Development of Poly(lactic acid)-Based Biocomposites with Silver Nanoparticles and Investigation of Their Characteristics
Abstract
1. Introduction
2. Materials and Methods
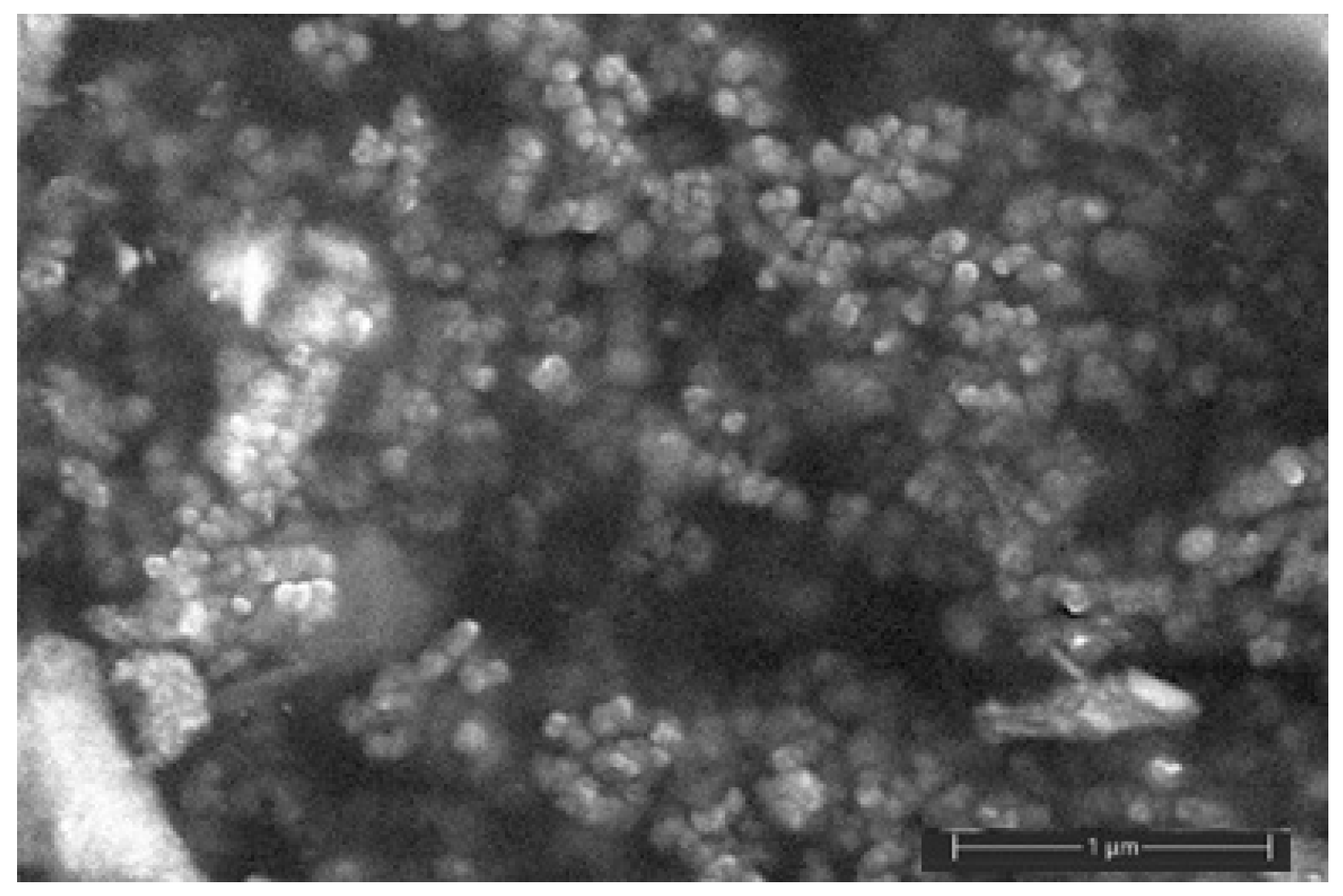
2.1. Synthesis of Silver Nanoparticles
2.2. Biocomposite Production
2.3. Pressing and Mechanical Tests
2.4. Water Absorption
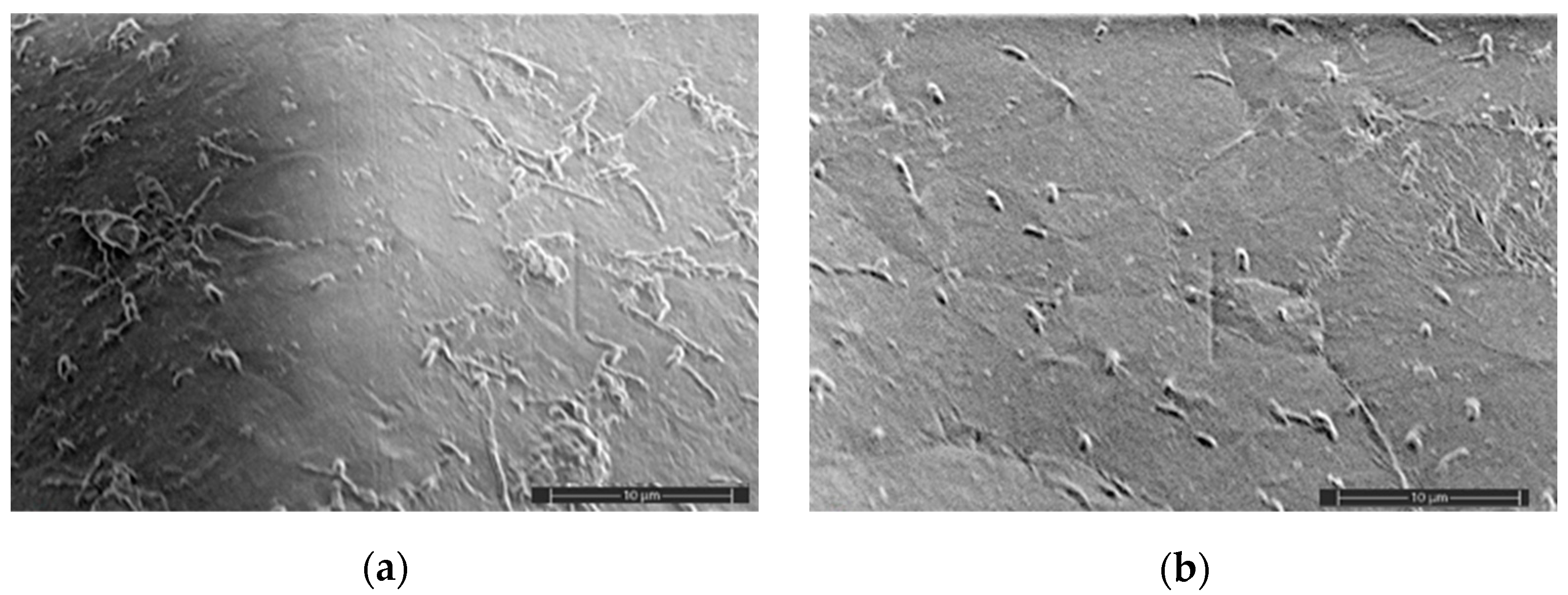
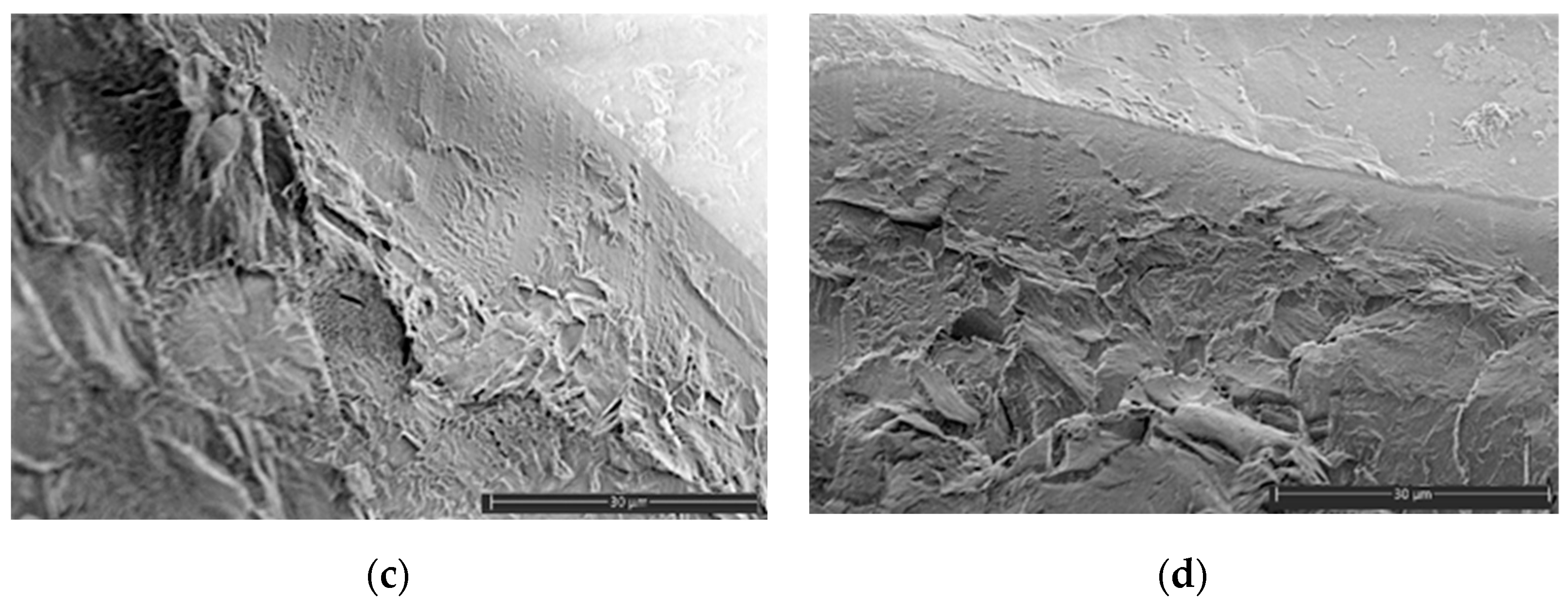
2.5. SEM Analysis
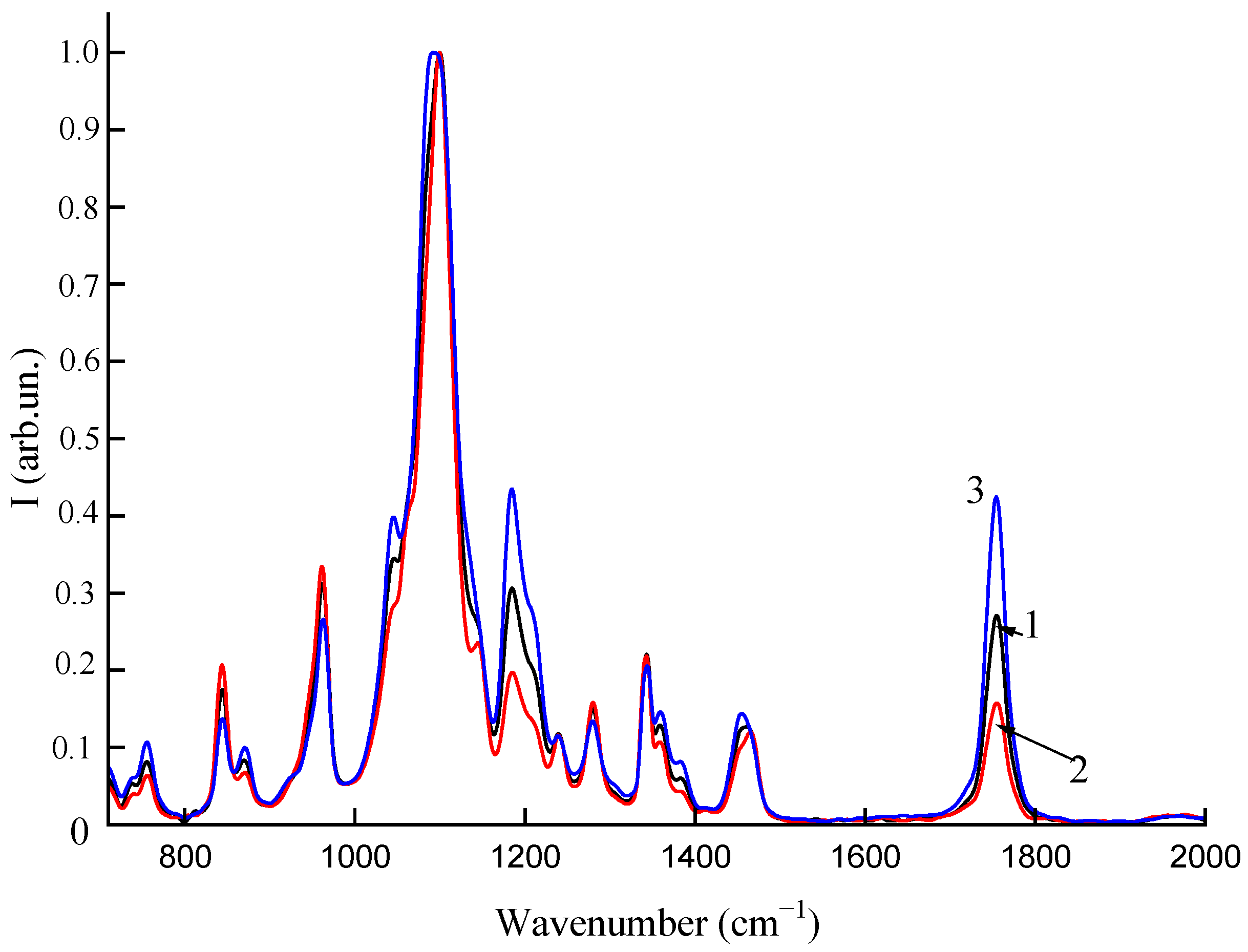
2.6. Spectral Analysis
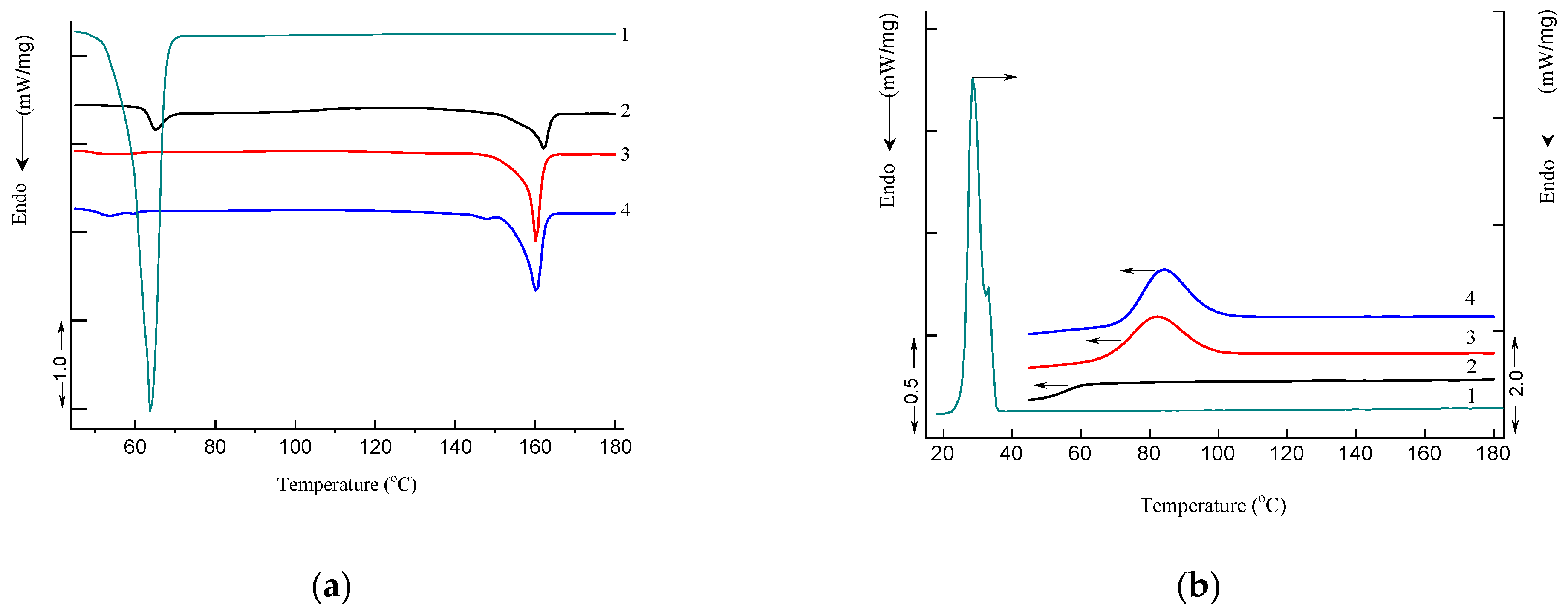
2.7. DSC Analysis
2.8. Test of Antibacterial Activity
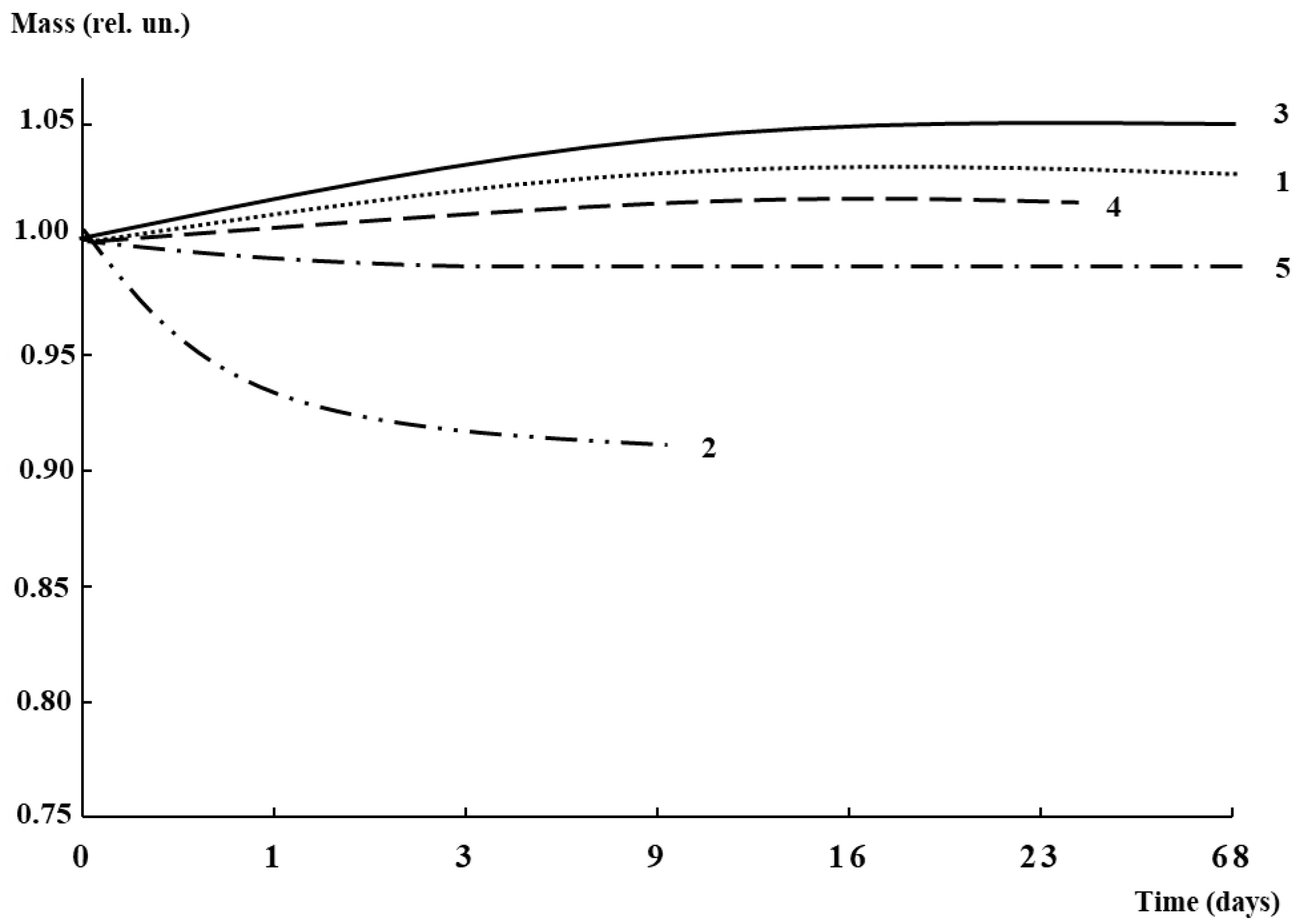
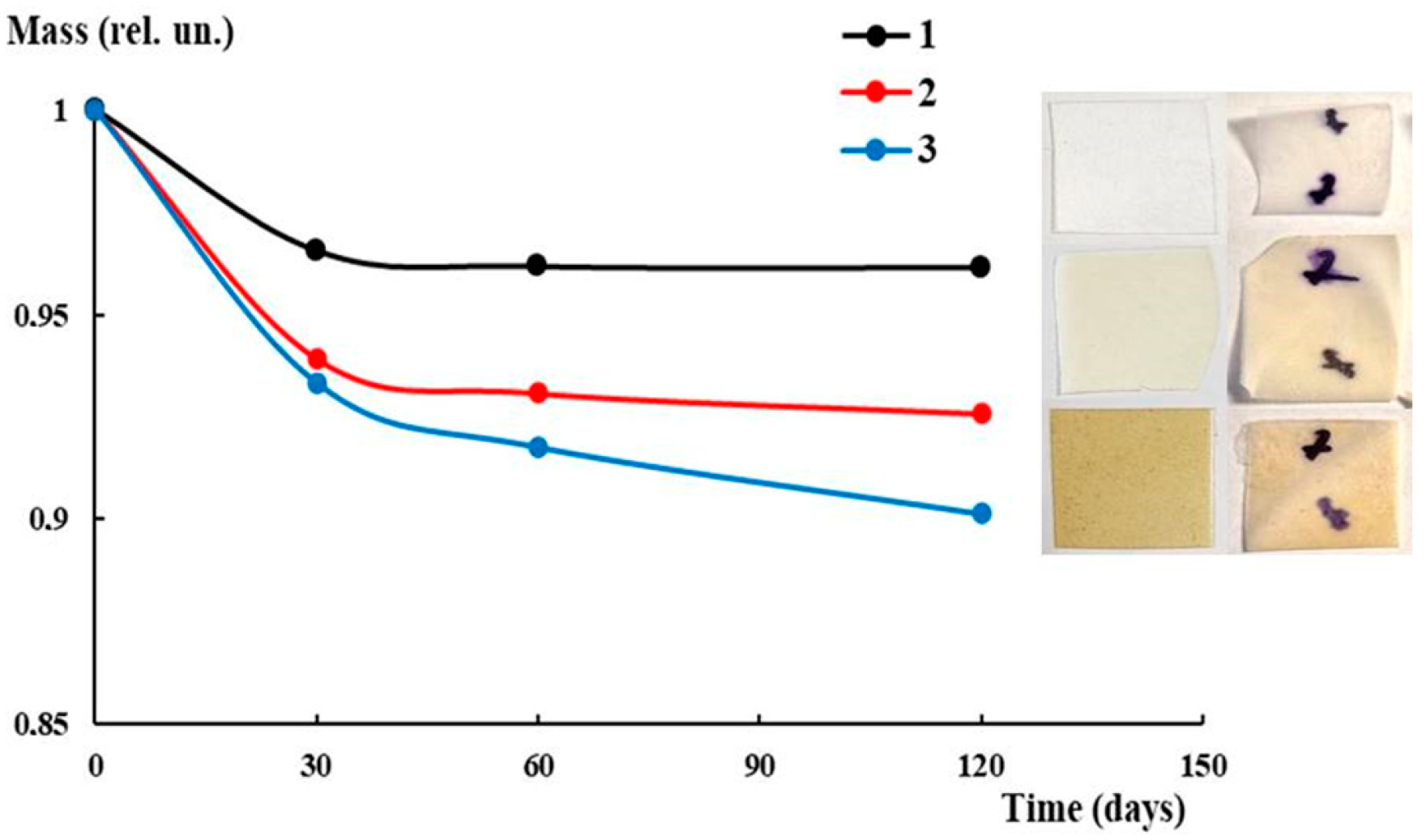
2.9. Biodegradability
3. Results and Discussion
3.1. PLA-Based Biocomposites
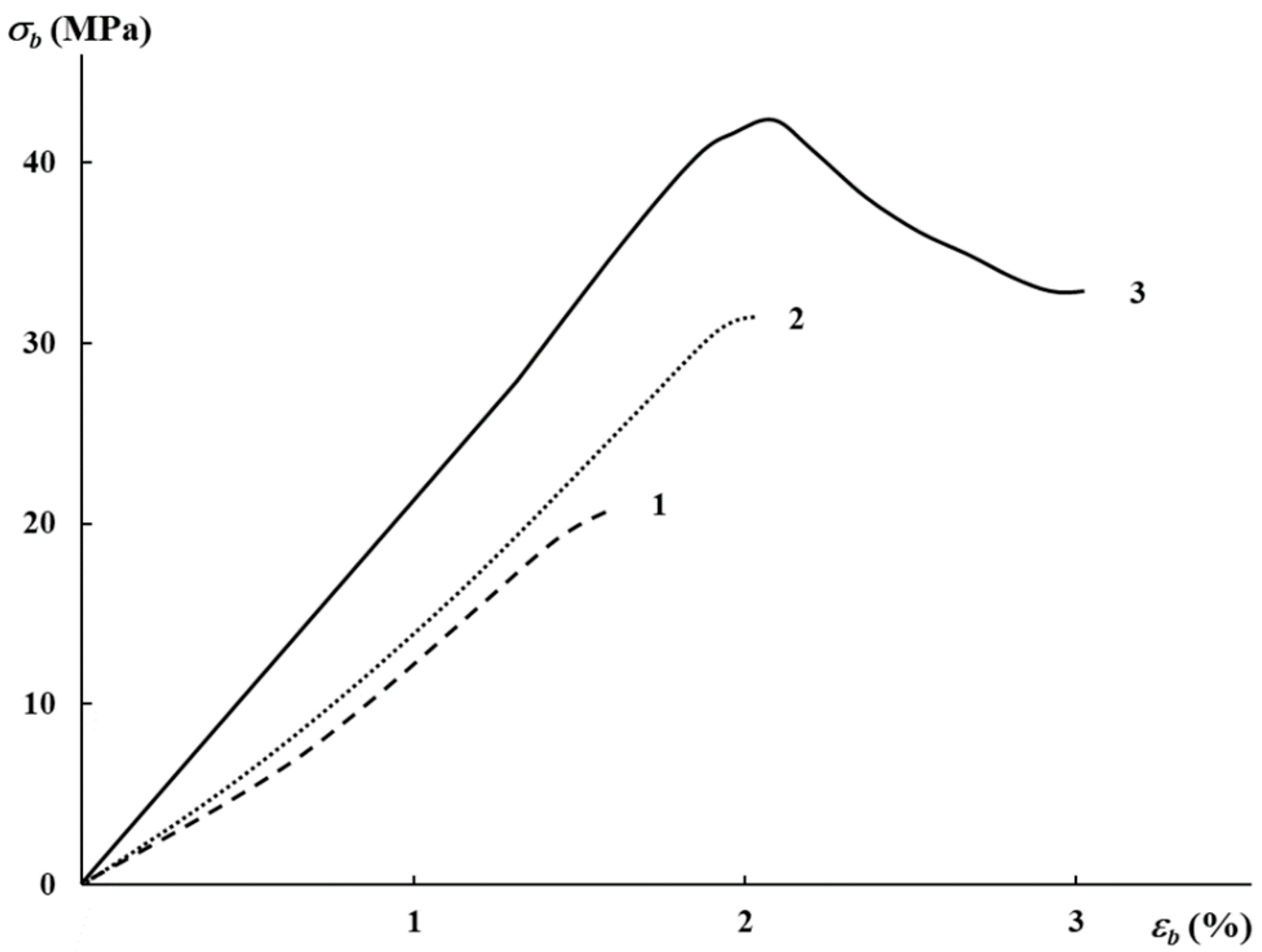
3.2. Mechanical Characteristics, Water Absorption, and Structural Features
3.3. Spectral Analysis
3.4. Differential Scanning Calorimetry
3.5. Antibacterial Activity and Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly(lactic acid) production for tissue engineering. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Khusairy Bin Bakri, M.; Mirza Bin Julaihi, M.R.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Diniz, M.S.d.F.; Mourão, M.M.; Xavier, L.P.; Santos, A.V. Recent biotechnological applications of polyhydroxyalkanoates (PHA) in the biomedical sector—A review. Polymers 2023, 15, 4405. [Google Scholar] [CrossRef]
- Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly(lactic acid)-based blends: A comprehensive review. Polymers 2023, 13, 5148. [Google Scholar] [CrossRef]
- Ren, J. Biodegradable Poly(lactioc acid): Synthesis, Modification, Processing and Applications, 1st ed.; Springer: Heidelberg/Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Aleksanyan, K.V. Recent approaches to the plasticization of poly(lactic acid) (PLA) (A review). Polymers 2024, 16, 87. [Google Scholar] [CrossRef]
- Kulkarni, A.; Narayan, R. Effects of modified thermoplastic starch on crystallization kinetics and barrier properties of PLA. Polymers 2021, 13, 4125. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Grachev, A.V.; Berlin, A.A.; Prut, E.V. Investigation of mechanical and thermophysical properties of biodegradable compositions of polylactide with ethyl cellulose and chitosan containing poly(ethylene glycol). Mendeleev Commun. 2015, 25, 361–363. [Google Scholar] [CrossRef]
- Kozlowski, M.; Masirek, R.; Piorkowska, E.; Gazicki-Lipman, M. Biodegradable blends of poly(L-lactide) and starch. J. Appl. Polym. Sci. 2007, 105, 269–277. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Prut, E.V.; Aleksanyan, K.V.; Krasheninnikov, V.G.; Perepelitsina, E.O.; Shashkin, D.P.; Berlin, A.A. Composites Based on Starch and Polylactide. Polym. Sci. Ser. B 2019, 61, 226–232. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Prut, E.V.; Aleksanyan, K.V.; Krasheninnikov, V.G.; Perepelitsina, E.O.; Shashkin, D.P.; Ivanushkina, N.E.; Berlin, A.A. Production and investigation of structure and properties of polyethylene–polylactide composites. J. Appl. Polym. Sci. 2019, 136, 47598. [Google Scholar] [CrossRef]
- Medjdoub, N.; Guessoum, M.; Fois, M. Viscoelastic, thermal and environmental characteristics of poly(lactic acid), linear low-density polyethylene and low-density polyethylene ternary blends and composites. J. Adhes. Sci. Technol. 2017, 3, 787–805. [Google Scholar] [CrossRef]
- Gere, D.; Czigany, T. Future trends of plastic bottle recycling: Compatibilization of PET and PLA. Polym. Test. 2020, 81, 106160. [Google Scholar] [CrossRef]
- Wang, M.; He, C.; Yang, X.; Duan, G.; Wang, W. Preparation and properties of PLA/PBAT composites modified with different filler particles. Mater. Lett. 2024, 372, 136960. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Cao, Y.; Lan, T.; Chen, H.; Qin, Y. Effects of PLA film incorporated with ZnO nanoparticle on the quality attributes of fresh-cut apple. Nanomaterials 2017, 7, 207. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Al Hazza, A.; Jacob, H.; Joseph, A. Comparative effects of untreated and 3-methacryloxypropyltrimethoxysilane treated ZnO nanoparticle reinforcement on properties of polylactide-based nanocomposite films. Int. J. Biol. Macromol. 2017, 101, 1041–1050. [Google Scholar] [CrossRef]
- Rodrigues-Tobias, H.; Morales, G.; Grande, D. Improvement of mechanical properties and antibacterial of electrospun poly(D,L-lactide)-based mats by incorporation of ZnO-graft-poly(D,L-lactide) nanoparticles. Mater. Chem. Phys. 2016, 182, 324–331. [Google Scholar] [CrossRef]
- Vasile, C.; Rapa, M.; Stefan, M.; Stan, M.; Macavei, S.; Darie-Nita, R.N.; Barbu-Tudoran, L.; Vodnar, D.C.; Popa, E.E.; Stefan, R.; et al. New PLA/ZnO:Cu/Ag bionanocomposites for food packaging. Express Polym. Lett. 2017, 11, 531–544. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and antibacterial performance of novel polylactic acid-graphene oxide-silver nanoparticle hybrid nanocomposite mats prepared by electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef]
- Chartarrayawadee, W.; Too, C.O.; Ross, S.; Ross, G.M.; Hongsith, N.; Ratchawet, A. The role of stearic acid for silver nanoparticle formation on graphene and its composite with poly(lactic acid). Polym. Bull. 2018, 75, 3171–3187. [Google Scholar] [CrossRef]
- Liu, C.; Chan, K.W.; Shen, J.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Melt-compounded polylactic acid composite hybrids with hydroxyapatite nanorods and silver nanoparticles: Biodegradation, antibacterial ability, bioactivity and cytotoxicity. RSC Adv. 2015, 5, 72288–72299. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Kenny, J.M. Development and thermal behavior of ternary PLA matrix composites. Polym. Degrad. Stab. 2010, 95, 2200–2206. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Yang, X.-Y.; Lu, F.-F.; Chen, G.-Y.; Yao, J.-M. Fabrication of multifunctional cellulose nanocrystals/poly(lactic acid) nanocomposites with silver nanoparticles by spraying method. Carbohydr. Polym. 2016, 140, 109–219. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Barbale, M.; Zaccheo, S.; Scavone, M.; Visai, L.; Kenny, J.M. New multifunctional poly(lactide acid) composites: Mechanical, antibacterial, and degradation properties. J. Appl. Polym. Sci. 2012, 124, 87–98. [Google Scholar] [CrossRef]
- Cacciotti, I.; Fortunati, E.; Puglia, D.; Kenny, J.M. Effect of silver nanoparticles and cellulose nanocrystals on electrospun poly(lacric) acid mats: Mechanical, thermal properties and mechanical behavior. Carbohydr. Polym. 2014, 103, 22–31. [Google Scholar] [CrossRef]
- Yalcinkaya, E.E.; Puglia, D.; Fortunati, E.; Bertoglio, F.; Bruni, G.; Visai, L.; Kenny, J.M. Cellulose nanocrystals as templates for cetyltrimethylammonium bromide mediated synthesis of Ag nanoparticles and their novel use in PLA films. Carbohydr. Polym. 2017, 157, 1557–1567. [Google Scholar] [CrossRef]
- Alipilakkotte, S.; Sreejith, L. Green synthesized PLA/silver nanoparticle probe for sensing of hydrogen peroxide in biological samples. Mater. Lett. 2018, 217, 33–38. [Google Scholar] [CrossRef]
- Salas-Papayanopolos, H.; Morales-Cepeda, A.B.; Sanchez, S.; Lafleur, P.G.; Gomez, I. Synergistic effect of silver nanoparticle content on the optical and thermo-mechanical properties of poly(L-lactic acid)/glycerol triacetate blends. Polym. Bull. 2017, 74, 4799–4814. [Google Scholar] [CrossRef]
- Samoilova, N.; Kurskaya, E.; Krayukhina, M.; Askadsky, A.; Yamskov, I. Copolymers of Maleic Acid and Their Amphiphilic Derivatives as Stabilizers of Silver Nanoparticles. J. Phys. Chem. B 2009, 113, 3395–3403. [Google Scholar] [CrossRef]
- Lomakin, S.; Mikheev, Y.; Usachev, S.; Rogovina, S.; Zhorina, L.; Perepelitsina, E.; Levina, I.; Kuznetsova, O.; Shilkina, N.; Iordanskii, A.; et al. Evaluation and modeling of polylactide photodegradation under ultraviolet irradiation: Bio-based polyester photolysis mechanism. Polymers 2024, 16, 985. [Google Scholar] [CrossRef]
- Fischer, E.; Sterzel, H.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Colloid. Polym. Sci. 1973, 521, 980–990. [Google Scholar] [CrossRef]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, crystallization and thermal behaviors of PLA-based composites: Wonderful effects of hybrid GO/PEG via dynamic impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Naumkin, A.V.; Korlyukov, A.A.; Anuchina, N.M.; Popov, D.A. Polymer-stabilized silver (gold)-zinc oxide nanoheterodimer structures as antimicrobials. Appl. Sci. 2023, 13, 11121. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Korlyukov, A.A.; Klemenkova, Z.S.; Naumkin, A.V.; Mezhuev, Y.O. One-pot synthesis of colloidal hybrid Au (Ag)/ZnO nanostructures with the participation of maleic acid copolymers. Polymers 2023, 15, 1670. [Google Scholar] [CrossRef]
- Saleh, M.; Anwar, S.; AlFaify, A.Y.; Al-Ahmari, A.M.; Elgawad, A.E.E.A. Development of PLA/recycled-desized carbon fiber composites for 3D printing: Thermal, mechanical, and morphological analyses. J. Mater. Res. Technol. 2024, 29, 2768–2780. [Google Scholar] [CrossRef]
- Sahan, Y.; Gurbuz, O.; Goncagul, G.; Kara, A.; Ozakin, C. Antimicrobial effect of PEG–PLA on food-spoilage microorganisms. Food Sci Biotechnol 2017, 26, 1123–1128. [Google Scholar] [CrossRef]
- Nalawade, T.M.; Bhat, K.; Sogi, S.H.P. Bactericidal activity of propylene glycol, glycerine, polyethylene glycol 400, and polyethylene glycol 1000 against selected microorganisms. J. Int. Soc. Prev. Community Dent. 2015, 5, 114–119. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Fathi, F. Biocontrol of Fusarium oxysporum in cucumber by some antagonist bacteria under drought stress. J. Crop Prot. 2018, 7, 375–385. [Google Scholar]
- Alexandrino, M.; Costa, R.; Canário, A.V.; Costa, M.C. Clostridia initiate heavy metal bioremoval in mixed sulfidogenic cultures. Environ. Sci. Technol. 2014, 48, 3378–3385. [Google Scholar] [CrossRef]
- Maleke, M.; Valverde, A.; Gomez-Arias, A.; Cason, E.D.; Vermeulen, J.; Coetsee-Hugo, L.; Swart, H.; van Heerden, E.; Castillo, J. Anaerobic reduction of europium by a Clostridium strain as a strategy for rare earth biorecovery. Sci. Rep. 2019, 9, 14339. [Google Scholar] [CrossRef]




| Microorganism | Type of Microorganism |
|---|---|
| Bacillus subtilis VKM B-501 | Gram-positive obligate aerobic bacterium |
| Escherichia coli VKM B-3674 | Gram-negative facultative anaerobic bacterium |
| Micrococcus luteus VKM Ac-2230 | Gram-positive obligate aerobic bacterium |
| Clostridium sporogenes VKM B-2623 | Gram-positive obligate anaerobic bacterium |
| Groenewaldozyma auringiensis VKM Y-2623 | Facultative anaerobic yeast |
| AgNP Content, wt% | E, MPa | σb, MPa | εb, % |
|---|---|---|---|
| 0.01 | 2130 | 19.8 | 1.5 |
| 0.03 | 2370 | 31.7 | 2 |
| 0.5 | 3050 | 32.0 | 3 |
| Sample | Tg, °C | Tr, °C | Tm, °C PEG | Tcr, °C PEG | Tcr, °C PLA | Tm, °C PLA | ΔHcr, J/g | ΔHm, J/g | χ, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| PLA | heating | 63.7 | 65.1 | - | - | - | 162.0 | - | −17.5 PLA | 18.8 |
| cooling | 57.0 | - | - | - | - | - | - | - | - | |
| PEG | heating | - | - | 63.9 | - | - | - | - | −212.4 PEG | 99.7 |
| cooling | - | - | - | 32.7/28.4 | - | - | 170.1 PEG | - | - | |
| PLA–PEG4000 (90:10 wt%) | heating | 50.8 | - | - | - | - | 160.2 | - | −42.1 PLA | 45.0 |
| cooling | - | - | - | - | 82.6 | - | 33.2 PLA | - | - | |
| PLA–PEG4000 + AgNPs (90:10 wt% + 0.2 wt%) | heating | 51.9 | - | 59.8 | - | - | 160.3/ 148.0 * | - | −42.4 PLA | 45.3 |
| cooling | - | - | - | - | 83.9 | - | 33.1 PLA | - | - | |
| Sample | G. auringiensis | E. coli | M. luteus | B. subtilis | C. sporogenes | |
|---|---|---|---|---|---|---|
| PLA–PEG1000 + AgNPs | 90:10 wt% + 0.1 wt% | no growth | weak growth | no growth | no growth | weak growth |
| 90:10 wt% + 0.5 wt% | no growth | no growth | no growth | no growth | weak growth | |
| PLA–PEG4000 + AgNPs | 90:10 wt% + 0.1 wt% | no growth | no growth | no growth | no growth | weak growth |
| 90:10 wt% + 0.5 wt% | no growth | no growth | no growth | no growth | weak growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksanyan, K.V.; Smykovskaya, R.S.; Samoilova, N.A.; Novikov, V.A.; Shakhov, A.M.; Aybush, A.V.; Kuznetsova, O.P.; Lomakin, S.M.; Ryzhmanova, Y.V. Development of Poly(lactic acid)-Based Biocomposites with Silver Nanoparticles and Investigation of Their Characteristics. Polymers 2024, 16, 2758. https://doi.org/10.3390/polym16192758
Aleksanyan KV, Smykovskaya RS, Samoilova NA, Novikov VA, Shakhov AM, Aybush AV, Kuznetsova OP, Lomakin SM, Ryzhmanova YV. Development of Poly(lactic acid)-Based Biocomposites with Silver Nanoparticles and Investigation of Their Characteristics. Polymers. 2024; 16(19):2758. https://doi.org/10.3390/polym16192758
Chicago/Turabian StyleAleksanyan, Kristine V., Regina S. Smykovskaya, Nadezhda A. Samoilova, Viktor A. Novikov, Aleksander M. Shakhov, Arseny V. Aybush, Olga P. Kuznetsova, Sergey M. Lomakin, and Yana V. Ryzhmanova. 2024. "Development of Poly(lactic acid)-Based Biocomposites with Silver Nanoparticles and Investigation of Their Characteristics" Polymers 16, no. 19: 2758. https://doi.org/10.3390/polym16192758
APA StyleAleksanyan, K. V., Smykovskaya, R. S., Samoilova, N. A., Novikov, V. A., Shakhov, A. M., Aybush, A. V., Kuznetsova, O. P., Lomakin, S. M., & Ryzhmanova, Y. V. (2024). Development of Poly(lactic acid)-Based Biocomposites with Silver Nanoparticles and Investigation of Their Characteristics. Polymers, 16(19), 2758. https://doi.org/10.3390/polym16192758








