Highly Transparent, Mechanically Robust, and Conductive Eutectogel Based on Oligoethylene Glycol and Deep Eutectic Solvent for Reliable Human Motions Sensing
Abstract
1. Introduction
2. Results and Discussion
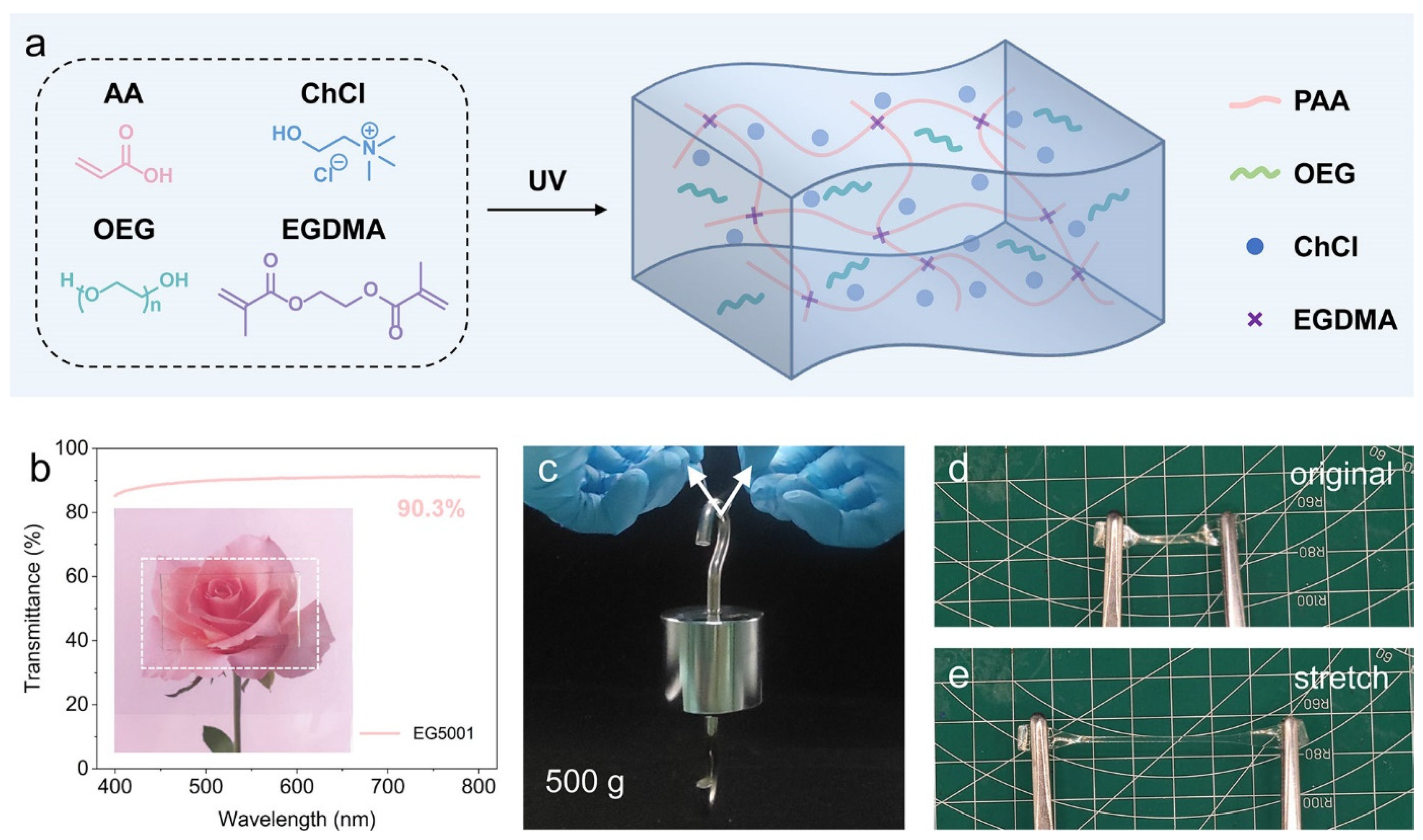
2.1. Design and Preparation of the P(AA-ChCl)/OEG Eutectogels
2.2. Characteristic and Anti-Freezing Properties of the P(AA-ChCl)/OEG Eutectogels
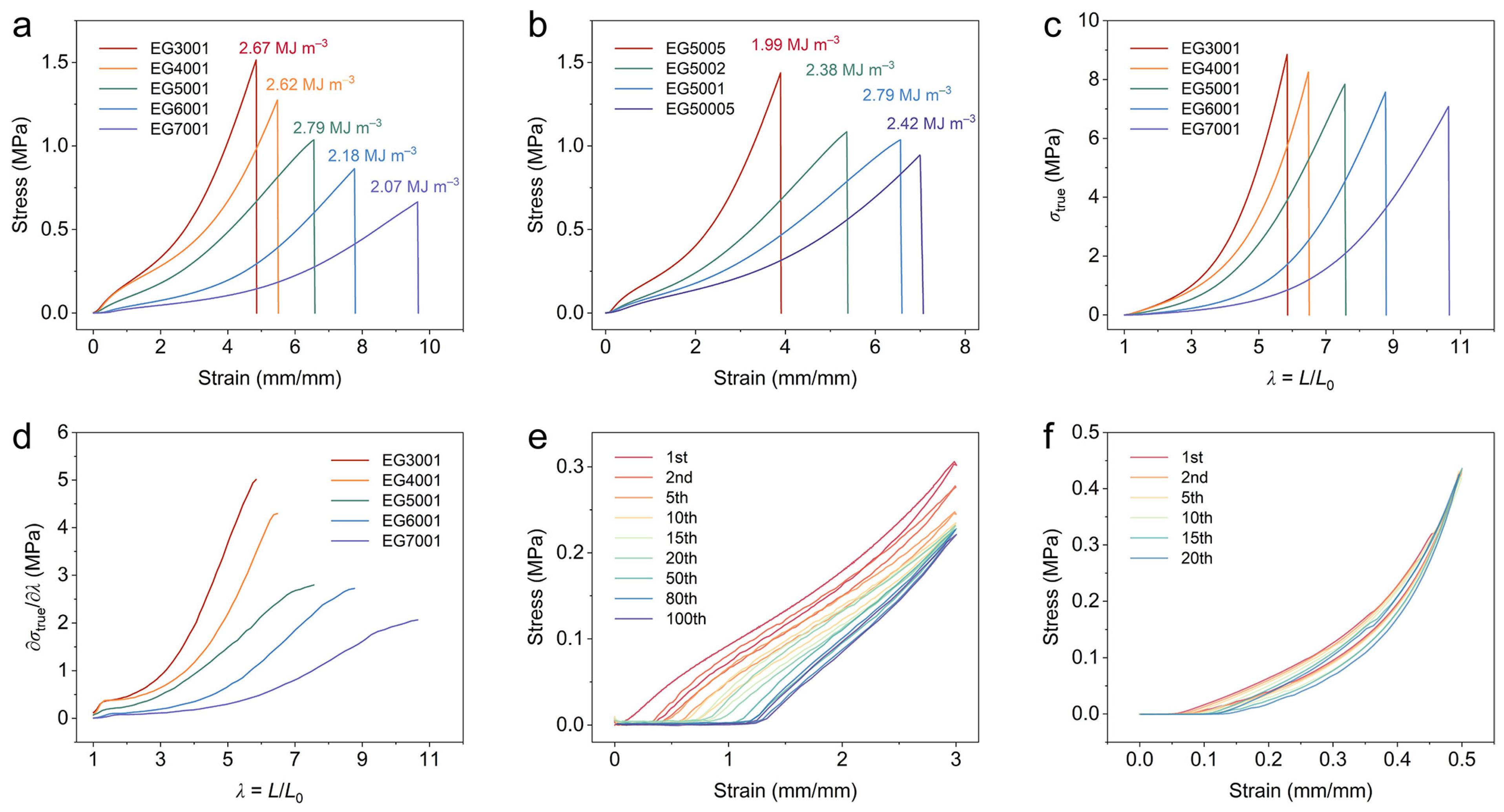
2.3. Mechanical Properties of the P(AA-ChCl)/OEG Eutectogels
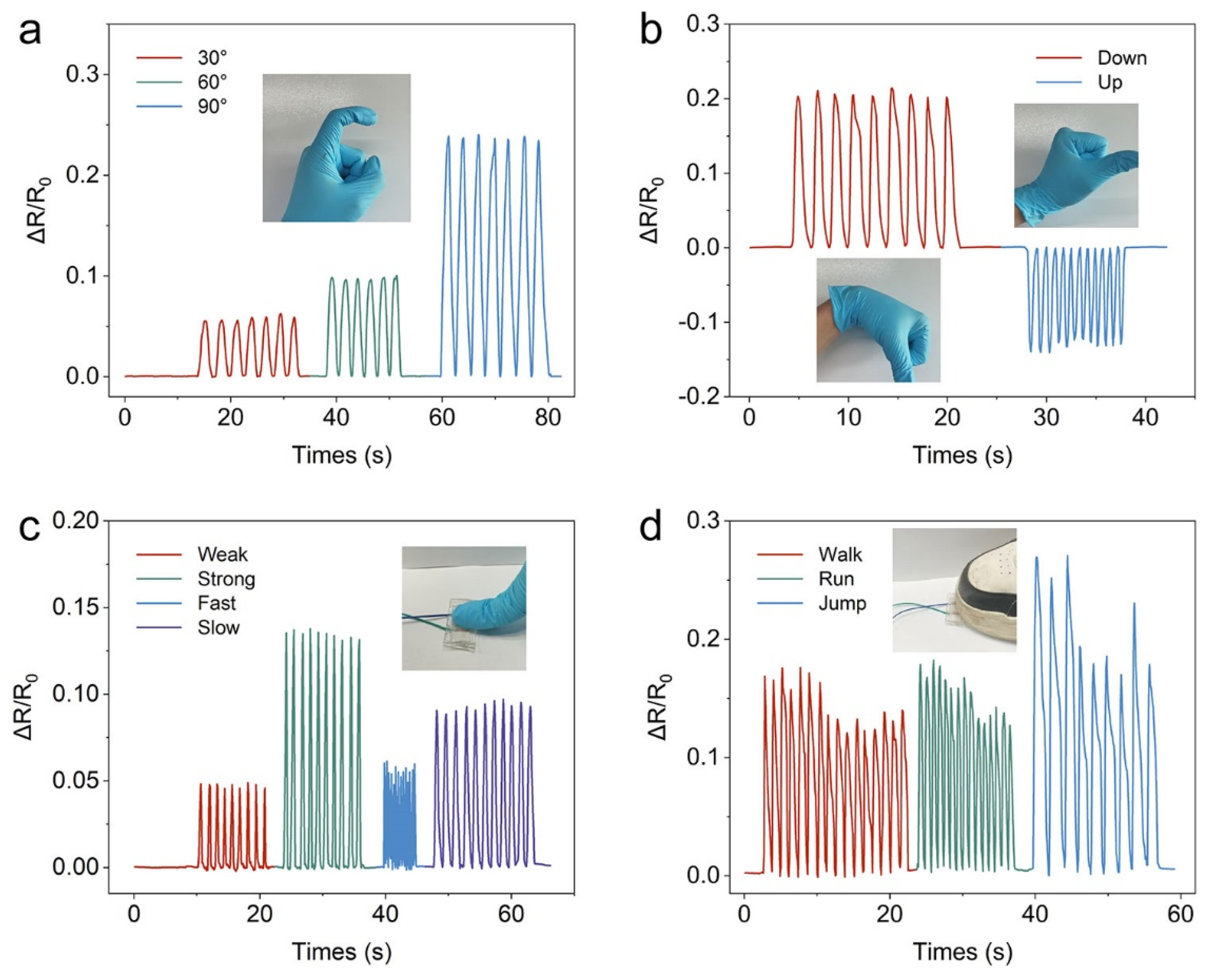
2.4. Electrical Sensing Performance
2.5. Strain Sensor Application
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Keplinger, C.; Sun, J.-Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, B.; Sun, S.; Wu, P. Skin-like mechanoresponsive self-healing ionic elastomer from supramolecular zwitterionic network. Nat. Commun. 2021, 12, 4082. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A Bioinspired Mineral Hydrogel as a Self-Healable, Mechanically Adaptable Ionic Skin for Highly Sensitive Pressure Sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, C.; Jiang, Y.; Yuan, Y.; Kim, M.-g.; Nishio, Y.; Shih, C.-C.; Wang, W.; Lai, J.-C.; Ji, X.; et al. High-speed and large-scale intrinsically stretchable integrated circuits. Nature 2024, 627, 313–320. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Zheng, D.; Xie, Z.; Zhuang, X.; Zhao, D.; Chen, Y.; Su, N.; Chen, H.; Pankow, R.M.; et al. Highly stretchable organic electrochemical transistors with strain-resistant performance. Nat. Mater. 2022, 21, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sun, W.; Li, J.; Chen, J.-P.; Wang, X.; Mei, Z.; Jin, G.; Lei, Y.; Xin, R.; Yang, M.; et al. N-type semiconducting hydrogel. Science 2024, 384, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Koo, J.H.; Yoo, J.; Kim, M.S.; Choi, M.K.; Kim, D.-H.; Song, Y.M. Flexible and Stretchable Light-Emitting Diodes and Photodetectors for Human-Centric Optoelectronics. Chem. Rev. 2024, 124, 768–859. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Jia, S.; Fan, C. Body-conformable light-emitting materials and devices. Nat. Photon. 2024, 18, 114–126. [Google Scholar] [CrossRef]
- Shi, X.; Zuo, Y.; Zhai, P.; Shen, J.; Yang, Y.; Gao, Z.; Liao, M.; Wu, J.; Wang, J.; Xu, X.; et al. Large-area display textiles integrated with functional systems. Nature 2021, 591, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Godaba, H.; Chen, G.; Tan, S.T.M.; Wan, G.; Li, G.; Lee, P.M.; Cai, Y.; Li, S.; Shepherd, R.F.; et al. A transparent, self-healing and high-κ dielectric for low-field-emission stretchable optoelectronics. Nat. Mater. 2020, 19, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.K.; Deng, Z.S.; Liu, X.; Huang, T.R.; Hu, Y.J.; Chen, Y.T.; Liu, Y.H.; Guo, Z.H.; Yue, K. Highly stretchable, strain-stiffening, self-healing ionic conductors for wearable sensors. Chem. Eng. J. 2022, 449, 137633. [Google Scholar] [CrossRef]
- Fu, M.; Sun, Z.X.; Liu, X.B.; Huang, Z.K.; Luan, G.F.; Chen, Y.T.; Peng, J.P.; Yue, K. Highly Stretchable, Resilient, Adhesive, and Self-Healing Ionic Hydrogels for Thermoelectric Application. Adv. Funct. Mater. 2023, 33, 2306086. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K.; Bai, R.B.; Suo, Z.G.; Vlassak, J.J. Highly Stretchable and Tough Hydrogels below Water Freezing Temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Chen, Y.; Huang, Z.; Fu, M.; Ou, W.; Huang, T.; Yue, K. A Fully Self-Healing and Highly Stretchable Liquid-Free Ionic Conductive Elastomer for Soft Ionotronics. Adv. Funct. Mater. 2023, 33, 2304486. [Google Scholar] [CrossRef]
- Wan, H.; Wu, B.; Hou, L.; Wu, P. Amphibious Polymer Materials with High Strength and Superb Toughness in Various Aquatic and Atmospheric Environments. Adv. Mater. 2024, 36, 2307290. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Yang, N.; Chu, X.; Sun, F.C.; Ali, M.U.; Zhang, Y.; Yang, B.; Cai, Y.L.; Liu, M.Y.; Gasparini, N.; et al. Wide-Humidity Range Applicable, Anti-Freezing, and Healable Zwitterionic Hydrogels for Ion-Leakage-Free Iontronic Sensors. Adv. Mater. 2023, 35, 2211617. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, H.; Li, Z.; Dai, J.; Cong, H.-P.; Yu, S.-H. Highly compressible and environmentally adaptive conductors with high-tortuosity interconnected cellular architecture. Nat. Synth. 2022, 1, 975–986. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Z.; Deng, Z.; Du, Z.; Sun, T.L.; Guo, Z.-H.; Yue, K. A Transparent, Highly Stretchable, Solvent-Resistant, Recyclable Multifunctional Ionogel with Underwater Self-Healing and Adhesion for Reliable Strain Sensors. Adv. Mater. 2021, 33, 2105306. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, L.; Liu, P.; Peng, J. Transparent, mechanically robust, conductive, self-healable, and recyclable ionogels for flexible strain sensors and electroluminescent devices. RSC Adv. 2024, 14, 28234–28243. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, X.; Liu, Z.; Zou, X.; Shen, Z.; Liu, D.; Li, L.; Guo, Y.; Yan, F. Nanoconfined polymerization limits crack propagation in hysteresis-free gels. Nat. Mater. 2024, 23, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, S.; Jia, Z.; Koh, J.J.; Yeo, J.C.C.; Wang, C.-G.; Surat’man, N.E.; Loh, X.J.; Le Bideau, J.; He, C.; et al. Ionogels: Recent advances in design, material properties and emerging biomedical applications. Chem. Soc. Rev. 2023, 52, 2497–2527. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-C.; Li, W.; Liu, Z.; Zheng, S.; Hu, Y.; Zhou, Y.; Guo, J.; Ou, X.; Li, Q.; Yu, J.; et al. Ionogels: Preparation, Properties and Applications. Adv. Funct. Mater. 2024, 34, 2314408. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Morales-Narváez, E. Transforming nature into the next generation of bio-based flexible devices: New avenues using deep eutectic systems. Matter 2021, 4, 2141–2162. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D. Emerging Ionic Soft Materials Based on Deep Eutectic Solvents. J. Phys. Chem. B 2020, 124, 8465–8478. [Google Scholar] [CrossRef]
- Shaibuna, M.; Theresa, L.V.; Sreekumar, K. Neoteric deep eutectic solvents: History, recent developments, and catalytic applications. Soft Matter 2022, 18, 2695–2721. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Yao, M.; Chen, S.; Yu, Q.; Liang, L.; Yu, C.; Liu, M.; Hao, H.; Zhang, H.; Yao, F.; et al. Environment-Tolerant Conductive Eutectogels for Multifunctional Sensing. Adv. Funct. Mater. 2024, 34, 2315656. [Google Scholar] [CrossRef]
- Li, R.a.; Chen, G.; He, M.; Tian, J.; Su, B. Patternable transparent and conductive elastomers towards flexible tactile/strain sensors. J. Mater. Chem. C 2017, 5, 8475–8481. [Google Scholar] [CrossRef]
- Li, X.; Yan, M.; Xiao, J.; Lian, H. Ultrafast fabrication of deep eutectic solvent flexible ionic gel with high-transmittance, freeze-resistant and conductivity by frontal polymerization. J. Colloid Interface Sci. 2023, 650, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, L.; Ge, Y.; Gao, Y.; Ma, X.; Fang, Y. High-strength, stretchable, and self-recoverable copolymer-supported deep eutectic solvent gels based on dense and dynamic hydrogen bonding for high-voltage and safe flexible supercapacitors. Polym. Bull. 2023, 80, 5587–5605. [Google Scholar] [CrossRef]
- Tang, N.; Jiang, Y.; Wei, K.; Zheng, Z.; Zhang, H.; Hu, J. Evolutionary Reinforcement of Polymer Networks: A Stepwise-Enhanced Strategy for Ultrarobust Eutectogels. Adv. Mater. 2024, 36, 2309576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Song, X.; Wei, R.; Liu, S.; Wu, Z.; Chen, H. A conductive ionogel with Stretchability, low hysteresis and adjustable adhesion for Air/Underwater mechanosensing. Chem. Eng. J. 2024, 499, 155992. [Google Scholar] [CrossRef]
- Li, R.A.; Chen, G.; Fan, T.; Zhang, K.; He, M. Transparent conductive elastomers with excellent autonomous self-healing capability in harsh organic solvent environments. J. Mater. Chem. A 2020, 8, 5056–5061. [Google Scholar] [CrossRef]
- Hu, F.; Huang, Z.; Luo, C.; Yue, K. High-sensitivity and ultralow-hysteresis fluorine-rich ionogel strain sensors for multi-environment contact and contactless sensing. Mater. Horiz. 2023, 10, 5907–5919. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Peng, J.; Huang, T.; Hu, F.; Liu, X.; Xu, L.; Yue, K. Highly stretchable ionotronic pressure sensors with broad response range enabled by microstructured ionogel electrodes. J. Mater. Chem. A 2023, 11, 7201–7212. [Google Scholar] [CrossRef]
- Zeng, Q.; Lai, X.; Li, H.; Chen, Z.; Zeng, X.; Zhang, L. High Fire-Safety and Multifunctional Eutectogel for Flexible Quasi-Solid-State Supercapacitors. Adv. Funct. Mater. 2024, 2411029. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Z.; Zheng, S.; Li, W.; Ren, Y.; Li, L.; Yan, F. Three-Dimensional Printable, Highly Conductive Ionic Elastomers for High-Sensitivity Iontronics. ACS Appl. Mater. Interfaces 2022, 14, 26068–26076. [Google Scholar] [CrossRef]
- He, X.; Zhang, B.; Liu, Q.; Chen, H.; Cheng, J.; Jian, B.; Yin, H.; Li, H.; Duan, K.; Zhang, J.; et al. Highly conductive and stretchable nanostructured ionogels for 3D printing capacitive sensors with superior performance. Nat. Commun. 2024, 15, 6431. [Google Scholar] [CrossRef]
- Dong, M.; Han, Y.; Hao, X.P.; Yu, H.C.; Yin, J.; Du, M.; Zheng, Q.; Wu, Z.L. Digital Light Processing 3D Printing of Tough Supramolecular Hydrogels with Sophisticated Architectures as Impact-Absorption Elements. Adv. Mater. 2022, 34, 2204333. [Google Scholar] [CrossRef]
- Yiming, B.; Guo, X.; Ali, N.; Zhang, N.; Zhang, X.; Han, Z.; Lu, Y.; Wu, Z.; Fan, X.; Jia, Z.; et al. Ambiently and Mechanically Stable Ionogels for Soft Ionotronics. Adv. Funct. Mater. 2021, 31, 2102773. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, J.; Liu, Z.; Sun, Z.; Wu, Y.; Liu, L.; Yan, F. Ionic liquid–based click-ionogels. Sci. Adv. 2019, 5, eaax0648. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Li, S.; Liu, X.; Sun, J. Mechanically Robust, Elastic, and Healable Ionogels for Highly Sensitive Ultra-Durable Ionic Skins. Adv. Mater. 2020, 32, 2002706. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Zhao, Y.; Sato, K.; Ihsan, A.B.; Li, X.; Guo, H.; Gong, J.P. Oppositely Charged Polyelectrolytes Form Tough, Self-Healing, and Rebuildable Hydrogels. Adv. Mater. 2015, 27, 2722–2727. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zheng, Z.; Zhao, D.; Liu, J.; Ouyang, J.; Wang, H. Tough and super-resilient hydrogels synthesized by using peroxidized polymer chains as polyfunctional initiating and cross-linking centers. Soft Matter 2013, 9, 2837–2844. [Google Scholar] [CrossRef]
- Si, L.; Zheng, X.; Nie, J.; Yin, R.; Hua, Y.; Zhu, X. Silicone-based tough hydrogels with high resilience, fast self-recovery, and self-healing properties. Chem. Commun. 2016, 52, 8365–8368. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, J.; Ma, Y.; Yan, B.; Pan, M.; Peng, Q.; Wang, W.; Han, L.; Liu, J.; Zeng, H. Ultra elastic, stretchable, self-healing conductive hydrogels with tunable optical properties for highly sensitive soft electronic sensors. J. Mater. Chem. A 2020, 8, 24718–24733. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Xie, J.; Li, T.; Xu, L.; Liu, P.; Peng, J. Highly Transparent, Mechanically Robust, and Conductive Eutectogel Based on Oligoethylene Glycol and Deep Eutectic Solvent for Reliable Human Motions Sensing. Polymers 2024, 16, 2761. https://doi.org/10.3390/polym16192761
Huang Z, Xie J, Li T, Xu L, Liu P, Peng J. Highly Transparent, Mechanically Robust, and Conductive Eutectogel Based on Oligoethylene Glycol and Deep Eutectic Solvent for Reliable Human Motions Sensing. Polymers. 2024; 16(19):2761. https://doi.org/10.3390/polym16192761
Chicago/Turabian StyleHuang, Zhenkai, Jiahuan Xie, Tonggen Li, Liguo Xu, Peijiang Liu, and Jianping Peng. 2024. "Highly Transparent, Mechanically Robust, and Conductive Eutectogel Based on Oligoethylene Glycol and Deep Eutectic Solvent for Reliable Human Motions Sensing" Polymers 16, no. 19: 2761. https://doi.org/10.3390/polym16192761
APA StyleHuang, Z., Xie, J., Li, T., Xu, L., Liu, P., & Peng, J. (2024). Highly Transparent, Mechanically Robust, and Conductive Eutectogel Based on Oligoethylene Glycol and Deep Eutectic Solvent for Reliable Human Motions Sensing. Polymers, 16(19), 2761. https://doi.org/10.3390/polym16192761







