Aromatic Biobased Polymeric Materials Using Plant Polyphenols as Sustainable Alternative Raw Materials: A Review
Abstract
1. Introduction
2. Plant Polyphenol Chemistry
2.1. Historical Outline
2.2. Classification and Structure
2.2.1. Hydrolysable Tannins
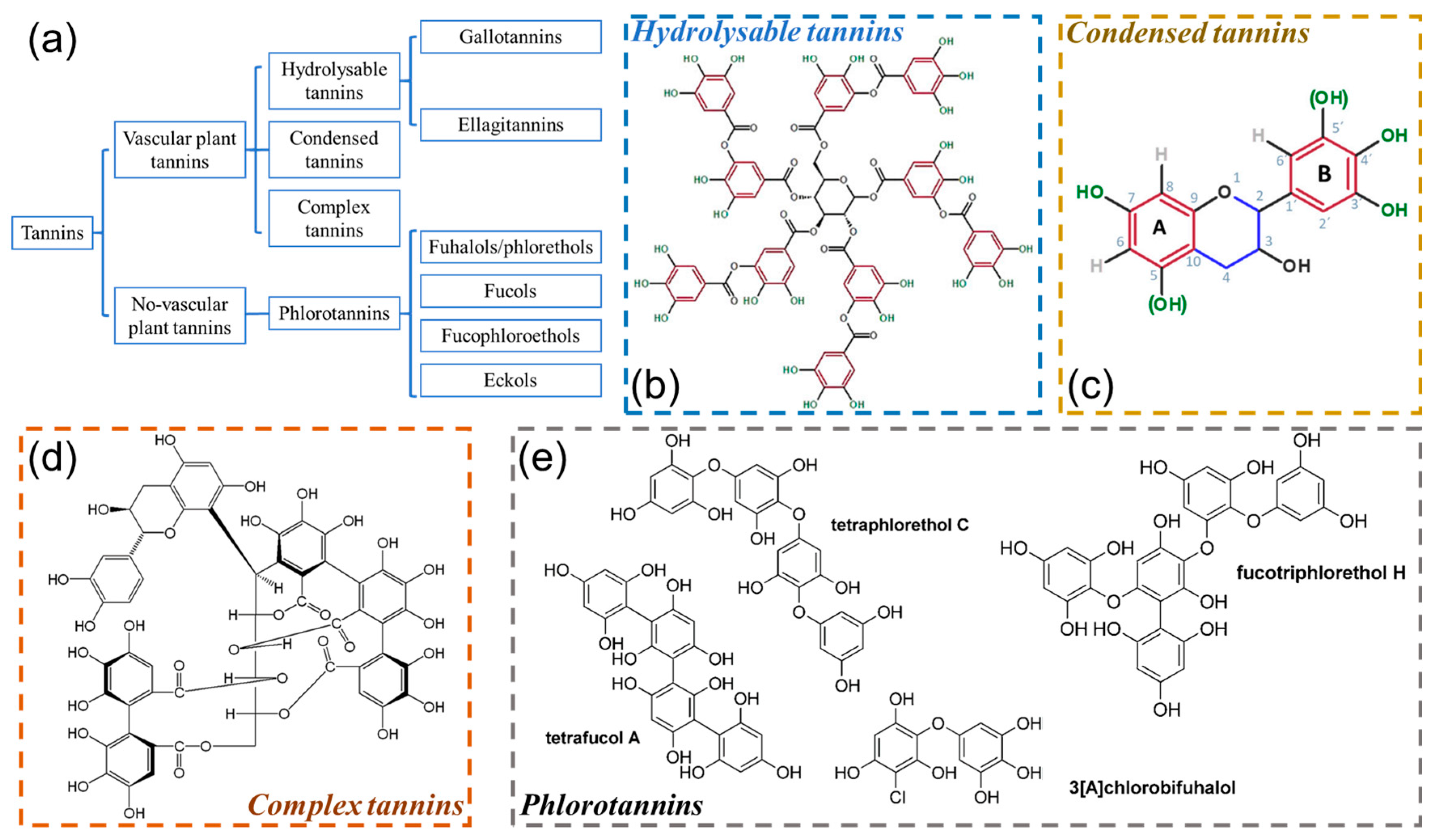
2.2.2. Condensed Tannins
2.2.3. Complex Tannins
2.2.4. Phlorotannins
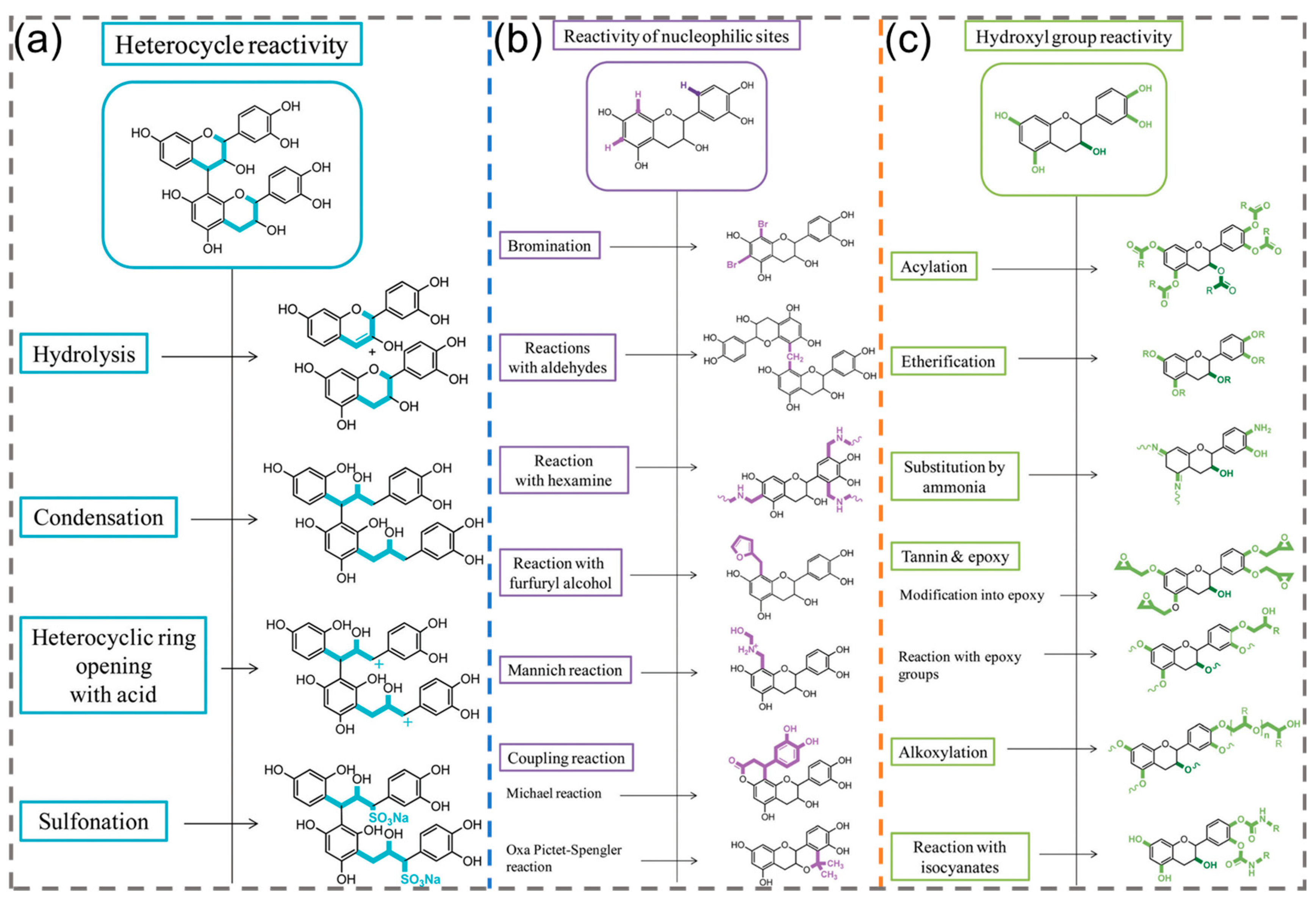
2.3. Reactivity of Plant Polyphenols
3. Toward Plant Polyphenol-Derived Materials
3.1. Materials Based on Polyurethane
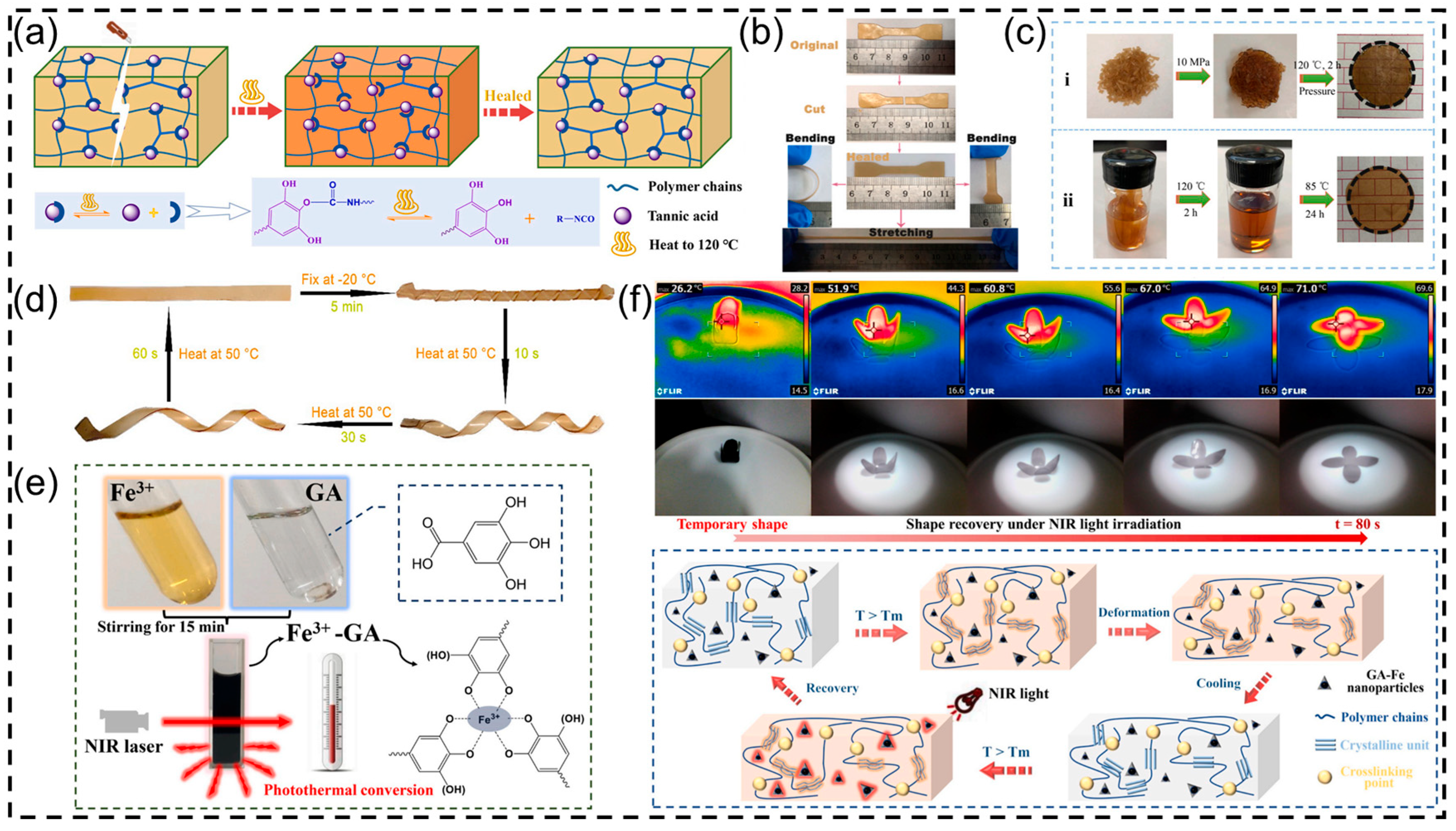
3.1.1. Polyurethane Films from Plant Polyphenols
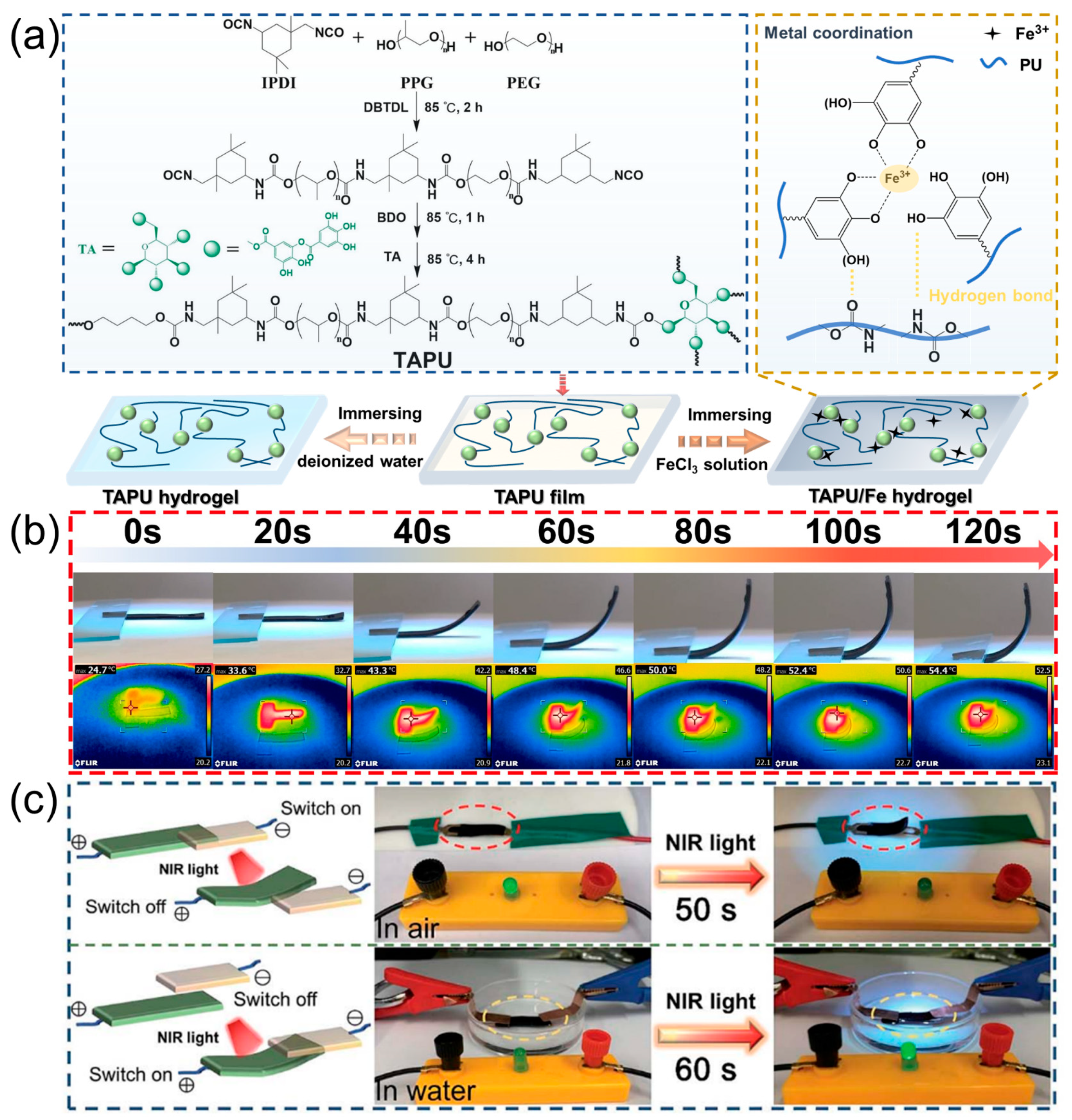
3.1.2. Polyurethane Hydrogels from Plant Polyphenols
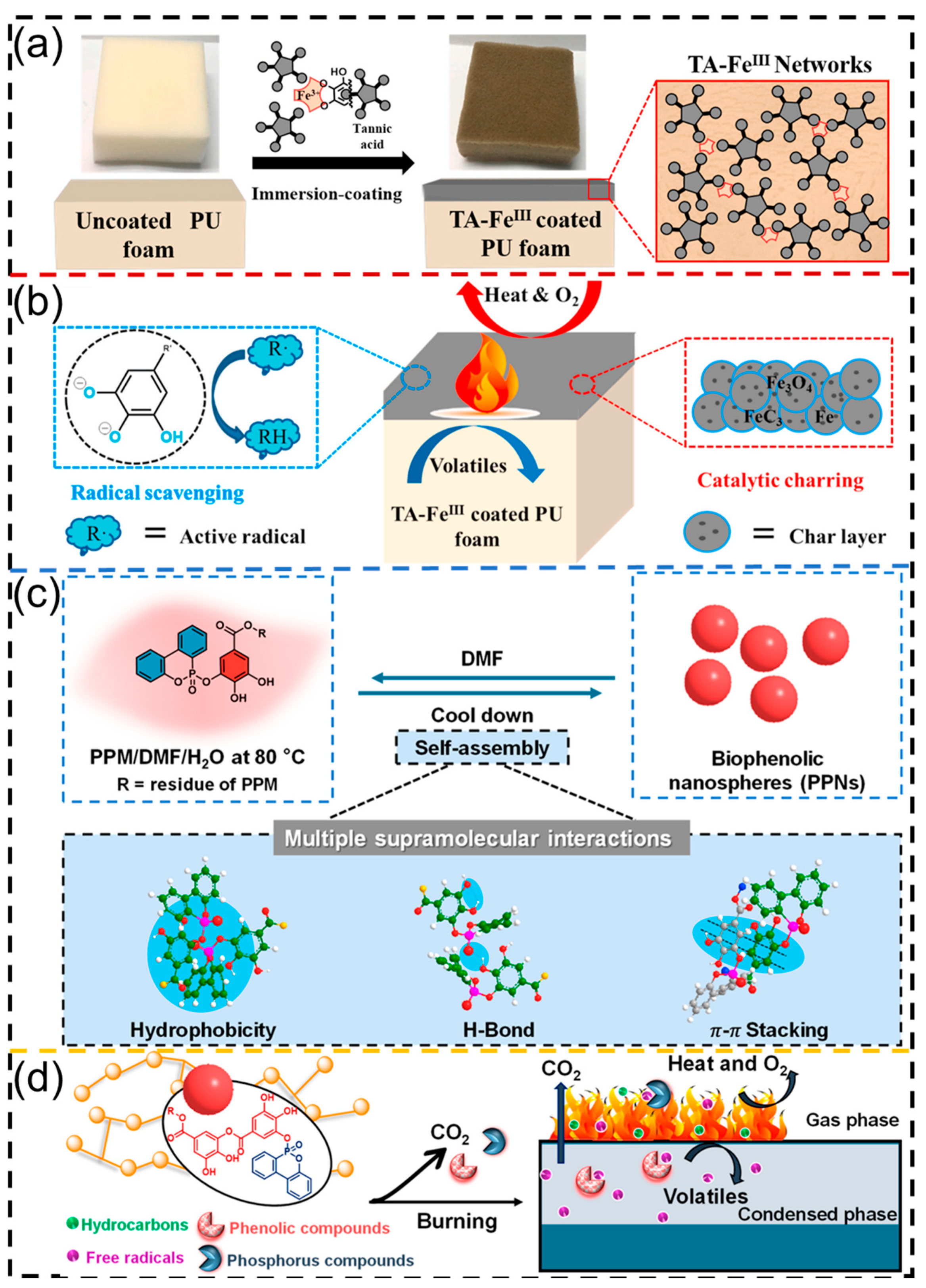
3.1.3. Polyurethane Foams from Plant Polyphenols
3.2. Materials Based on Epoxy Resin
3.3. Materials Based on Phenol-Aldehyde Polymer
3.4. Materials Based on Polyester
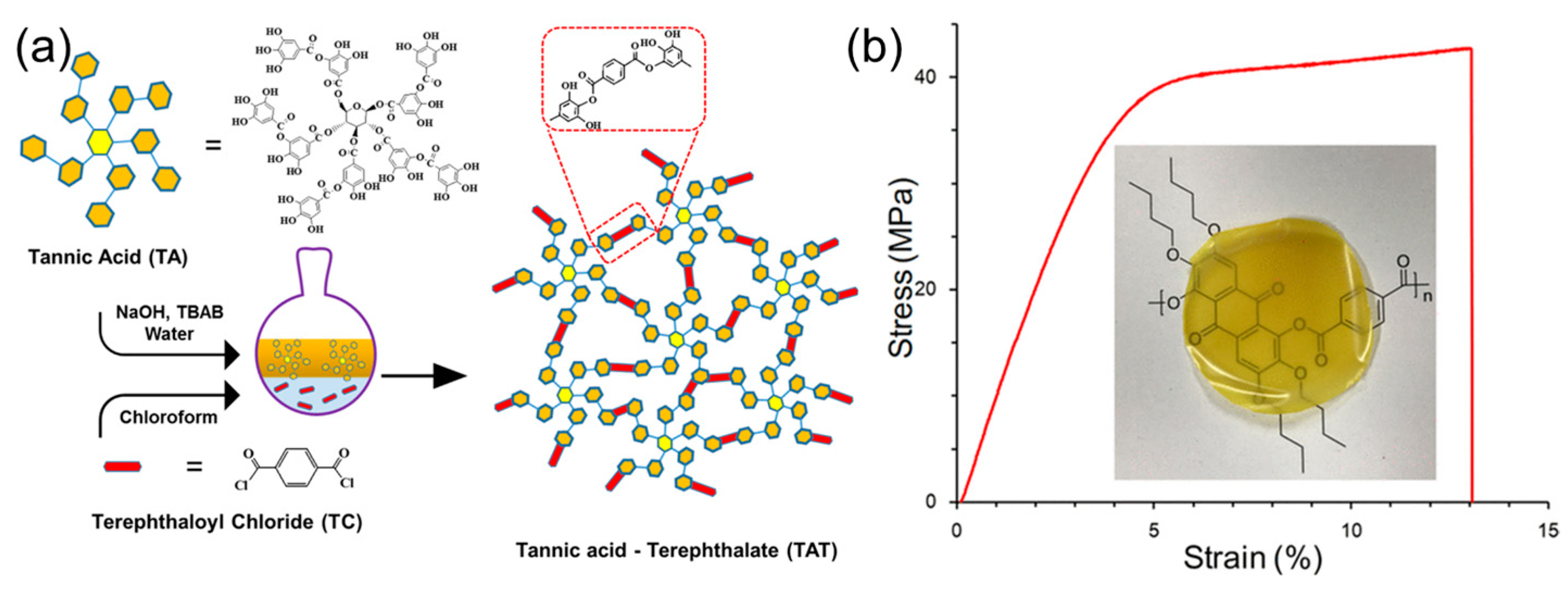
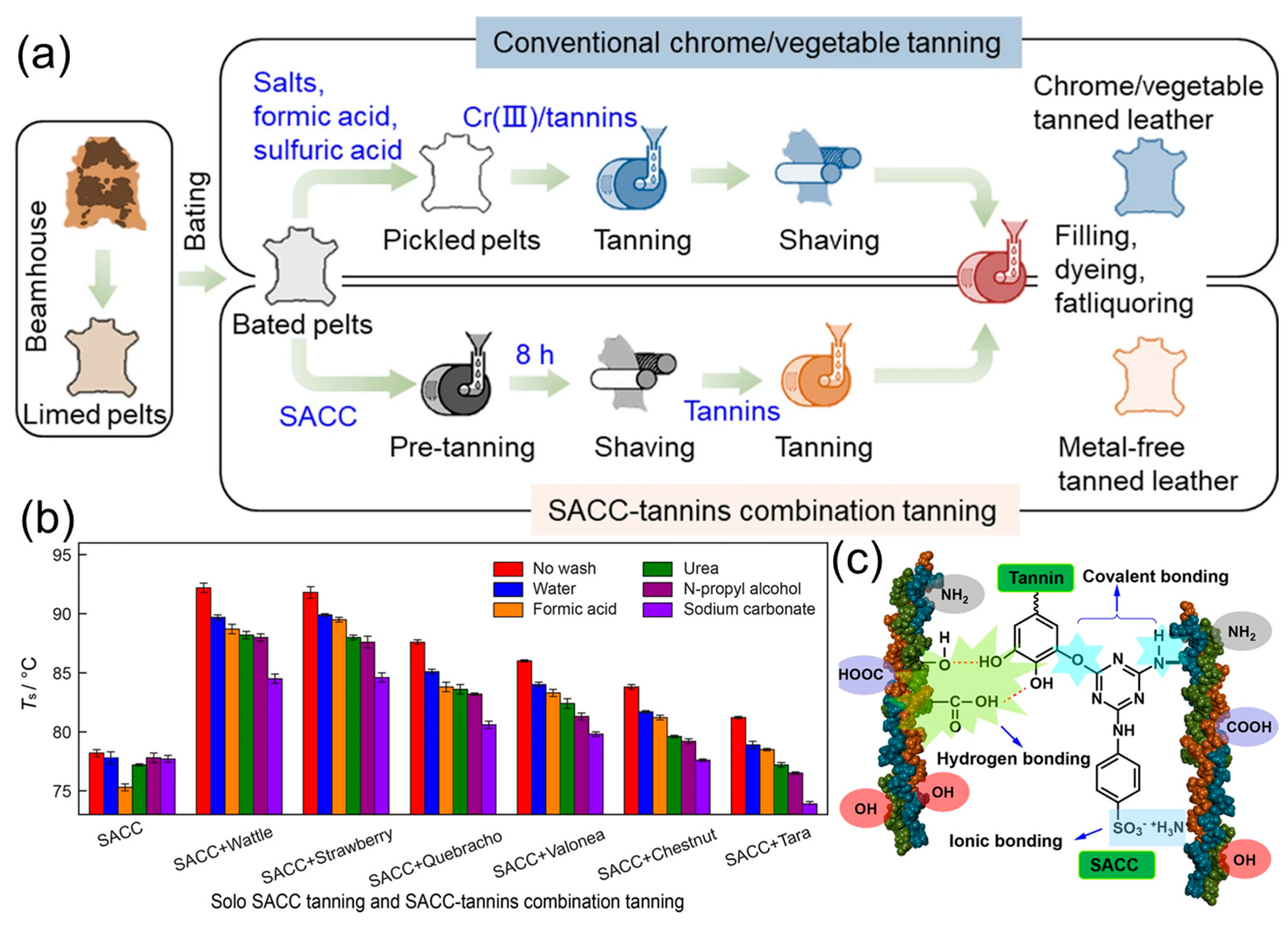
3.5. Materials Based on Leather
3.6. Materials Based on Hydrogel
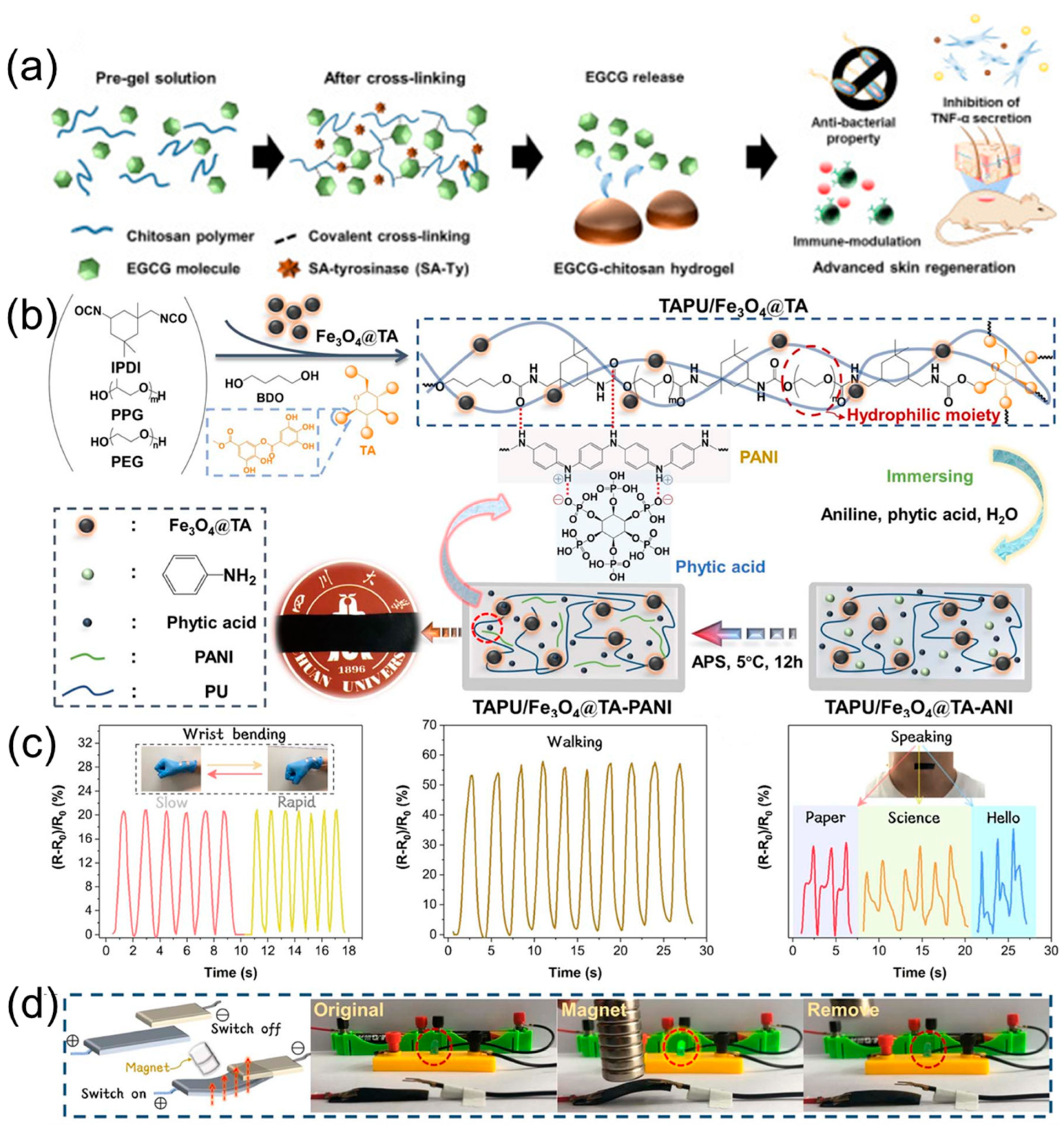
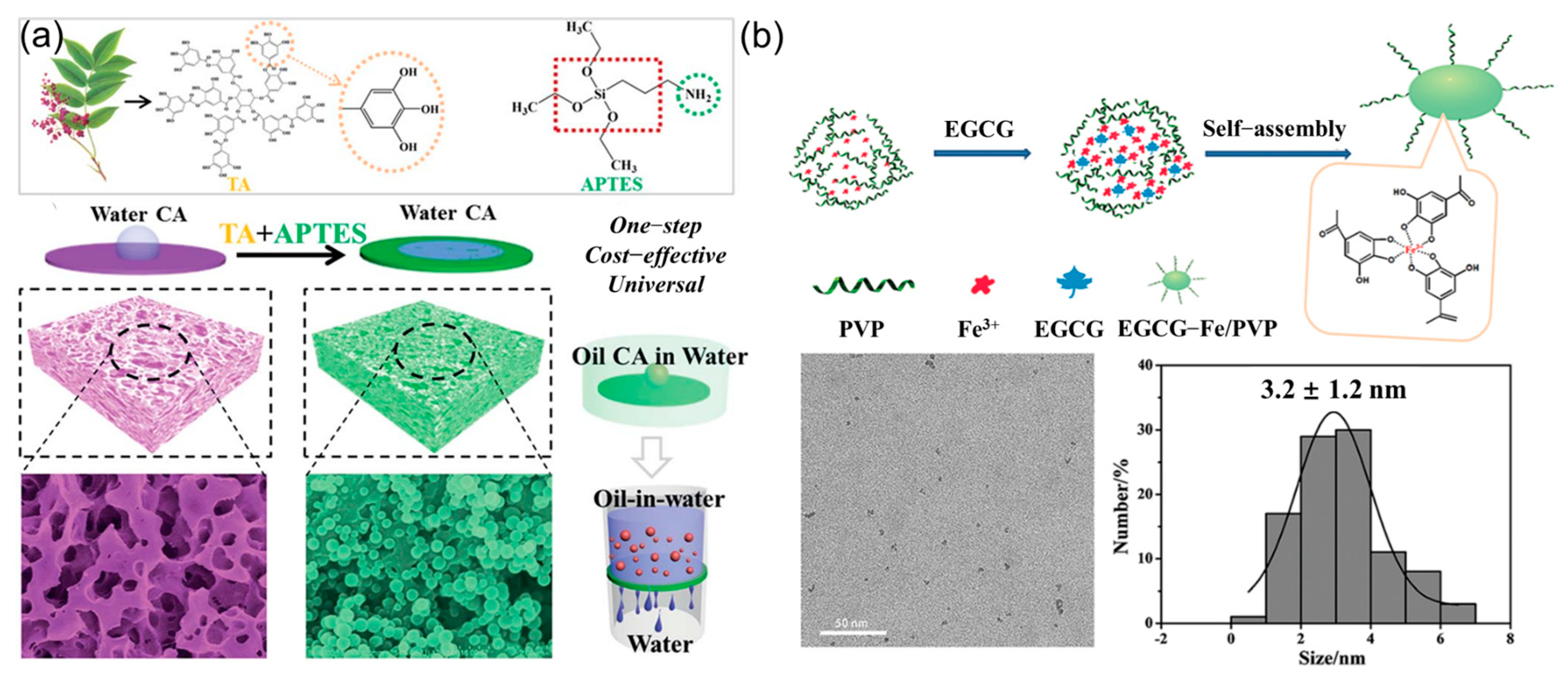
3.7. Materials Based on Nanoparticles
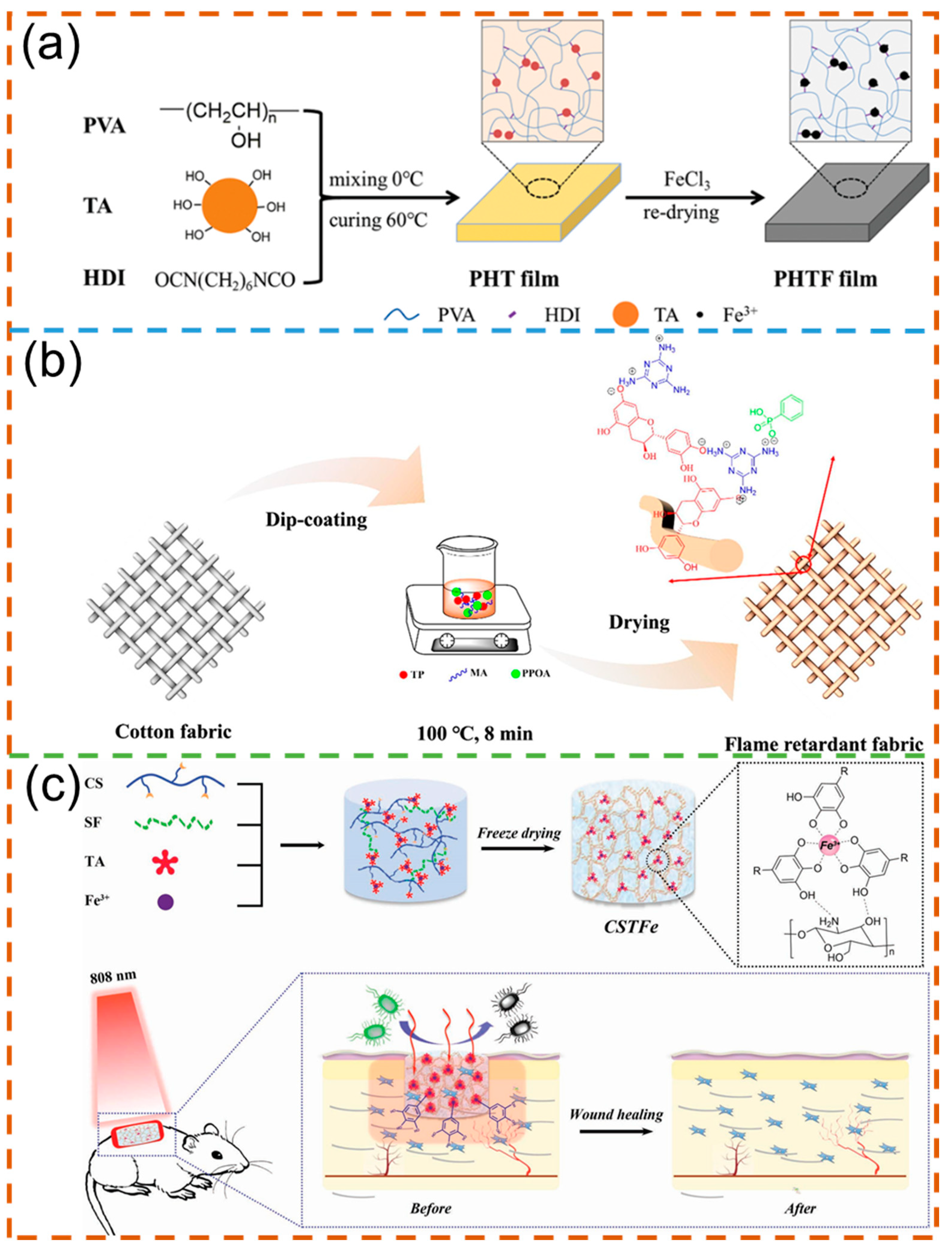
3.8. Other Functional Materials
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Hernes, P.J.; Hedges, J.I. Tannin signatures of barks, needles, leaves, cones, and wood at the molecular level11Associate editor: C. Arnosti. Geochim. Cosmochim. Acta 2004, 68, 1293–1307. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Yang, T.; Sun, B. High-speed countercurrent chromatography as an efficient technique for large separation of plant polyphenols: A review. Food Res. Int. 2022, 153, 110956. [Google Scholar] [CrossRef]
- Vera, M.; Silva, C.; Li, N.-N.; García, Y.; Jiménez, V.A.; Urbano, B.F. Laccase-mediated polymerization of tannins from a pine bark extract: Toward an eco-friendly valorization of forest wastes. J. Appl. Polym. Sci. 2024, 141, e55437. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Tondi, G.; Thevenon, M.F.; Mies, B.; Standfest, G.; Petutschnigg, A.; Wieland, S. Impregnation of Scots pine and beech with tannin solutions: Effect of viscosity and wood anatomy in wood infiltration. Wood Sci. Technol. 2013, 47, 615–626. [Google Scholar] [CrossRef]
- Fairhead, V.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biol. 2005, 28, 680–686. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E.A. The occurrence and cellular significance of physodes in brown algae. Phycologia 2002, 41, 125–139. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Bacelo, H.A.M.; Santos, S.C.R.; Botelho, C.M.S. Tannin-based biosorbents for environmental applications-A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier-El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Ebrahimnezhad-Khaljiri, H.; Ghadi, A. Recent advancement in synthesizing bio-epoxy nanocomposites using lignin, plant oils, saccharides, polyphenols, and natural rubbers: A review. Int. J. Biol. Macromol. 2024, 256, 128041. [Google Scholar] [CrossRef]
- Vera, M.; Urbano, B.F. Tannin polymerization: An overview. Polym. Chem. 2021, 12, 4272–4290. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M.E.M. Vegetable Tannins Used in the Manufacture of Historic Leathers. Molecules 2018, 23, 1081. [Google Scholar] [CrossRef]
- Qiao, D.-W.; Yao, J.; Song, L.-J.; Yang, J.-Y. Migration of leather tannins and chromium in soils under the effect of simulated rain. Chemosphere 2021, 284, 131413. [Google Scholar] [CrossRef]
- Vuthiganond, N.; Nakpathom, M.; Mongkholrattanasit, R. Azoic Deep Dyeing of Silk and UV Protection Using Plant Polyphenols and Diazonium Coupling. Fibers Polym. 2020, 21, 1052–1060. [Google Scholar] [CrossRef]
- Lund, M.N. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Senusi, F.; Nasuha, N.; Husain, A.; Ismail, S. Synthesis of catechol-amine coating solution for membrane surface modification. Environ. Sci. Pollut. Res. 2022, 30, 124585–124595. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-Y.; Chen, Y.-Q.; Wang, Z.; Wei, J. Synthesis of mesoporous carbon materials from renewable plant polyphenols for environmental and energy applications. New Carbon Mater. 2022, 37, 196–222. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, F.; Li, X.-S.; Qu, D.; Xu, Y.-L. A systematic review of tea pigments: Prevention of major diseases, protection of organs, and potential mechanisms and applications. Food Sci. Nutr. 2023, 11, 6830–6844. [Google Scholar] [CrossRef]
- Peng, H.; Du, X.; Cheng, X.; Wang, H.; Du, Z. Room-temperature self-healable and stretchable waterborne polyurethane film fabricated via multiple hydrogen bonds. Prog. Org. Coat. 2021, 151, 106081. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Lei, H.; Zhou, X.; Du, G.; Pizzi, A.; Xi, X. Preparation and fire resistance modification on tannin-based non-isocyanate polyurethane (NIPU) rigid foams. Int. J. Biol. Macromol. 2024, 258, 128994. [Google Scholar] [CrossRef]
- Ren, L.; Ma, X.; Zhang, J.; Qiang, T. Preparation of gallic acid modified waterborne polyurethane made from bio-based polyol. Polymer 2020, 194, 122370. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, J.; Zhang, X.; Fan, H.; Zhang, J.; Hu, D.; Jin, P.; Wang, D.-Y. Epoxy thermosets and materials derived from bio-based monomeric phenols: Transformations and performances. Prog. Polym. Sci. 2020, 108, 101287. [Google Scholar] [CrossRef]
- Ristić, M.; Samaržija-Jovanović, S.; Jovanović, V.; Kostić, M.; Erceg, T.; Jovanović, T.; Marković, G.; Marinović-Cincović, M. Hydrolytic and thermal stability of urea-formaldehyde resins based on tannin and betaine bio-fillers. J. Vinyl Addit. Technol. 2023, 29, 1082–1092. [Google Scholar] [CrossRef]
- Santiago-Medina, F.-J.; Pizzi, A.; Basso, M.C.; Delmotte, L.; Celzard, A. Polycondensation Resins by Flavonoid Tannins Reaction with Amines. Polymers 2017, 9, 37. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, S.-Y.; Qu, Y.; Yang, B.; Zhang, Q.; Lin, Y.; Lu, G.-P. Modified Gallic Acids as Both Reactive Flame Retardants and Cross-Linkers for the Fabrication of Flame-Retardant Polyurethane Elastomers. ChemistrySelect 2023, 8, e202302496. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, J.; Wang, F.; Wu, H.; Zhou, J.; Dai, Z.; Guo, H. Recent advances in membranes modified with plant polyphenols in wastewater treatment: A review. Sep. Purif. Technol. 2024, 334, 125861. [Google Scholar] [CrossRef]
- Meng, X.; Chen, L.; Lv, R.; Liu, M.; He, N.; Wang, Z. A metal–phenolic network-based multifunctional nanocomposite with pH-responsive ROS generation and drug release for synergistic chemodynamic/photothermal/chemo-therapy. J. Mater. Chem. B 2020, 8, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols- A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Mukherjee, A.G.; Gopalakrishnan, A.V.; Wanjari, U.R.; Kannampuzha, S.; Murali, R.; Veeraraghavan, V.P.; Vinayagam, S.; Paz-Montelongo, S.; George, A.; et al. Protective effects of macromolecular polyphenols, metals (zinc, selenium, and copper)—Polyphenol complexes, and different organs with an emphasis on arsenic poisoning: A review. Adv. Colloid Interface Sci. 2023, 253, 126715. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, J.; Yang, X.; Ke, Y.; Ou, R.; Wang, Y.; Madbouly, S.A.; Wang, Q. From plant phenols to novel bio-based polymers. Prog. Polym. Sci. 2022, 125, 101473. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Y.; Tong, Y.; Ding, W.; Li, W. Plant-inspired biomimetic hybrid PVDF membrane co-deposited by tea polyphenols and 3-amino-propyl-triethoxysilane for high-efficiency oil-in-water emulsion separation. J. Chem. Eng. 2023, 53, 170–180. [Google Scholar] [CrossRef]
- Xiao, S.; Wei, J.; Jin, S.; Xia, X.; Yuan, L.; Zou, Q.; Zuo, Y.; Li, J.; Li, Y. A Multifunctional Coating Strategy for Promotion of Immunomodulatory and Osteo/Angio-Genic Activity. Adv. Funct. Mater. 2023, 33, 2208968. [Google Scholar] [CrossRef]
- Nicollin, A.; Zhou, X.; Pizzi, A.; Grigsby, W.; Rode, K.; Delmotte, L. MALDI-TOF and 13C NMR analysis of a renewable resource additive-Thermoplastic acetylated tannins. Ind. Crops Prod. 2013, 49, 851–857. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Celzard, A. Nitrogen-doped carbon materials produced from hydrothermally treated tannin. Carbon 2012, 50, 5411–5420. [Google Scholar] [CrossRef]
- Hashida, K.; Makino, R.; Ohara, S. Amination of pyrogallol nucleus of condensed tannins and related polyphenols by ammonia water treatment. Holzforschung 2009, 63, 319–326. [Google Scholar] [CrossRef]
- Braghiroli, F.; Fierro, V.; Pizzi, A.; Rode, K.; Radke, W.; Delmotte, L.; Parmentier, J.; Celzard, A. Reaction of condensed tannins with ammonia. Ind. Crops Prod. 2013, 44, 330–335. [Google Scholar] [CrossRef]
- Soto, R.; Freer, J.; Baeza, J. Evidence of chemical reactions between di- and poly-glycidyl ether resins and tannins isolated from Pinus radiata D. Don bark. Bioresour. Technol. 2005, 96, 95–101. [Google Scholar] [CrossRef]
- Luo, S.; Yang, K.; Zhong, Z.; Wu, X.; Ren, T. Facile preparation of degradable multi-arm-star-branched waterborne polyurethane with bio-based tannic acid. RSC Adv. 2018, 8, 37765–37773. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for their Antioxidant Activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin complexation with metal ions and its implication on human health, environment and industry: An overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, Z.; Yang, C.; Ning, J.; Zhou, Z.; Li, G. Novel magnetic graphene oxide decorated with persimmon tannins for efficient adsorption of malachite green from aqueous solutions. Colloids Surf. A 2019, 566, 48–57. [Google Scholar] [CrossRef]
- Cao, H.; Yang, L.; Tian, R.; Wu, H.; Gu, Z.; Li, Y. Versatile polyphenolic platforms in regulating cell biology. Chem. Soc. Rev. 2022, 51, 4175–4198. [Google Scholar] [CrossRef]
- Yin, X.; Huang, Z.; Liu, X.; Sun, Y.; Lin, X.; Lin, W.; Yi, G. Room-temperature self-healing polyurethanes with high mechanical strength and superior toughness for sensor application. J. Appl. Polym. Sci. 2024, 141, e55917. [Google Scholar] [CrossRef]
- Li, X.-C.; Bi, Y.-S.; Bi, G.-H. A comparative study of polyurethane foam by substituting LBA using green polyurethane foam CFA-1. J. Appl. Polym. Sci. 2024, 141, e55177. [Google Scholar] [CrossRef]
- Seydibeyoǧlu, M.Ö.; İşçi, S.; Güngör, N.; Ece, O.I.; Güner, F.S. Preparation of polyurethane/hectorite, polyurethane/montmorillonite, and polyurethane/laponite nanocomposites without organic modifiers. J. Appl. Polym. Sci. 2010, 116, 832–837. [Google Scholar] [CrossRef]
- Gogoi, S.; Karak, N. Biobased Biodegradable Waterborne Hyperbranched Polyurethane as an Ecofriendly Sustainable Material. ACS Sustain. Chem. Eng. 2014, 2, 2730–2738. [Google Scholar] [CrossRef]
- Luo, S.; Fan, L.; Yang, K.; Zhong, Z.; Wu, X.; Ren, T. In situ and controllable synthesis of Ag NPs in tannic acid-based hyperbranched waterborne polyurethanes to prepare antibacterial polyurethanes/Ag NPs composites. RSC Adv. 2018, 8, 36571–36578. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Wang, J.; Xie, T.; Sun, L.; Yang, K.; Li, Z. Renewable tannic acid based self-healing polyurethane with dynamic phenol-carbamate network: Simultaneously showing robust mechanical properties, reprocessing ability and shape memory. Polymer 2021, 228, 123860. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Zhang, Z.; Wang, J.; Sun, L.; Xie, T. Thermal-driven self-healing waterborne polyurethane with robust mechanical properties based on reversible phenol-carbamate network and Fe3+-catechol coordination bond. Prog. Org. Coat. 2021, 153, 106153. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Fan, W.; Yang, K.; Li, Z. Preparation of renewable gallic acid-based self-healing waterborne polyurethane with dynamic phenol-carbamate network: Toward superior mechanical properties and shape memory function. J. Mater. Sci. 2022, 57, 5679–5696. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Yang, K.; Chen, D.; Li, Z. Novel near-infrared light-induced shape memory nonionic waterborne polyurethane composites based on iron gallate and dynamic phenol-carbamate network. Polymer 2022, 247, 124749. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, J.; Gao, F.; Du, X.; Du, Z.; Cheng, X.; Wang, H. Self-healable and recyclable polyurethane-polyaniline hydrogel toward flexible strain sensor. Compos. Part B 2021, 219, 108965. [Google Scholar] [CrossRef]
- Dalton, E.; Morris, Z.; Ayres, N. Synthesis and characterization of sulfated-lactose polyurethane hydrogels. Polym. Chem. 2022, 13, 2933–2940. [Google Scholar] [CrossRef]
- Divakaran, A.V.; Azad, L.B.; Surwase, S.S.; Torris A. T., A.; Badiger, M.V. Mechanically Tunable Curcumin Incorporated Polyurethane Hydrogels as Potential Biomaterials. Chem. Mater. 2016, 28, 2120–2130. [Google Scholar] [CrossRef]
- Wen, J.; Pan, M.; Yuan, J.; Wang, J.; Zhu, L.; Jia, Z.; Song, S. Facile fabrication of dual-crosslinked single network heterostructural polyurethane hydrogels with superior mechanical and fluorescent performance. React. Funct. Polym. 2020, 146, 104433. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Liang, Z.; Yong, Y.; Yang, C.; Li, Z. Multifunctional polyurethane hydrogel based on a phenol–carbamate network and an Fe3+–polyphenol coordination bond toward NIR light triggered actuators and strain sensors. J. Mater. Chem. A 2022, 10, 16928–16940. [Google Scholar] [CrossRef]
- Arbenz, A.; Frache, A.; Cuttica, F.; Avérous, L. Advanced biobased and rigid foams, based on urethane-modified isocyanurate from oxypropylated gambier tannin polyol. Polym. Degrad. Stab. 2016, 132, 62–68. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Nicolas, V.; Marie, Z.; Celzard, A.; Fierro, V. A Review of Rigid Polymeric Cellular Foams and Their Greener Tannin-Based Alternatives. Polymers 2022, 14, 3974. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Shi, X.; Cai, M.; Wu, R.; Wang, M. A novel biodegradable antimicrobial PU foam from wattle tannin. J. Appl. Polym. Sci. 2003, 90, 2756–2763. [Google Scholar] [CrossRef]
- Oliviero, M.; Stanzione, M.; D’Auria, M.; Sorrentino, L.; Iannace, S.; Verdolotti, L. Vegetable Tannin as a Sustainable UV Stabilizer for Polyurethane Foams. Polymers 2019, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, W.; Yang, P.; Hu, J.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Metal-phenolic network green flame retardants. Polymer 2021, 221, 123627. [Google Scholar] [CrossRef]
- Zeng, F.-R.; Liu, B.-W.; Wang, Z.-H.; Zhang, J.-Y.; Chen, X.-L.; Zhao, H.-B.; Wang, Y.-Z. Recyclable Biophenolic Nanospheres for Sustainable and Durable Multifunctional Applications in Thermosets. ACS Mater. Lett. 2023, 5, 1692–1702. [Google Scholar] [CrossRef]
- Guan, J.; Song, Y.; Lin, Y.; Yin, X.; Zuo, M.; Zhao, Y.; Tao, X.; Zheng, Q. Progress in Study of Non-Isocyanate Polyurethane. Ind. Eng. Chem. Res. 2011, 50, 6517–6527. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Dumarçay, S.; Gerardin, P.; Fredon, E.; Delmotte, L. Polyurethanes from hydrolysable tannins obtained without using isocyanates. Ind. Crops Prod. 2014, 59, 329–336. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Xi, X.; Pizzi, A.; Zhou, X.; Fredon, E.; Du, G.; Gerardin, C. Condensed tannin-glucose-based NIPU bio-foams of improved fire retardancy. Polym. Degrad. Stab. 2020, 175, 109121. [Google Scholar] [CrossRef]
- Sternberg, J.; Sequerth, O.; Pilla, S. Green chemistry design in polymers derived from lignin: Review and perspective. Prog. Polym. Sci. 2021, 113, 101344. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.; Ren, T.; Luo, L.; Li, D.; Dai, J.; Xu, Y.; Yuan, C.; Zeng, B.; Dai, L. Green construction of multi-functional fire resistant epoxy resins based on boron nitride with core-shell structure. Polym. Degrad. Stab. 2022, 203, 110059. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.-Y.; Jian, R.-K.; Liu, Z.; Huang, G. Chemical structure construction of DOPO-containing compounds for flame retardancy of epoxy resin: A review. Prog. Org. Coat. 2023, 175, 107316. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Chen, Y.; Li, W.; Chang, Q.; He, Z.; Zhu, Y.; Huang, J.; Nie, X. Construction of multiple sacrificial bonds between epoxy resin and tung oil-based modifier towards mechanical performance enhancement. Ind. Crops Prod. 2024, 210, 118091. [Google Scholar] [CrossRef]
- Rad, E.R.; Vahabi, H.; de Anda, A.R.; Saeb, M.R.; Thomas, S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coat. 2019, 135, 608–612. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins: A review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in Epoxy Resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- Gonçalves, F.A.M.M.; Ferreira, P.; Alves, P. Synthesis and characterization of itaconic-based epoxy resin: Chemical and thermal properties of partially biobased epoxy resins. Polymer 2021, 235, 124285. [Google Scholar] [CrossRef]
- Liang, D.; Yang, J.; Li, X.; Wang, W.; Qi, J.; Lu, Z. Preparation and physical properties of CeO2 doped and modified epoxy resin composites. Polym. Compos. 2024, 45, 5968–5979. [Google Scholar] [CrossRef]
- Brand, S.; Veith, L.; Baier, R.; Dietrich, C.; Schmid, M.J.; Ternes, T.A. New methodical approaches for the investigation of weathered epoxy resins used for corrosion protection of steel constructions. J. Hazard. Mater. 2020, 395, 122289. [Google Scholar] [CrossRef]
- Benyahya, S.; Aouf, C.; Caillol, S.; Boutevin, B.; Pascault, J.P.; Fulcrand, H. Functionalized green tea tannins as phenolic prepolymers for bio-based epoxy resins. Ind. Crops Prod. 2014, 53, 296–307. [Google Scholar] [CrossRef]
- Long, Y.; Han, W.; Xing, Z.; Hao, C. Synthesis of pyrogallol triglycidyl ether: A bio-based epoxy resin monomer with low viscosity, high activity, and good thermomechanical properties. Polymer 2024, 297, 126851. [Google Scholar] [CrossRef]
- Esmaeili, N.; Salimi, A.; Zohuriaan-Mehr, M.J.; Vafayan, M.; Meyer, W. Bio-based thermosetting epoxy foam: Tannic acid valorization toward dye-decontaminating and thermo-protecting applications. J. Hazard. Mater. 2018, 357, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-O.; Cho, J.; Yeo, H.; Lee, B.W.; Moon, B.J.; Ha, Y.-M.; Jo, Y.R.; Jung, Y.C. Flame Retardant Epoxy Derived from Tannic Acid as Biobased Hardener. ACS Sustain. Chem. Eng. 2019, 7, 3858–3865. [Google Scholar] [CrossRef]
- Feng, X.; Fan, J.; Li, A.; Li, G. Biobased Tannic Acid Cross-Linked Epoxy Thermosets with Hierarchical Molecular Structure and Tunable Properties: Damping, Shape Memory, and Recyclability. ACS Sustain. Chem. Eng. 2020, 8, 874–883. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, L.; Zhang, H.; Zhai, H.; Ren, H. Research status, industrial application demand and prospects of phenolic resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, W.; Zhang, S.; Gao, Q.; Xia, C.; Zhang, W.; Li, J. Depolymerization and characterization of Acacia mangium tannin for the preparation of mussel-inspired fast-curing tannin-based phenolic resins. Chem. Eng. J. 2019, 370, 420–431. [Google Scholar] [CrossRef]
- Hafiz, N.L.M.; Tahir, P.M.; Hua, L.S.; Abidin, Z.Z.; Sabaruddin, F.A.; Yunus, N.M.; Abdullah, U.H.; Abdul Khalil, H.P.S. Curing and thermal properties of co-polymerized tannin phenol-formaldehyde resin for bonding wood veneers. J. Mater. Res. Technol. 2020, 9, 6994–7001. [Google Scholar] [CrossRef]
- Santiago-Medina, F.; Foyer, G.; Pizzi, A.; Caillol, S.; Delmotte, L. Lignin-derived non-toxic aldehydes for ecofriendly tannin adhesives for wood panels. Int. J. Adhes. Adhes. 2016, 70, 239–248. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Laborie, M.P.; Garcia, D.; Celzard, A. Bioresourced pine tannin/furanic foams with glyoxal and glutaraldehyde. Ind. Crops Prod. 2013, 45, 401–405. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Stauber, M.; Celzard, A. Carbon meringues derived from flavonoid tannins. Carbon 2013, 65, 214–227. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Bridson, J.H.; Lomas, C.; Elliot, J.-A. Esterification of Condensed Tannins and Their Impact on the Properties of Poly(Lactic Acid). Polymers 2013, 5, 344–360. [Google Scholar] [CrossRef]
- Xia, Z.; Kiratitanavit, W.; Facendola, P.; Thota, S.; Yu, S.; Kumar, J.; Mosurkal, R.; Nagarajan, R. Fire resistant polyphenols based on chemical modification of bio-derived tannic acid. Polym. Degrad. Stab. 2018, 153, 227–243. [Google Scholar] [CrossRef]
- Song, P.; Jiang, S.; Ren, Y.; Zhang, X.; Qiao, T.; Song, X.; Liu, Q.; Chen, X. Synthesis and characterization of tannin grafted polycaprolactone. J. Colloid Interface Sci. 2016, 479, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Iwata, T.; Abe, H. Synthesis and Characterization of Biobased Polyesters Containing Anthraquinones Derived from Gallic Acid. Biomacromolecules 2019, 20, 318–325. [Google Scholar] [CrossRef]
- Gazzotti, S.; Hakkarainen, M.; Adolfsson, K.H.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. One-Pot Synthesis of Sustainable High-Performance Thermoset by Exploiting Eugenol Functionalized 1,3-Dioxolan-4-one. ACS Sustain. Chem. Eng. 2018, 6, 15201–15211. [Google Scholar] [CrossRef]
- Teklemedhin, T.B.; Gebretsadik, T.T.; Gebrehiwet, T.B.; Gebrekidan, G.A.; Edris, M.; Teklegiorgis, N.T.; Hagos, K.B. Vegetable Tannins as Chrome-Free Leather Tanning. Adv. Mater. Sci. Eng. 2023, 2023, 6220778. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, J.; Wang, C.; Zhang, J.; Radnaeva, V.D.; Lin, W. Sustainable metal-free leather manufacture via synergistic effects of triazine derivative and vegetable tannins. Collagen Leather 2023, 5, 2. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, J.; Xie, T.; Sun, L.; Li, Z. A chrome-free combination tanning strategy: Based on silicic acid and plant tannin. J. Leather Sci. Eng. 2021, 3, 15. [Google Scholar] [CrossRef]
- Marsal, A.; Cuadros, S.; Manich, A.M.; Izquierdo, F.; Font, J. Reduction of the formaldehyde content in leathers treated with formaldehyde resins by means of plant polyphenols. J. Clean. Prod. 2017, 148, 518–526. [Google Scholar] [CrossRef]
- Yu, R.; Wang, H.; Wang, R.; Zhao, P.; Chen, Y.; Liu, G.; Liao, X. Polyphenol modified natural collagen fibrous network towards sustainable and antibacterial microfiltration membrane for efficient water disinfection. Water Res. 2022, 218, 118469. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, J.; Li, H.; Zhong, R.; Zhuang, J.; Xu, X.; Wang, Y.; Liao, X.; Shi, B. Wearable synthetic leather-based high-performance X-ray shielding materials enabled by the plant polyphenol- and hierarchical structure-facilitated dispersion. Collagen Leather 2023, 5, 12. [Google Scholar] [CrossRef]
- Bahrami, Z.; Akbari, A.; Eftekhari-Sis, B. Double network hydrogel of sodium alginate/polyacrylamide cross-linked with POSS: Swelling, dye removal and mechanical properties. Int. J. Biol. Macromol. 2019, 129, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, C.; Zhou, J.; Hao, C.; Liu, W.; Liu, H.; Wang, J.; Huang, M.; He, S.; Yang, M. Rapid Photothermal Responsive Conductive MXene Nanocomposite Hydrogels for Soft Manipulators and Sensitive Strain Sensors. Macromol. Rapid Commun. 2021, 42, 2100499. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Y.; Sun, D.; Ren, W.; Shao, C.; Sun, R. Highly Conductive and Mechanically Robust Cellulose Nanocomposite Hydrogels with Antifreezing and Antidehydration Performances for Flexible Humidity Sensors. ACS Appl. Mater. Interfaces 2022, 14, 10886–10897. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Lin, T.; Gao, J.; Hu, Y.; Zhang, Z.; Peng, J.; Li, J.; Zhai, M. Mechanically Robust and Highly Conductive Poly(ionic liquid)/Polyacrylamide Double-Network Hydrogel Electrolytes for Flexible Symmetric Supercapacitors with a Wide Operating Voltage Range. ACS Appl. Mater. Interfaces 2024, 16, 12586–12598. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Liu, R.; Wang, H.; Wang, S.; Fan, B.; Hu, C.; Wang, H. Mussel-inspired PDA@PEDOT nanocomposite hydrogel with excellent mechanical strength, self-adhesive, and self-healing properties for a flexible strain sensor. J. Mater. Chem. B 2024, 12, 3092–3102. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, K.; Huang, J.; Sun, X.; Li, J.; Cheng, Y.; Sun, Y.; Shi, Y.; Pan, L. Mechanically Robust, Flexible, Fast Responding Temperature Sensor and High-Resolution Array with Ionically Conductive Double Cross-Linked Hydrogel. Adv. Funct. Mater. 2024, 34, 2314433. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, C.; Liu, W.; Liu, H.; Liu, J.; Hao, R.; Huang, M.; He, S. Two-Dimensional MoO2 Nanosheet Composite Hydrogels with High Transmittance and Excellent Photothermal Property for Near-Infrared Responsive Actuators and Microvalves. ACS Appl. Mater. Interfaces 2021, 13, 33404–33416. [Google Scholar] [CrossRef]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering multifunctional dynamic hydrogel for biomedical and tissue regenerative applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Q.; Cheng, Y.-F. Fabrication, characterization and application of chitosan/tea polyphenols blending hydrogels. Bull. Mater. Sci. 2022, 45, 161. [Google Scholar]
- Kim, B.S.; Kim, S.H.; Kim, K.; An, Y.H.; So, K.H.; Kim, B.G.; Hwang, N.S. Enzyme-mediated one-pot synthesis of hydrogel with the polyphenol cross-linker for skin regeneration. Mater. Today Bio 2020, 8, 100079. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yu, Z.; Chen, S.; Fei, L.; Sha, Q.; Zhou, N.; Chen, Z.; Xu, C. Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydr. Polym. 2020, 230, 115565. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhao, L.; Ling, Q.; Gu, H. Tough, Self-Adhesive, Antibacterial, and Recyclable Supramolecular Double Network Flexible Hydrogel Sensor Based on PVA/Chitosan/Cyclodextrin. Ind. Eng. Chem. Res. 2022, 61, 3620–3635. [Google Scholar] [CrossRef]
- Liu, J.; Bao, S.; Ling, Q.; Fan, X.; Gu, H. Ultra-fast preparation of multifunctional conductive hydrogels with high mechanical strength, self-healing and self-adhesive properties based on Tara Tannin-Fe3+ dynamic redox system for strain sensors applications. Polymer 2022, 240, 124513. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Zhang, C.; Liao, L. Robust Near-Infrared-Responsive Composite Hydrogel Actuator Using Fe3+/Tannic Acid as the Photothermal Transducer. ACS Appl. Mater. Interfaces 2021, 13, 18175–18183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Yang, X.; Li, F.; Liang, Z.; Yong, Y.; Dai, S.; Li, Z. A stretchable, environmentally stable, and mechanically robust nanocomposite polyurethane organohydrogel with anti-freezing, anti-dehydration, and electromagnetic shielding properties for strain sensors and magnetic actuators. J. Mater. Chem. A 2023, 11, 6603–6614. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Kong, F.; Yu, L. Design and preparation of poly(tannic acid) nanoparticles with intrinsic fluorescence: A sensitive detector of picric acid. Chem. Eng. J. 2021, 416, 129090. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, X.; Zhang, J.; Zhong, J.; Zhu, F.; Liu, X.; Gu, Z.; Li, Y. Natural polyphenol fluorescent polymer dots. Green Chem. 2021, 23, 1834–1839. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, J.; Hu, Y.; Lin, Z.; Ma, Y.; Richardson, J.J.; Caruso, F. Polyphenol-Based Nanoparticles for Intracellular Protein Delivery via Competing Supramolecular Interactions. ACS Nano 2020, 14, 12972–12981. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, S.; He, F.; Cao, M.; Peng, S.; Li, Y. One-step transformation of highly hydrophobic membranes into superhydrophilic and underwater superoleophobic ones for high-efficiency separation of oil-in-water emulsions. J. Mater. Chem. A 2018, 6, 3391–3396. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Wu, X.; Zhu, C. A dual-inhibitor system for the effective antifibrillation of Aβ40 peptides by biodegradable EGCG–Fe(iii)/PVP nanoparticles. J. Mater. Chem. B 2019, 7, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Cheng, M.; Wang, Y.; Wen, L.; Chen, L.; Li, Z.; Wu, Y.; Gao, M.; Chai, Z. pH-Responsive Fe(III)–Gallic Acid Nanoparticles for In Vivo Photoacoustic-Imaging-Guided Photothermal Therapy. Adv. Healthc. Mater. 2016, 5, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Yan, N.; Sheng, S.; Xu, C.; Tian, H.; Chen, X. Exploration of FeIII-Phenol Complexes for Photothermal Therapy and Photoacoustic Imaging. ACS Biomater. Sci. Eng. 2019, 5, 4700–4707. [Google Scholar] [CrossRef]
- Pan, T.G.; Wu, Y.N.; He, S.; Wu, Z.; Jin, R. Food allergenic protein conjugation with plant polyphenols for allergenicity reduction. Curr. Opin. Food Sci. 2022, 43, 36–42. [Google Scholar] [CrossRef]
- Feng, Y.; Li, P.; Wei, J. Engineering functional mesoporous materials from plant polyphenol based coordination polymers. Coord. Chem. Rev. 2022, 468, 214649. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(vinyl alcohol)-Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P.; et al. Soft Conducting Polymer Hydrogels Cross-Linked and Doped by Tannic Acid for Spinal Cord Injury Repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Biao, Y.; Yuxuan, C.; Qi, T.; Ziqi, Y.; Yourong, Z.; McClements, D.J.; Chongjiang, C. Enhanced performance and functionality of active edible films by incorporating tea polyphenols into thin calcium alginate hydrogels. Food Hydrocoll. 2019, 97, 105197. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, Y.; Zhang, X.; Tao, K.; Li, G. High-strength collagen/delphinidin film incorporated with Vaccinium oxycoccus pigment for active and intelligent food packaging. Collagen Leather 2023, 5, 11. [Google Scholar] [CrossRef]
- Deng, Z.; Yi, Z.; Chen, G.; Ma, X.; Tang, Y.; Li, X. Green tea polyphenol nanoparticle as a novel adsorbent to remove Pb2+ from wastewater. Mater. Lett. 2021, 284, 128986. [Google Scholar] [CrossRef]
- Bai, Y.; Ionov, L. A thermo-, near-infrared light- and water-induced shape memory polymer with healing fatigued shape memory performance. Mater. Chem. Front. 2022, 6, 1218–1227. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, P.; Liu, Y.; Zhu, P. Flame retardant cotton fabrics with anti-UV properties based on tea polyphenol-melamine-phenylphosphonic acid. J. Colloid Interface Sci. 2023, 629, 392–403. [Google Scholar] [CrossRef]
- Yu, Y.; Li, P.; Zhu, C.; Ning, N.; Zhang, S.; Vancso, G.J. Multifunctional and Recyclable Photothermally Responsive Cryogels as Efficient Platforms for Wound Healing. Adv. Funct. Mater. 2019, 29, 1904402. [Google Scholar] [CrossRef]
- Kohli, S.; Bhatia, S.; Banavar, S.R.; Al-Haddad, A.; Kandasamy, M.; Qasim, S.S.B.; Kit-Kay, M.; Pichika, M.R.; Daood, U. In-vitro evaluation of the effectiveness of polyphenols based strawberry extracts for dental bleaching. Sci. Rep. 2023, 13, 4181. [Google Scholar] [CrossRef]
- Kim, K.H.; Ki, M.-R.; Min, K.H.; Pack, S.P. Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy. Antioxidants 2023, 12, 1048. [Google Scholar] [CrossRef]
- Chen, G.; Yi, Z.; Chen, X.; Tong, Q.; Ran, Y.; Ma, L.; Li, X. Polymerization-Induced Self-Assembly of Tea Polyphenols into Open-Mouthed Nanoparticles for Active Delivery Systems and Stable Carbon Bowls. ACS Appl. Nano Mater. 2021, 4, 13510–13522. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Yan, J.; Yang, M. Engineering polyphenol-based polymeric nanoparticles for drug delivery and bioimaging. Chem. Eng. J. 2022, 439, 135661. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Li, S.; Lei, W.; Liu, Y.; Sommer, T.; Friederich, P.; Sobek, C.; Messersmith, P.B.; Levkin, P.A. High-throughput screening of multifunctional nanocoatings based on combinations of polyphenols and catecholamines. Mater. Today Bio 2021, 10, 100108. [Google Scholar] [CrossRef] [PubMed]
- Centurion, F.; Hassan, M.M.; Tang, J.; Allioux, F.-M.; Chakraborty, S.; Chen, R.; Mao, G.; Kumar, N.; Kalantar-Zadeh, K.; Rahim, M.A. Assembly of surface-independent polyphenol/liquid gallium composite nanocoatings. Nanoscale 2022, 14, 14760–14769. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Wang, Q.; Wang, T.; Zhou, Z.; Mehling, M.; Guo, T.; Zou, H.; Xiao, X.; He, Y.; et al. Flowthrough Capture of Microplastics through Polyphenol-Mediated Interfacial Interactions on Wood Sawdust. Adv. Mater. 2023, 35, 2301531. [Google Scholar] [CrossRef]
- Trisha, A.T.; Shakil, M.H.; Talukdar, S.; Rovina, K.; Huda, N.; Zzaman, W. Tea Polyphenols and Their Preventive Measures against Cancer: Current Trends and Directions. Foods 2022, 11, 3349. [Google Scholar] [CrossRef] [PubMed]
- Farghadani, R.; Naidu, R. The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, Q.; Kang, M.; Jiang, S.; Yang, F.; Gong, J.; Ou, G.; Wang, S. Regulation of main ncRNAs by polyphenols: A novel anticancer therapeutic approach. Phytomedicine 2023, 120, 155072. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, J.; Sun, Z. Aromatic Biobased Polymeric Materials Using Plant Polyphenols as Sustainable Alternative Raw Materials: A Review. Polymers 2024, 16, 2752. https://doi.org/10.3390/polym16192752
Liu Y, Wang J, Sun Z. Aromatic Biobased Polymeric Materials Using Plant Polyphenols as Sustainable Alternative Raw Materials: A Review. Polymers. 2024; 16(19):2752. https://doi.org/10.3390/polym16192752
Chicago/Turabian StyleLiu, Yang, Junsheng Wang, and Zhe Sun. 2024. "Aromatic Biobased Polymeric Materials Using Plant Polyphenols as Sustainable Alternative Raw Materials: A Review" Polymers 16, no. 19: 2752. https://doi.org/10.3390/polym16192752
APA StyleLiu, Y., Wang, J., & Sun, Z. (2024). Aromatic Biobased Polymeric Materials Using Plant Polyphenols as Sustainable Alternative Raw Materials: A Review. Polymers, 16(19), 2752. https://doi.org/10.3390/polym16192752







