Copper Nanoparticle Loaded Electrospun Patches for Infected Wound Treatment: From Development to In-Vivo Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospun Patches Synthesis and Structural Assessment
2.3. Investigation of Antibacterial Properties
2.4. Bacteriological Experiment In Vitro
2.5. In-Vivo Experiment Design
2.6. Microbiological Assessment of the Wound
2.7. Histological and Immunohistochemical Assessment
3. Results
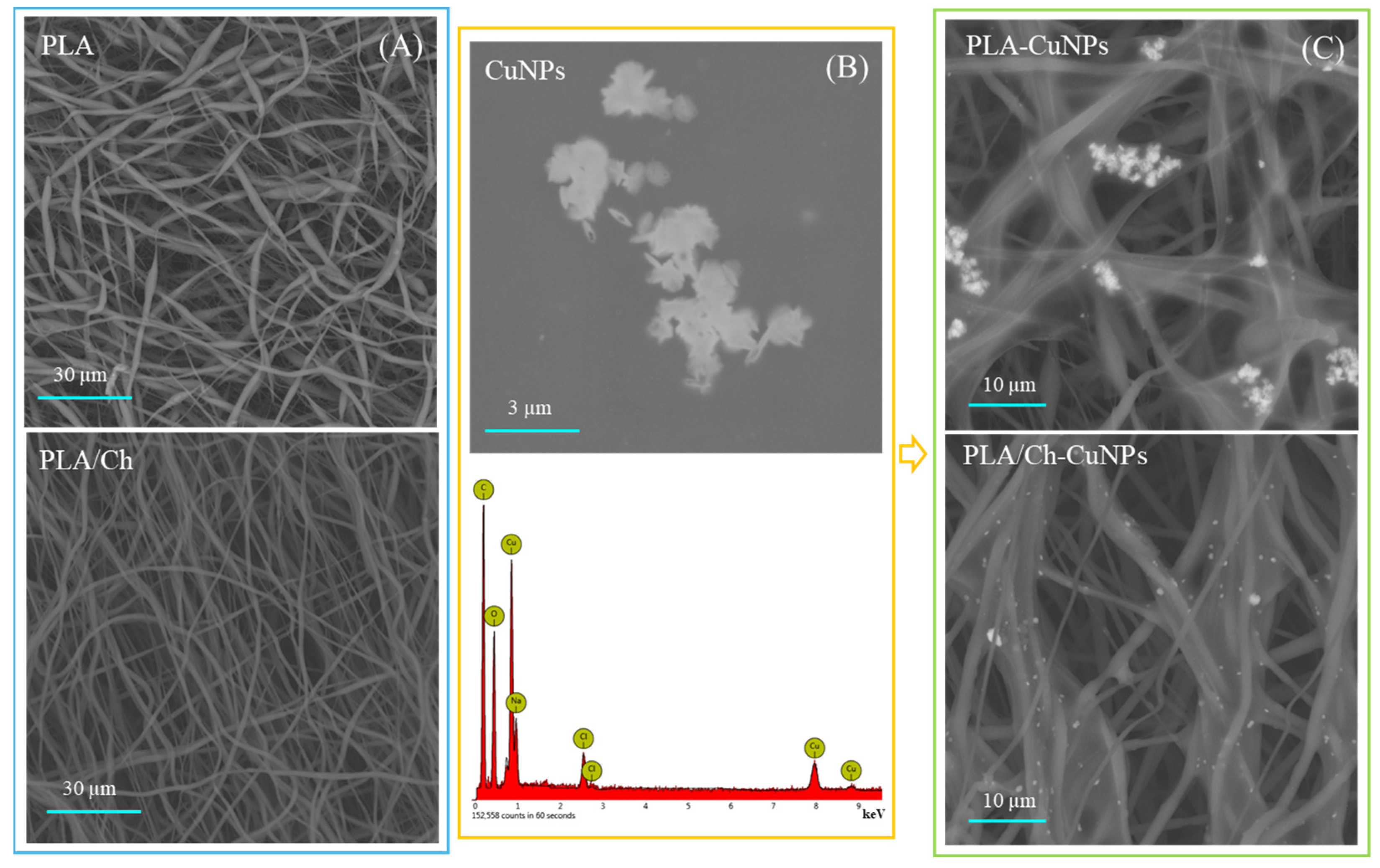
3.1. Patches Structure
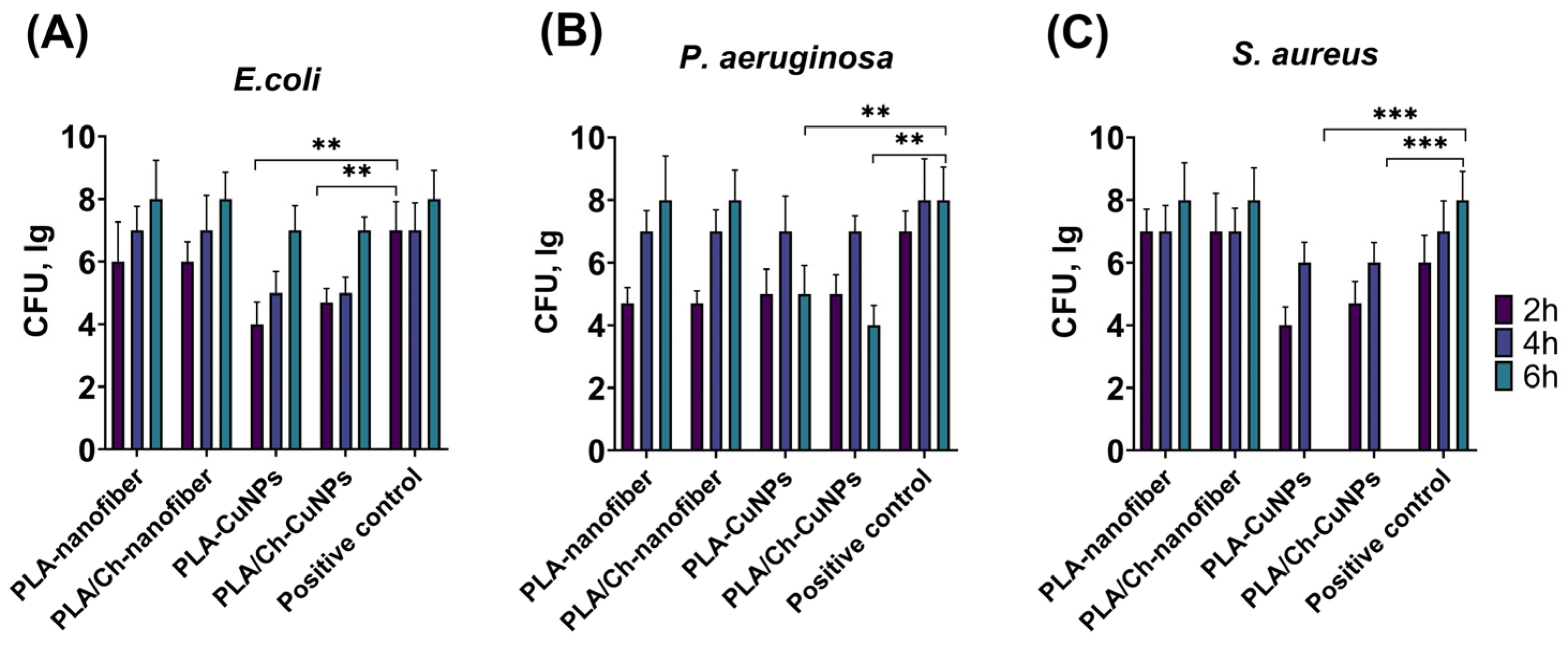
3.2. Antibacterial Activity of Novel Electrospun Patches
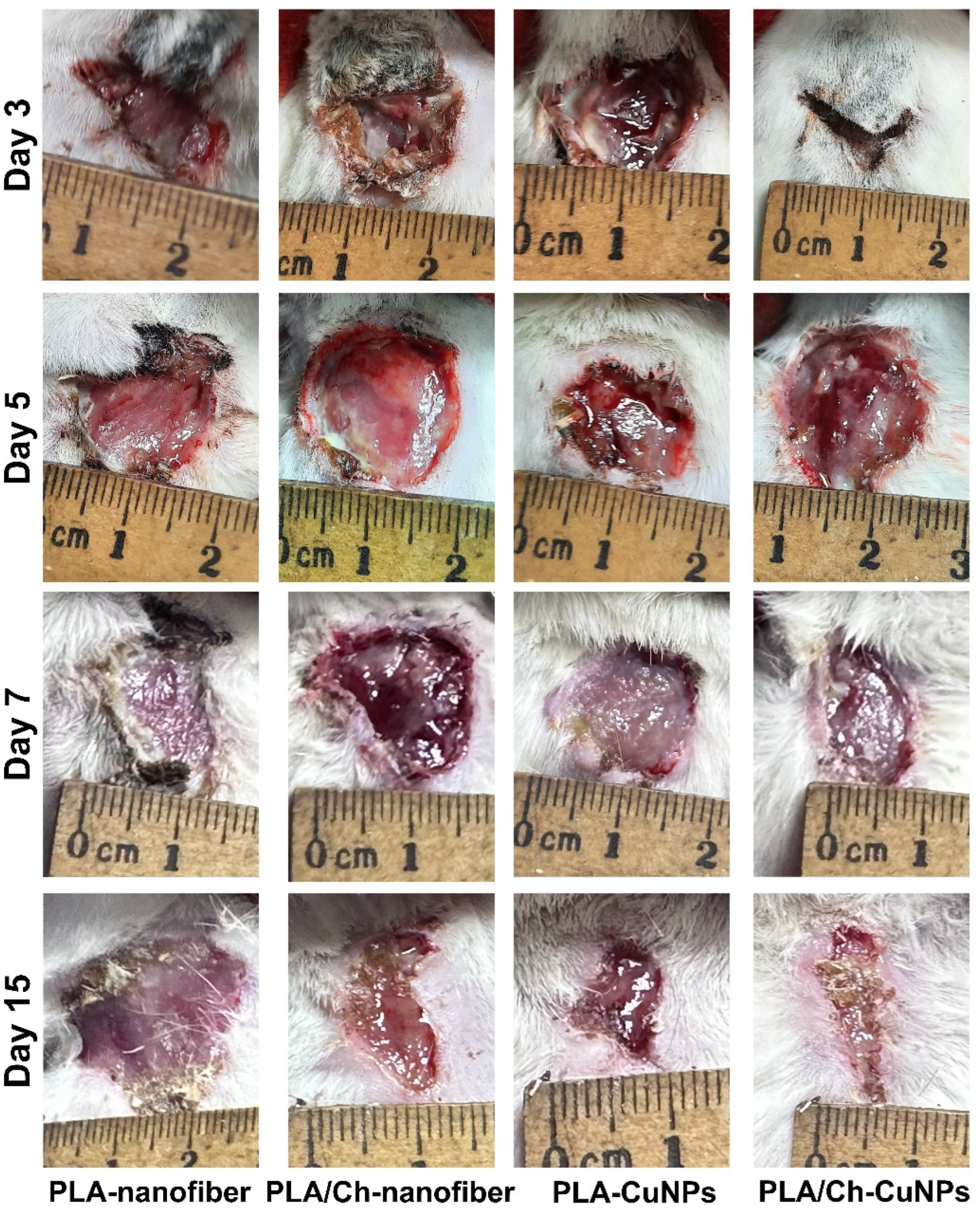
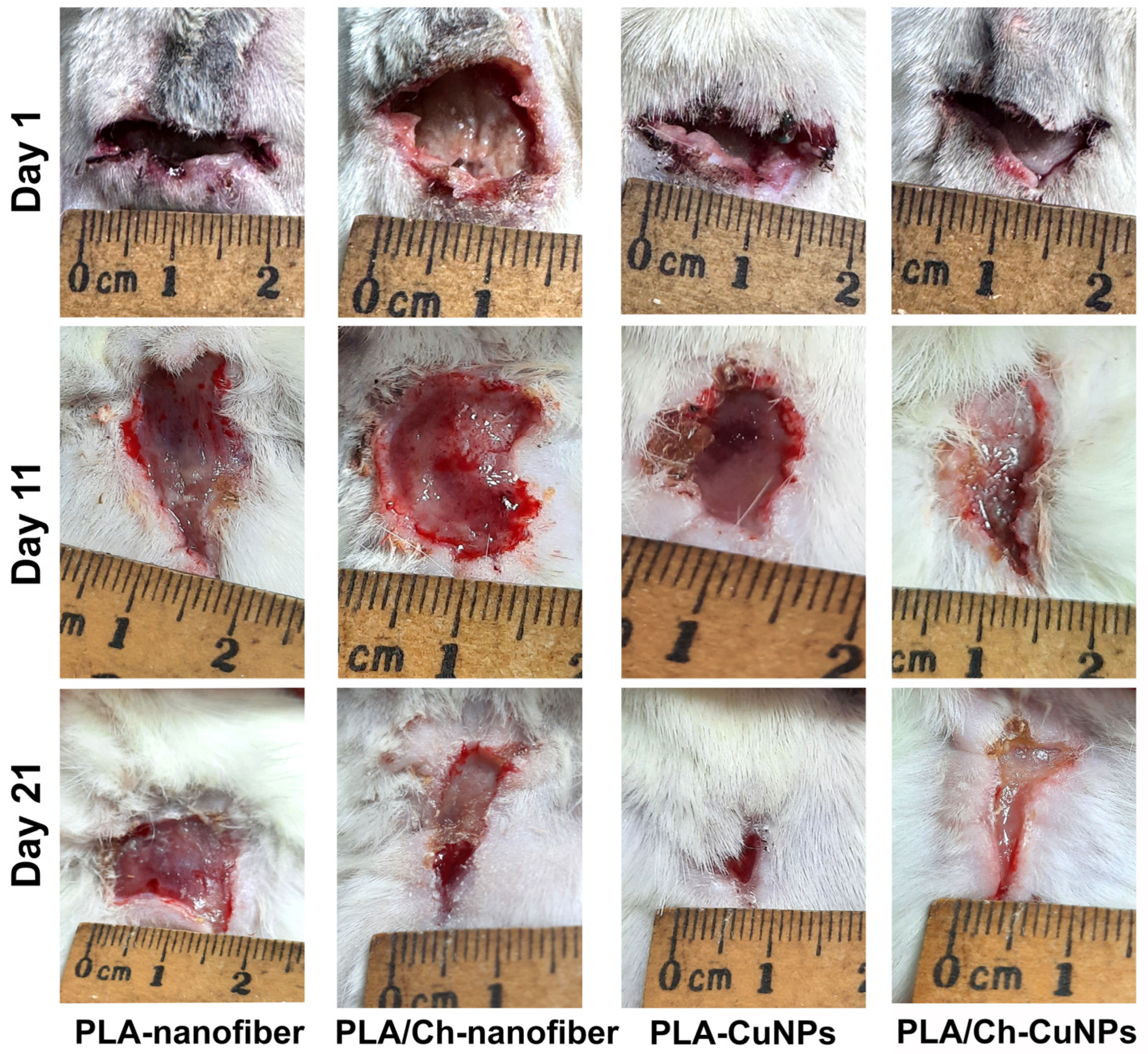
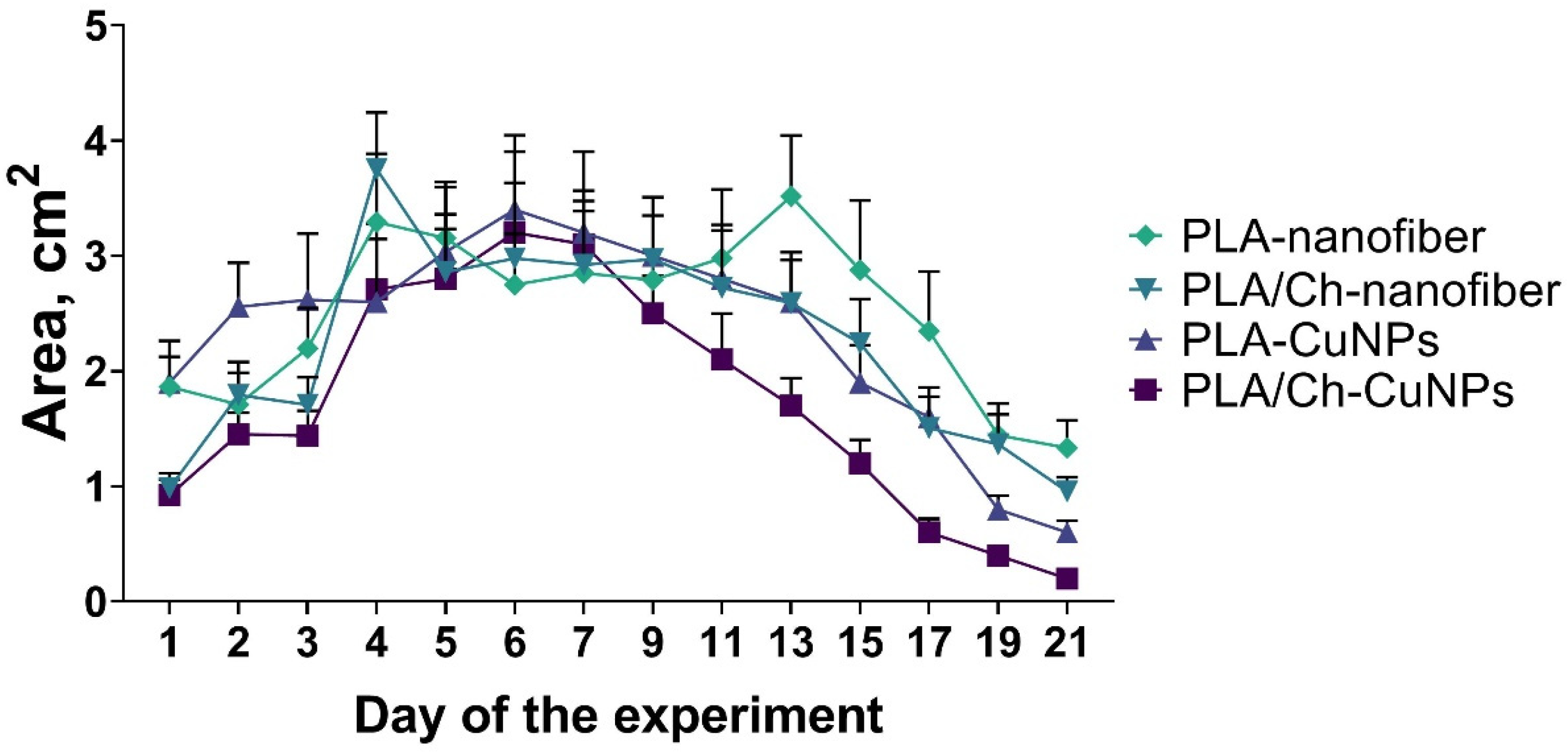
3.3. Wound Size Evaluation
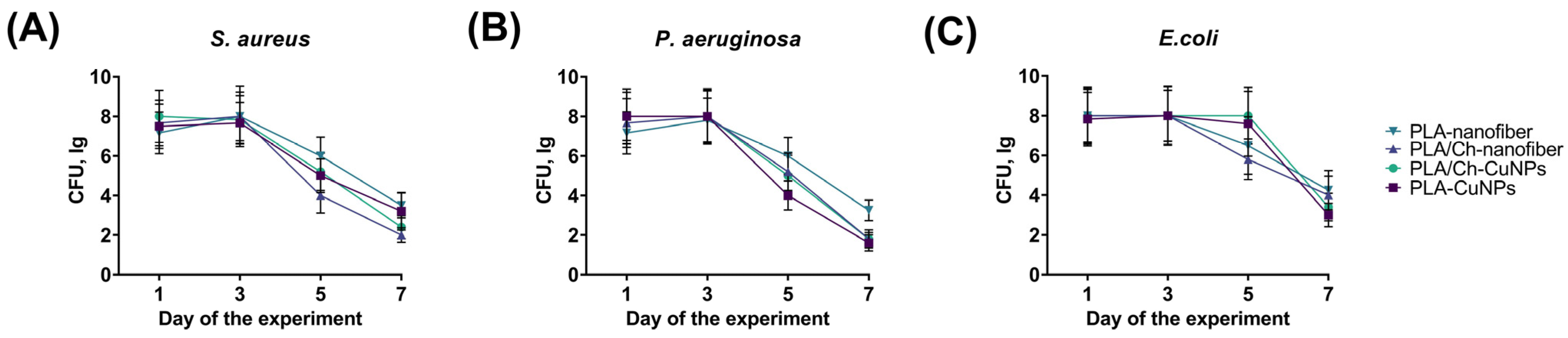
3.4. Microbiological Evaluation
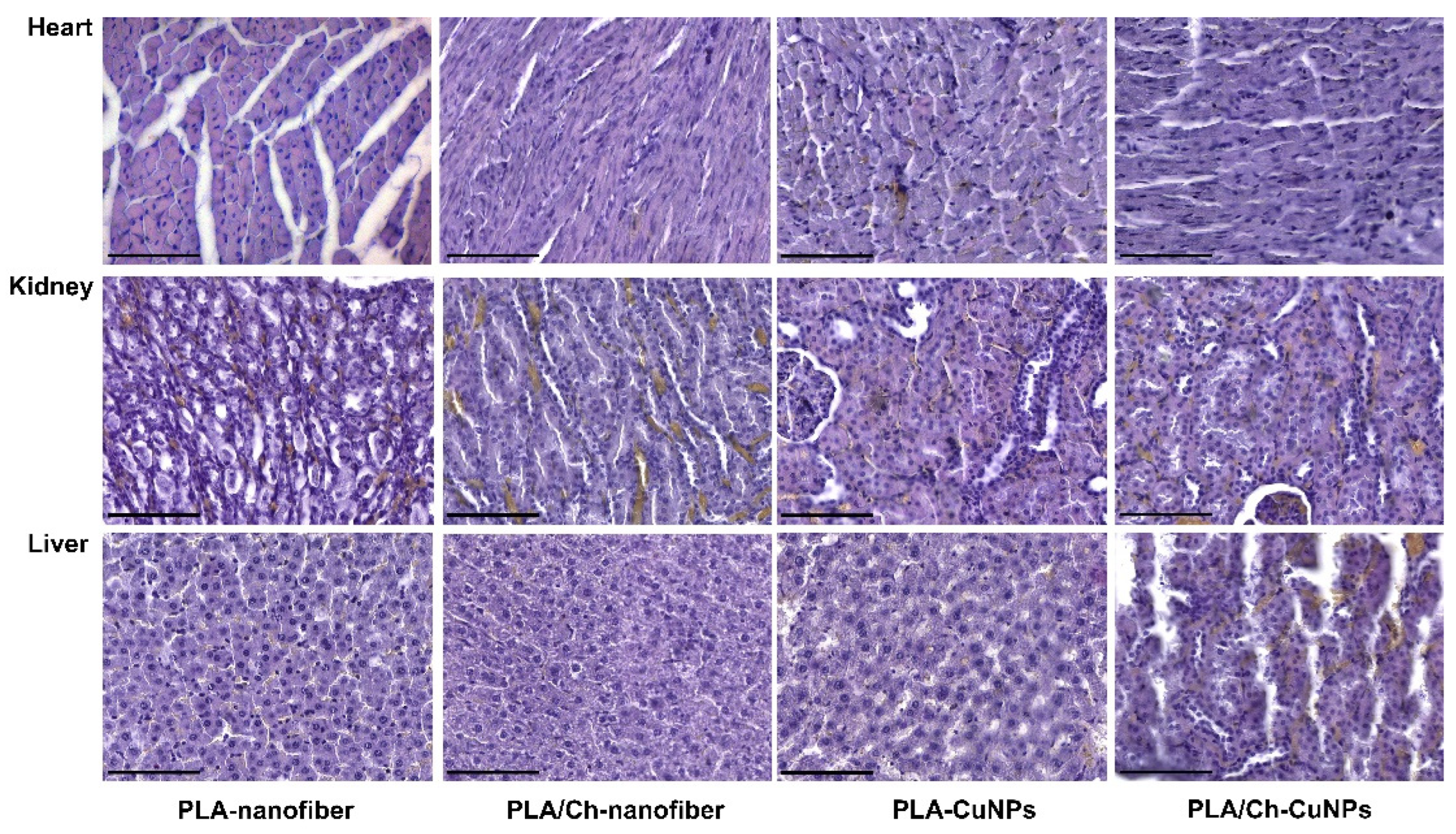
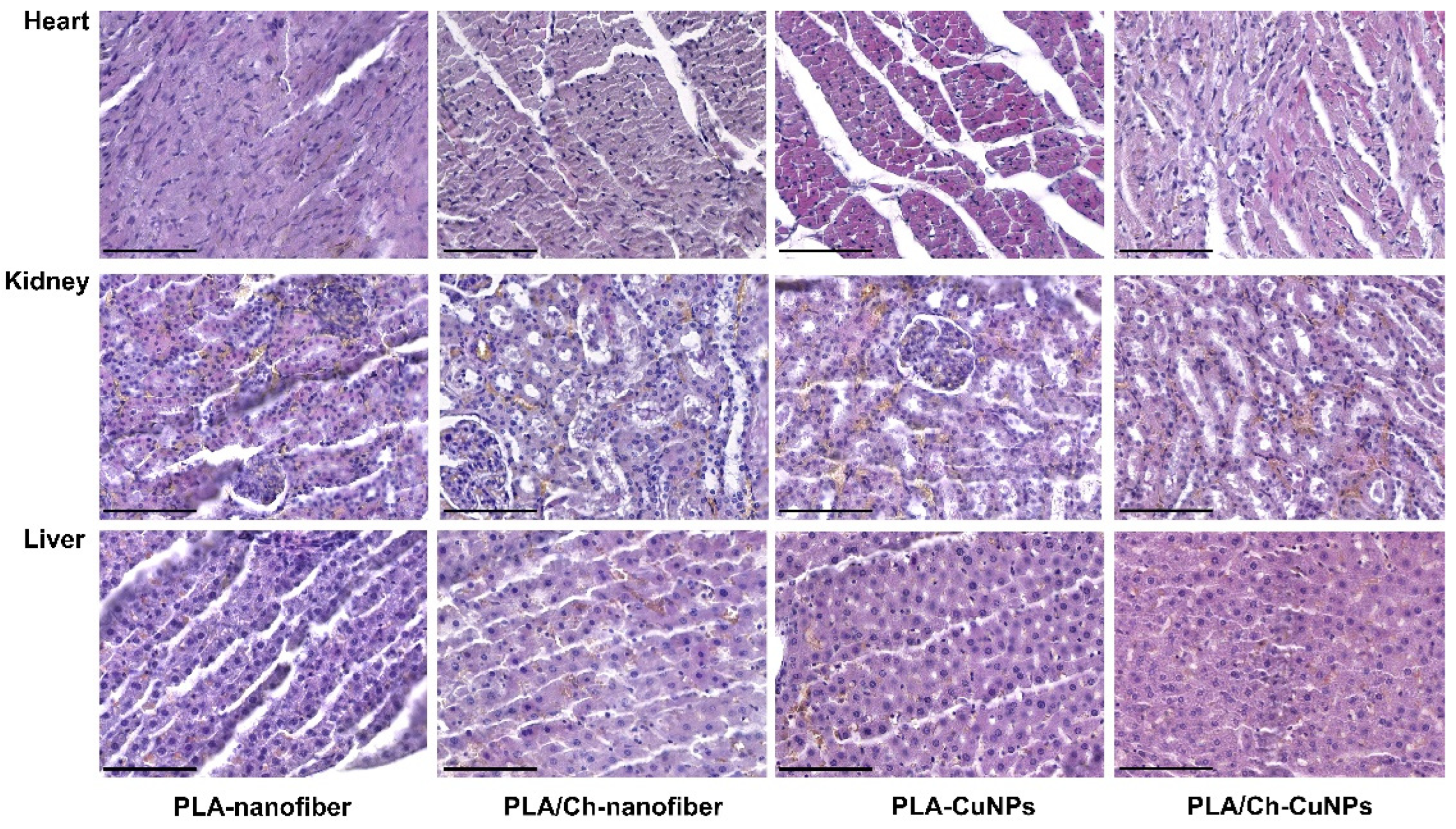
3.5. Histological and Immunohistochemical Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Dou, J.L.; Jiang, Y.W.; Xie, J.Q.; Zhang, X.G. New Is Old, and Old Is New: Recent Advances in Antibiotic-Based, Antibiotic-Free and Ethnomedical Treatments against Methicillin-Resistant Staphylococcus aureus Wound Infections. Int. J. Mol. Sci. 2016, 17, 617. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; LeFrock, J.L.; Lew, D.P.; Mader, J.T.; Norden, C.; et al. Diagnosis and treatment of diabetic foot infections. Plast. Reconstr. Surg. 2006, 117, 212s–238s. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qin, Z.; Qu, Y.; Wang, F.; Huang, B.; Chen, G.; Liu, X.; Yin, L. Cell electrospinning and its application in wound healing: Principles, techniques and prospects. Burns Trauma 2023, 11, tkad028. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of Silver Nanoparticles in Hydrogel Matrices for Controlling Wound Infection. J. Burn Care Res. 2021, 42, 785–793. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- González López, E.J.; Palacios, Y.B.; Martinez, S.R.; Durantini, A.M.; Durantini, E.N.; Abraham, G.A.; Bongiovanni Abel, S.; Heredia, D.A. Light-Activated Antibacterial Ethylcellulose Electrospun Nanofibrous Mats Containing Fluorinated Zn(II) Porphyrin. ACS Appl. Polym. Mater. 2024, 6, 7691–7704. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef]
- Kirsipuu, T.; Zadorožnaja, A.; Smirnova, J.; Friedemann, M.; Plitz, T.; Tõugu, V.; Palumaa, P. Copper(II)-binding equilibria in human blood. Sci. Rep. 2020, 10, 5686. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Oztekin, Y.; Tok, M.; Bilici, E.; Mikoliunaite, L.; Yazicigil, Z.; Ramanaviciene, A.; Ramanavicius, A. Copper nanoparticle modified carbon electrode for determination of dopamine. Electrochim. Acta 2012, 76, 201–207. [Google Scholar] [CrossRef]
- Sharif, M.; Rahman, M.A.; Ahmed, B.; Abbas, R.Z.; Hassan, F.U. Copper Nanoparticles as Growth Promoter, Antioxidant and Anti-Bacterial Agents in Poultry Nutrition: Prospects and Future Implications. Biol. Trace Elem. Res. 2021, 199, 3825–3836. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Pérez, A.; Durán-Armenta, L.F.; Pérez-Loredo, M.G.; Torres-Huerta, A.L. Biosynthesis of Copper Nanoparticles with Medicinal Plants Extracts: From Extraction Methods to Applications. Micromachines 2023, 14, 1882. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry-a narrative review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef] [PubMed]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burns Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef]
- Song, L.; Connolly, M.; Fernández-Cruz, M.L.; Vijver, M.G.; Fernández, M.; Conde, E.; de Snoo, G.R.; Peijnenburg, W.J.; Navas, J.M. Species-specific toxicity of copper nanoparticles among mammalian and piscine cell lines. Nanotoxicology 2014, 8, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Hedberg, Y.; Tornberg, M.; De Battice, L.; Svedhem, S.; Wallinder, I.O. Cell membrane damage and protein interaction induced by copper containing nanoparticles--importance of the metal release process. Toxicology 2013, 313, 59–69. [Google Scholar] [CrossRef]
- Sharma, P.; Goyal, D.; Baranwal, M.; Chudasama, B. Oxidative Stress Induced Cytotoxicity of Colloidal Copper Nanoparticles on RAW 264.7 Macrophage Cell Line. J. Nanosci. Nanotechnol. 2021, 21, 5066–5074. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Clapper, J.C. Antibacterial Activity of Electrospun Polyacrylonitrile Copper Nanoparticle Nanofibers on Antibiotic Resistant Pathogens and Methicillin Resistant Staphylococcus aureus (MRSA). Nanomaterials 2022, 12, 2139. [Google Scholar] [CrossRef]
- Eslaminezhad, S.; Moradi, F.; Hojjati, M.R. Evaluation of the wound healing efficacy of new antibacterial polymeric nanofiber based on polyethylene oxide coated with copper nanoparticles and defensin peptide: An in-vitro to in-vivo assessment. Heliyon 2024, 10, e29542. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Satei, P.; Ganji, A.; Abtahi, H. Wound healing performance of PVA/PCL based electrospun nanofiber incorporated green synthetized CuNPs and Quercus infectoria extracts. J. Biomater. Sci. Polym. Ed. 2023, 34, 277–301. [Google Scholar] [CrossRef]
- Iranpour Mobarakeh, A.; Shahmoradi Ramsheh, A.; Khanshan, A.; Aghaei, S.; Mirbagheri, M.S.; Esmaeili, J. Fabrication and evaluation of a bi-layered electrospun PCL/PVA patch for wound healing: Release of vitamins and silver nanoparticle. Heliyon 2024, 10, e33178. [Google Scholar] [CrossRef] [PubMed]
- Korniienko, V.; Husak, Y.; Radwan-Pragłowska, J.; Holubnycha, V.; Samokhin, Y.; Yanovska, A.; Varava, J.; Diedkova, K.; Janus, Ł.; Pogorielov, M. Impact of Electrospinning Parameters and Post-Treatment Method on Antibacterial and Antibiofilm Activity of Chitosan Nanofibers. Molecules 2022, 27, 3343. [Google Scholar] [CrossRef] [PubMed]
- Samokhin, Y.; Varava, Y.; Diedkova, K.; Yanko, I.; Husak, Y.; Radwan-Pragłowska, J.; Pogorielova, O.; Janus, Ł.; Pogorielov, M.; Korniienko, V. Fabrication and Characterization of Electrospun Chitosan/Polylactic Acid (CH/PLA) Nanofiber Scaffolds for Biomedical Application. J. Funct. Biomater. 2023, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Deineka, V.; Sulaieva, O.; Pernakov, M.; Korniienko, V.; Husak, Y.; Yanovska, A.; Yusupova, A.; Tkachenko, Y.; Kalinkevich, O.; Zlatska, A.; et al. Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials. Biomedicines 2021, 9, 588. [Google Scholar] [CrossRef]
- Korniienko, V.; Varava, Y.; Banasiuk, R.; Korniienko, V.; Diedkova, K.; Petricenko, O.; Arora, D.; Denysenko, A.; Moskalenko, R.; Pogorielov, M. Layer-by-Layer Chitosan/PCL Electrospun Membrane Loaded with Copper Nanoparticles as Antibacterial Wound Healing Dressing. In Nanocomposite and Nanocrystalline Materials and Coatings: Microstructure, Properties and Applications; Pogrebnjak, A.D., Bing, Y., Sahul, M., Eds.; Springer Nature: Singapore, 2024; pp. 149–162. [Google Scholar] [CrossRef]
- Korniienko, V.; Husak, Y.; Diedkova, K.; Varava, Y.; Grebnevs, V.; Pogorielova, O.; Bērtiņš, M.; Korniienko, V.; Zandersone, B.; Ramanaviciene, A.; et al. Antibacterial Potential and Biocompatibility of Chitosan/Polycaprolactone Nanofibrous Membranes Incorporated with Silver Nanoparticles. Polymers 2024, 16, 1729. [Google Scholar] [CrossRef] [PubMed]
- Korniienko, V.; Husak, Y.; Yanovska, A.; Banasiuk, R.; Yusupova, A.; Savchenko, A.; Holubnycha, V.; Pogorielov, M. Functional and biological characterization of chitosan electrospun nanofibrous membrane nucleated with silver nanoparticles. Appl. Nanosci. 2022, 12, 1061–1070. [Google Scholar] [CrossRef]
- Pernakov, M.; Ermini, M.L.; Sulaieva, O.; Cassano, D.; Santucci, M.; Husak, Y.; Korniienko, V.; Giannone, G.; Yusupova, A.; Liubchak, I.; et al. Complementary Effect of Non-Persistent Silver Nano-Architectures and Chlorhexidine on Infected Wound Healing. Biomedicines 2021, 9, 1215. [Google Scholar] [CrossRef]
- Chyzhma, R.; Piddubnyi, A.; Romaniuk, A.; Moskalenko, R. Case report: Metastasis of Merkel cell carcinoma in the small intestine. Pathol. Res. Pract. 2023, 248, 154594. [Google Scholar] [CrossRef]
- Skibniewski, M.; Skibniewska, E.M.; Kośla, T.; Olbrych, K. Relationship between Cd and Zn concentration in the kidneys, liver, and muscles of moose (Alces alces) from north-eastern Poland. Environ. Sci. Pollut. Res. Int. 2017, 24, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Saylor, J.; Ma, Z.; Goodridge, H.S.; Huang, F.; Cress, A.E.; Pandol, S.J.; Shiao, S.L.; Vidal, A.C.; Wu, L.; Nickols, N.G.; et al. Spatial Mapping of Myeloid Cells and Macrophages by Multiplexed Tissue Staining. Front. Immunol. 2018, 9, 2925. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a Therapeutic Target to Promote Diabetic Wound Healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef]
- Kolomiiets, O.; Moskalenko, R. Immunohistochemical Study of M1 and M2 Macrophages in Breast Cancer with Microcalcifications. East. Ukr. Med. J. 2023, 11, 155–163. [Google Scholar] [CrossRef]
- Gauchotte, G.; Bochnakian, A.; Campoli, P.; Lardenois, E.; Brix, M.; Simon, E.; Colomb, S.; Martrille, L.; Peyron, P.A. Myeloperoxydase and CD15 with Glycophorin C Double Staining in the Evaluation of Skin Wound Vitality in Forensic Practice. Front. Med. 2022, 9, 910093. [Google Scholar] [CrossRef]
- Neville, S.L.; Cunningham, B.A.; Maunders, E.A.; Tan, A.; Watts, J.A.; Ganio, K.; Eijkelkamp, B.A.; Pederick, V.G.; Gonzalez de Vega, R.; Clases, D.; et al. Host-Mediated Copper Stress Is Not Protective against Streptococcus pneumoniae D39 Infection. Microbiol. Spectr. 2022, 10, e02495-22. [Google Scholar] [CrossRef]
- Jończy, A.; Mazgaj, R.; Smuda, E.; Żelazowska, B.; Kopeć, Z.; Starzyński, R.R.; Lipiński, P. The Role of Copper in the Regulation of Ferroportin Expression in Macrophages. Cells 2021, 10, 2259. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in Research of Chitosan Chemical Modification Technologies and Their Applications. Mar. Drugs 2022, 20, 536. [Google Scholar] [CrossRef] [PubMed]
- Weißpflog, J.; Gündel, A.; Vehlow, D.; Steinbach, C.; Müller, M.; Boldt, R.; Schwarz, S.; Schwarz, D. Solubility and Selectivity Effects of the Anion on the Adsorption of Different Heavy Metal Ions onto Chitosan. Molecules 2020, 25, 2482. [Google Scholar] [CrossRef]
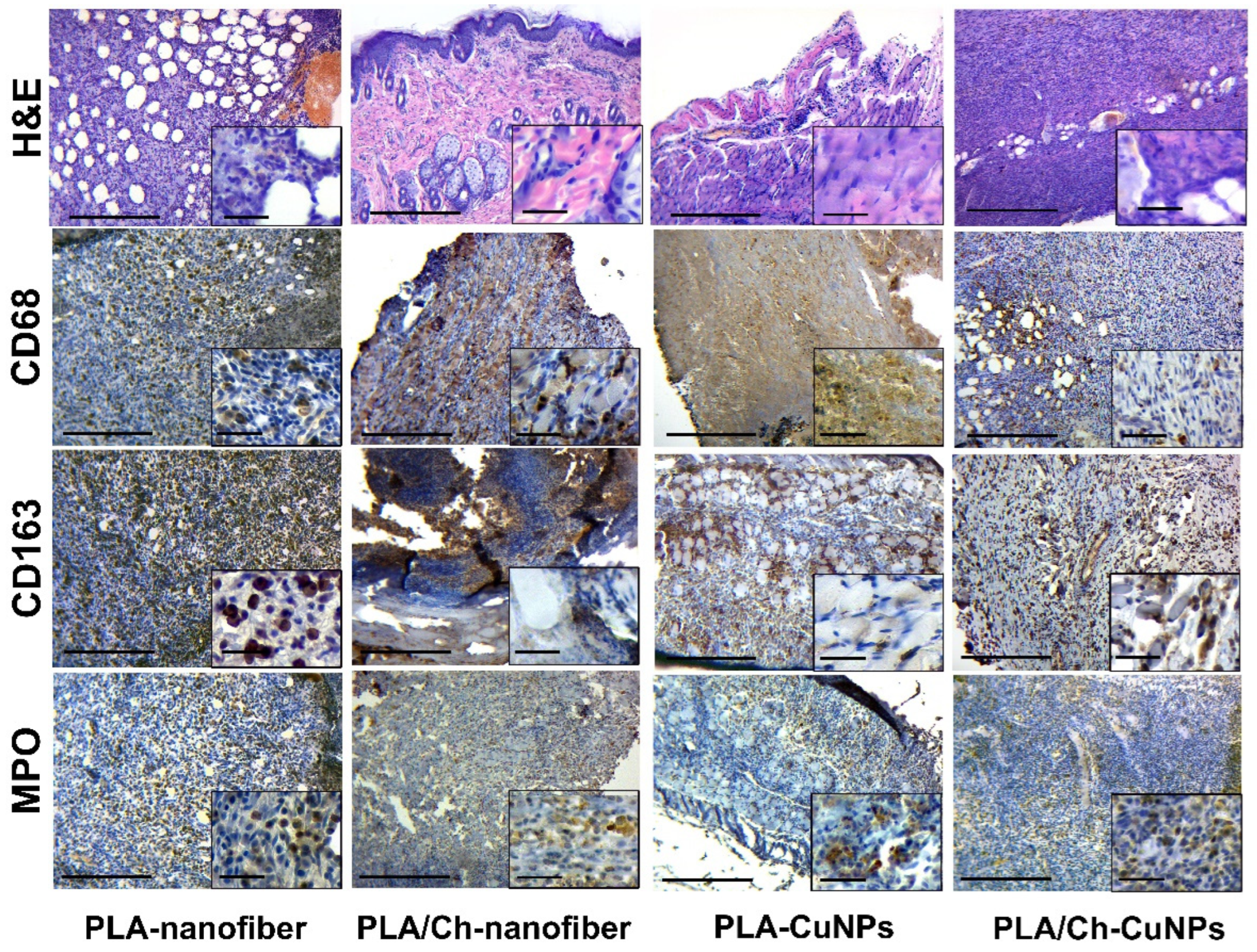
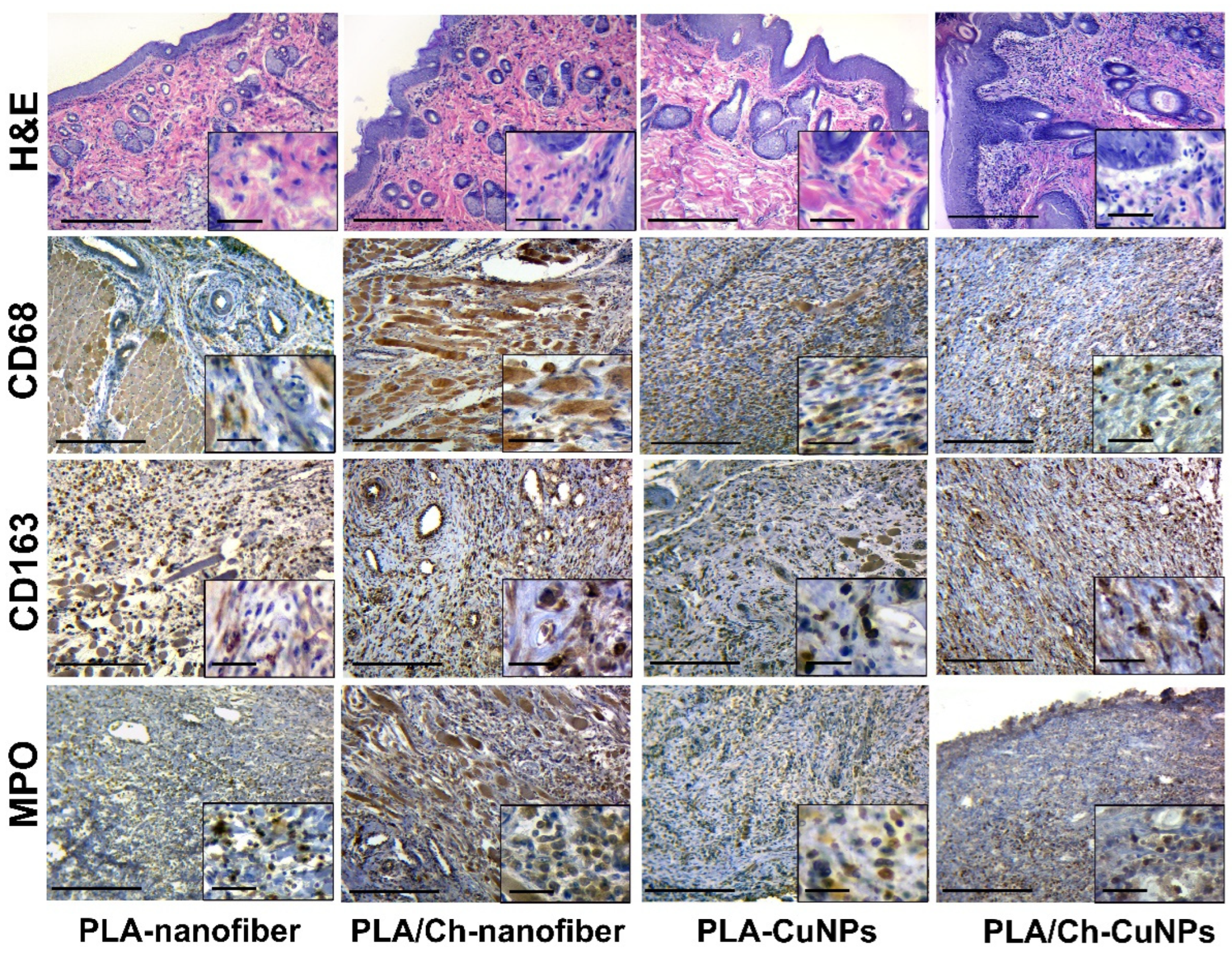
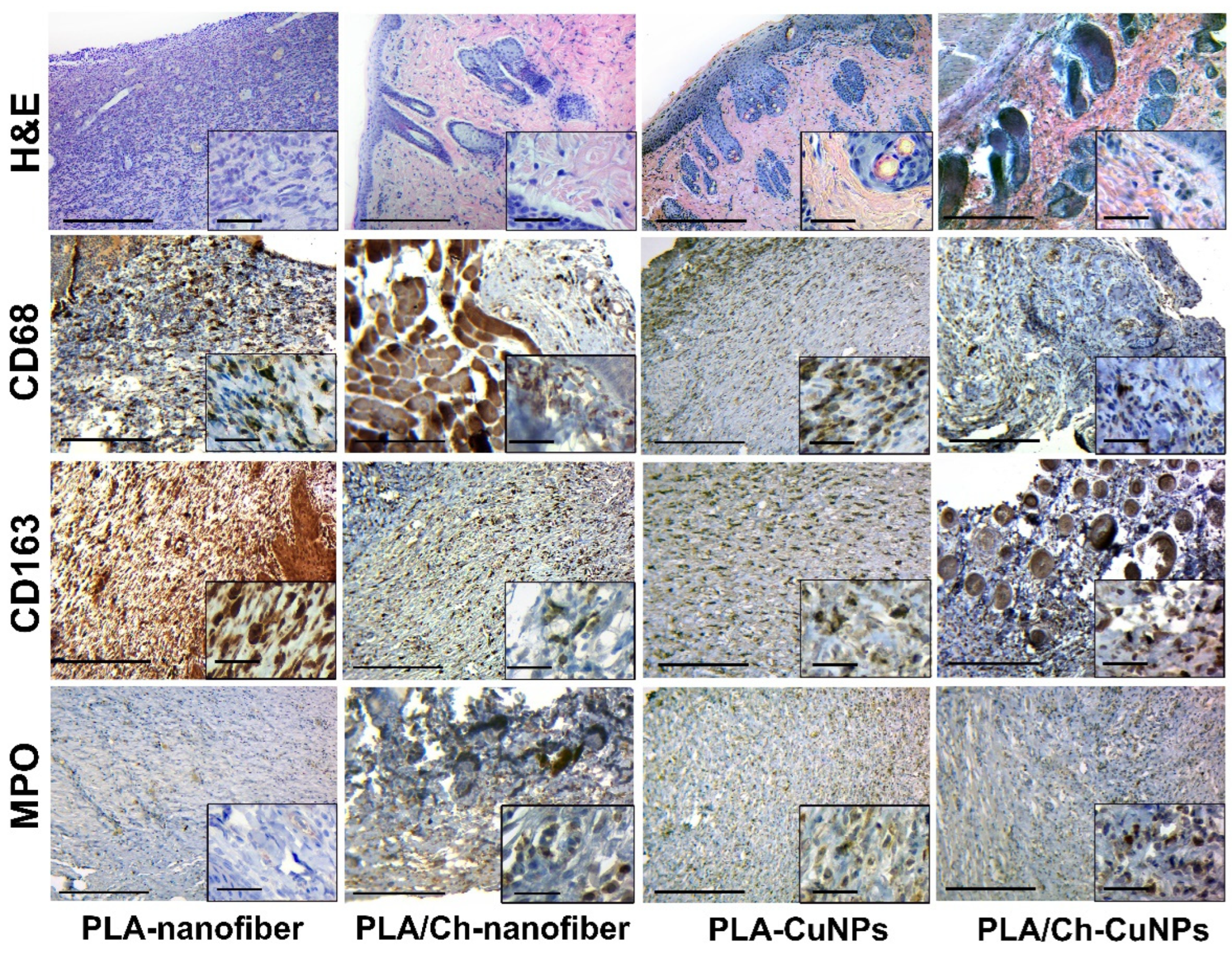
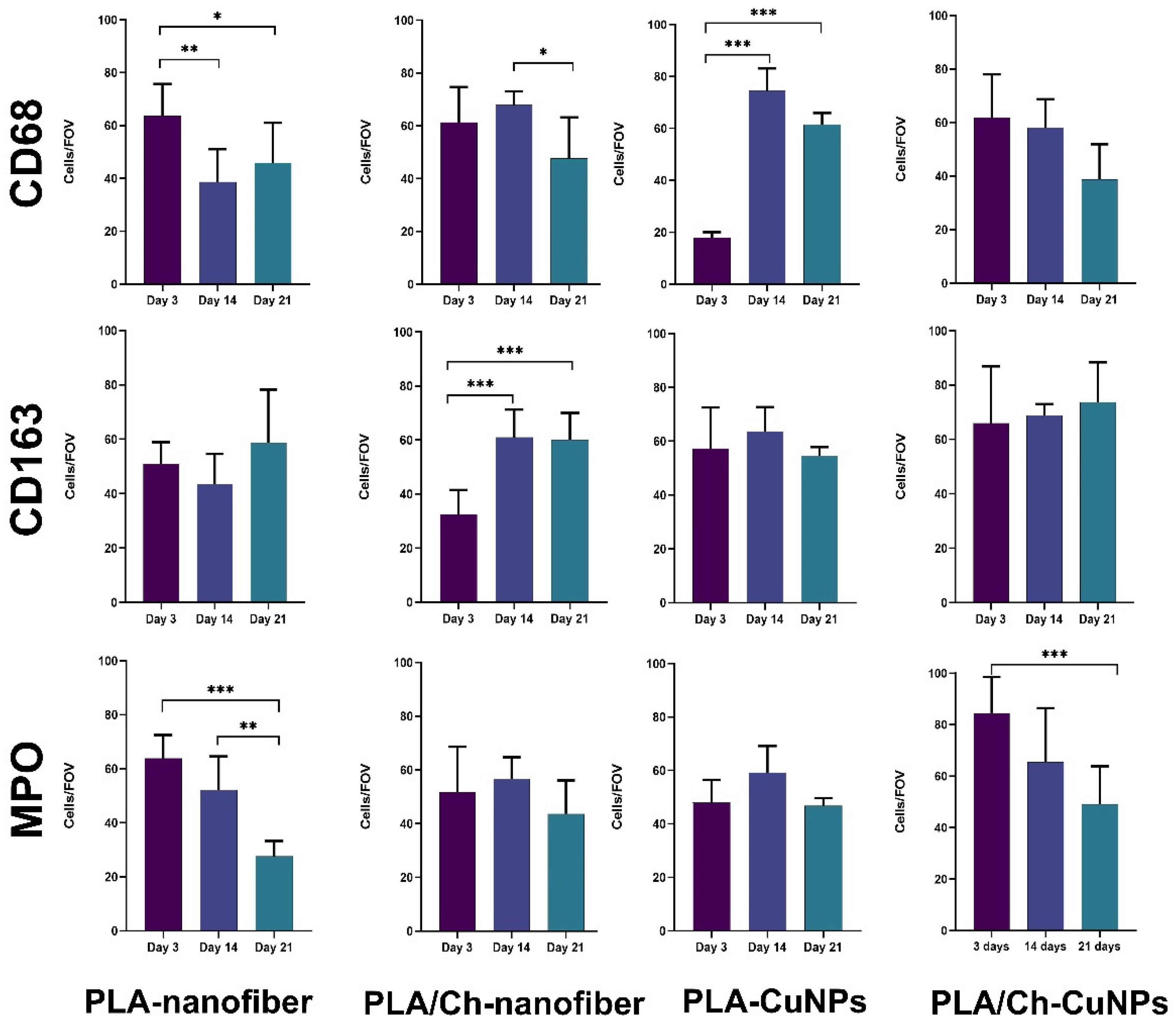
| Group Number | Dressing Material | Concentration of CuNPs, µg/mL |
|---|---|---|
| 1 | PLA-nanofiber | 0 |
| 2 | PLA/Ch-nanofiber | 0 |
| 3 | PLA-CuNPs | 100 |
| 4 | PLA/Ch-CuNPs | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butsyk, A.; Varava, Y.; Moskalenko, R.; Husak, Y.; Piddubnyi, A.; Denysenko, A.; Korniienko, V.; Ramanaviciute, A.; Banasiuk, R.; Pogorielov, M.; et al. Copper Nanoparticle Loaded Electrospun Patches for Infected Wound Treatment: From Development to In-Vivo Application. Polymers 2024, 16, 2733. https://doi.org/10.3390/polym16192733
Butsyk A, Varava Y, Moskalenko R, Husak Y, Piddubnyi A, Denysenko A, Korniienko V, Ramanaviciute A, Banasiuk R, Pogorielov M, et al. Copper Nanoparticle Loaded Electrospun Patches for Infected Wound Treatment: From Development to In-Vivo Application. Polymers. 2024; 16(19):2733. https://doi.org/10.3390/polym16192733
Chicago/Turabian StyleButsyk, Anna, Yulia Varava, Roman Moskalenko, Yevheniia Husak, Artem Piddubnyi, Anastasiia Denysenko, Valeriia Korniienko, Agne Ramanaviciute, Rafal Banasiuk, Maksym Pogorielov, and et al. 2024. "Copper Nanoparticle Loaded Electrospun Patches for Infected Wound Treatment: From Development to In-Vivo Application" Polymers 16, no. 19: 2733. https://doi.org/10.3390/polym16192733
APA StyleButsyk, A., Varava, Y., Moskalenko, R., Husak, Y., Piddubnyi, A., Denysenko, A., Korniienko, V., Ramanaviciute, A., Banasiuk, R., Pogorielov, M., Ramanavicius, A., & Korniienko, V. (2024). Copper Nanoparticle Loaded Electrospun Patches for Infected Wound Treatment: From Development to In-Vivo Application. Polymers, 16(19), 2733. https://doi.org/10.3390/polym16192733







