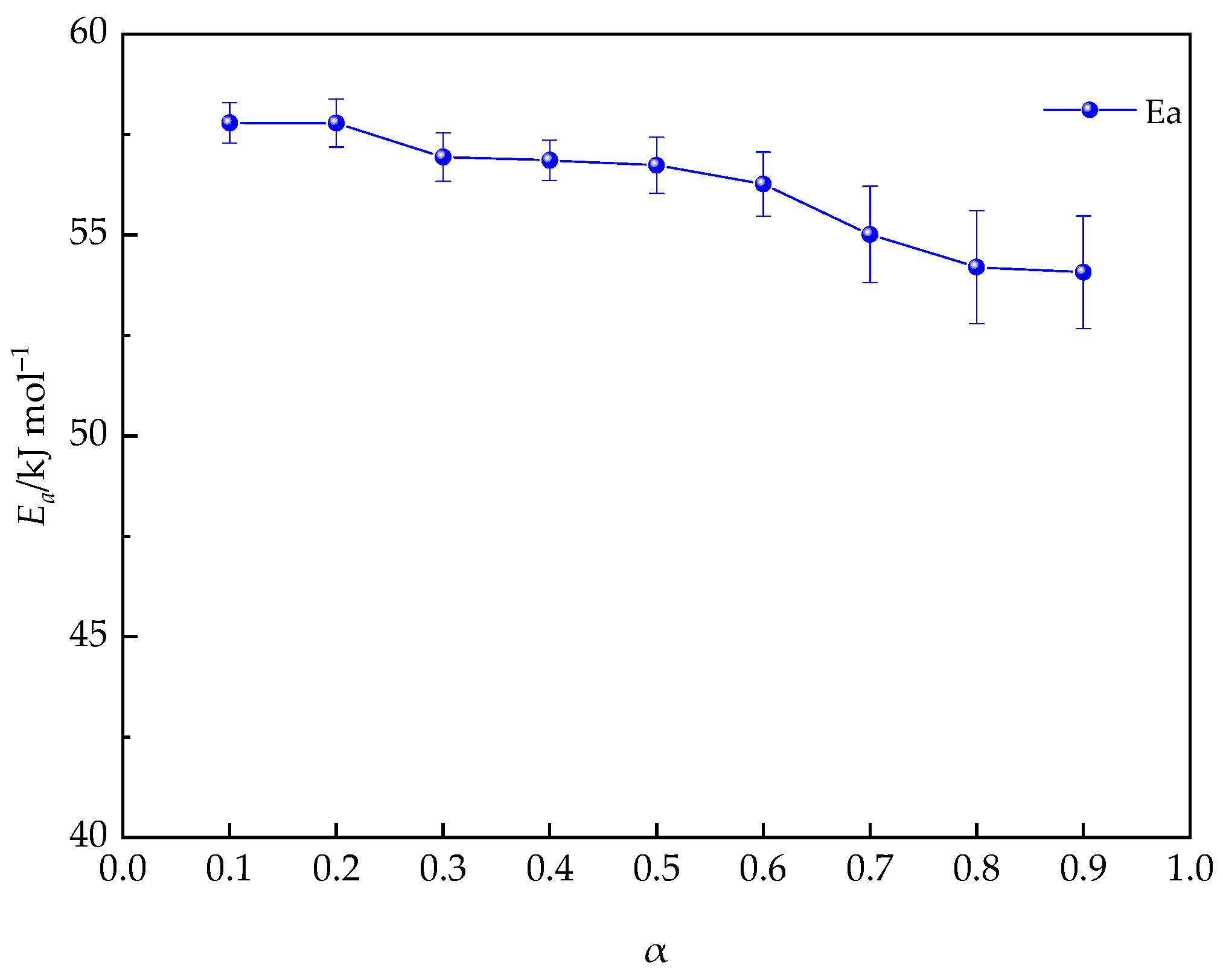
3.2.5. Evaluation of the Activation Energy (Eα)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, H.; Park, J.E.; Cho, W.; Jeon, J.; Wie, J.J. Curing kinetics and structure-property relationship of moisture-cured one-component polyurethane adhesives. Eur. Polym. J. 2023, 201, 112579. [Google Scholar] [CrossRef]
- Ghosh, B.; Gogoi, S.; Thakur, S.; Karak, N. Bio-based waterborne polyurethane/carbon dot nanocomposite as a surface coating material. Prog. Org. Coat. 2016, 90, 324–330. [Google Scholar] [CrossRef]
- Kozakiewicz, J.; Rokicki, G.; Przybylski, J.; Sylwestrzak, K.; Parzuchowski, P.G.; Tomczyk, K.M. Studies on the effect of curing conditions on the curing rate and mechanical properties of moisture-cured poly(urethane-urea) elastomers containing oligocarbonate segments. Polimery 2011, 56, 564–570. [Google Scholar] [CrossRef]
- Song, X.; Lin, Y.; Zhu, C.; Huang, J.; Bai, X.; Zhang, H.; Hu, H.; Li, G. Biooxazolidines-enabled improvement of monocomponent polyurethane coatings. Macromol. Mater. Eng. 2022, 307, 2100667. [Google Scholar] [CrossRef]
- Nomura, Y.; Sato, A.; Sato, S.; Mori, H.; Endo, T. Synthesis of novel moisture-curable polyurethanes end-capped with trialkoxysilane and their application to one-component adhesives. J. Polym. Sci. Pol. Chem. 2007, 45, 2689–2704. [Google Scholar] [CrossRef]
- John, G.; Nagarajan, S.; Vemula, P.K.; Silverman, J.R.; Pillai, C.K.S. Natural monomers: A mine for functional and sustainable materials—Occurrence, chemical modification and polymerization. Prog. Polym. Sci. 2019, 92, 158–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.T.; Shiu, B.C.; Lin, J.H.; Lou, C.W. Multifunctional sodium alginate@ urushiol fiber with targeted antibacterial, acid corrosion resistance and flame retardant properties for personal protection based on wet spinning. Appl. Surf. Sci. 2022, 584, 152573. [Google Scholar] [CrossRef]
- Fang, Y.; Yan, L.; Liu, H. Facile preparation of hydrophobic melamine sponges using naturally derived urushiol for efficient oil/water separation. ACS Appl. Polym. 2020, 2, 3781–3788. [Google Scholar] [CrossRef]
- Xia, J.; Lin, J.; Xu, Y.; Chen, Q. On the UV-induced polymeric behavior of Chinese lacquer. ACS Appl. Mater. Inter. 2011, 3, 482–489. [Google Scholar] [CrossRef]
- Yang, W.; Liang, B.; Tan, W.; He, X.; Lv, J.; Xiao, H.; Zeng, K.; Hu, J.; Yang, G. Rheological study on the cure kinetics of dicyanimidazole resin. Thermochim. Acta 2020, 694, 178801. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Madsen, B. Chemorheological study of a polyfurfuryl alcohol resin system-pre-gel curing stage. Ind. Crops. Prod. 2014, 52, 321–328. [Google Scholar] [CrossRef]
- He, W.; He, L.; Ma, Z.; Guo, Y. Using Rheometry to study the curing kinetics of glycidyl azide polymer spherical propellant by non-isothermal method. Rheol. Acta 2016, 55, 365–371. [Google Scholar]
- Malkin, A.Y.; Kulichikhin, S.G. Rheokinetics: Rheological Transformations in Synthesis and Reactions of Oligomers and Polymers, 1st ed.; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Lucio, B.; De La Fuente, J.L. Rheological cure characterization of an advanced functional polyurethane. Thermochim. Acta 2014, 596, 6–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, R.; Xue, H.; Xia, J.; Lin, Q. Investigation of DSC curing kinetic model fitting and rheological behavior of urushiol/IPDI System. Thermochim. Acta 2022, 711, 179210. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, R.; Xue, H.; Ye, Y.; Lin, J.; Lin, Q.; Xia, J. Study on rheological behaviors and rheokinetics of urushiol/MDI resin system during curing process. Thermochim. Acta 2023, 721, 179451. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Zhang, G. Synthesis and properties of thermosetting resin based on urushiol. RSC Adv. 2012, 2, 2768. [Google Scholar] [CrossRef]
- Latimer, G.W. (Ed.) Official Methods of Analysis of AOAC International, 22nd ed.; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- GB/T 8807-1988; Test Method for Gloss of Plastic Mirror Surface. China National Standardization Administration Committee: Beijing, China, 1988.
- GB/T 6739-2006; Paints and Varnishes–Determination of Film Hardness by Pencil Test. China National Standardization Administration Committee: Beijing, China, 2006.
- GB/T 9286-2021; Paints and Varnishes–Cross-Cut Test. China National Standardization Administration Committee: Beijing, China, 2021.
- GB/T 6742-2007; Paints and Varnishes–Bend Test (Cylindrical Mandrel). China National Standardization Administration Committee: Beijing, China, 2007.
- GB/T 1732-1993; Paints and Varnishes–Determination of Impact Resistance of Film. China National Standardization Administration Committee: Beijing, China, 1993.
- Yin, F.; Lin, J.; Mao, X.; Deng, Y.; Chen, Q. Preparation and characterization of bamboo fibers coated with Titanium urushiol and its composite materials with polypropylene. Int. Polym. Proc. 2013, 28, 489–495. [Google Scholar] [CrossRef]
- Lee, H.; Han, H.; Kim, D.; Lee, B.; Cho, J.H.; Lee, Y.; Lee, S.-S.; Lim, J.A. Mixed urushiol and laccol compositions in natural lacquers: Convenient evaluation method and its effect on the physicochemical properties of lacquer coatings. Prog. Org. Coat. 2021, 154, 106195. [Google Scholar] [CrossRef]
- Lu, R.; Honda, T.; Ishimura, T.; Miyakoshi, T. Study of a naturally drying lacquer hybridized with organic silane. Polym. J. 2005, 37, 309–315. [Google Scholar] [CrossRef]
- Xia, J.; Xu, Y.; Lin, J.; Hu, B. UV-induced polymerization of urushiol without photoinitiator. Prog. Org. Coat. 2008, 61, 7–10. [Google Scholar] [CrossRef]
- Lu, M.G.; Lee, J.Y.; Shim, M.J.; Kim, S.W. Thermal degradation of film cast from aqueous polyurethane dispersions. J. Appl. Polym. Sci. 2002, 85, 2552–2558. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, K.; Li, S.; Zhou, Y. FTIR spectroscopy and thermal analysis of polyurethanes. Spectrosc. Spectr. Anal. 2006, 26, 624–628. [Google Scholar]
- Jin, X.; Guo, N.; You, Z.; Wang, L.; Wen, Y.; Tan, Y. Rheological properties and micro-characteristics of polyurethane composite modified asphalt. Constr. Build. Mater. 2020, 234, 117395. [Google Scholar] [CrossRef]
- Zheng, S.X.; Chen, H.S. Correlations of rheological methods to coatings’ performance. Prog. Org. Coat. 2023, 177, 107403. [Google Scholar] [CrossRef]
- Patel, A.; Maiorana, A.; Yue, L.; Gross, R.A.; Manas-Zloczower, I. Curing kinetics of biobased epoxies for tailored applications. Macromolecules 2016, 49, 5315–5324. [Google Scholar] [CrossRef]
- Halley, P.J.; Mackay, M.E. Chemorheology of thermosets—An overview. Polym. Eng. Sci. 1996, 36, 593–609. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Yang, Y.; Zheng, Q. Rheologic studies on chemical cross-linking kinetics for LDPE. Chin. J. Polym. Sci. 2012, 30, 378–386. [Google Scholar]
- Li, J.; Si, Z.; Shang, K.; Feng, Y.; Wang, S.; Li, S. Kinetic and chemorheological evaluation on the crosslinking process of peroxide-initiated low-density polyethylene. Polymer 2023, 266, 125627. [Google Scholar] [CrossRef]
- Thomas, R.; Yumei, D.; Yuelong, H.; Le, Y.; Moldenaers, P.; Weimin, Y.; Czigany, T.; Thomas, S. Miscibility, morphology, thermal, and mechanical properties of a DGEBA based epoxy resin toughened with a liquid rubber. Polymer 2008, 49, 278–294. [Google Scholar] [CrossRef]
- Chiou, B.S.; English, R.J.; Khan, S.A. Rheology and photo-cross-linking of Thiol-Ene Polymers. Macromolecules 1996, 29, 5368–5374. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Chen, L.A.; McDowell, C.; Khan, S.A.; Hwang, R.; White, S. Rheological study of crosslinking and gelation in chlorobutyl elastomer systems. Polymer 1996, 37, 5869–5875. [Google Scholar] [CrossRef]
- Weng, L.; Chen, X.; Chen, W. Rheological characterization of in situ crosslinkable hydrogels formulated from oxidized dextran and N -carboxyethyl chitosan. Biomacromolecules 2007, 8, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, S.A.; Xia, Y.; Kessler, M.R. Rheological behavior of environmentally friendly castor oil-based waterborne polyurethane dispersions. Macromolecules 2013, 46, 4606–4616. [Google Scholar] [CrossRef]
- Chambon, F.; Winter, H.H. Linear viscoelasticity at the gel point of a crosslinking PDMS with imbalanced stoichiometry. J. Rheol. 1987, 31, 683–697. [Google Scholar] [CrossRef]
- Winter, H.H. Can the gel point of a cross-linking polymer be detected by the G′-G″ crossover? Polym. Eng. Sci. 1987, 27, 1698–1702. [Google Scholar] [CrossRef]
- Tokita, M.; Nishinari, K. (Eds.) Gels: Structures, Properties, and Functions; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Henning Winter, H.; Izuka, A.; De Rosa, M.E. Experimental observation of the molecular weight dependence of the critical exponents for the rheology near the gel point. Polym. Gels Netw. 1994, 2, 239–245. [Google Scholar] [CrossRef]
- Takenaka, M.; Kobayashi, T.; Hashimoto, T.; Takahashi, M. Time evolution of dynamic shear moduli in a physical gelation process of 1, 3:2, 4-Bis-O-(p-Methylbenzylidene)-D-sorbitol in polystyrene melt: Critical exponent and gel strength. Phys. Rev. E 2002, 65, 041401. [Google Scholar] [CrossRef]
- Park, J.O.; Yoon, B.J.; Srinivasarao, M. Effect of chemical structure on the crosslinking behavior of bismaleimides: Rheological study. J. Non-Newton. Fluid Mech. 2011, 166, 925–931. [Google Scholar] [CrossRef]
- Lucio, B.; Fuente, J.L. Non-isothermal DSC and rheological curing of ferrocene-functionalized, hydroxyl-terminated polybutadiene polyurethane. React. Funct. Polym. 2016, 107, 60–68. [Google Scholar]
- He, W.; Tao, B.; Yang, Z.; Yang, G.; Guo, X.; Liu, P.J.; Yan, Q.L. Mussel-inspired polydopamine-directed crystal growth of core-shell n-Al@PDA@CuO metastable intermixed composites. Chem. Eng. J. 2019, 369, 1093–1101. [Google Scholar] [CrossRef]
- Ma, Z.; Qi, L.; He, W.; He, L. A novel approach on the study of cure kinetics for rheological isothermal and non-isothermal methods. Compos. Part B Eng. 2019, 162, 242–249. [Google Scholar]
- He, W.; Guo, J.H.; Cao, C.K.; Liu, X.K.; Lv, J.Y.; Chen, S.W.; Liu, P.J.; Yan, Q.L. Catalytic reactivity of graphene oxide stabilized transition metal complexes of triaminoguanidine on thermolysis of RDX. J. Phys. Chem. C 2018, 122, 14714–14724. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rojo, E.; Rodríguez, F. Kinetic study of a phenolic-novolac resin curing process by rheological and DSC analysis. Thermochim. Acta 2010, 498, 39–44. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Beghishev, V.P.; Keapin, I.A.; Andrianova, Z.S. General treatment of polymer crystallization kinetics-Part 2. The kinetics of nonisothermal crystallization. Polym. Eng. Sci. 1984, 24, 1402–1408. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stan. Sect. A. 1966, 70A, 487. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 2006, 38, 1881–1886. [Google Scholar] [CrossRef]
- Vyazovkin, S. Advanced isoconversional method. J. Therm. Anal. 1997, 49, 1493–1499. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar]
- Akahira, T.; Sunose, T. Method of determining activation deterioration constant of electrical insulating materials. Res. Rep. Chiba. Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Stanko, M.; Stommel, M. Kinetic prediction of fast curing polyurethane resins by model-free isoconversional methods. Polymers 2018, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Milanese, A.C.; Cioffi, M.O.H.; Voorwald, H.J.C.; Shigue, C.Y. Cure kinetic of castor oil-based polyurethane. J. Appl. Polym. Sci. 2011, 122, 3168–3171. [Google Scholar] [CrossRef]
- Liang, T.; Li, C.; Pan, D.; Song, G.; Mai, X.; Naik, N.; Vupputuri, S.; Guo, Z. Rheological non-isothermal mechanistic investigation on the curing of glycidyl azide polymer with solid nanofillers. React. Funct. Polym. 2020, 158, 104796. [Google Scholar] [CrossRef]
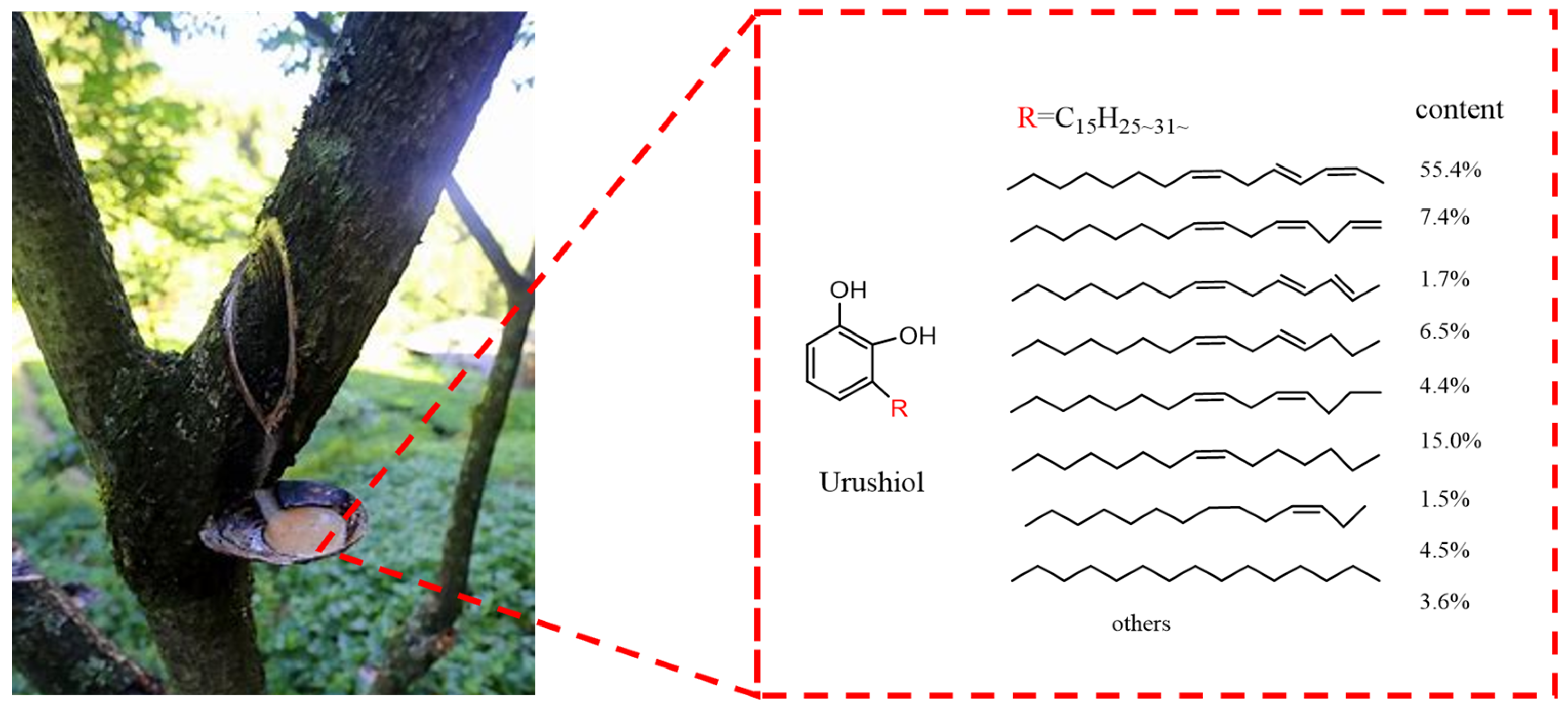

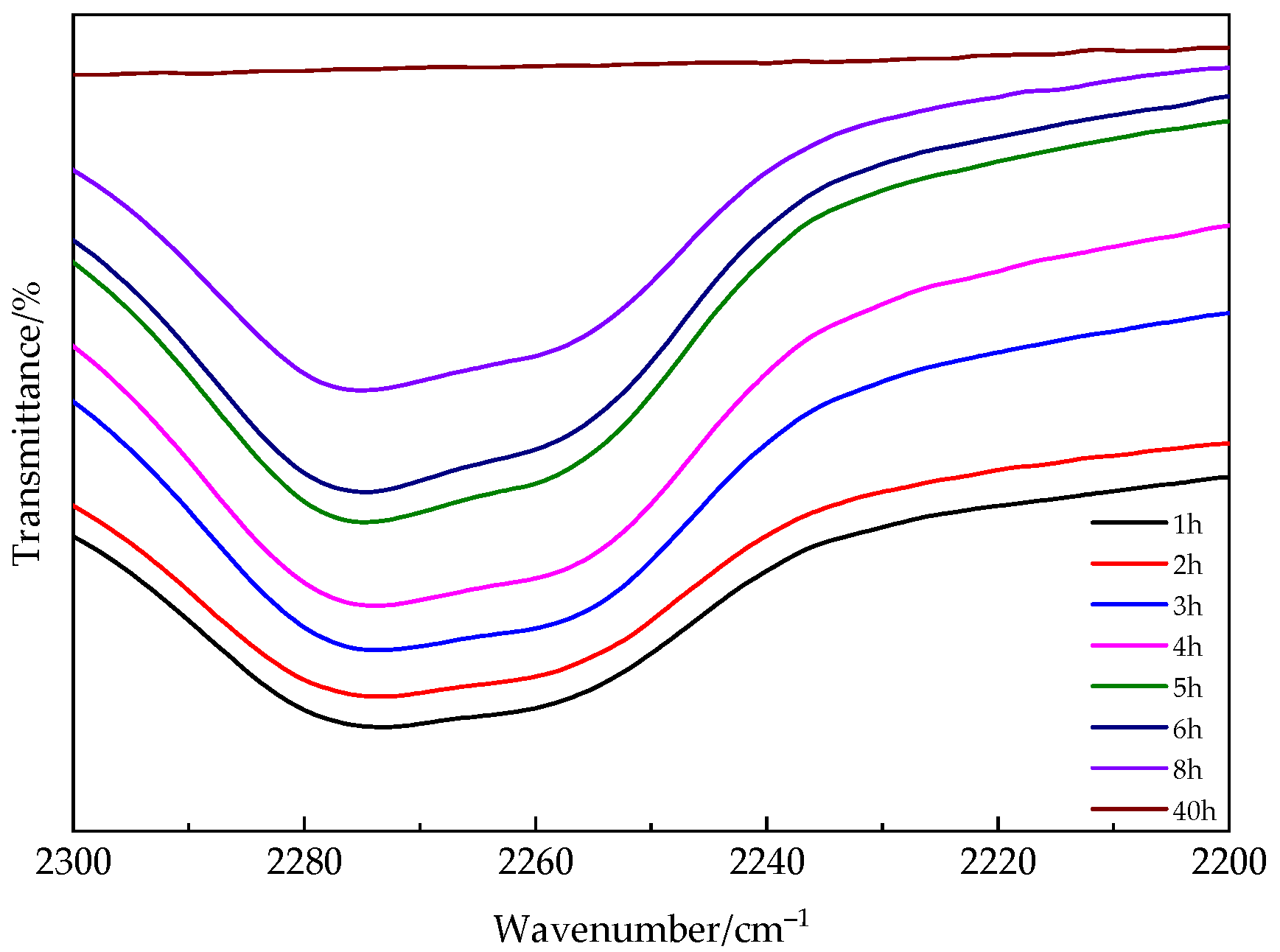
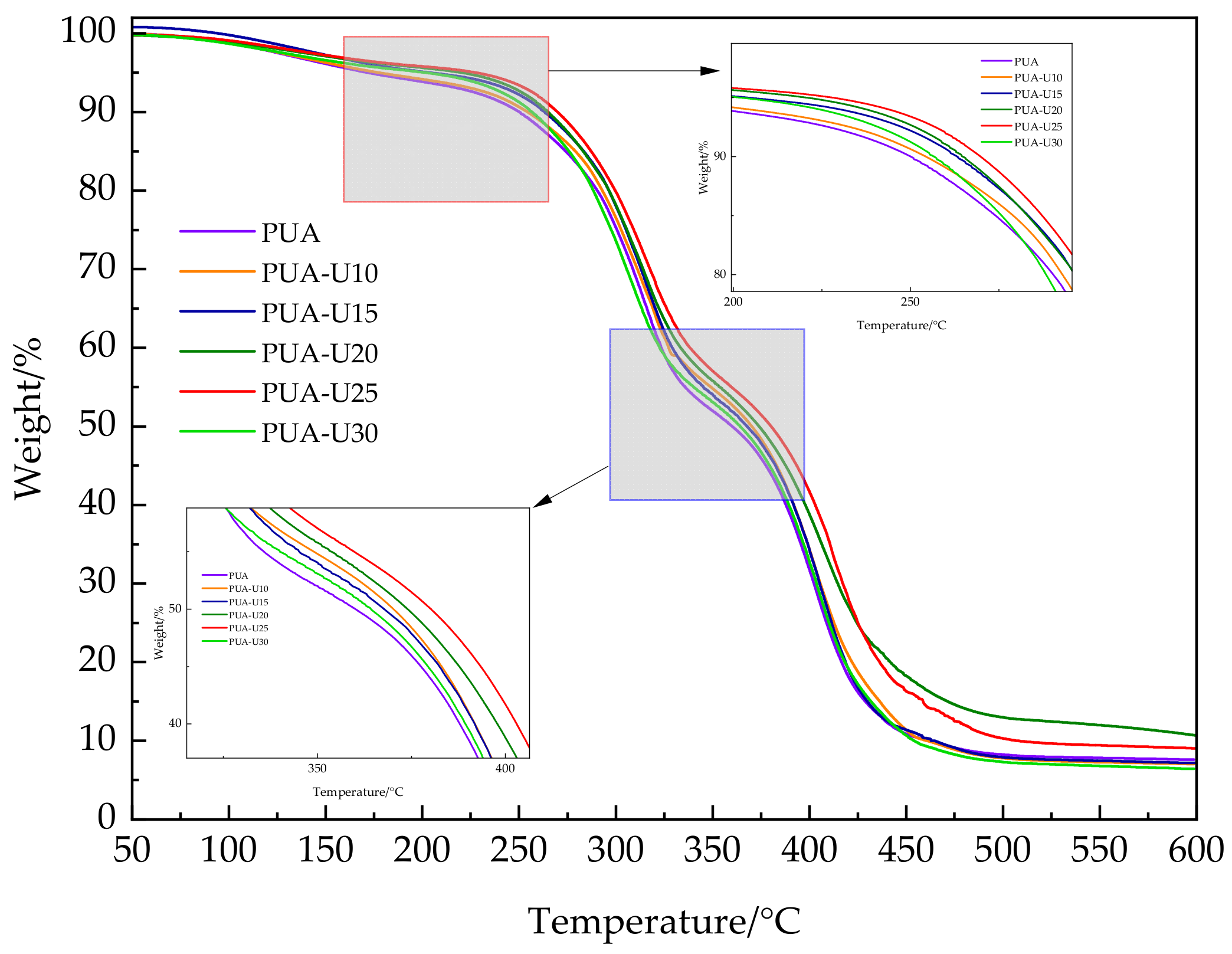
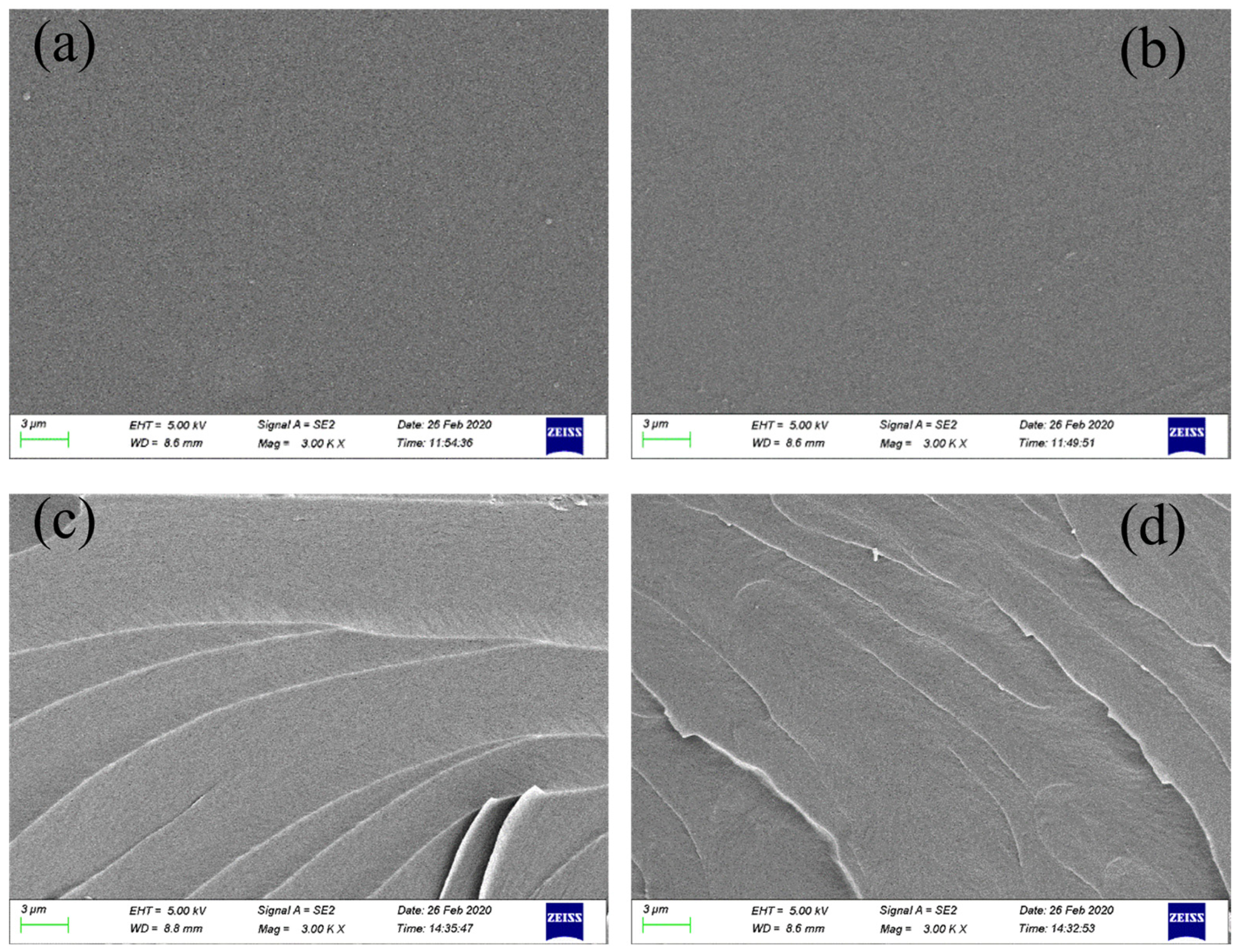



| Physicochemical Parameter | Value |
|---|---|
| Appearance | Transparent liquid |
| Solid content (105 °C for 1 h) | 70 ± 2% |
| Acid value | ≤7 |
| Viscosity | 1500–2300 CPS |
| Isocyanate (NCO) value | 7 ± 0.5% |
| Solvent | Butyl Acetate/Ethyl Acetate/ Propylene Glycol Methyl Ether Acetate |
| Sample | PUA | PUA-U10 | PUA-U15 | PUA-U20 | PUA-U25 | PUA-U30 |
|---|---|---|---|---|---|---|
| PUA (g) | 100 | 100 | 100 | 100 | 100 | 100 |
| Urushiol (g) | 0 | 10 | 15 | 20 | 25 | 30 |
| Type | Gloss/GU | Pencil Hardness | Adhesion | Bending Test/mm | Frontal Impact/kg·cm |
|---|---|---|---|---|---|
| PUA | 200 | 2H | 0 | <2 | 50 |
| PUA-U10 | 200 | 3H | 0 | <2 | 50 |
| PUA-U15 | 200 | 4H | <1 | <2 | 50 |
| PUA-U20 | 200 | 4H | <1 | <2 | 50 |
| PUA-U25 | 200 | 5H | <1 | <2 | 50 |
| PUA-U30 | 200 | 5H | 2 | <3 | 40 |
| Type | NaCl, 10% (w/w) | H2SO4, 10% (w/w) | NaOH, 10% (w/w) | C2H5OH, 95% (v/v) |
|---|---|---|---|---|
| PUA | + | + | + | + |
| PUA-U10 | + | + | + | + |
| PUA-U15 | + | + | + | + |
| PUA-U20 | + | + | + | + |
| PUA-U25 | + | + | + | + |
| PUA-U30 | + | + | − | + |
| Heat Rate °C/min | k | m | n | R2 |
|---|---|---|---|---|
| 1 | 0.0954 | 0.5844 | 0.6071 | 0.9975 |
| 1.5 | 0.1802 | 0.6392 | 0.8220 | 0.9966 |
| 2 | 0.1863 | 0.5481 | 0.7875 | 0.9936 |
| 3 | 0.2592 | 0.5401 | 0.7236 | 0.9999 |
| 4 | 0.2638 | 0.4896 | 0.6311 | 0.9999 |
| 5 | 0.3534 | 0.5389 | 0.5635 | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Fang, R.; Xue, H.; Ye, Y.; Chen, L.; Xia, J. Performance Enhancement of Polyurethane Acrylate Resin by Urushiol: Rheological and Kinetic Studies. Polymers 2024, 16, 2716. https://doi.org/10.3390/polym16192716
Zhang Y, Fang R, Xue H, Ye Y, Chen L, Xia J. Performance Enhancement of Polyurethane Acrylate Resin by Urushiol: Rheological and Kinetic Studies. Polymers. 2024; 16(19):2716. https://doi.org/10.3390/polym16192716
Chicago/Turabian StyleZhang, Yuchi, Run Fang, Hanyu Xue, Yuansong Ye, Li Chen, and Jianrong Xia. 2024. "Performance Enhancement of Polyurethane Acrylate Resin by Urushiol: Rheological and Kinetic Studies" Polymers 16, no. 19: 2716. https://doi.org/10.3390/polym16192716






