Recent Advances in Propylene-Based Elastomers Polymerized by Homogeneous Catalysts
Abstract
:1. Introduction
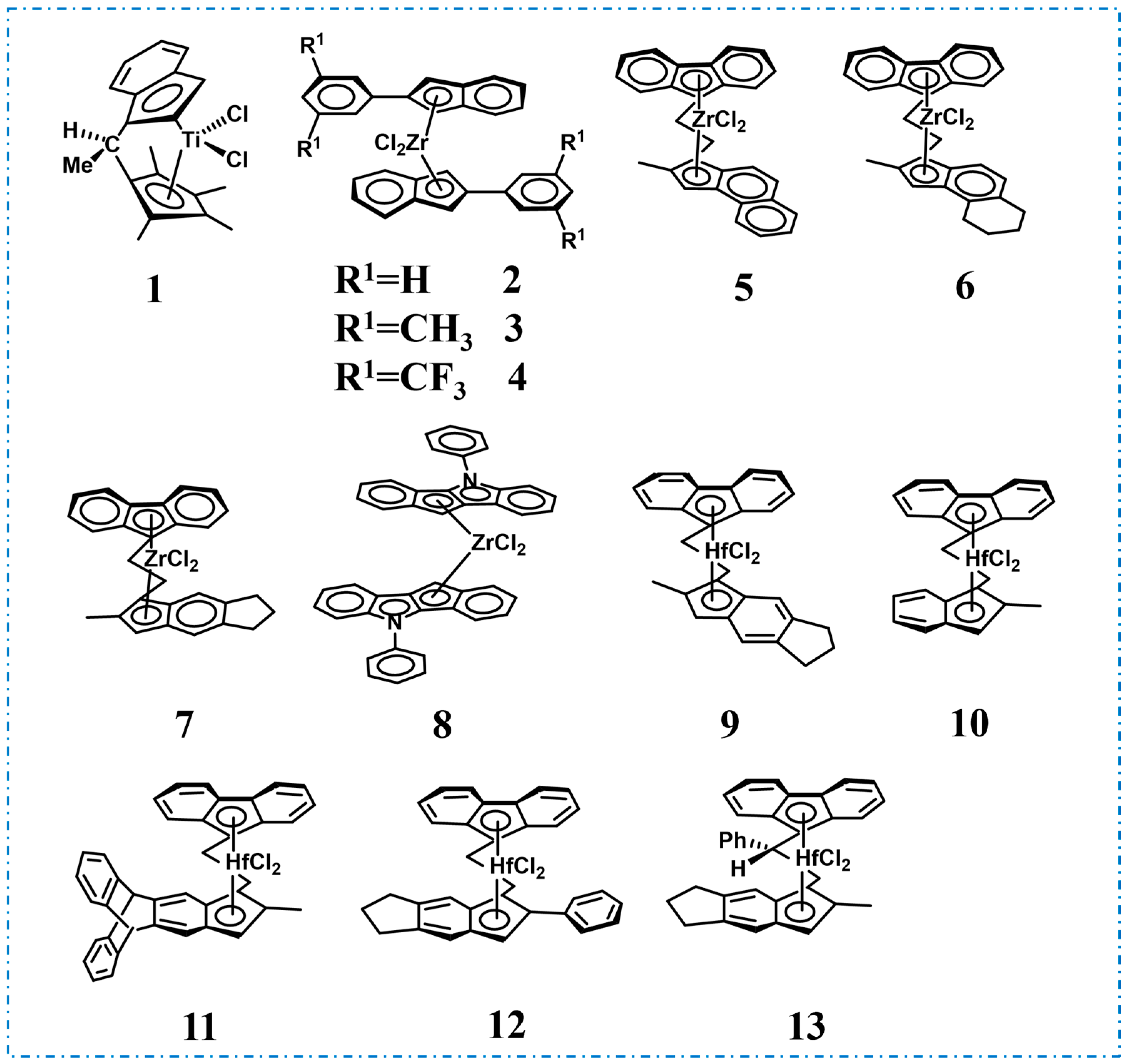
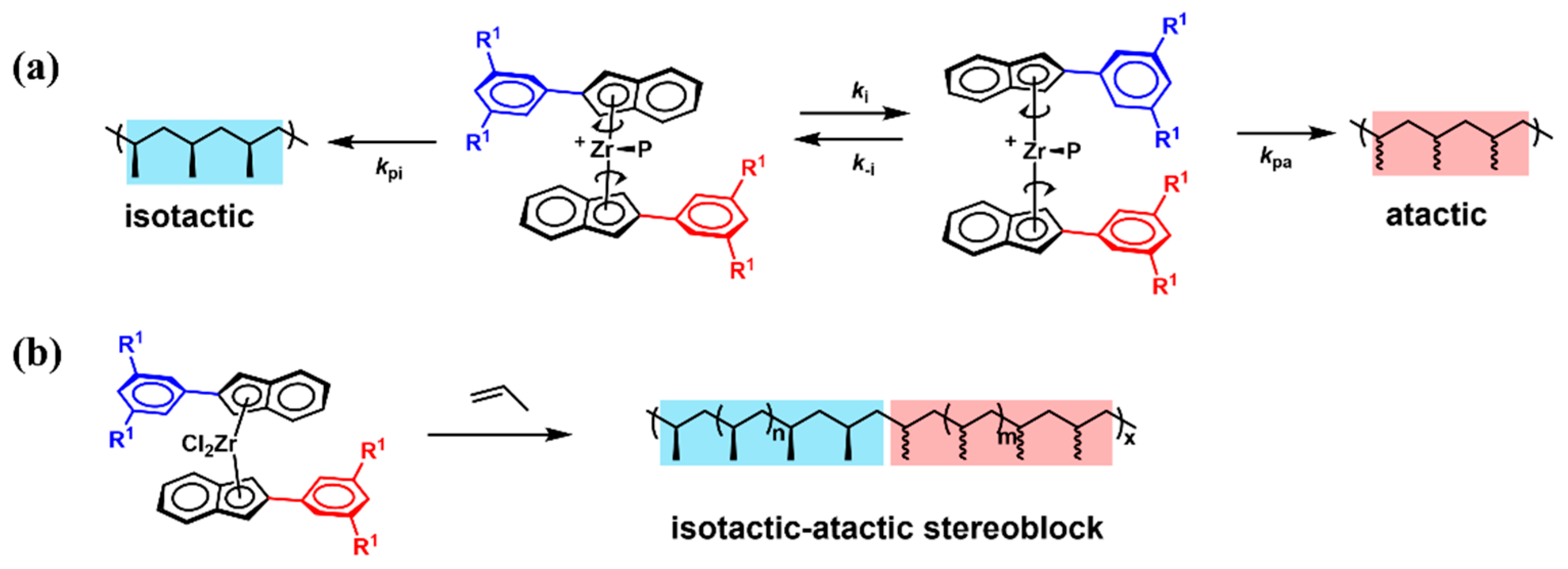
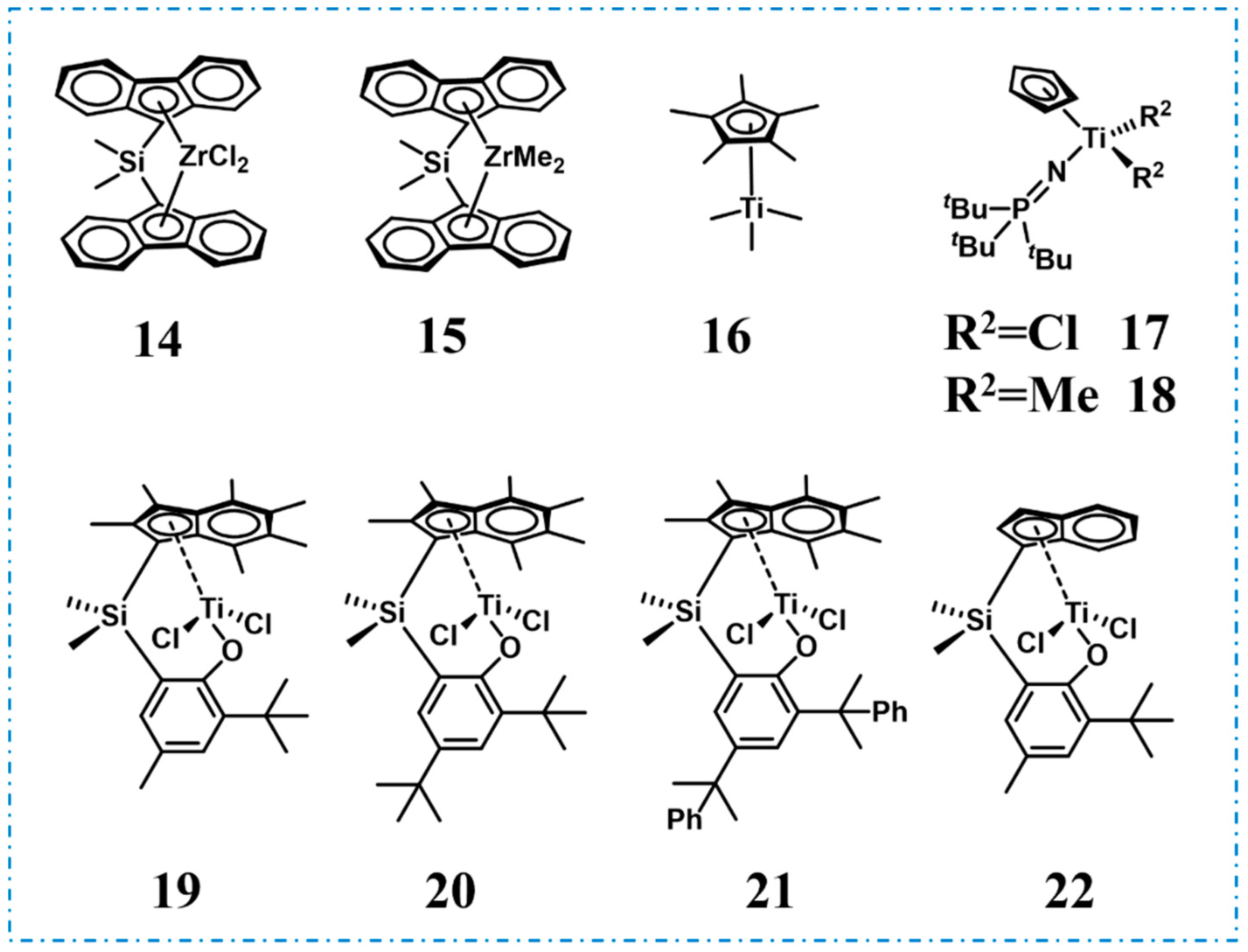
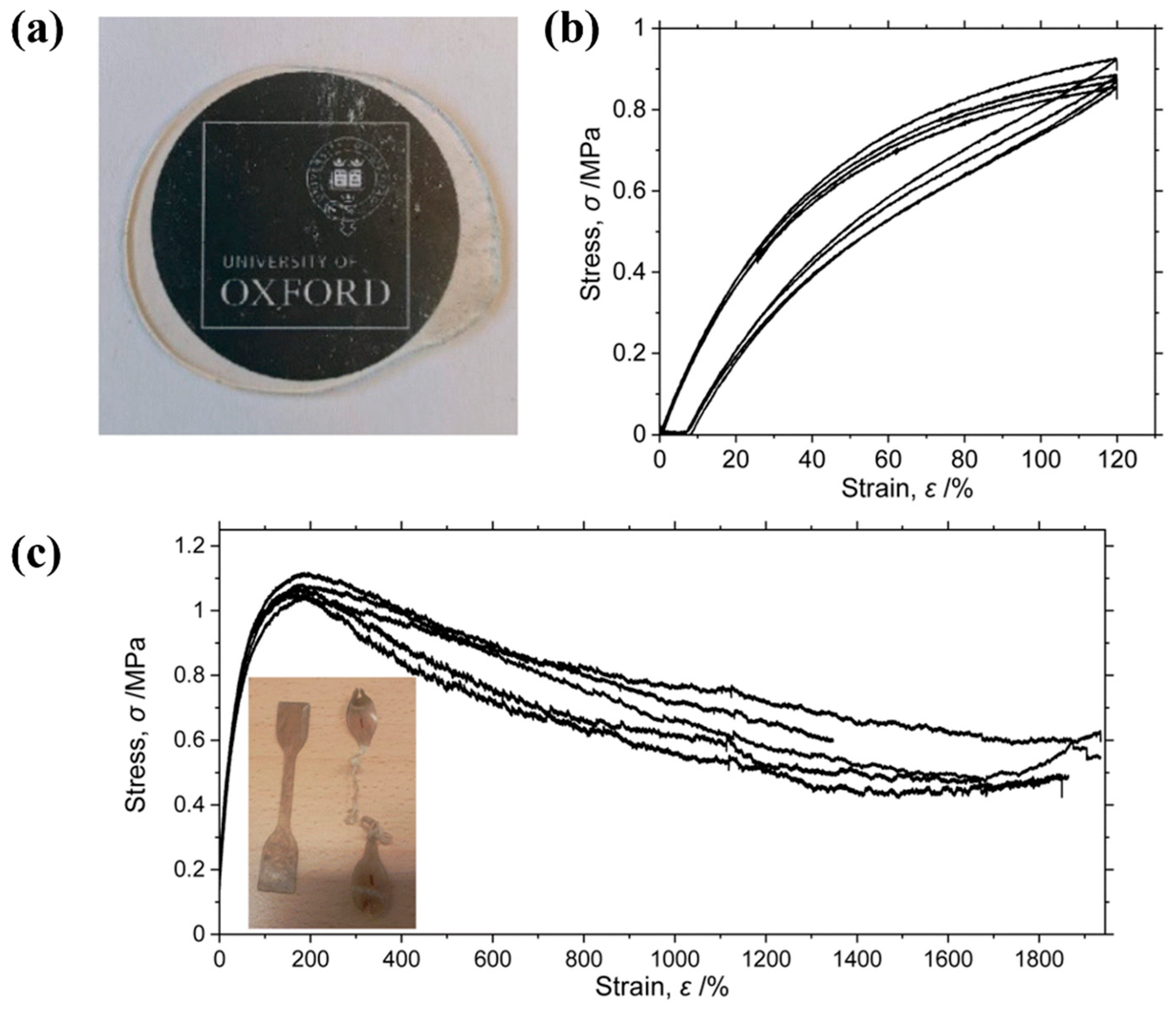
2. Homopolymer Polypropylene-Based Elastomers
| Run | Catalyst | Tp (°C) | Pressure (bar) | Activity (kg/(mol·bar·h)) | Mw (kg/mol) | Ð | [mmmm] (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 50 | 1.5 | 250 | 127 | 1.9 | n.d. | [32] |
| 2 | 2 | 0 | 1.3 | 208 | 213 | 1.5 | 11.6 | [33] |
| 3 | 0 | 6.1 | 280 | 604 | 1.8 | 17.4 | ||
| 4 | −25 | 1 | 1100 | 330 | 2.2 | 60 | ||
| 5 | 25 | 2.4 | 213 | 203 | 3.2 | 22 | ||
| 6 | 45 | 1 | 190 | 24 | 2.8 | 52 | ||
| 7 | 3 | 25 | 2.4 | 63 | 81 | 2.5 | 15 | [34] |
| 8 | 4 | 25 | 2.4 | 208 | 243 | 3.2 | 51 | [34] |
| 9 | 17 | 20 | 5 | 12 | 442 | 1.7 | n.d. | [41] |
| 10 | 40 | 5 | 650 | 206 | 1.9 | n.d. | ||
| 11 | 18 | 30 | 1 | 250 | 126 | 1.8 | n.d. | [41] |
| 12 | 19 | 60 | 2 | 2600 | 470 | 2.2 | n.d. | [42] |
| 13 | 20 | 30 | 2 | 11,790 | 1512 | 2.6 | n.d. | [42] |
| 14 | 21 | 60 | 2 | 1700 | 370 | 2.3 | n.d. | [42] |
| 15 | 22 | 60 | 2 | 100 | 96 | 4.5 | n.d. | [42] |
| Run | Catalyst | Tp (°C) | [C3] (mol/L) | Activity (kg/(mol·[C3]·h)) | Mw (kg/mol) | Ð | [mmmm] (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 70 | 1.1 | 12,540 | 32.4 | 3.04 | 66.3 | [36] |
| 2 | 6 | 70 | 1.1 | 8870 | 19.9 | 1.98 | 78.6 | [36] |
| 3 | 7 | 30 | 1.1 | 2880 | 71.0 | 1.88 | 47.8 | [36] |
| 4 | 30 | 6.1 | 2570 | 230.0 | 1.91 | 24.9 | ||
| 5 | 70 | 1.1 | 32,020 | 24.8 | 2.05 | 64.0 | ||
| 6 | 8 | 25 | bulk | 1115 kg/mol | 85.9 | 6.1 | 26 | [37] |
| 7 | 9 | 0 | 2.14 | 1100 | 2860 | 2.2 | 24 | [38] |
| 8 | 10 | 0 | 2.14 | 1200 | 2730 | 2.1 | 15 | [38] |
| 9 | 11 | 0 | 2.14 | 940 | 2880 | 2.4 | 11 | [38] |
| 10 | 12 | 0 | 2.14 | 1400 | 1650 | 1.5 | 74 | [38] |
| 11 | 13 | 0 | 2.14 | 1300 | 1680 | 1.4 | 3 | [38] |
| 12 | 14 | 50 | 10.56 | 1060 | 440 | n.d. | n.d. | [39] |
| 13 | 16 | -45 | bulk | 5250 kg/(mol·h) | 3966 | 2.0 | n.d. | [40] |
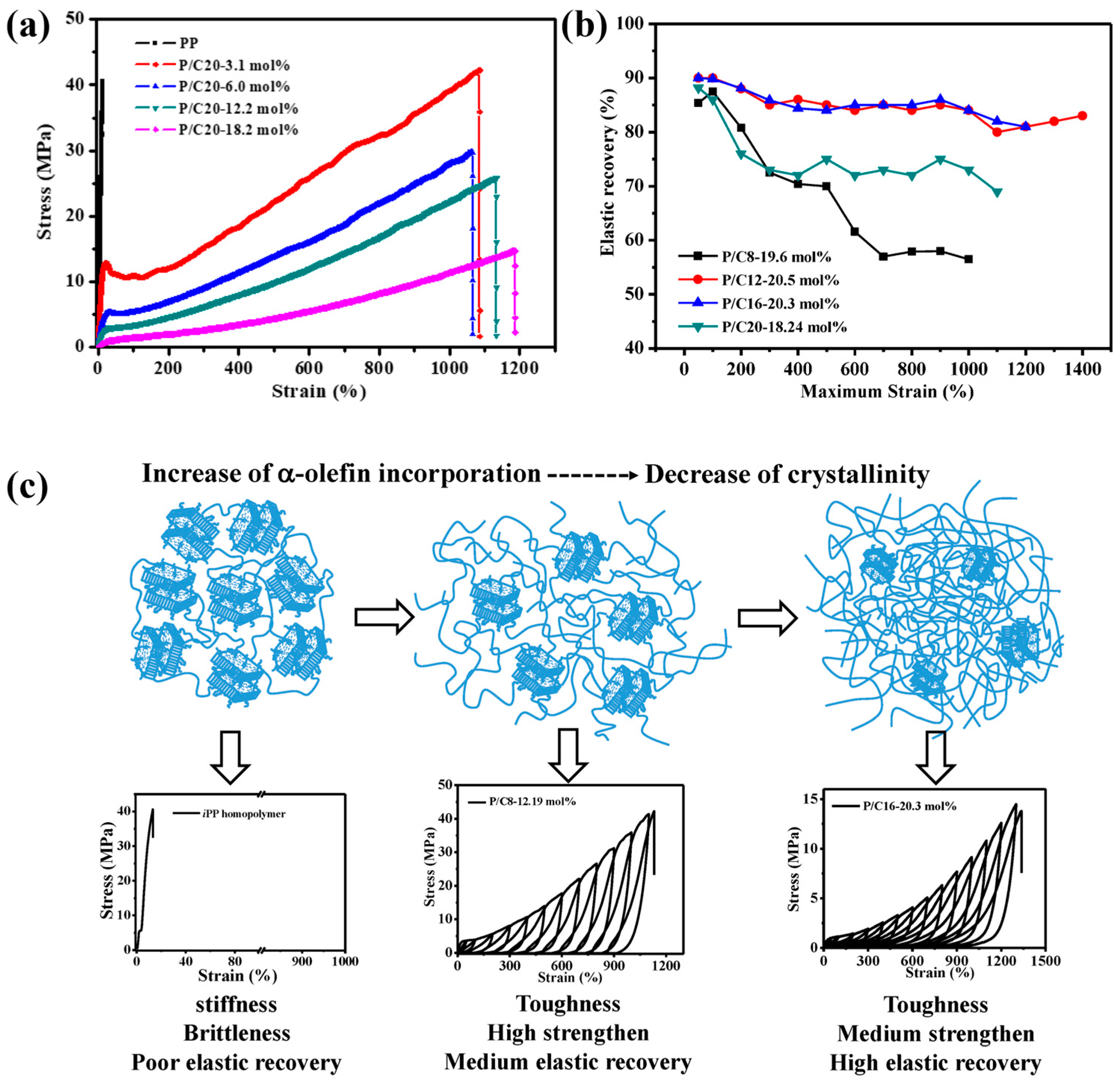
3. Random Copolymer Propylene-Based Elastomers
| Run | Catalyst | Comonomer | [Comonomer] (mol/L) | Tp (°C) | Pressure (bar) | Activity | Mw (kg/mol) | Ð | Tm | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | 1-butene | 0.97 | 70 | 7 | 21,300 kg/(mol·bar·h) | n.d. | 2.05 | n.d. | [44] |
| 2 | 33 | ethylene | 4.0 | 60–70 | bulk | n.d. | 292 | 2.1 | 130 | [50] |
| 3 | 7.4 | 60–70 | bulk | n.d. | 288 | 2.1 | 115 | [50] | ||
| 4 | 36 | 13.1 | 60–70 | bulk | n.d. | 193 | 2.0 | 55 | [50] | |
| 5 | 33 | 1-butene | 4.3 | 60–70 | bulk | n.d. | 228 | 2.1 | 137 | [50] |
| 6 | 8.2 | 60–70 | bulk | n.d. | 178 | 2.0 | 125 | [50] | ||
| 7 | 34 | 1.6 | 60–70 | bulk | n.d. | 225 | 2.0 | 139 | [50] | |
| 8 | 2.8 | 60–70 | bulk | n.d. | 251 | 2.0 | 134 | [50] | ||
| 9 | 6.0 | 60–70 | bulk | n.d. | 229 | 2.0 | 123 | [50] | ||
| 10 | 35 | 1.3 | 60–70 | bulk | n.d. | 173 | 2.1 | 135 | [50] | |
| 11 | 4.6 | 60–70 | bulk | n.d. | 176 | 2.0 | 125 | [50] | ||
| 12 | 8.2 | 60–70 | bulk | n.d. | 177 | 2.0 | 115 | [50] | ||
| 13 | 36 | 1.4 | 60–70 | bulk | n.d. | 214 | 2.1 | 128 | [50] | |
| 14 | 2.2 | 60–70 | bulk | n.d. | 215 | 2.0 | 124 | [50] | ||
| 15 | 6.4 | 60–70 | bulk | n.d. | 214 | 2.0 | 114 | [50] | ||
| 16 | 33 | 1-hexene | 1.2 | 60–70 | bulk | n.d. | 700 | 2.3 | 139 | [50] |
| 17 | 4.2 | 60–70 | bulk | n.d. | 292 | 2.0 | 109 | [50] | ||
| 18 | 11.2 | 60–70 | bulk | n.d. | 266 | 1.9 | 70 | [50] | ||
| 19 | 37 | ethylene 1-hexene | 1 | 90 | 19 mol/L | 2490 kg/(mol·h)) | n.d. | n.d. | 117 | [51] |
| 20 | 38 | 3,3-dimethyl-3-sila-1,5-hexadiene | 2.5 × 10−4 | 25 | 1 | 2940 kg/(mol·bar·h) | 252 | 2.10 | 156.8 | [54] |
| 21 | 2 × 10−3 | 25 | 1 | 670 kg/(mol·bar·h) | 726 | 2.13 | 100 | |||
| 22 | 3 × 10−3 | 25 | 1 | 300 kg/(mol·bar·h) | 700 | 1.56 | n.d. | |||
| 23 | 5-(N,N-diisopropylamino)-1-pentene | 1.9 × 10−2 | 25 | 1 | 1080 kg/(mol·bar·h) | 156 | 2.1 | 129.7 | [55] | |
| 24 | 5-(N,N-diphenylamino)-1-pentene | 1.9 × 10−2 | 25 | 1 | 6580 kg/(mol·bar·h) | 185 | 2.4 | 147.1 | ||
| 25 | 1-octene | 0.15 | 25 | 1 | 50,000 kg/(mol·bar·h) | 519 | 1.4 | 46 | [56] | |
| 26 | 1-dodecane | 0.15 | 25 | 1 | 51,000 kg/(mol·bar·h) | 960 | 1.4 | 33 | ||
| 27 | 1-hexadecane | 0.15 | 25 | 1 | 52,000 kg/(mol·bar·h) | 1416 | 1.5 | 34 | ||
| 28 | 1-eicosene | 0.15 | 25 | 1 | 57,000 kg/(mol·bar·h) | 1678 | 1.4 | 80 |
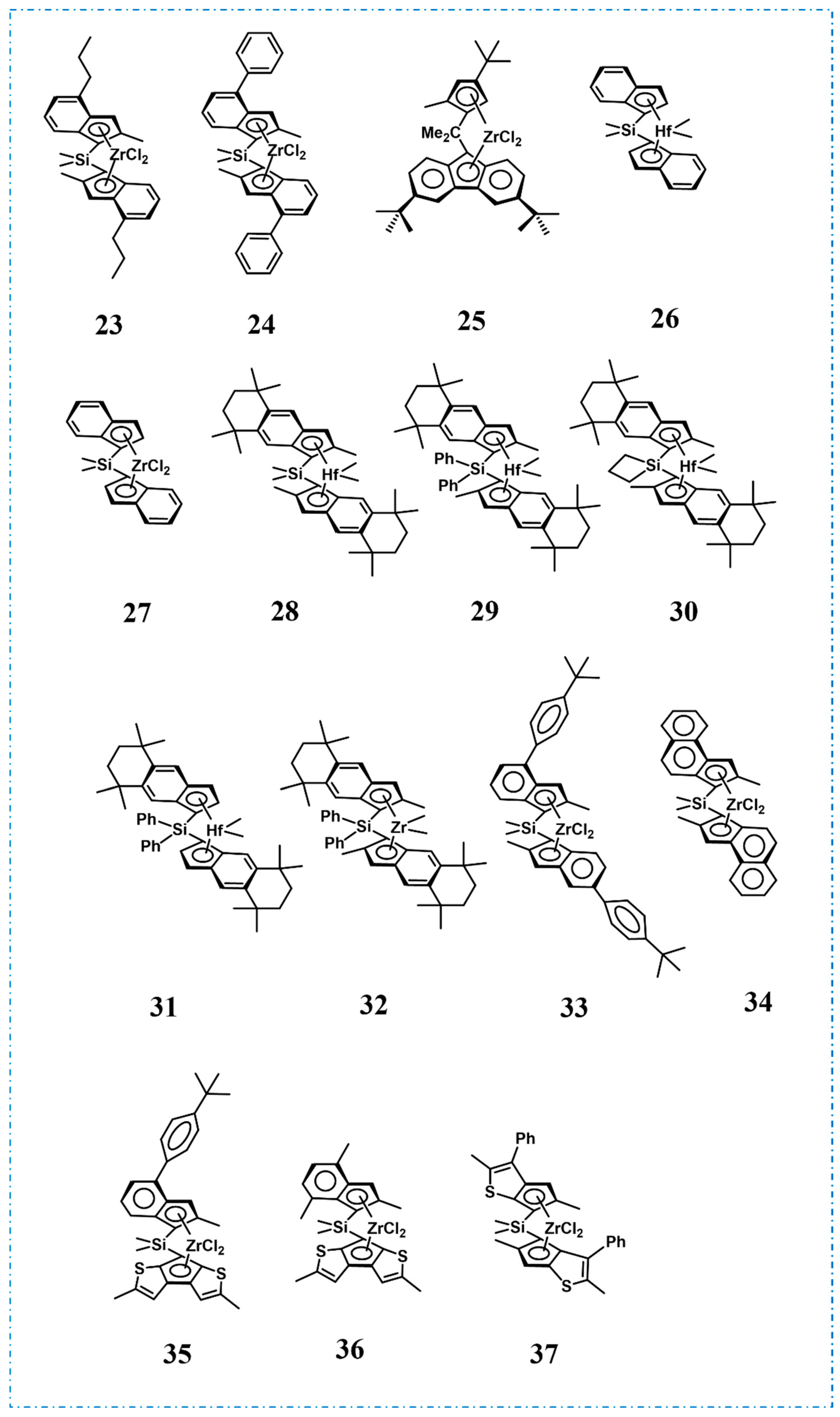
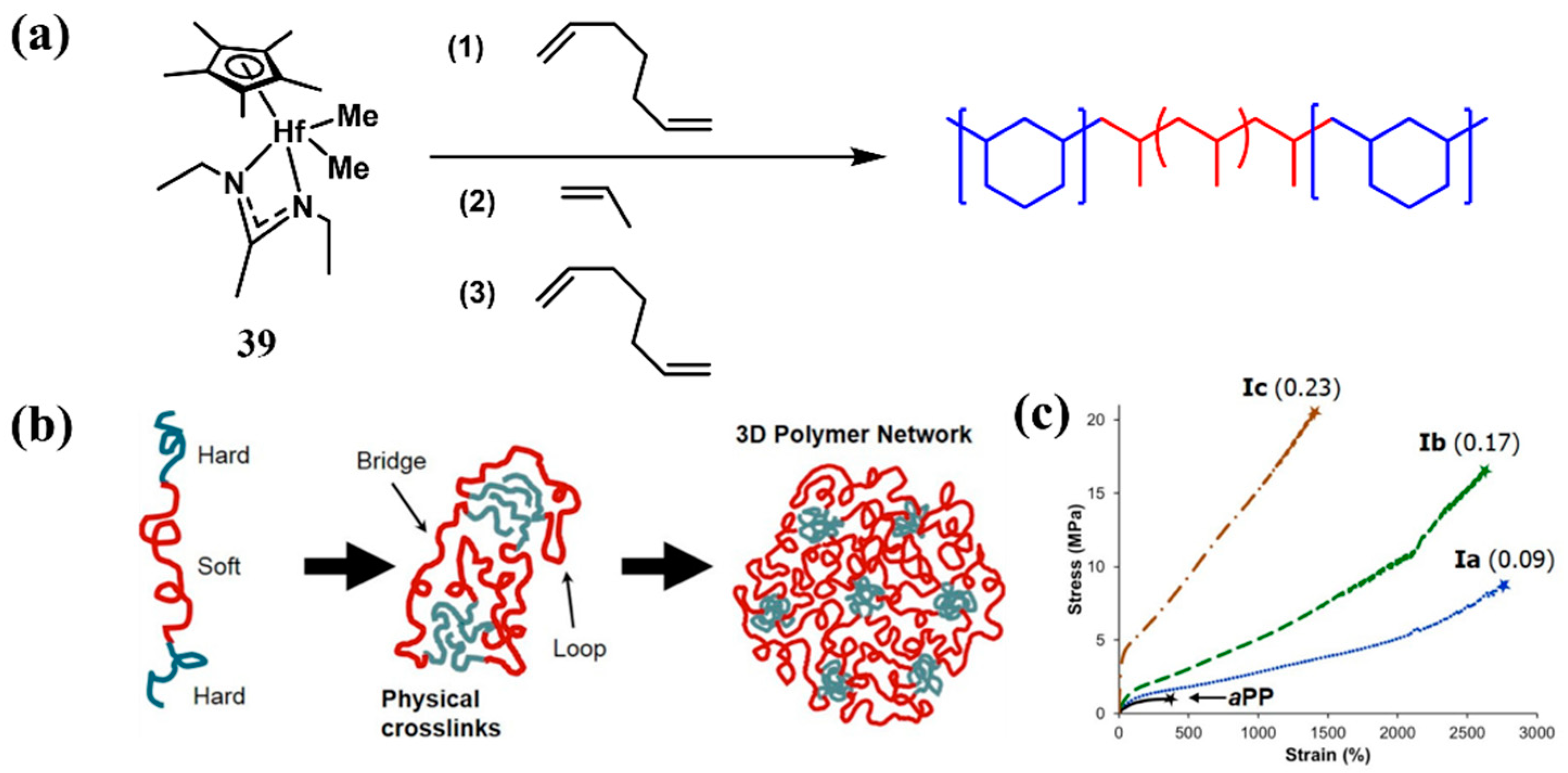
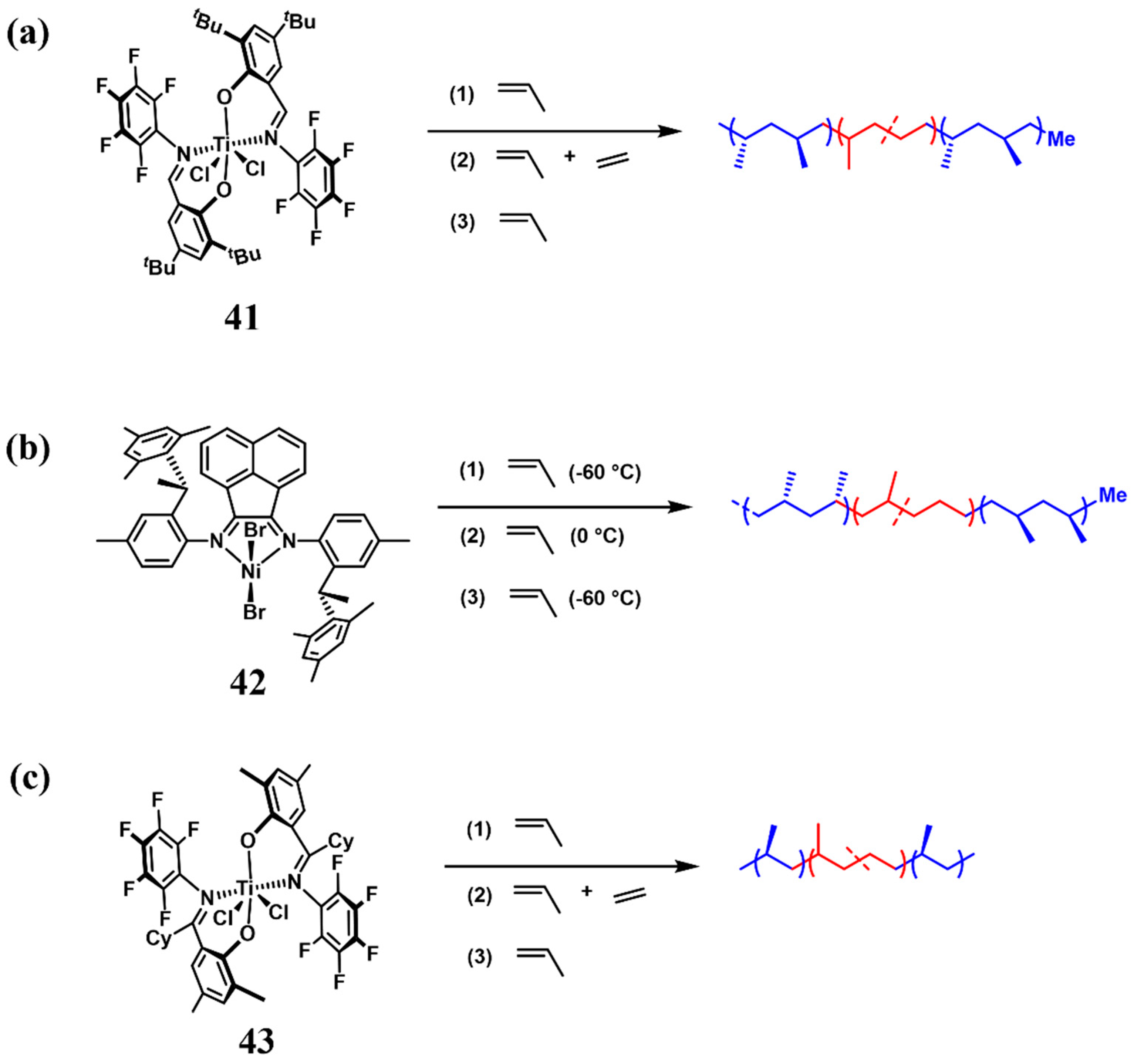
4. Block Copolymer Propylene-Based Elastomers
| Run | Catalyst | Tp (°C) | Mw (kg/mol) | Ð | Tm (°C) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 39 | −15 | 403 | 1.18 | n.d. | [61] |
| 2 | 40 | 30/20 | 335 | 1.11 | n.d. | [62] |
| 3 | 41 | 0 | 358 | 1.20 | 36, 134 | [64] |
| 4 | 42 | 0/−60 | 124 | 1.14 | 130 | [64] |
| 5 | 43 | 0 | 306 | 1.30 | 95 | [65] |
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.Y.; Gao, Y.; Tang, Y. Sustainable developments in polyolefin chemistry: Progress, challenges, and outlook. Prog. Polym. Sci. 2023, 143, 101713. [Google Scholar] [CrossRef]
- Sauter, D.; Taoufik, M.; Boisson, C. Polyolefins, a success story. Polymers 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev. 2015, 116, 1398–1433. [Google Scholar] [CrossRef]
- Kaminsky, W. Trends in polyolefin chemistry. Macromol. Chem. Phys. 2008, 209, 459–466. [Google Scholar] [CrossRef]
- Dong, J.Y.; Hu, Y. Design and synthesis of structurally well-defined functional polyolefins via transition metal-mediated olefin polymerization chemistry. Coord. Chem. Rev. 2006, 250, 47–65. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Jin, G.X. Polymerized metallocene catalysts and late transition metal catalysts for ethylene polymerization. Coord. Chem. Rev. 2006, 250, 95–109. [Google Scholar] [CrossRef]
- Zanchin, G.; Leone, G. Polyolefin thermoplastic elastomers from polymerization catalysis: Advantages, pitfalls and future challenges. Prog. Polym. Sci. 2021, 113, 101342. [Google Scholar] [CrossRef]
- Mohite, A.S.; Rajpurkar, Y.D.; More, A.P. Bridging the gap between rubbers and plastics: A review on thermoplastic polyolefin elastomers. Polym. Bull. 2021, 79, 1309–1343. [Google Scholar] [CrossRef]
- Mahmood, Q.; Sun, W.H. N,N-chelated nickel catalysts for highly branched polyolefin elastomers: A survey. R. Soc. Open Sci. 2018, 5, 180367. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Wang, L.; Xing, Y.; Bilal Ul, A. Advances in the synthesis of polyolefin elastomers with “chain-walking” catalysts and electron spin resonance research of related catalytic systems. Curr. Org. Chem. 2021, 25, 935–949. [Google Scholar] [CrossRef]
- Muller, G.; Rieger, B. Propene based thermoplastic elastomers by early and late transition metal catalysis. Prog. Polym. Sci. 2002, 27, 815–851. [Google Scholar] [CrossRef]
- Cobzaru, C.; Hild, S.; Boger, A.; Troll, C.; Rieger, B. “Dual-side” catalysts for high and ultrahigh molecular weight homopolypropylene elastomers and plastomers. Coord. Chem. Rev. 2006, 250, 189–211. [Google Scholar] [CrossRef]
- Shcherbina, M.A.; Meshchankina, M.Y.; Odarchenko, Y.I.; Machat, M.; Rieger, B.; Chvalun, S.N. From elastomers to thermoplasts—Precise control of isotactic propylene structure and properties and the role of different structural elements in its mechanical behaviour. Polymer 2017, 133, 213–222. [Google Scholar] [CrossRef]
- Tsou, A.H.; Norman, A.I.; Lu, Y.; Throckmorton, J.A.; Hsiao, B.S. Sequence distribution and elastic properties of propylene-based elastomers. Polymer 2017, 111, 115–122. [Google Scholar] [CrossRef]
- Heidari, A.; Fasihi, M. Cell structure-impact property relationship of polypropylene/thermoplastic elastomer blend foams. eXPRESS Polym. Lett. 2019, 13, 429–442. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Yuan, Y.; Huang, S.; Du, B. Trap distribution and dielectric breakdown of isotactic polypropylene/propylene based elastomer with improved flexibility for dc cable insulation. IEEE Access 2018, 6, 58645–58661. [Google Scholar] [CrossRef]
- Wen, Z.; Wu, C.; Chen, J.; Qu, S.; Li, X.; Wang, W. Homogeneous non-metallocene group 4 metals ligated with [N,N] bidentate ligand(s) for olefin polymerization. Polymers 2024, 16, 406. [Google Scholar] [CrossRef]
- Ullah Khan, W.; Mazhar, H.; Shehzad, F.; Al-Harthi, M.A. Recent advances in transition metal-based catalysts for ethylene copolymerization with polar comonomer. Chem. Rec. 2023, 23, e202200243. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Komarov, P.D.; Kostomarova, O.D.; Kolosov, N.A.; Ivchenko, P.V. MAO- and borate-free activating supports for group 4 metallocene and post-metallocene catalysts of α-olefin polymerization and oligomerization. Polymers 2023, 15, 3095. [Google Scholar] [CrossRef]
- Mishra, A.; Patil, H.R.; Gupta, V. Progress in propylene homo- and copolymers using advanced transition metal catalyst systems. New J. Chem. 2021, 45, 10577–10588. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Marks, T.J. Early transition metal catalysis for olefin–polar monomer copolymerization. Angew. Chem. Int. Ed. 2020, 59, 14726–14735. [Google Scholar] [CrossRef] [PubMed]
- Schöbel, A.; Winkenstette, M.; Anselment, T.M.J.; Rieger, B. Copolymerization of alkenes and polar monomers by early and late transition metal catalysts. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 779–823. [Google Scholar]
- De Rosa, C.; Auriemma, F. Single site metallorganic polymerization catalysis as a method to probe the properties of polyolefins. Polym. Chem. 2011, 2, 2155. [Google Scholar] [CrossRef]
- Wang, B. Ansa-metallocene polymerization catalysts: Effects of the bridges on the catalytic activities. Coord. Chem. Rev. 2006, 250, 242–258. [Google Scholar] [CrossRef]
- Muldoon, M.J. Modern multiphase catalysis: New developments in the separation of homogeneous catalysts. Dalton Trans. 2010, 39, 337–348. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef]
- Arranz-Andrés, J.; Suárez, I.; Peña, B.; Benavente, R.; Pérez, E.; Cerrada, M.L. Metallocenic isotactic poly(propylene) and its copolymers with 1-hexene and ethylene. Macromol. Chem. Phys. 2007, 208, 1510–1521. [Google Scholar] [CrossRef]
- Corradini, P. The discovery of isotactic polypropylene and its impact on pure and applied science. J. Polym. Sci. Part A Polym. Chem. 2003, 42, 391–395. [Google Scholar] [CrossRef]
- Balboni, D.; Moscardi, G.; Baruzzi, G.; Braga, V.; Camurati, I.; Piemontesi, F.; Resconi, L.; Nifant’ev, I.E.; Venditto, V.; Antinucci, S. C2-symmetric zirconocenes for high molecular weight amorphous poly(propylene). Macromol. Chem. Phys. 2001, 202, 2010–2028. [Google Scholar] [CrossRef]
- Natta, G. Properties of isotactic, atactic, and stereoblock homopolymers, random and block copolymers of a-olefins. J. Polym Sci. 1959, 34, 531–549. [Google Scholar] [CrossRef]
- Pino, P.; Miilhaupt, R. Stereospecific polymerization of propylene: An outlook 25 years after its discovery. Angew. Chem. Int. Ed. 1980, 19, 857–875. [Google Scholar] [CrossRef]
- Mallin, D.T.; Rausch, M.D.; Lin, Y.G.; Dong, S.; Chien, J.C.W. Rac-[ethylidene(1-η5-tetramethylcyclopentadienyl) (l-η5-indenyl)]dichlorotitanium and its homopolymerization of propylene to crystalline-amorphous block thermoplastic elastomers. J. Am. Chem. Soc. 1990, 112, 2030–2031. [Google Scholar] [CrossRef]
- Coates, G.W.; Waymouth, R.M. Oscillating stereocontrol: A strategy for the synthesis of thermoplastic elastomeric polypropylene. Science 1995, 267, 217–219. [Google Scholar] [CrossRef]
- Hauptman, E.; Waymouth, R.M. Stereoblock polypropylene: Ligand effects on the stereospecificity of 2-arylindene zirconocene catalysts. J. Am. Chem. Soc. 1995, 117, 11586–11587. [Google Scholar] [CrossRef]
- Bruce, M.D.; Coates, G.W.; Hauptman, E.; Waymouth, R.M.; Ziller, J.W. Effect of metal on the stereospecificity of 2-arylindene catalysts for elastomeric polypropylene. J. Am. Chem. Soc. 1997, 119, 11174–11182. [Google Scholar] [CrossRef]
- Dietrich, U.; Hackmann, M.; Rieger, B.; Klinga, M.; Leskela, M. Control of stereoerror formation with high-activity “dual-side” zirconocene catalysts: A novel strategy to design the properties of thermoplastic elastic polypropenes. J. Am. Chem. Soc. 1999, 121, 4348–4355. [Google Scholar] [CrossRef]
- Meverden, C.C.; Nagy, S. Polypropylene Preparation. CA2444902C, 6 July 2010. [Google Scholar]
- Machat, M.R.; Lanzinger, D.; Drees, M.; Altmann, P.J.; Herdtweck, E.; Rieger, B. High-melting, elastic polypropylene: A one-pot, one-catalyst strategy toward propylene-based thermoplastic elastomers. Macromolecules 2018, 51, 914–929. [Google Scholar] [CrossRef]
- Resconi, L.; Jones, R.L.; Rheingold, A.L.; Yap, G.P.A. High-molecular-weight atactic polypropylene from metallocene catalysts. 1. Me2Si(9-Flu)2ZrX2 (X = Cl, Me). Organometallics 1996, 15, 998–1005. [Google Scholar] [CrossRef]
- Safimannshausen, J.; Bochmarrn, M.; Riisch, J.; Lilge, D. Titanium-catalysed formation of high molecular weight elastomeric polypropene: Evidence for living propene polymerisation. J. Organomet. Chem. 1997, 548, 23–28. [Google Scholar] [CrossRef]
- Chen, J.J.; Wang, T.S.; Tang, Z.W.; Xu, Y.B.; Cao, M.S.; Feng, Z.G. Half-titanocenes catalysts based on titamium-phosphinimide for producing atactic elastomeric polypropylenes with high molecular weight. Acta Polym. Sin. 2017, 1294–1303. [Google Scholar]
- Collins Rice, C.G.; Buffet, J.C.; Turner, Z.R.; O’Hare, D. Efficient synthesis of thermoplastic elastomeric amorphous ultra-high molecular weight atactic polypropylene (UHMWaPP). Polym. Chem. 2022, 13, 5597–5603. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, Y.C.; Li, P. Propylene-Based Elastomer As Well As Preparation Method and Application Thereof. CN116874676A, 13 October 2023. [Google Scholar]
- Takashi, U.; Akira, M.; Masahiro, K.; Toshiyuki, T. Propylene Elastomer. EP682042, 26 March 1997. [Google Scholar]
- Winter, A.; Kuber, F.; Bachmann, B. High Molecular Weight Copolymers of Propylene and Olefins Having 2 or 4 to 32 Carbon Atoms. US6057408, 2 May 2000. [Google Scholar]
- Kawahara, N.; Kojoh, S.; Kashiwa, N.; Mori, R.; Ikenaga, S.; Okada, K.; Takayasu, H.; Inoue, N.; Hirota, N.; Kaneyoshi, H.; et al. Propylene Copolymer, Polypropylene Composition, Use Thereof, Transition Metal Compounds, and Catalysts for Olefin Polymerization. WO2004087775, 14 October 2004. [Google Scholar]
- Cozewith, C.; Datta, S.; Hu, W. Propylene Ethylene Polymers. US6525157, 25 February 2003. [Google Scholar]
- Quijada, R.; Guevara, J.L.; Galland, G.B.; Rabagliati, F.M.; Lopez-Majada, J.M. Synthesis and properties coming from the copolymerization of propene with α-olefins using different metallocene catalysts. Polymer 2005, 46, 1567–1574. [Google Scholar] [CrossRef]
- Rix, F.C.; Kacker, S.; Datta, S.; Zhao, R.; Eswaran, V.R. Olefin Polymerization Catalyst System and Process for Use Thereof. US2006009595, 12 January 2006. [Google Scholar]
- Rosa, C.D.; Auriemma, F.; Ballesteros, O.R.d.; Resconi, L.; Camurati, I. Tailoring the physical properties of isotactic polypropylene through incorporation of comonomers and the precise control of stereoand regioregularity by metallocene catalysts. Chem. Mater. 2007, 19, 5122–5130. [Google Scholar] [CrossRef]
- Han, X.; Wang, X.; Kang, W.; Huang, C.; Gao, L.; Li, Y.; Zhang, C. Polyolefin Elastomer, Preparation Method Thereof and Metallocene Catalyst Used in Polyolefin Elastomer. CN116410385A, 7 November 2023. [Google Scholar]
- Tau, L.-M.; Chum, P.-W.S.; Karande, S.; Bosnyak, C. Blends and Sealant Compositions Comprising Isotactic Propylene Copolymers. US6919407, 19 July 2005. [Google Scholar]
- Thomas, C.A.C. Propylene-Based Elastomeric Composition. US7893161, 22 February 2011. [Google Scholar]
- Wang, B.; Long, Y.Y.; Li, Y.G.; Men, Y.F.; Li, Y.S. Cyclic olefin copolymers of propylene with asymmetric Si-containing α,ω-diolefins: The tailored thermal and mechanical properties. Polymer 2015, 61, 108–114. [Google Scholar] [CrossRef]
- Shang, R.; Gao, H.; Luo, F.; Li, Y.; Wang, B.; Ma, Z.; Pan, L.; Li, Y. Functional isotactic polypropylenes via efficient direct copolymerizations of propylene with various amino-functionalized α-olefins. Macromolecules 2019, 52, 9280–9290. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Ma, Z.; Wang, B.; Pan, L.; Li, Y. Copolymerization of propylene with higher α-olefins by a pyridylamidohafnium catalyst: An effective approach to polypropylene-based elastomer. Polymers 2020, 12, 89. [Google Scholar] [CrossRef]
- Rishina, L.A.; Kissin, Y.V.; Lalayan, S.S.; Krasheninnikov, V.G. Synthesis of atactic polypropylene: Propylene polymerization reactions with TiCl4–Al(C2H5)2Cl/Mg(C4H9)2 catalyst. J. Appl. Polym. Sci. 2019, 136, 47692. [Google Scholar] [CrossRef]
- Garcı’a-Martı’nez, J.M.; Cofrades, A.G.S.; Areso, E.P.C. Chemical modification process of an atactic polypropylene by p-phenylen-bismaleamic acid in the melt. J. Appl. Polym. Sci. 2003, 88, 2202–2209. [Google Scholar] [CrossRef]
- Collar, E.P.; Marco, C.; Laguna, O.; Areso, S.; García-Martínez, J.M. On the changes in glass transition temperatures of atactic polypropylenes induced by grafting of polar groups. J. Therm. Anal. Calorim. 1999, 58, 541–550. [Google Scholar] [CrossRef]
- Wu, Q.; Su, Q.; Ye, L.; Li, G.; Mu, Y. Propylene polymerization to high molecular weight atactic polypropylene and copolymerization with 1-hexene using monocyclopentadienyl titanium catalysts. Dalton Trans. 2010, 39, 2525. [Google Scholar] [CrossRef]
- Crawford, K.E.; Sita, L.R. De novo design of a new class of “hard–soft” amorphous, microphase-separated, polyolefin block copolymer thermoplastic elastomers. ACS Macro Lett. 2015, 4, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cao, L.; Tanaka, R.; Shiono, T.; Cai, Z. Optically transparent functional polyolefin elastomer with excellent mechanical and thermal properties. ACS Macro Lett. 2019, 8, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Suenaga, T.; Cai, Z.; Nakayama, Y.; Shiono, T. Synthesis and thermal, mechanical, and optical properties of A–B–A or A–B block copolymers containing poly(norbornene-co-1-octene). J. Polym. Sci., Part A Polym. Chem. 2013, 52, 267–271. [Google Scholar] [CrossRef]
- Hotta, A.; Cochran, E.; Ruokolainen, J.; Khanna, V.; Fredrickson, G.H.K.; Edward, J.; Shin, Y.W.; Shimizu, F.; Cherian, A.E.; Hustad, P.D.; et al. Semicrystalline thermoplastic elastomeric polyolefins: Advances through catalyst development and macromolecular design. PNAS 2006, 103, 15327–15332. [Google Scholar] [CrossRef]
- Edson, J.B.; Wang, Z.; Kramer, E.J.; Coates, G.W. Fluorinated bis(phenoxyketimine titanium complexes for the living, isoselective polymerization of propylene: Multiblock isotactic polypropylene copolymers via sequential monomer addition. J. Am. Chem. Soc. 2008, 130, 4968–4977. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, E.J.; Park, I.S.; Lee, C.H.; Kim, B.S. Propylene-Based Elastomer. EP3031834, 15 June 2016. [Google Scholar]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Fan, G.; Zheng, G.; Gao, R.; Liu, L. Recent Advances in Propylene-Based Elastomers Polymerized by Homogeneous Catalysts. Polymers 2024, 16, 2717. https://doi.org/10.3390/polym16192717
Li C, Fan G, Zheng G, Gao R, Liu L. Recent Advances in Propylene-Based Elastomers Polymerized by Homogeneous Catalysts. Polymers. 2024; 16(19):2717. https://doi.org/10.3390/polym16192717
Chicago/Turabian StyleLi, Chengkai, Guoqiang Fan, Gang Zheng, Rong Gao, and Li Liu. 2024. "Recent Advances in Propylene-Based Elastomers Polymerized by Homogeneous Catalysts" Polymers 16, no. 19: 2717. https://doi.org/10.3390/polym16192717






