Evaluation of Additives on the Cell Metabolic Activity of New PHB/PLA-Based Formulations by Means of Material Extrusion 3D Printing for Scaffold Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of PHB/PLA Biocomposites
2.3. 3D Printing of PHB/PLA Biocomposites
2.4. Mechanical Characterization
2.5. Thermal Properties
2.6. Water Contact Angle (WCA)
2.7. Morphological Characterization
2.8. Cell Seeding and Culture
2.9. Cell Metabolic Activity Evaluation
3. Results
3.1. Effect of Additives in PHB/PLA Blend
3.1.1. Mechanical Properties of the PHB/PLA Blends
3.1.2. Thermal Properties
3.2. Scaffold Characterization
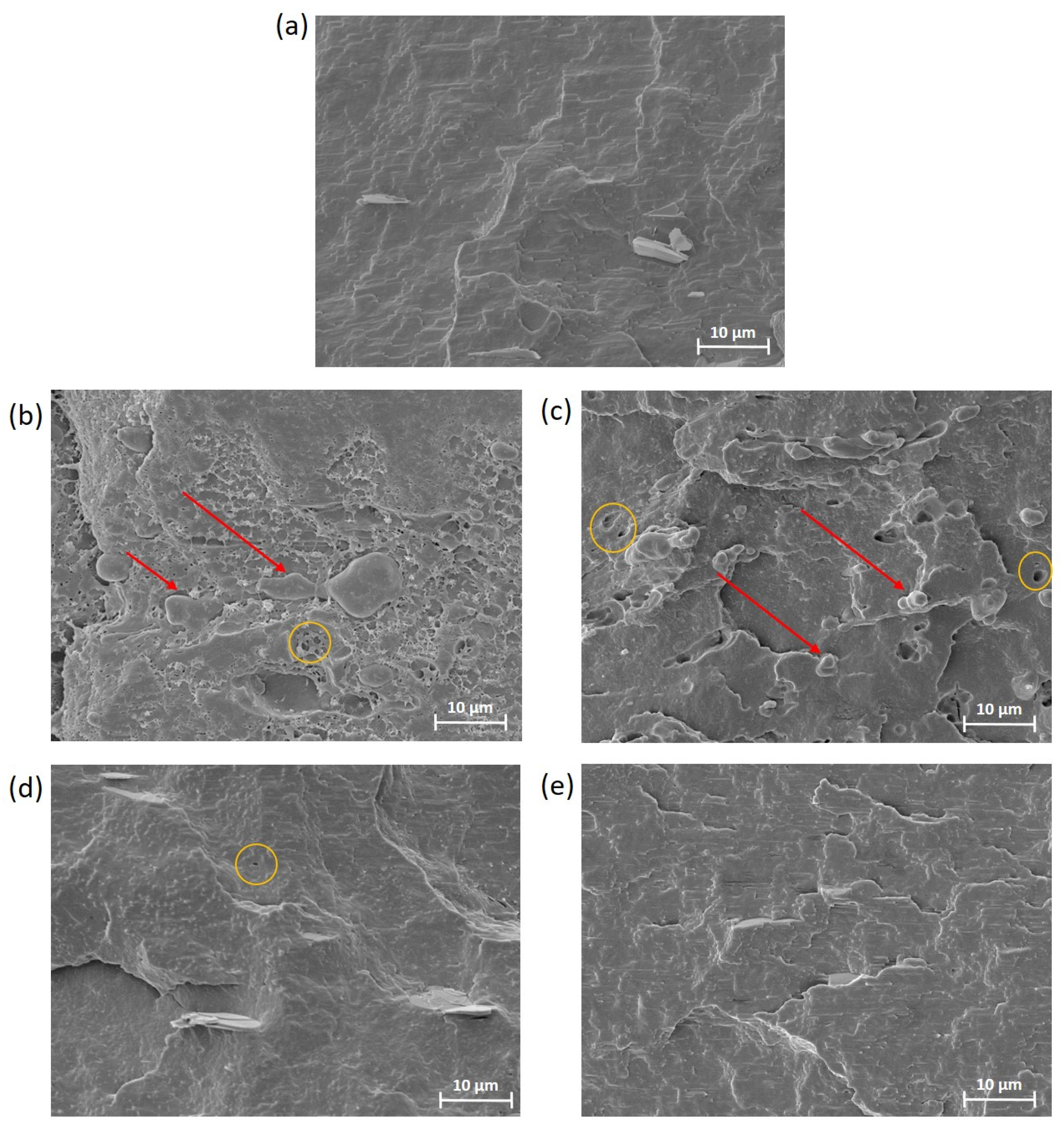
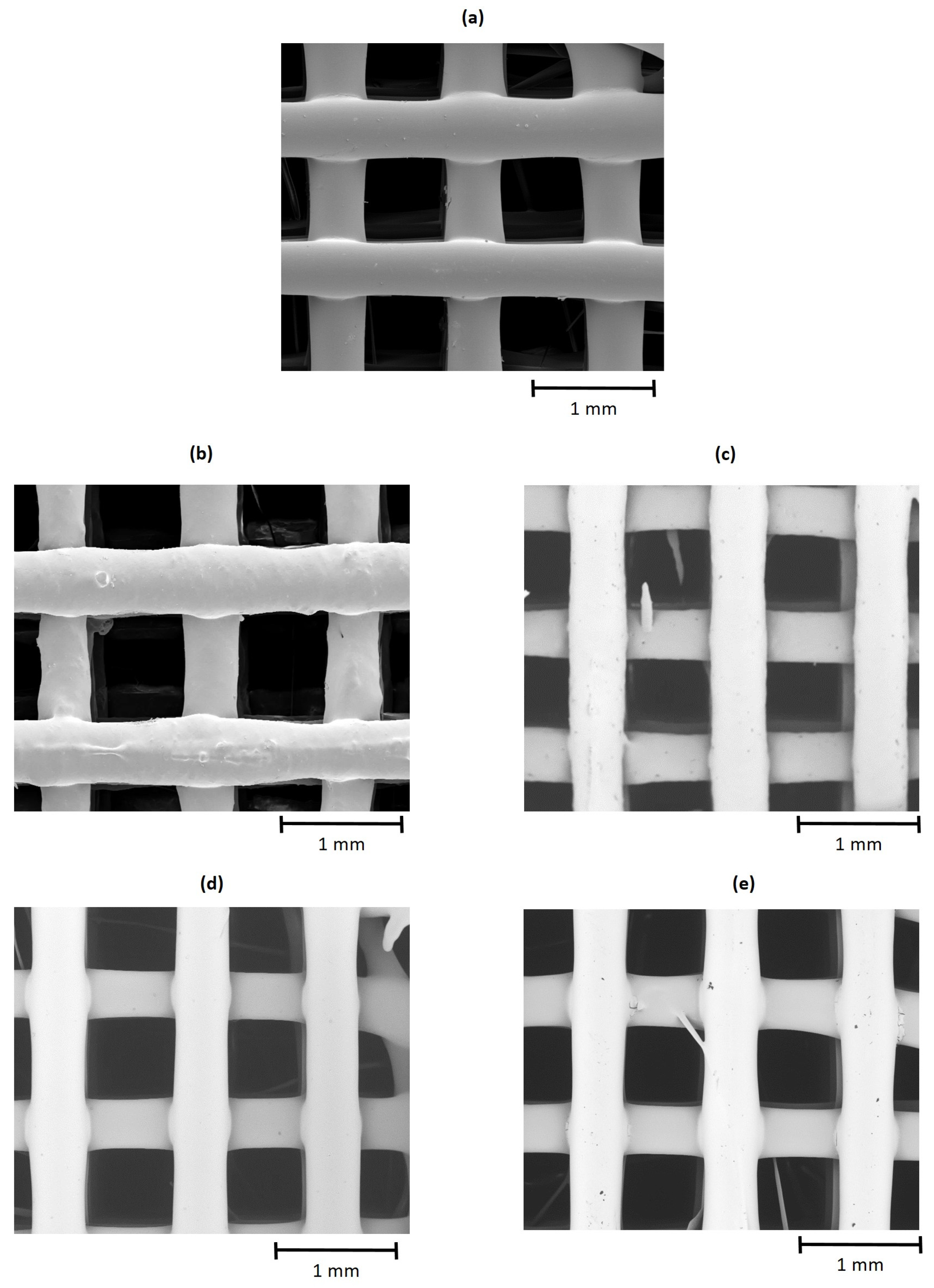
3.2.1. Porosity, Pore Size and Surface Morphology
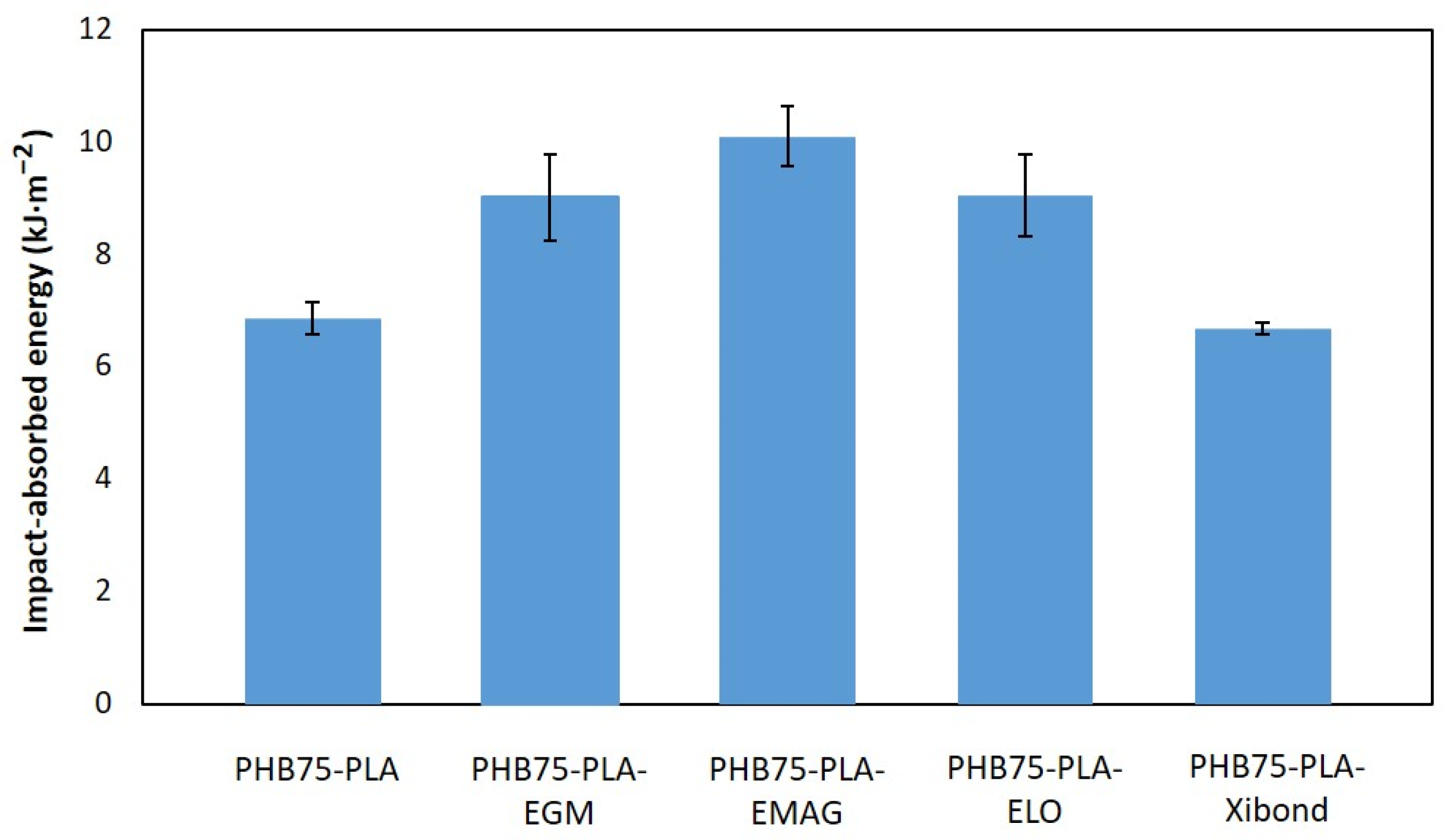
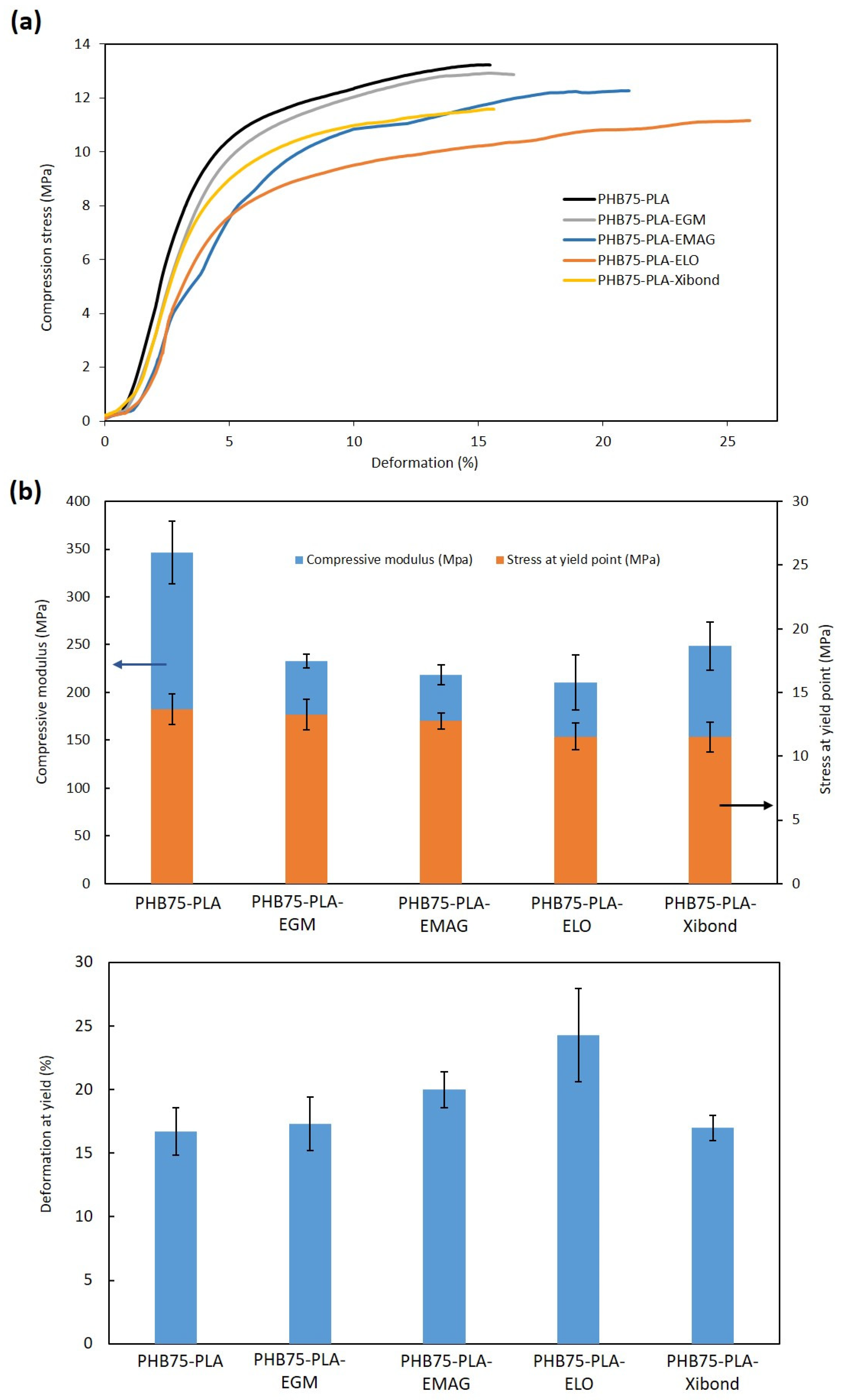
3.2.2. Compression Test
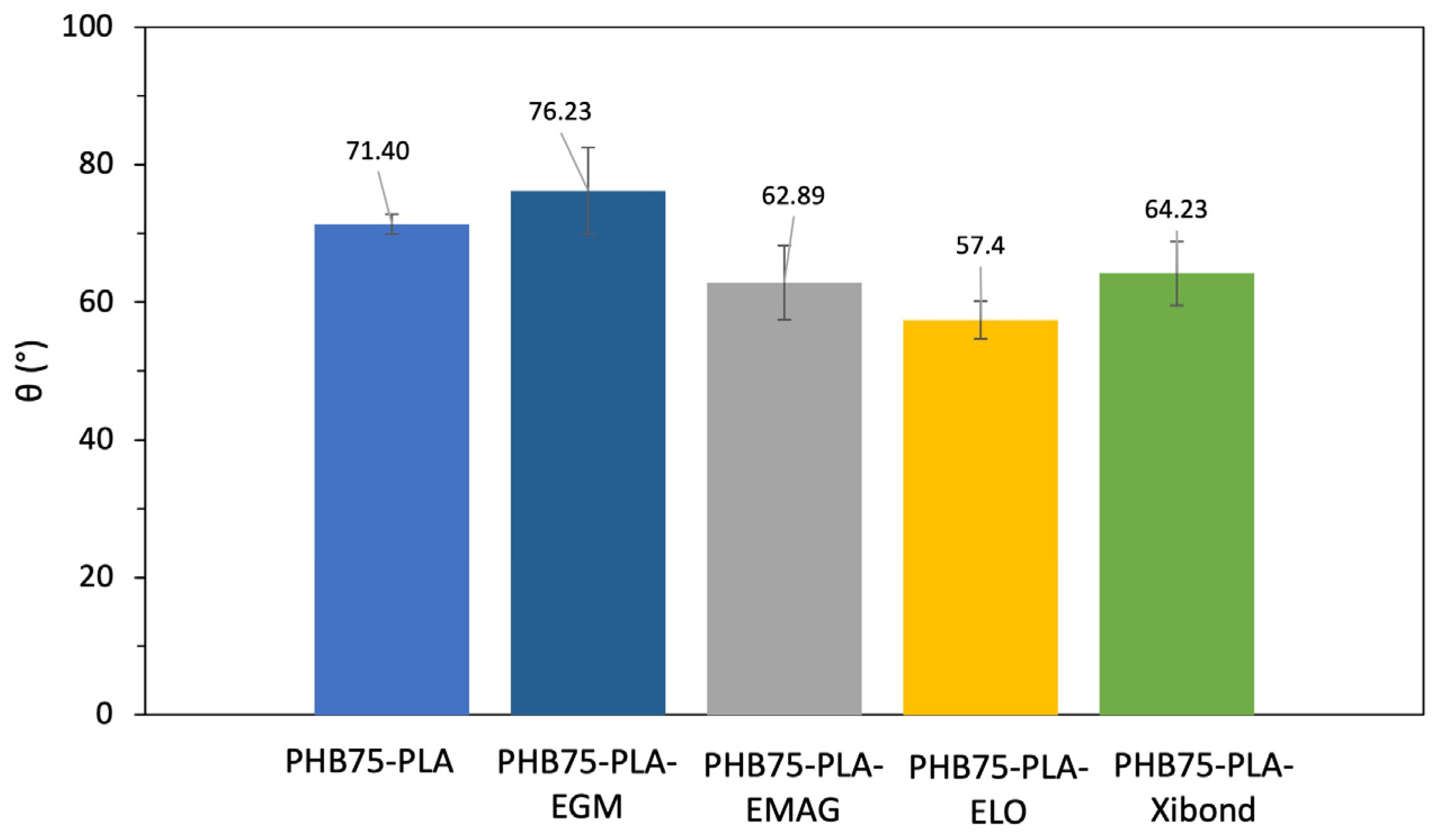
3.2.3. Water Contact Angle Measurement (WCA)
3.2.4. Cell Metabolic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigil Fuentes, M.A.; Thakur, S.; Wu, F.; Misra, M.; Gregori, S.; Mohanty, A.K. Study on the 3D Printability of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Poly(Lactic Acid) Blends with Chain Extender Using Fused Filament Fabrication. Sci. Rep. 2020, 10, 11804. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.; Rosen, D.W.; Stucker, B.; Khorasani, M.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Wang, S.; Li, R.; Qing, Y.; Wei, Y.; Wang, Q.; Zhang, T.; Sun, C.; Qin, Y.; Li, D.; Yu, J. Antibacterial Activity of Ag-Incorporated Zincosilicate Zeolite Scaffolds Fabricated by Additive Manufacturing. Inorg. Chem. Commun. 2019, 105, 31–35. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA Scaffolds for Bone Tissue Replacement Fabricated by Ordinary Commercial 3D Printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Laschke, M.W.; Augustin, V.A.; Sahin, F.; Anschütz, D.; Metzger, W.; Scheuer, C.; Bischoff, M.; Aktas, C.; Menger, M.D. Surface Modification by Plasma Etching Impairs Early Vascularization and Tissue Incorporation of Porous Polyethylene (Medpor®) Implants. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1738–1748. [Google Scholar] [CrossRef]
- Rebia, R.A.; Rozet, S.; Tamada, Y.; Tanaka, T. Biodegradable PHBH/PVA Blend Nanofibers: Fabrication, Characterization, in Vitro Degradation, and in Vitro Biocompatibility. Polym. Degrad. Stab. 2018, 154, 124–136. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold Degradation in Bone Tissue Engineering: An Overview. Int. Biodeterior. Biodegrad. 2023, 180, 105599. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- Sabino, M.; Fermín, Z.; Marielys, L.; Moret, J.; Rodríguez, D.; Rezende, R.A.; Neto, P.I.; Pereira, F.D.S.; Silva, J.V.L.; Alvarez, J. In Vitro Biocompatibility Study of Biodegradable Polyester Scaffolds Constructed Using Fused Deposition Modeling (FDM). IFAC Proc. Vol. 2013, 46, 356–360. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Daculsi, G.; Goyenvalle, E.; Cognet, R.; Aguado, E.; Suokas, E.O. Osteoconductive Properties of Poly(96L/4D-Lactide)/Beta-Tricalcium Phosphate in Long Term Animal Model. Biomaterials 2011, 32, 3166–3177. [Google Scholar] [CrossRef] [PubMed]
- Abert, J.; Amella, A.; Weigelt, S.; Fischer, H. Degradation and Swelling Issues of Poly-(d,l-Lactide)/β-Tricalcium Phosphate/Calcium Carbonate Composites for Bone Replacement. J. Mech. Behav. Biomed. Mater. 2016, 54, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.-H.; Wu, Q.; Zhang, K.-Y.; Chen, G.Q. In Vivo Studies of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Based Polymers: Biodegradation and Tissue Reactions. Biomaterials 2006, 27, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Kontárová, S.; Přikryl, R.; Melčová, V.; Menčík, P.; Horálek, M.; Figalla, S.; Plavec, R.; Feranc, J.; Sadílek, J.; Pospíšilová, A. Printability, Mechanical and Thermal Properties of Poly(3-Hydroxybutyrate)-Poly(Lactic Acid)-Plasticizer Blends for Three-Dimensional (3D) Printing. Materials 2020, 13, 4736. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Akoulina, E.A.; Chesnokova, D.V.; Wenhao, Y.; Makhina, T.K.; Demyanova, I.V.; Zhuikova, Y.V.; Voinova, V.V.; Belishev, N.V.; Surmenev, R.A.; et al. The Growth of 3T3 Fibroblasts on PHB, PLA and PHB/PLA Blend Films at Different Stages of Their Biodegradation In Vitro. Polymers 2021, 13, 108. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Mokrane, N.; Kaci, M.; Lopez-Cuesta, J.-M.; Dehouche, N. Combined Effect of Poly(Lactic Acid)-Grafted Maleic Anhydride Compatibilizer and Halloysite Nanotubes on Morphology and Properties of Polylactide/Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Blends. Materials 2023, 16, 6438. [Google Scholar] [CrossRef]
- Frone, A.N.; Batalu, D.; Chiulan, I.; Oprea, M.; Gabor, A.R.; Nicolae, C.-A.; Raditoiu, V.; Trusca, R.; Panaitescu, D.M. Morpho-Structural, Thermal and Mechanical Properties of PLA/PHB/Cellulose Biodegradable Nanocomposites Obtained by Compression Molding, Extrusion, and 3D Printing. Nanomaterials 2020, 10, 51. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Quiles-Carrillo, L.; Richart, N.; Torres-Giner, S.; Montanes, N. Enhanced Interfacial Adhesion of Polylactide/Poly(ε-Caprolactone)/Walnut Shell Flour Composites by Reactive Extrusion with Maleinized Linseed Oil. Polymers 2019, 11, 758. [Google Scholar] [CrossRef]
- Lopera-Valle, A.; Caputo, J.V.; Leão, R.; Sauvageau, D.; Luz, S.M.; Elias, A. Influence of Epoxidized Canola Oil (ECO) and Cellulose Nanocrystals (CNCs) on the Mechanical and Thermal Properties of Polyhydroxybutyrate (PHB)—Poly(Lactic Acid) (PLA) Blends. Polymers 2019, 11, 933. [Google Scholar] [CrossRef]
- Garcia-Campo, M.J.; Quiles-Carrillo, L.; Sanchez-Nacher, L.; Balart, R.; Montanes, N. High Toughness Poly(Lactic Acid) (PLA) Formulations Obtained by Ternary Blends with Poly(3-Hydroxybutyrate) (PHB) and Flexible Polyesters from Succinic Acid. Polym. Bull. 2019, 76, 1839–1859. [Google Scholar] [CrossRef]
- Xiao, X.; Chevali, V.S.; Song, P.; He, D.; Wang, H. Polylactide/Hemp Hurd Biocomposites as Sustainable 3D Printing Feedstock. Compos. Sci. Technol. 2019, 184, 107887. [Google Scholar] [CrossRef]
- Jorda, M.; Montava-Jorda, S.; Balart, R.; Lascano, D.; Montanes, N.; Quiles-Carrillo, L. Functionalization of Partially Bio-Based Poly(Ethylene Terephthalate) by Blending with Fully Bio-Based Poly(Amide) 10,10 and a Glycidyl Methacrylate-Based Compatibilizer. Polymers 2019, 11, 1331. [Google Scholar] [CrossRef]
- Bin, Y.; Yang, B.; Wang, H. The Effect of a Small Amount of Modified Microfibrillated Cellulose and Ethylene–Glycidyl Methacrylate Copolymer on the Crystallization Behaviors and Mechanical Properties of Polylactic Acid. Polym. Bull. 2018, 75, 3377–3394. [Google Scholar] [CrossRef]
- Garcia-Campo, M.J.; Quiles-Carrillo, L.; Masia, J.; Reig-Pérez, M.J.; Montanes, N.; Balart, R. Environmentally Friendly Compatibilizers from Soybean Oil for Ternary Blends of Poly(Lactic Acid)-PLA, Poly(ε-Caprolactone)-PCL and Poly(3-Hydroxybutyrate)-PHB. Materials 2017, 10, 1339. [Google Scholar] [CrossRef]
- Kilic, N.T.; Can, B.N.; Kodal, M.; Ozkoc, G. Compatibilization of PLA/PBAT Blends by Using Epoxy-POSS. J. Appl. Polym. Sci. 2019, 136, 47217. [Google Scholar] [CrossRef]
- Vernaez, O.; Neubert, K.J.; Kopitzky, R.; Kabasci, S. Compatibility of Chitosan in Polymer Blends by Chemical Modification Of-Based Polyesters. Polymers 2019, 11, 1939. [Google Scholar] [CrossRef]
- ISO 527-1:2019; Plastics—Determination of Tensile Propertiespart 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2019.
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric Materials for Bone and Cartilage Repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- ISO 178: 2019-08; Plastics–Determination of Flexural Properties. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- ISO 604: 2002; UNE-EN-ISO, UNE Norma Española. 604. Plásticos, Determinación de Las Propiedades En Compresión. International Organization for Standardization (ISO): Geneva, Switzerland, 2003.
- Ivorra-Martinez, J.; Ferrer, I.; Aguado, R.; Delgado-Aguilar, M.; Garcia-Romeu, M.L.; Boronat, T. Development of P (3HB-Co-3HHx) Nanohydroxyapatite (NHA) Composites for Scaffolds Manufacturing by Means of Fused Deposition Modeling. Int. J. Bioprint. 2024, 10, 274–293. [Google Scholar] [CrossRef]
- Loureiro, N.C.; Ghosh, S.; Viana, J.C.; Esteves, J.L. Thermal Characterization of Polyhydroxyalkanoates and Poly(Lactic Acid) Blends Obtained by Injection Molding. Polym. Plast. Technol. Eng. 2015, 54, 350–356. [Google Scholar] [CrossRef]
- Meng, B.; Deng, J.; Liu, Q.; Wu, Z.; Yang, W. Transparent and Ductile Poly(Lactic Acid)/Poly(Butyl Acrylate) (PBA) Blends: Structure and Properties. Eur. Polym. J. 2012, 48, 127–135. [Google Scholar] [CrossRef]
- Maziad, N.A.; EL-Nashar, D.E.; Sadek, E.M. The Effects of a Silane Coupling Agent on Properties of Rice Husk-Filled Maleic Acid Anhydride Compatibilized Natural Rubber/Low-Density Polyethylene Blend. J. Mater. Sci. 2009, 44, 2665–2673. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Donate, R.; Monzón, M.; Ortega, Z.; Wang, L.; Ribeiro, V.; Pestana, D.; Oliveira, J.M.; Reis, R.L. Comparison between Calcium Carbonate and β-Tricalcium Phosphate as Additives of 3D Printed Scaffolds with Polylactic Acid Matrix. J. Tissue Eng. Regen. Med. 2020, 14, 272–283. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Gomez-Caturla, J.; Sanchez-Nacher, L.; Montanes, N.; Quiles-Carrillo, L. Improved Toughness of Polylactide by Binary Blends with Polycarbonate with Glycidyl and Maleic Anhydride-Based Compatibilizers. Macromol. Mater. Eng. 2021, 306, 2100480. [Google Scholar] [CrossRef]
- Chang, B.P.; Mohanty, A.K.; Misra, M. Tuning the Compatibility to Achieve Toughened Biobased Poly(Lactic Acid)/Poly(Butylene Terephthalate) Blends. RSC Adv. 2018, 8, 27709–27724. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Gomez-Caturla, J.; Cardona, S.C.; Lora-García, J.; Fombuena, V. Novel Compatibilizers and Plasticizers Developed from Epoxidized and Maleinized Chia Oil in Composites Based on PLA and Chia Seed Flour. Eur. Polym. J. 2022, 173, 111289. [Google Scholar] [CrossRef]
- Han, Y.; Shi, J.; Mao, L.; Wang, Z.; Zhang, L. Improvement of Compatibility and Mechanical Performances of PLA/PBAT Composites with Epoxidized Soybean Oil as Compatibilizer. Ind. Eng. Chem. Res. 2020, 59, 21779–21790. [Google Scholar] [CrossRef]
- Graupner, N.; Müssig, J. A Comparison of the Mechanical Characteristics of Kenaf and Lyocell Fibre Reinforced Poly(Lactic Acid) (PLA) and Poly(3-Hydroxybutyrate) (PHB) Composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2010–2019. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Fenollar, O.; Balart, R.; Torres-Giner, S.; Rallini, M.; Dominici, F.; Torre, L. A Comparative Study on the Reactive Compatibilization of Melt-Processed Polyamide 1010/Polylactide Blends by Multi-Functionalized Additives Derived from Linseed Oil and Petroleum. Express Polym. Lett. 2020, 14, 583–604. [Google Scholar] [CrossRef]
- Iglesias-Montes, M.L.; Soccio, M.; Siracusa, V.; Gazzano, M.; Lotti, N.; Cyras, V.P.; Manfredi, L.B. Chitin Nanocomposite Based on Plasticized Poly(Lactic Acid)/Poly(3-Hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials. Polymers 2022, 14, 3177. [Google Scholar] [CrossRef]
- D’Amico, D.A.; Iglesias Montes, M.L.; Manfredi, L.B.; Cyras, V.P. Fully Bio-Based and Biodegradable Polylactic Acid/Poly(3-Hydroxybutirate) Blends: Use of a Common Plasticizer as Performance Improvement Strategy. Polym. Test 2016, 49, 22–28. [Google Scholar] [CrossRef]
- Sui, G.; Wang, K.; Xu, S.; Liu, Z.; Zhang, Q.; Du, R.; Fu, Q. The Combined Effect of Reactive and High-Shear Extrusion on the Phase Morphologies and Properties of PLA/OBC/EGMA Ternary Blends. Polymer 2019, 169, 66–73. [Google Scholar] [CrossRef]
- Perez-Nakai, A.; Lerma-Canto, A.; Dominguez-Candela, I.; Ferri, J.M.; Fombuena, V. Novel Epoxidized Brazil Nut Oil as a Promising Plasticizing Agent for PLA. Polymers 2023, 15, 1997. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Sarkar, B.; Samantaray, D.P.; Daware, A.; Maity, S.; Pattnaik, S.; Bhattacharjee, S. Bioconversion of Fish Solid Waste into PHB Using Bacillus Subtilis Based Submerged Fermentation Process. Environ. Technol. 2017, 38, 3201–3208. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Ferri, J.M.; Cardona, S.C.; Lora, J.; Fombuena, V. Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (Salvia hispanica L.) to Improve Ductility and Thermal Properties of Poly(Lactic Acid). Polymers 2021, 13, 1283. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and Characterization of High Environmental Efficiency Composites Based on PLA and Hazelnut Shell Flour (HSF) with Biobased Plasticizers Derived from Epoxidized Linseed Oil (ELO). Compos. B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Lai, S.-M.; Li, H.-C.; Liao, Y.-C. Properties and Preparation of Compatibilized Nylon 6 Nanocomposites/ABS Blends: Part II—Physical and Thermal Properties. Eur. Polym. J. 2007, 43, 1660–1671. [Google Scholar] [CrossRef]
- Dong, W.; Ma, P.; Wang, S.; Chen, M.; Cai, X.; Zhang, Y. Effect of Partial Crosslinking on Morphology and Properties of the Poly(β-Hydroxybutyrate)/Poly(d,l-Lactic Acid) Blends. Polym. Degrad. Stab. 2013, 98, 1549–1555. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Montanes, N.; Lopez-Martinez, J.; Balart, R. Plasticization Effects of Epoxidized Vegetable Oils on Mechanical Properties of Poly(3-Hydroxybutyrate). Polym. Int. 2016, 65, 1157–1164. [Google Scholar] [CrossRef]
- Benwood, C.; Anstey, A.; Andrzejewski, J.; Misra, M.; Mohanty, A.K. Improving the Impact Strength and Heat Resistance of 3D Printed Models: Structure, Property, and Processing Correlationships during Fused Modeling (FDM) of Poly(Lactic Acid). ACS Omega 2018, 3, 4400–4411. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg Stephan and Kukla, C.; Sapkota, J.; Holzer, C. Additive Manufacturing of Metallic and Ceramic Components by the Material Extrusion of Highly-Filled Polymers: A Review and Future. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Jia, J.; Yu, L.; Min, A.; Yang, S.; Shuai, C. Accelerated Degradation of Poly(L-Lactide) Bone Scaffold: Crystallinity Hydrophilicity. Mater. Chem. Phys. 2021, 266, 124545. [Google Scholar] [CrossRef]
- Hablot, E.; Bordes, P.; Pollet, E.; Avérous, L. Thermal and Thermo-Mechanical Degradation of Poly(3-Hydroxybutyrate)-Based Multiphase Systems. Polym. Degrad. Stab. 2008, 93, 413–421. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Generalized Kinetic Master Plots for the Thermal Degradation of Polymers Following a Random Scission Mechanism. J. Phys. Chem. A 2010, 114, 7868–7876. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of Flexible Materials Based on Plasticized Electrospun PLA–PHB Blends: Structural, Thermal, Mechanical and Disintegration Properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, C.; Cai, J.; Yan, H.; Huang, X.; Yang, H.; Cheng, R. Effects of Macromolecular Compatibilizers Containing Epoxy Groups on the Properties of Linear Low-Density Polyethylene/Magnesium Hydroxide Composites. Ind. Eng. Chem. Res. 2010, 49, 6291–6301. [Google Scholar] [CrossRef]
- Jebrane, M.; Cai, S.; Panov, D.; Yang, X.; Terziev, N. Synthesis and Characterization of New Vinyl Acetate Grafting onto Epoxidized Linseed Oil in Aqueous Media. J. Appl. Polym. Sci. 2015, 132, 42089. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Taylor, S.; Misra, M.; Mohanty, A.K. Thermo-Mechanical Characterization of Bioblends from Polylactide and Poly(Butylene Adipate-Co-Terephthalate) and Lignin. Macromol. Mater. Eng. 2015, 300, 299–311. [Google Scholar] [CrossRef]
- Caputo, M.R.; Fernandez, M.; Aguirresarobe Robert and Kovalcik, A.; Sardon, H.; Candal, M.V.; Mueller, A.J. Influence of FFF Process Conditions on the Thermal, Mechanical, and Rheological Properties of Poly(Hydroxybutyrate-Co-Hydroxy Hexanoate). Polymers 2023, 15, 1817. [Google Scholar] [CrossRef]
- Ivorra-Martinez, J.; Quiles-Carrillo, L.; Boronat, T.; Torres-Giner, S.; Covas, J.A. Assessment of the Mechanical and Thermal Properties of Injection-Molded Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate)/Hydroxyapatite Nanoparticles Parts for Use in Bone Tissue Engineering. Polymers 2020, 12, 1389. [Google Scholar] [CrossRef]
- McGregor, M.; Patel, S.; McLachlin, S.; Vlasea, M. Architectural Bone Parameters and the Relationship to Titanium Lattice Design for Powder Bed Fusion Additive Manufacturing. Addit. Manuf. 2021, 47, 102273. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Roosa, S.M.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The Pore Size of Polycaprolactone Scaffolds Has Limited Influence on Bone Regeneration in an in Vivo Model. J. Biomed. Mater. Res. A 2010, 92A, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Alam, M.; Raja, M.; Abduljaleel, Z.; Dass, L.A. MWCNTs-Reinforced Epoxidized Linseed Oil Plasticized Polylactic Acid Nanocomposite and Its Electroactive Shape Memory Behaviour. Int. J. Mol. Sci. 2014, 15, 19924–19937. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (Lactic Acid)-Based Biomaterials for Orthopaedic Regenerative Engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current State of Fabrication Technologies and Materials for Bone Tissue Engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and Reactivity of Water at Biomaterial Surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Senatov, F.; Zimina, A.; Chubrik, A.; Kolesnikov, E.; Permyakova, E.; Voronin, A.; Poponova, M.; Orlova, P.; Grunina, T.; Nikitin, K.; et al. Effect of Recombinant BMP-2 and Erythropoietin on Osteogenic Properties of Biomimetic PLA/PCL/HA and PHB/HA Scaffolds in Critical-Size Cranial Defects Model. Biomater. Adv. 2022, 135, 112680. [Google Scholar] [CrossRef]
- Darie-Niţă, R.N.; Vasile, C.; Irimia, A.; Lipşa, R.; Râpă, M. Evaluation of Some Eco-Friendly Plasticizers for PLA Films Processing. J. Appl. Polym. Sci. 2016, 133, 43223. [Google Scholar] [CrossRef]
- Lim, J.Y.; Taylor, A.F.; Li, Z.; Vogler, E.A.; Donahue, H.J. Integrin Expression and Osteopontin Regulation in Human Fetal Osteoblastic Cells Mediated by Substratum Surface Characteristics. Tissue Eng. 2005, 11, 19–29. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D Printing Smart Biomedical Scaffolds with Novel Soybean Oil Epoxidized Acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Kothapalli, C.R.; Kang, Q.K.; Ramamurthi, A. Characterization of Glycidyl Methacrylate—Crosslinked Hyaluronan Scaffolds Incorporating Elastogenic Hyaluronan Oligomers. Acta Biomater. 2011, 7, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell Lekeith and Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun Bio-Nanocomposite Scaffolds for Bone Tissue Engineering by Cellulose Nanocrystals Reinforcing Maleic Anhydride Grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef]
- Rahatuzzaman, M.; Mahmud, M.; Rahman, S.; Hoque, M.E. Design, Fabrication, and Characterization of 3D-Printed ABS and PLA Potentially for Tissue Engineering. Results Eng. 2024, 21, 101685. [Google Scholar] [CrossRef]
- Nemethova, M.; Auinger, S.; Small, J.V. Building the Actin Cytoskeleton: Filopodia Contribute to the Construction of Contractile Bundles in the Lamella. J. Cell Biol. 2008, 180, 1233–1244. [Google Scholar] [CrossRef]
- You, R.; Li, X.; Luo, Z.; Qu, J.; Li, M. Directional Cell Elongation through Filopodia-Steered Lamellipodial Extension on Patterned Silk Fibroin Films. Biointerphases 2015, 10, 011005. [Google Scholar] [CrossRef]
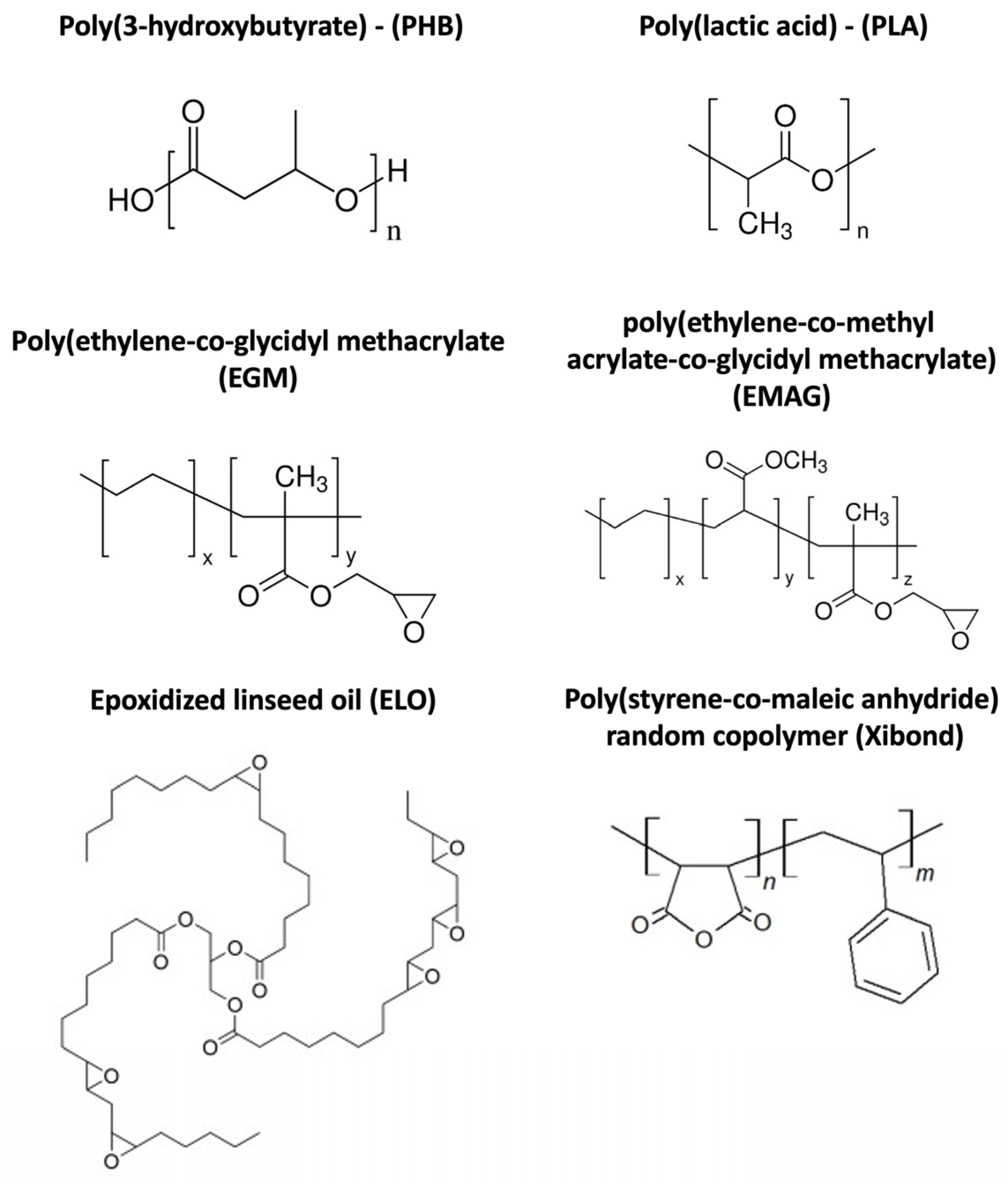





| Parts Per Hundred Resin (phr) | ||||||
|---|---|---|---|---|---|---|
| Sample Code | PHB (wt%) | PLA (wt%) | EGM | EMAG | ELO | Xibond |
| PHB75-PLA | 75 | 25 | - | - | - | - |
| PHB75-PLA-EGM | 75 | 25 | 5 | - | - | - |
| PHB75-PLA-EMAG | 75 | 25 | - | 5 | - | - |
| PHB75-PLA-ELO | 75 | 25 | - | - | 5 | - |
| PHB75-PLA-Xibond | 75 | 25 | - | - | - | 5 |
| Printing Parameters | Standards Geometries | Scaffolds |
|---|---|---|
| Nozzle (mm) | 0.4 | 0.4 |
| Printing temperature (°C) | 195 | 195 |
| Bed temperature (°C) | 45 | 45 |
| Printing speed (mm/s) | 50 | 15 |
| Layer height (mm) | 0.2 | 0.2 |
| Infill (%) | 100 | 30 |
| Raster angle (°) | 0/90 | 0/90 |
| Sample | Flexural Modulus (MPa) | Flexural Strength (MPa) | Flexural Strain (%) |
|---|---|---|---|
| PHB75-PLA | 3330 ± 120 | 53.5 ± 4.0 | 3.1 ± 0.3 |
| PHB75-PLA-EGM | 2900 ± 254 | 52.5 ± 1.7 | 3.4 ± 0.3 |
| PHB75-PLA-EMAG | 3050 ± 124 | 52.6 ± 4.7 | 3.7 ± 0.3 |
| PHB75-PLA-ELO | 3080 ± 207 | 54.1 ± 1.3 | 4.6 ± 0.2 |
| PHB75-PLA-Xibond | 3200 ± 135 | 57.0 ± 1.6 | 3.3 ± 0.4 |
| Sample | Tg (°C) | ΔHcc (J·g−1) | Tcc (°C) | ΔHm (J·g−1) | Tm (°C) | Xc (%) |
|---|---|---|---|---|---|---|
| PHB75-PLA | 60.6 ± 0.6 | 1.51 ± 0.1 | 91.0 ± 0.4 | 82.8 ± 1.2 | 174.3 ± 0.5 | 61.2 ± 0.5 |
| PHB75-PLA-EGM | 61.0 ± 0.4 | 1.64 ± 0.2 | 91.7 ± 0.5 | 76.8 ± 1.0 | 171.4 ± 0.4 | 56.6 ± 0.4 |
| PHB75-PLA-EMAG | 61.5 ± 0.5 | 1.69 ± 0.1 | 91.2 ± 0.5 | 75.1 ± 0.9 | 172.2 ± 0.6 | 55.3 ± 0.4 |
| PHB75-PLA-ELO | 58.0 ± 0.7 | 0.82 ± 0.1 | 87.8 ± 0.6 | 78.9 ± 1.1 | 171.5 ± 0.4 | 58.7 ± 0.3 |
| PHB75-PLA-Xibond | 60.9 ± 0.6 | 3.08 ± 0.3 | 91.4 ± 0.8 | 78.6 ± 0.9 | 171.9 ± 0.5 | 56.9 ± 0.5 |
| Sample | Bulk Density (g/cm3) | Apparent Density (g/cm3) | Porosity (%) | Pore Size (μm) |
|---|---|---|---|---|
| PHB75-PLA | 1.24 ± 0.01 | 0.57 ± 0.04 | 53.3 ± 3.7 | 710.7 ± 28.8 |
| PHB75-PLA-EGM | 1.20 ± 0.02 | 0.57 ± 0.03 | 52.5 ± 3.8 | 718.4 ± 40.5 |
| PHB75-PLA-EMAG | 1.21 ± 0.02 | 0.57 ± 0.01 | 52.9 ± 1.5 | 675.0 ± 25.0 |
| PHB75-PLA-ELO | 1.22 ± 0.02 | 0.60 ± 0.02 | 50.3 ± 2.1 | 712.3 ± 37.5 |
| PHB75-PLA-Xibond | 1.22 ± 0.01 | 0.58 ± 0.03 | 52.2 ± 3.5 | 696.5 ± 18.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez-Candela, I.; Sempere-José, L.; Sandoval-Perez, I.; Martínez-García, A. Evaluation of Additives on the Cell Metabolic Activity of New PHB/PLA-Based Formulations by Means of Material Extrusion 3D Printing for Scaffold Applications. Polymers 2024, 16, 2784. https://doi.org/10.3390/polym16192784
Dominguez-Candela I, Sempere-José L, Sandoval-Perez I, Martínez-García A. Evaluation of Additives on the Cell Metabolic Activity of New PHB/PLA-Based Formulations by Means of Material Extrusion 3D Printing for Scaffold Applications. Polymers. 2024; 16(19):2784. https://doi.org/10.3390/polym16192784
Chicago/Turabian StyleDominguez-Candela, Ivan, Lluc Sempere-José, Ignacio Sandoval-Perez, and Asunción Martínez-García. 2024. "Evaluation of Additives on the Cell Metabolic Activity of New PHB/PLA-Based Formulations by Means of Material Extrusion 3D Printing for Scaffold Applications" Polymers 16, no. 19: 2784. https://doi.org/10.3390/polym16192784
APA StyleDominguez-Candela, I., Sempere-José, L., Sandoval-Perez, I., & Martínez-García, A. (2024). Evaluation of Additives on the Cell Metabolic Activity of New PHB/PLA-Based Formulations by Means of Material Extrusion 3D Printing for Scaffold Applications. Polymers, 16(19), 2784. https://doi.org/10.3390/polym16192784








