Synthesis of Ultrahigh Molecular Weight Poly (Trifluoroethyl Methacrylate) Initiated by the Combination of Palladium Nanoparticles with Organic Halides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pd NPs
2.3. Synthesis of Ultrahigh Molecular Weight PTFEMA Initiated by the Combination of Pd NPs with Organic Halides
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of Ultrahigh Molecular Weight PTFEMA Using Organic Halides Combined with Pd NPs as Initiators
3.2. Synthesis of Ultrahigh Molecular Weight PTFEMA Using EBP as an Initiator Combined with Pd NPs
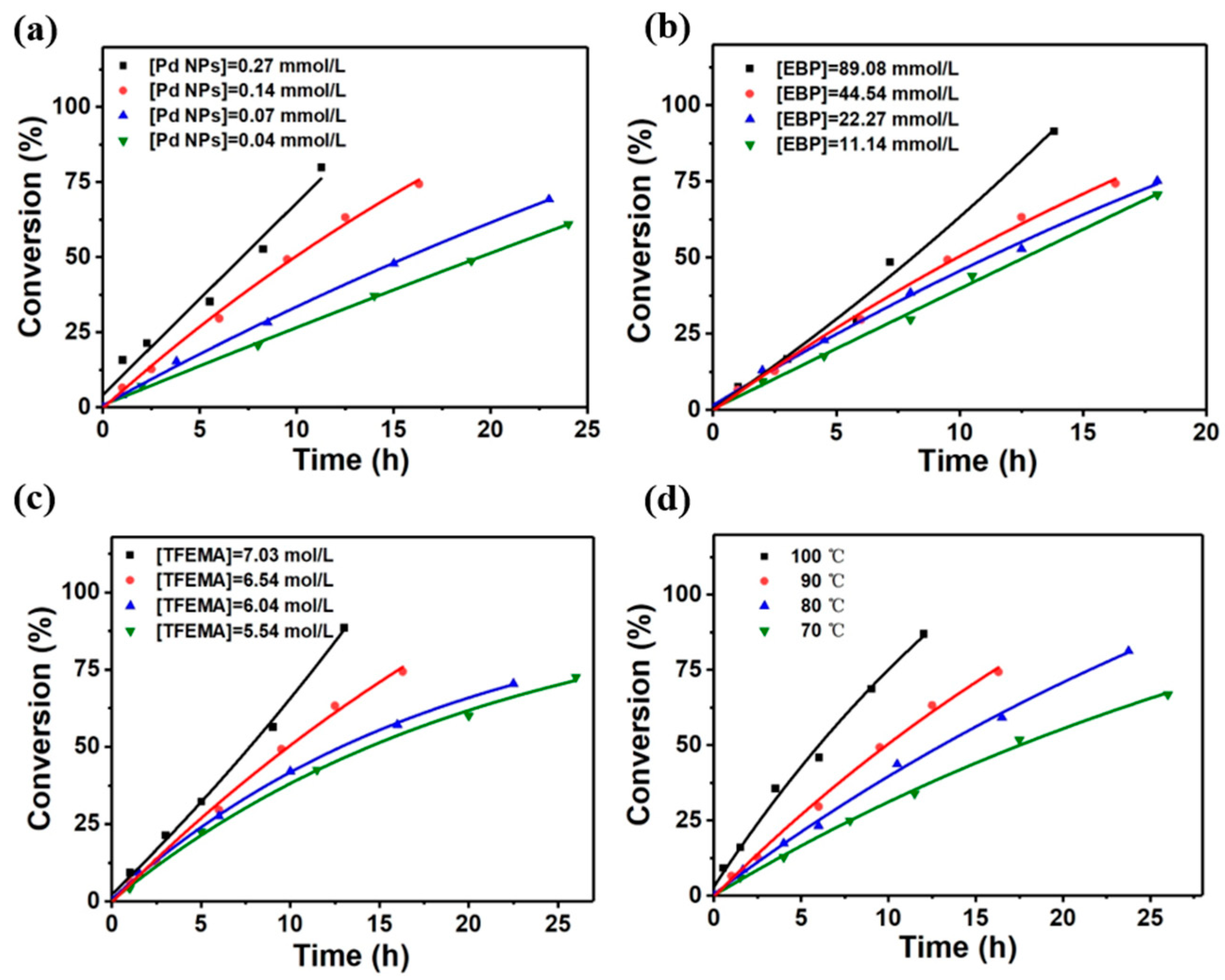
3.3. Kinetics of the Polymerization of TFEMA Initiated by the Combination of Pd NPs with EBP
3.4. Preliminary Investigation of the Mechanism of TFEMA Polymerization Initiated by the Combination of Pd NPs with EBP
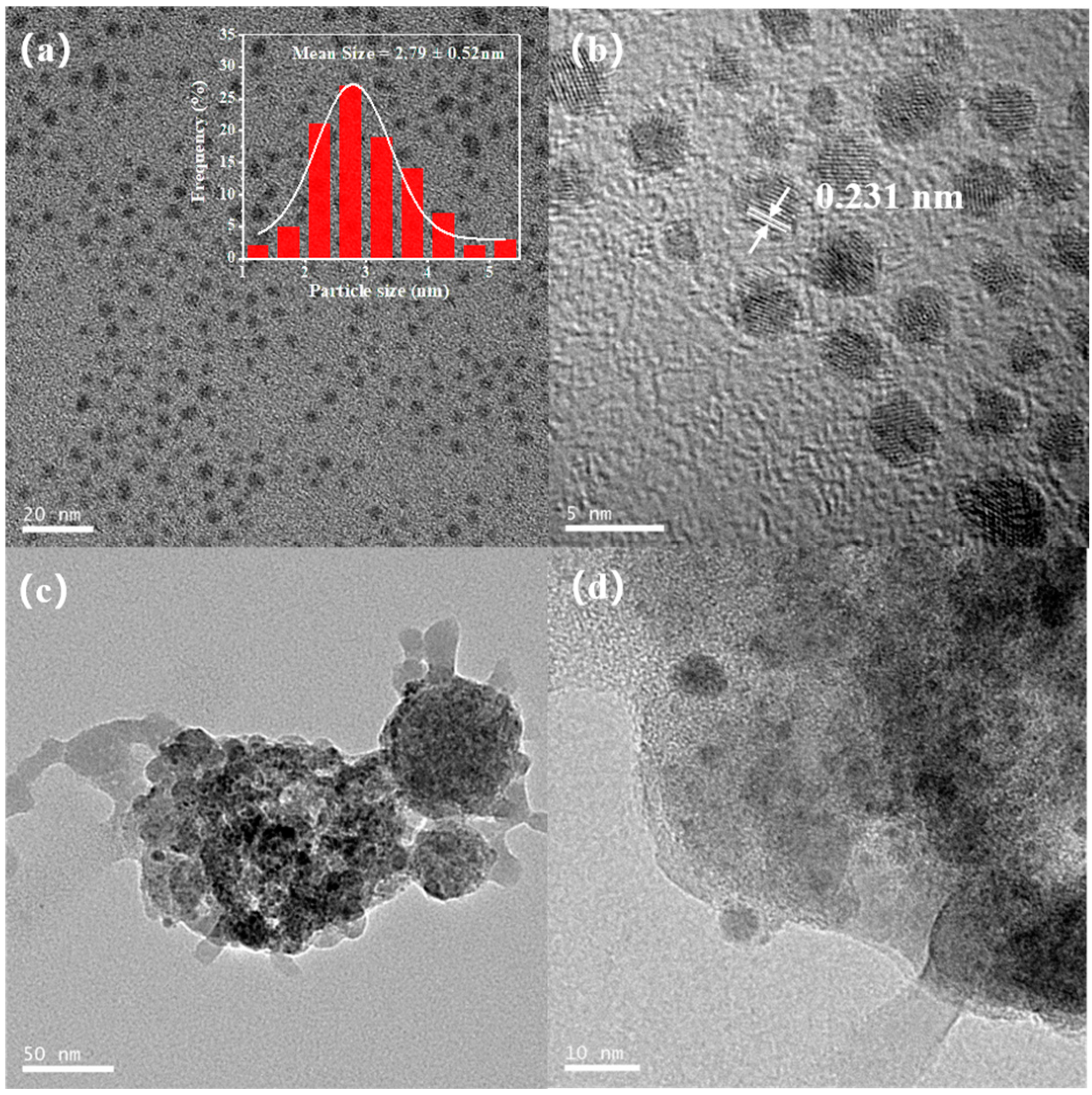
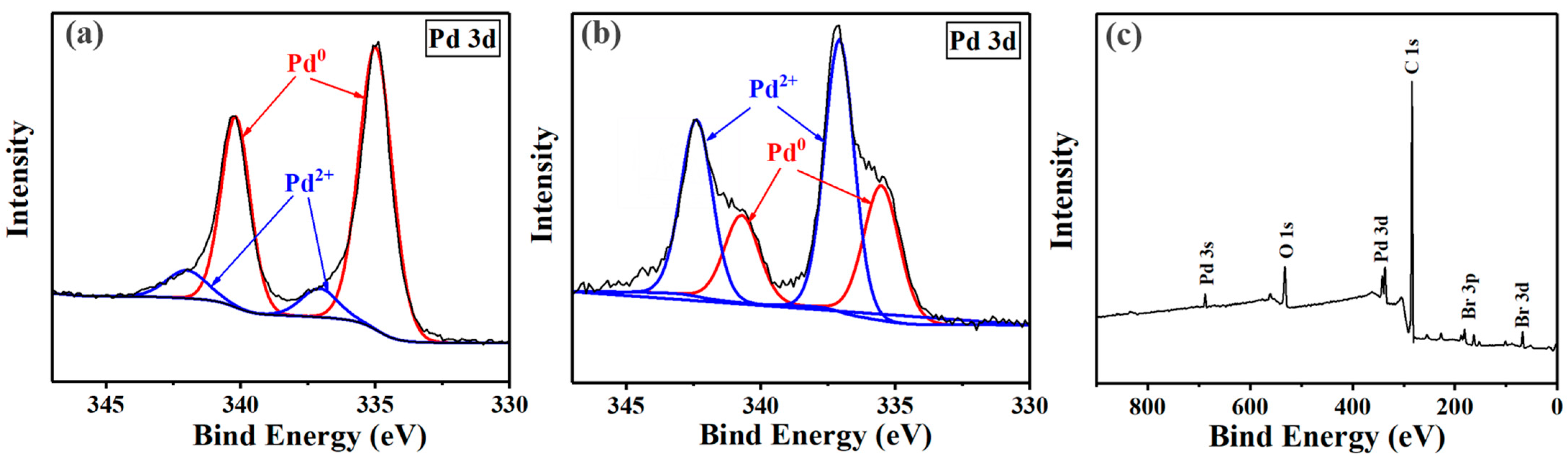
3.4.1. TEM Images and XPS Analysis of the Pd NPs before and after Polymerization
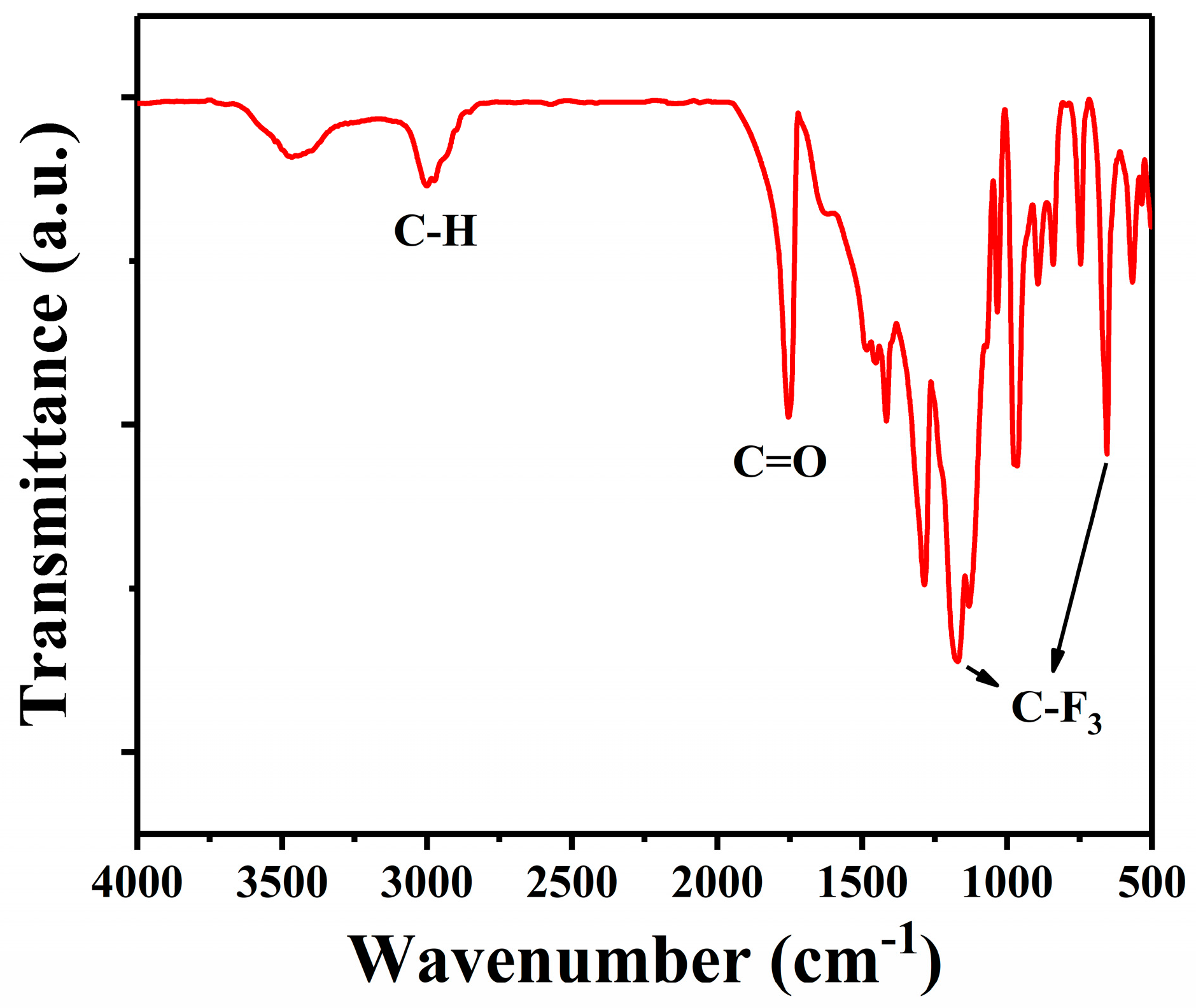
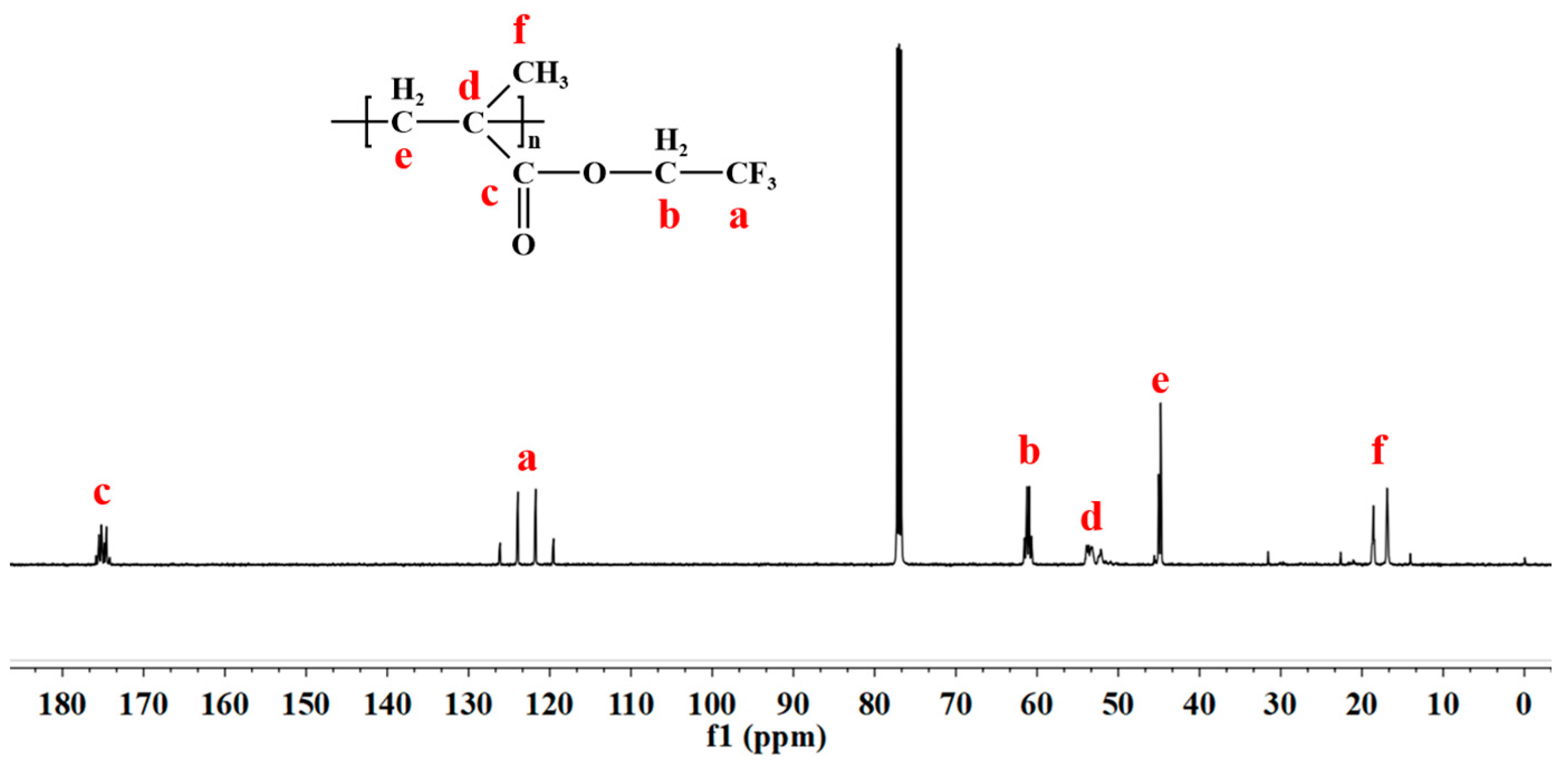
3.4.2. Characterization of PTFEMA: Infrared and Carbon Nuclear Magnetic Resonance Spectroscopy Analysis
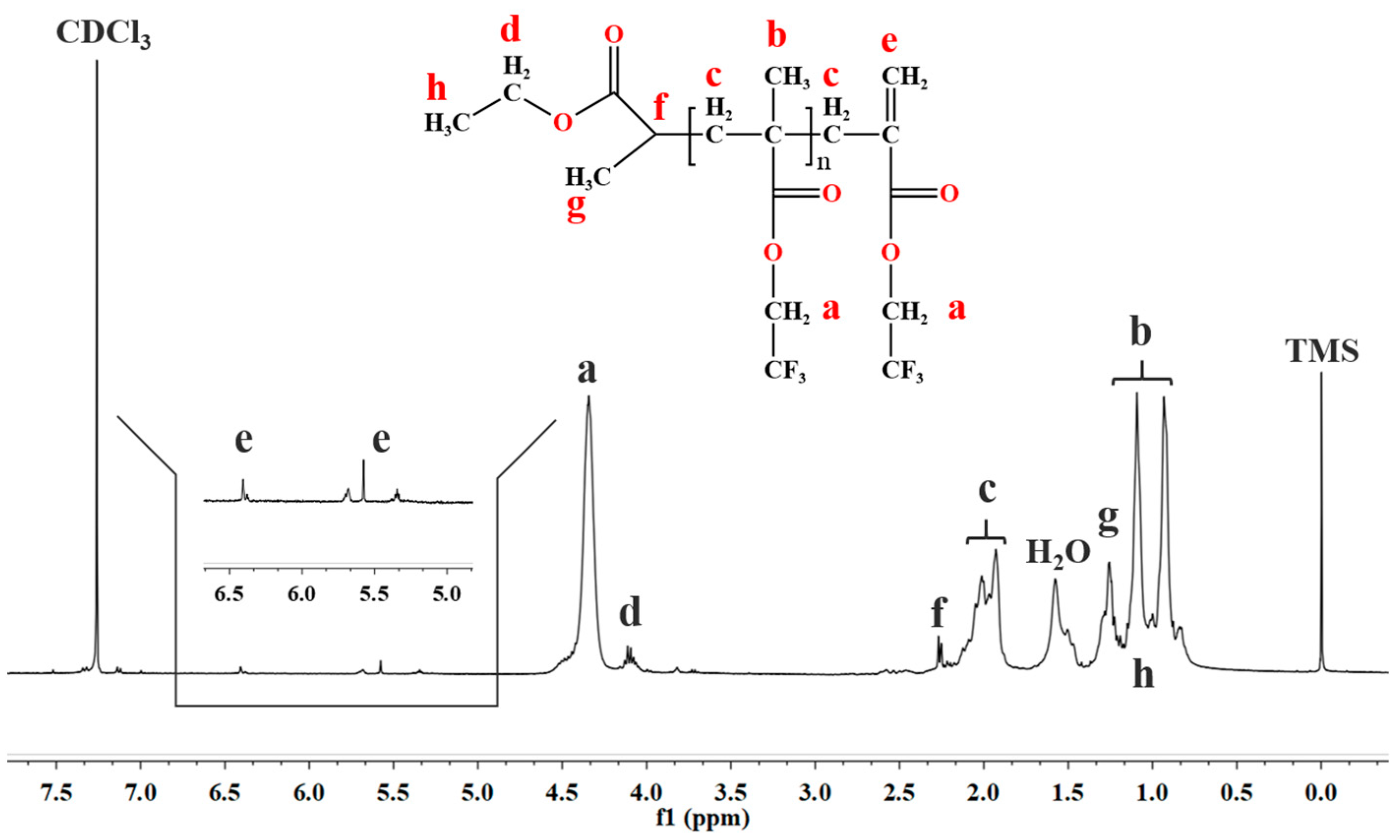
3.4.3. End-Group Analysis of the Prepared Low Molecular Weight PTFEMA
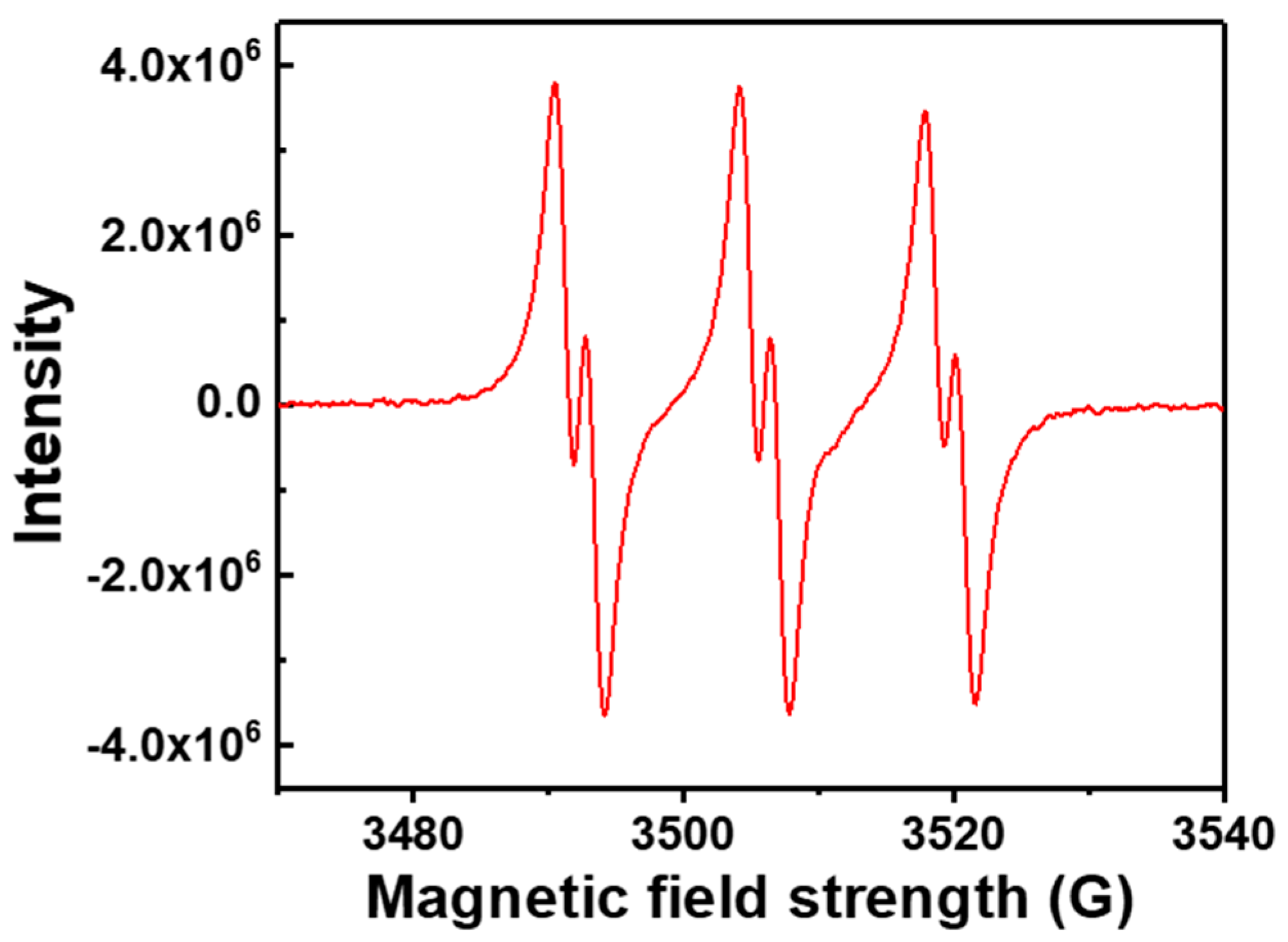
3.4.4. ESR Analysis for the Polymerization Initiated by EBP with Pd NPs
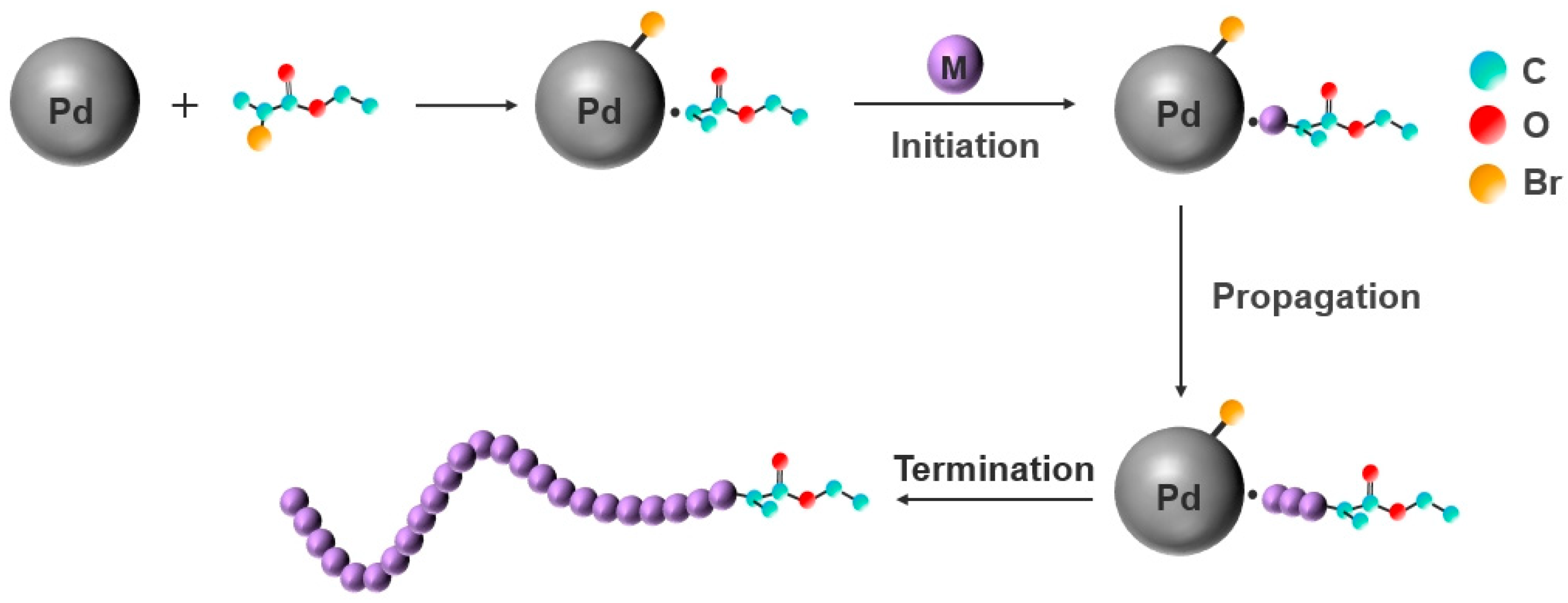
3.4.5. Mechanism Model for the Polymerization of PTFEMA Initiated by EBP with Pd NPs
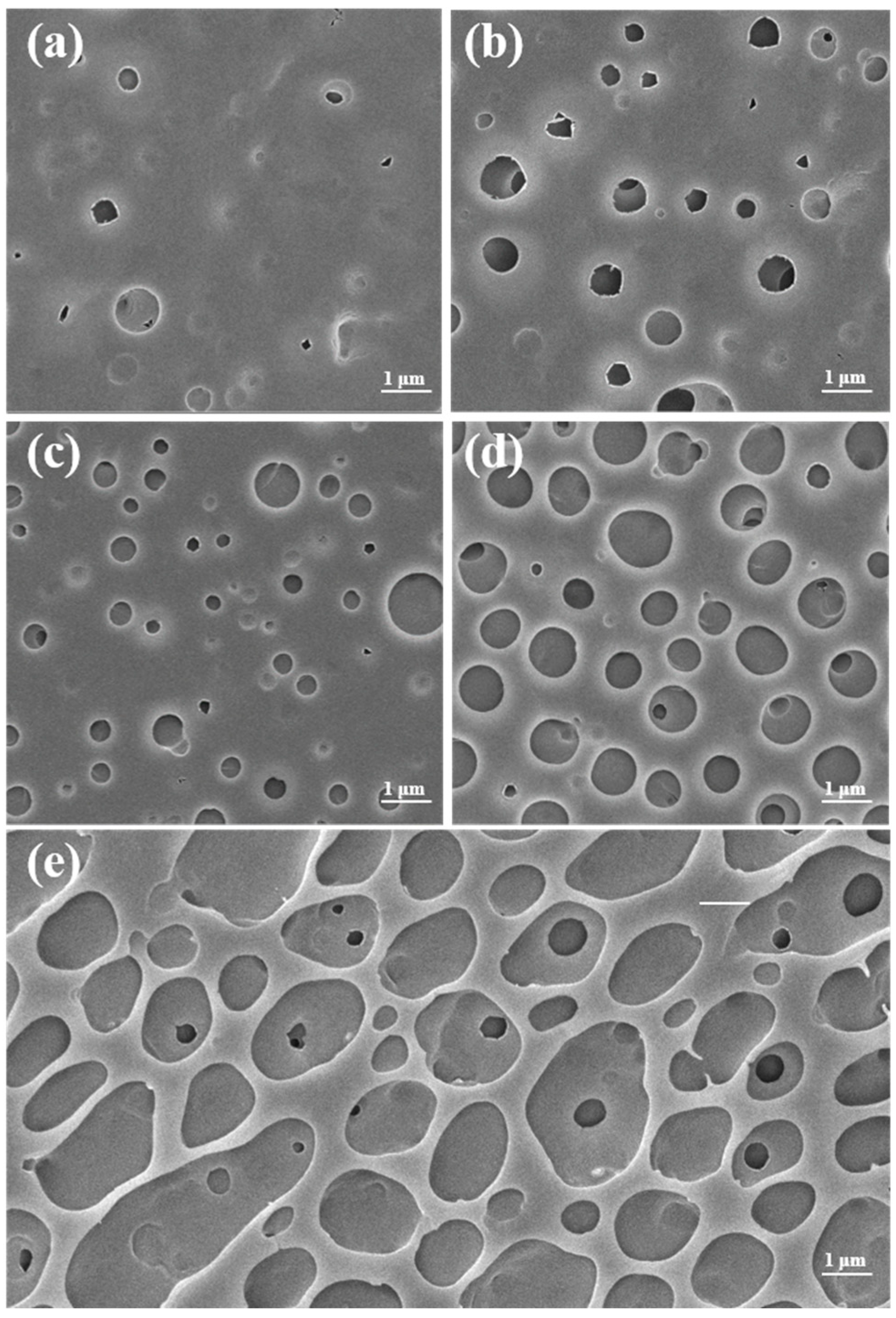
3.5. Hydrophobicity Test of PTFEMA Films with Different Molecular Weights
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, W.; Li, Y.; Huang, X. Fluorinated poly(meth)acrylate: Synthesis and properties. Polymer 2014, 55, 6197–6211. [Google Scholar] [CrossRef]
- Ameduri, B. Fluoropolymers: The Right Material for the Right Applications. Chem. Eur. J. 2018, 24, 18830–18841. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Higaki, Y.; Mukai, M.; Takahara, A. Molecular aggregation structure and water repellency of Poly(perfluorohexyl acrylate) with a carbamate linkage. Polymer 2019, 182, 121846. [Google Scholar] [CrossRef]
- Moon, S.J.; Kim, Y.J.; Kang, D.R.; Lee, S.Y.; Kim, J.H. Fluorine-Containing, Self-Assembled Graft Copolymer for Tuning the Hydrophilicity and Antifouling Properties of PVDF Ultrafiltration Membranes. Polymers 2023, 15, 3623. [Google Scholar] [CrossRef]
- Wu, X.W.; Hong, Y.L.; Xu, B.Q.; Nishiyama, Y.; Jiang, W.; Zhu, J.W.; Zhang, G.; Kitagawa, S.; Horike, S. Perfluoroalkyl-Functionalized Covalent Organic Frameworks with Superhydrophobicity for Anhydrous Proton Conduction. J. Am. Chem. Soc. 2020, 142, 14357–14364. [Google Scholar] [CrossRef] [PubMed]
- Han, D.J.; Kim, S.; Heo, H.J.; Park, I.J.; Kang, H.S.; Lee, S.G.; Lee, J.-C.; Sohn, E.-H. Access to Fluorinated Polymer Surfaces with Outstanding Mechanical Property, High Optical Transparency, and Low Surface Energy via Nonafluoro-tert-Butyl Group Introduction. ACS Appl. Polym. Mater. 2020, 2, 3957–3965. [Google Scholar] [CrossRef]
- Simonov-Emel’yanov, I.D.; Shirshin, K.V.; Motsinov, P.V.; Vlasov, S.V. The Influence of Molecular Weight on the Orientation and Properties of Polymethyl Methacrylate Sheets. Int. Polym. Sci. Technol. 2018, 45, 47–51. [Google Scholar] [CrossRef]
- Sifri, R.J.; Padilla-Velez, O.; Coates, G.W.; Fors, B.P. Controlling the Shape of Molecular Weight Distributions in Coordination Polymerization and Its Impact on Physical Properties. J. Am. Chem. Soc. 2020, 142, 1443–1448. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M. Precise Control of Molecular Weight and Stereospecificity in Lewis Pair Polymerization of Semifluorinated Methacrylates: Mechanistic Studies and Stereocomplex Formation. Macromolecules 2020, 53, 4659–4669. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, D.; Ma, Y.; Yang, W. Radical Polymerization of TFEMA and Relationship Between Surface Hydrophobicity and Molecular Weight of Poly-TFEMA. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 263–270. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, X.; Yu, Q.; Li, C.; He, W.; Zhang, L.; Cheng, Z. Facile grafting modification of main-chain-type semi-fluorinated alternating fluoropolymers via simultaneous CuAAC reaction and ATRP in one pot at ambient temperature. Polym. Chem. 2023, 14, 1036–1042. [Google Scholar] [CrossRef]
- Whitfield, R.; Parkatzidis, K.; Rolland, M.; Truong, N.P.; Anastasaki, A. Tuning Dispersity by Photoinduced Atom Transfer Radical Polymerisation: Monomodal Distributions with ppm Copper Concentration. Angew. Chem. Int. Ed. 2019, 58, 13323–13328. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, C.; Hu, F.; Huang, Z.; Yue, K. Recent advances in fluorinated polymers: Synthesis and diverse applications. Sci. China Chem. 2023, 66, 3347–3359. [Google Scholar] [CrossRef]
- Gong, H.; Gu, Y.; Chen, M. Controlled/Living Radical Polymerization of Semifluorinated (Meth)acrylates. Synlett 2018, 29, 1543–1551. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, Y.; Shen, X.; Lin, J.; Chen, M. Organocatalyzed Photocontrolled Radical Polymerization of Semifluorinated (Meth)acrylates Driven by Visible Light. Angew. Chem. Int. Ed. 2018, 57, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Chen, K.X.; Ameduri, B.; Chen, M. Fluoropolymer Nanoparticles Synthesized via Reversible-Deactivation Radical Polymerizations and Their Applications. Chem. Rev. 2023, 123, 12431–12470. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Matyjaszewski, K. Iron-Catalyzed Atom Transfer Radical Polymerization of Semifluorinated Methacrylates. ACS Macro Lett. 2019, 8, 1110–1114. [Google Scholar] [CrossRef]
- Su, Y.; Rong, X.; Gao, A.; Liu, Y.; Li, J.; Mao, M.; Qi, X.; Chai, G.; Zhang, Q.; Suo, L.; et al. Rational design of a topological polymeric solid electrolyte for high-performance all-solid-state alkali metal batteries. Nat. Commun. 2022, 13, 4181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, M.; Chen, C.; Li, Y.; Zhang, Z.; Zhou, Y.; Wang, S. Preparation and performance study of the self-brittle composite detergent with controllable morphology for radioactive decontamination of surface layer of various materials by RAFT one-pot synthesis. React. Funct. Polym. 2023, 187, 105591. [Google Scholar] [CrossRef]
- Reyhani, A.; Allison-Logan, S.; Ranji-Burachaloo, H.; McKenzie, T.G.; Bryant, G.; Qiao, G.G. Synthesis of ultra-high molecular weight polymers by controlled production of initiating radicals. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1922–1930. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Z.; Gong, H.; Chen, M. Investigations into CTA-differentiation-involving polymerization of fluorous monomers: Exploitation of experimental variances in fine-tuning of molecular weights. Polym. Chem. 2020, 11, 7402–7409. [Google Scholar] [CrossRef]
- Mohammad, S.A.; Shingdilwar, S.; Banerjee, S.; Ameduri, B. Macromolecular engineering approach for the preparation of new architectures from fluorinated olefins and their applications. Prog. Polym. Sci. 2020, 106, 101255. [Google Scholar] [CrossRef]
- Jiang, K.M.; Han, S.T.; Ma, M.Y.; Zhang, L.; Zhao, Y.C.; Chen, M. Photoorganocatalyzed Reversible-Deactivation Alternating Copolymerization of Chlorotrifluoroethylene and Vinyl Ethers under Ambient Conditions: Facile Access to Main-Chain Fluorinated Copolymers. J. Am. Chem. Soc. 2020, 142, 7108–7115. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Zhang, S.D.; Li, Q.S.; Qiao, G.G.; An, Z.S. An Atom-Economic Enzymatic Cascade Catalysis for High-Throughput RAFT Synthesis of Ultrahigh Molecular Weight Polymers. Angew. Chem. Int. Ed. 2022, 61, e202213396. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, V.J.; Derry, M.J.; Fielding, L.A.; Musa, O.M.; Armes, S.P. RAFT Aqueous Dispersion Polymerization of N-(2-(Methacryloyloxy)ethyl)pyrrolidone: A Convenient Low Viscosity Route to High Molecular Weight Water-Soluble Copolymers. Macromolecules 2016, 49, 4520–4533. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, Q.Y.; Pan, X.C. Controlled Radical Polymerization toward Ultra-High Molecular Weight by Rationally Designed Borane Radical Initiators. Cell Rep. Phys. Sci. 2020, 1, 100073. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Kasahara, H.; Yamashita, Y. Polymerization of methylmethacrylate initiated by hydrogenation catalysts. Macromol. Chem. Phys. 1967, 104, 254–262. [Google Scholar] [CrossRef]
- Otsu, T.; Aoki, S.; Nishimura, M.; Yamaguchi, M.; Kusuki, Y. Initiation of vinyl polymerization by systems of activated metals and organic halides having various types of halogen bonds. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 835–837. [Google Scholar] [CrossRef]
- Otsu, T.; Yamaguchi, M.; Takemura, Y.; Kusuki, Y.; Aoki, S. New initiator systems for radical polymerization of vinyl monomers. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 697–701. [Google Scholar] [CrossRef]
- Otsu, T.; Yamaguchi, M. Metal-containing initiator systems. IV. Polymerization of methyl methacrylate by systems of some activated metals and organic halides. J. Polym. Sci. Part A Polym. Chem. 1968, 6, 3075–3085. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, L.; Cui, X.; Lv, J.; Zhang, P.; Tang, H. Facile Synthesis of Ultrahigh Molecular Weight Poly(Methyl Methacrylate) by Organic Halides in the Presence of Palladium Nanoparticles. Polymers 2020, 12, 2747. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, Q.; Mao, W.; Lv, J.; Tang, H.; Tang, H. Synthesis of Ultrahigh Molecular Weight Poly(methyl Methacrylate) via the Polymerization of MMA Initiated by the Combination of Palladium Carboxylates with Thiols. Polymers 2023, 15, 2501. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, Y.; Su, H.; Liu, S.; Liu, Y.; Li, Q.; Xia, C. Novel insights into the mechanism for protic solvent promoting Pd/C-catalyzed hydrodechlorination of chlorinated organic compounds. Chem. Eng. J. 2022, 431, 133729. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.M.; Friedrich, H.B. The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions. Molecules 2018, 23, 1676. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. Chemicals from Alkynes with Palladium Catalysts. Chem. Rev. 2014, 114, 1783–1826. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Z.; Wu, X.; Jiang, X.; Li, H.; Xu, J.; Yang, K.; Lin, D. Few-Atomic Zero-Valent Palladium Ensembles for Efficient Reductive Dehydrogenation and Dehalogenation Catalysis. ACS Nano 2023, 17, 22859–22871. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, H.M.; Fang, L.P.; Xu, C.H.; He, Z.L.; Lai, Y.J.; Zhao, H.C.; Bekana, D.; Liu, J.F. Au@Pd Bimetallic Nanocatalyst for Carbon-Halogen Bond Cleavage: An Old Story with New Insight into How the Activity of Pd is Influenced by Au. Environ. Sci. Technol. 2018, 52, 4244–4255. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.; Freemantle, R.G.; Obare, S.O. Monodisperse Thioether-Stabilized Palladium Nanoparticles: Synthesis, Characterization, and Reactivity. Chem. Mater. 2007, 19, 3464–3471. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Chernikova, E.V.; Kostina, Y.V.; Kulichikhin, V.G.; Malkin, A.Y. Viscosity of polyacrylonitrile solutions: The effect of the molecular weight. Polym. Sci. Ser. A 2015, 57, 494–500. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Tamate, R.; Hiroi, T.; Samitsu, S.; Fujii, K.; Ueki, T. Highly stretchable and self-healable polymer gels from physical entanglements of ultrahigh-molecular weight polymers. Sci. Adv. 2022, 8, eadd0226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Lv, Y.; An, Z.S. Enzymatic Cascade Catalysis for the Synthesis of Multiblock and Ultrahigh-Molecular-Weight Polymers with Oxygen Tolerance. Angew. Chem. Int. Ed. 2017, 56, 13852–13856. [Google Scholar] [CrossRef]
- An, Z. 100th Anniversary of Macromolecular Science Viewpoint: Achieving Ultrahigh Molecular Weights with Reversible Deactivation Radical Polymerization. ACS Macro Lett. 2020, 9, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Mouat, A.R.; Whitford, C.L.; Chen, B.-R.; Liu, S.; Perras, F.A.; Pruski, M.; Bedzyk, M.J.; Delferro, M.; Stair, P.C.; Marks, T.J. Synthesis of Supported Pd0 Nanoparticles from a Single-Site Pd2+ Surface Complex by Alkene Reduction. Chem. Mater. 2018, 30, 1032–1044. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Tsikourkitoudi, V.; Stathi, P.; Wegner, K.; Papavasiliou, J.; Louloudi, M. PdO/Pd0/TiO2 Nanocatalysts Engineered by Flame Spray Pyrolysis: Study of the Synergy of PdO/Pd0 on H2 Production by HCOOH Dehydrogenation and the Deactivation Mechanism. Energy Fuels 2020, 34, 15026–15038. [Google Scholar] [CrossRef]
- Peng, H.-C.; Xie, S.; Park, J.; Xia, X.; Xia, Y. Quantitative Analysis of the Coverage Density of Br–Ions on Pd{100} Facets and Its Role in Controlling the Shape of Pd Nanocrystals. J. Am. Chem. Soc. 2013, 135, 3780–3783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.T.; Chen, J.G.; Liu, Z.W.; Lu, J. New process for synthesizing fluorinated polymers in supercritical carbon dioxide. Macromolecules 2008, 41, 6987–6992. [Google Scholar] [CrossRef]
- Sofyane, A.; Atlas, S.; Lahcini, M.; Vidovic, E.; Ameduri, B.; Raihane, M. Insights into hydrophobic (meth)acrylate polymers as coating for slow-release fertilizers to reduce nutrient leaching. Polym. Chem. 2024, 15, 3327–3340. [Google Scholar] [CrossRef]
- Song, H.H.; Jiang, Y.H.; Chen, C.X.; Wen, S.M.; Zhou, Z.Z.; Yan, C.H.; Cong, W. Durable photobioreactor antibiofouling coatings for microalgae cultivation by photoreactive poly(2,2,2-trifluoroethyl methacrylate). Biofouling 2024, 40, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-F.; Jiang, F.-J.; Zhang, P.-P.; Luo, J.; Tang, H.-D. Atom transfer radical polymerization and copolymerization of isoprene catalyzed by copper bromide/2,2′-bipyridine. Chin. Chem. Lett. 2016, 27, 910–914. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Ma, Y.; Chen, H.; Yang, W. One-Pot Synthesis of PTFEMA-b-PMMA-b-PTFEMA by Controlled Radical Polymerization with a Difunctional Initiator in Conjugation with Photoredox Catalyst of Ir(ppy)3 Under Visible Light. Macromol. Chem. Phys. 2013, 214, 2624–2631. [Google Scholar] [CrossRef]
- Chen, J.-G.; Feng, X.; Wang, M.-X.; Shen, S.; Li, Y.; Wang, W.; Liu, Z.-T.; Liu, Z.-W.; Jiang, J.; Lu, J. Controlled radical polymerization of fluorinated methacrylates in supercritical CO2: Synthesis and application of a novel RAFT agent. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 825–834. [Google Scholar] [CrossRef]
- Borman, C.D.; Jackson, A.T.; Bunn, A.; Cutter, A.L.; Irvine, D.J. Evidence for the low thermal stability of poly(methyl methacrylate) polymer produced by atom transfer radical polymerisation. Polymer 2000, 41, 6015–6020. [Google Scholar] [CrossRef]
- Hatada, K.; Kitayama, T.; Ute, K.; Terawaki, Y.; Yanagida, T. End-Group Analysis of Poly(methyl methacrylate) Prepared with Benzoyl Peroxide by 750 MHz High-Resolution 1H NMR Spectroscopy. Macromolecules 1997, 30, 6754–6759. [Google Scholar] [CrossRef]
- Pafiti, K.S.; Loizou, E.; Patrickios, C.S.; Porcar, L. End-Linked Semifluorinated Amphiphilic Polymer Conetworks: Synthesis by Sequential Reversible Addition−Fragmentation Chain Transfer Polymerization and Characterization. Macromolecules 2010, 43, 5195–5204. [Google Scholar] [CrossRef]
- Cui, M.; Tian, X.; Zou, G.; Zhu, B.; Zhang, Q. Composite optical fiber polarizer with ternary copolymer overlay for large range modulation of phase difference. Opt. Mater. 2017, 66, 415–421. [Google Scholar] [CrossRef]
- McCay, P.B.; Lai, E.K.; Poyer, J.L.; DuBose, C.M.; Janzen, E.G. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J. Biol. Chem. 1984, 259, 2135–2143. [Google Scholar] [CrossRef]
- Yamada, B.; Westmoreland, D.G.; Kobatake, S.; Konosu, O. ESR spectroscopic studies of radical polymerization. Prog. Polym. Sci. 1999, 24, 565–630. [Google Scholar] [CrossRef]
- Imann, R.; Bandermann, F.; Korth, H.-G. Free-radical polymerization of acrylates and vinyl acetates initiated by transition metal/hydrosilane two-component initiation systems. Macromol. Chem. Phys. Suppl. 1996, 197, 921–935. [Google Scholar] [CrossRef]
- Haire, L.D.; Krygsman, P.H.; Janzen, E.G.; Oehler, U.M. Correlation of radical structure with EPR spin adduct parameters: Utility of the proton, carbon-13, and nitrogen-14 hyperfine splitting constants of aminoxyl adducts of PBN-nitronyl-13C for three-parameter scatter plots. J. Org. Chem. 1988, 53, 4535–4542. [Google Scholar] [CrossRef]
- Yang, C.; Tartaglino, U.; Persson, B.N.J. Influence of Surface Roughness on Superhydrophobicity. Phys. Rev. Lett. 2006, 97, 116103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of surface roughness on contact angle hysteresis and spreading work. Colloid. Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Bormashenko, E. Breath Figures; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lee, L.R.; Liu, C.T.; Tseng, H.F.; Lin, K.T.; Chu, C.W.; Chen, J.T. Hierarchical Polymer Structures Using Templates and the Modified Breath Figure Method. Langmuir 2018, 34, 7472–7478. [Google Scholar] [CrossRef] [PubMed]


| Entry | Initiator 2 | TFEMA/Init./Pd NPs (Molar Ratio) | Conv. (%) | Mn (Da) | Mw (Da) | PDI (95% CI) | η (dL/g) | Mv (Da) |
|---|---|---|---|---|---|---|---|---|
| 1 | - | 3.95 × 104:0:1 | - | - | - | - | - | - |
| 2 | α-EBP | 3.95 × 104:756:1 | 16.9 | 5.81 × 105 | 1.71 × 106 | 2.87 ± 0.07 | 1.26 | 1.98 × 106 |
| 3 | α-BPA | 3.95 × 104:756:1 | 5.6 | 4.75 × 105 | 1.11 × 106 | 2.35 ± 0.04 | 1.11 | 1.24 × 106 |
| 4 | ECP | 3.95 × 104:756:1 | 12.4 | 5.10 × 105 | 2.84 × 106 | 5.42 ± 0.41 | 1.59 | 3.63 × 106 |
| 5 | EBiB | 3.95 × 104:756:1 | 46.7 | 2.55 × 106 | 1.06 × 107 | 4.53 ± 0.19 | 3.88 | 1.47 × 107 |
| 6 | EBP | 3.95 × 104:756:1 | 43.0 | 3.03 × 106 | 1.54 × 107 | 4.99 ± 0.07 | 4.00 | 1.53 × 107 |
| Entry | TFEMA/Init./Pd NPs (Molar Ratio) | T (°C) | Time (h) | Conv. (%) | Mn (Da) | Mw (Da) | PDI (95% CI) | η (dL/g) | Mv (Da) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.95 × 104:756:2 | 80 | 18 | 98.7 | 1.30 × 106 | 6.37 × 106 | 4.97 ± 0.08 | 2.43 | 7.64 × 106 |
| 3.95 × 104:756:1.5 | 80 | 20 | 95.8 | 2.19 × 106 | 1.11 × 107 | 4.85 ± 0.21 | 3.69 | 1.38 × 107 | |
| 3.95 × 104:756:1 | 80 | 20 | 65.8 | 2.51 × 106 | 1.23 × 107 | 4.62 ± 0.19 | 3.76 | 1.41 × 107 | |
| 2 | 3.95 × 104:756:1.5 | 80 | 20 | 95.8 | 2.19 × 106 | 1.11 × 107 | 4.85 ± 0.21 | 3.69 | 1.38 × 107 |
| 3.95 × 104:527:1.5 | 80 | 20 | 81.2 | 2.36 × 106 | 1.36 × 107 | 4.74 ± 0.21 | 4.25 | 1.65 × 107 | |
| 3.95 × 104:298:1.5 | 80 | 20 | 58.3 | 1.32 × 106 | 9.50 × 106 | 8.37 ± 0.54 | 3.02 | 1.05 × 107 | |
| 3.95 × 104:69:1.5 | 80 | 20 | 49.8 | 1.17 × 106 | 9.66 × 106 | 6.55 ± 0.37 | 3.15 | 1.11 × 107 | |
| 3 | 3.95 × 104:527:1.5 | 70 | 24 | 76.7 | 2.48 × 106 | 1.35 × 107 | 5.70 ± 0.23 | 3.86 | 1.46 × 107 |
| 3.95 × 104:527:1.5 | 90 | 13 | 82.6 | 1.72 × 106 | 7.08 × 106 | 4.14 ± 0.06 | 3.55 | 1.31 × 107 | |
| 3.95 × 104:527:1.5 | 100 | 9 | 90.3 | 1.61 × 106 | 5.47 × 106 | 3.25 ± 0.07 | 3.34 | 1.21 × 107 |
| Entry | TFEMA/Init./Pd NPs (Molar Ratio) | DPPH (mol/L) | Time (h) | T (°C) | Conv. (%) |
|---|---|---|---|---|---|
| 1 | 3.95 × 104:756:1 | 0 | 17 | 90 | 81.1 |
| 2 | 3.95 × 104:756:1 | 3.2 × 10−3 | 17 | 90 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, J.; Yu, X.; He, M.; Han, W.; Li, Y.; Liu, Z.; Zhang, P.; Tang, H. Synthesis of Ultrahigh Molecular Weight Poly (Trifluoroethyl Methacrylate) Initiated by the Combination of Palladium Nanoparticles with Organic Halides. Polymers 2024, 16, 2764. https://doi.org/10.3390/polym16192764
Guan J, Yu X, He M, Han W, Li Y, Liu Z, Zhang P, Tang H. Synthesis of Ultrahigh Molecular Weight Poly (Trifluoroethyl Methacrylate) Initiated by the Combination of Palladium Nanoparticles with Organic Halides. Polymers. 2024; 16(19):2764. https://doi.org/10.3390/polym16192764
Chicago/Turabian StyleGuan, Jian, Xiaodi Yu, Minghui He, Wenfeng Han, Ying Li, Zongjian Liu, Panpan Zhang, and Haodong Tang. 2024. "Synthesis of Ultrahigh Molecular Weight Poly (Trifluoroethyl Methacrylate) Initiated by the Combination of Palladium Nanoparticles with Organic Halides" Polymers 16, no. 19: 2764. https://doi.org/10.3390/polym16192764
APA StyleGuan, J., Yu, X., He, M., Han, W., Li, Y., Liu, Z., Zhang, P., & Tang, H. (2024). Synthesis of Ultrahigh Molecular Weight Poly (Trifluoroethyl Methacrylate) Initiated by the Combination of Palladium Nanoparticles with Organic Halides. Polymers, 16(19), 2764. https://doi.org/10.3390/polym16192764







