Water Repellent Coating in Textile, Paper and Bioplastic Polymers: A Comprehensive Review
Abstract
1. Introduction
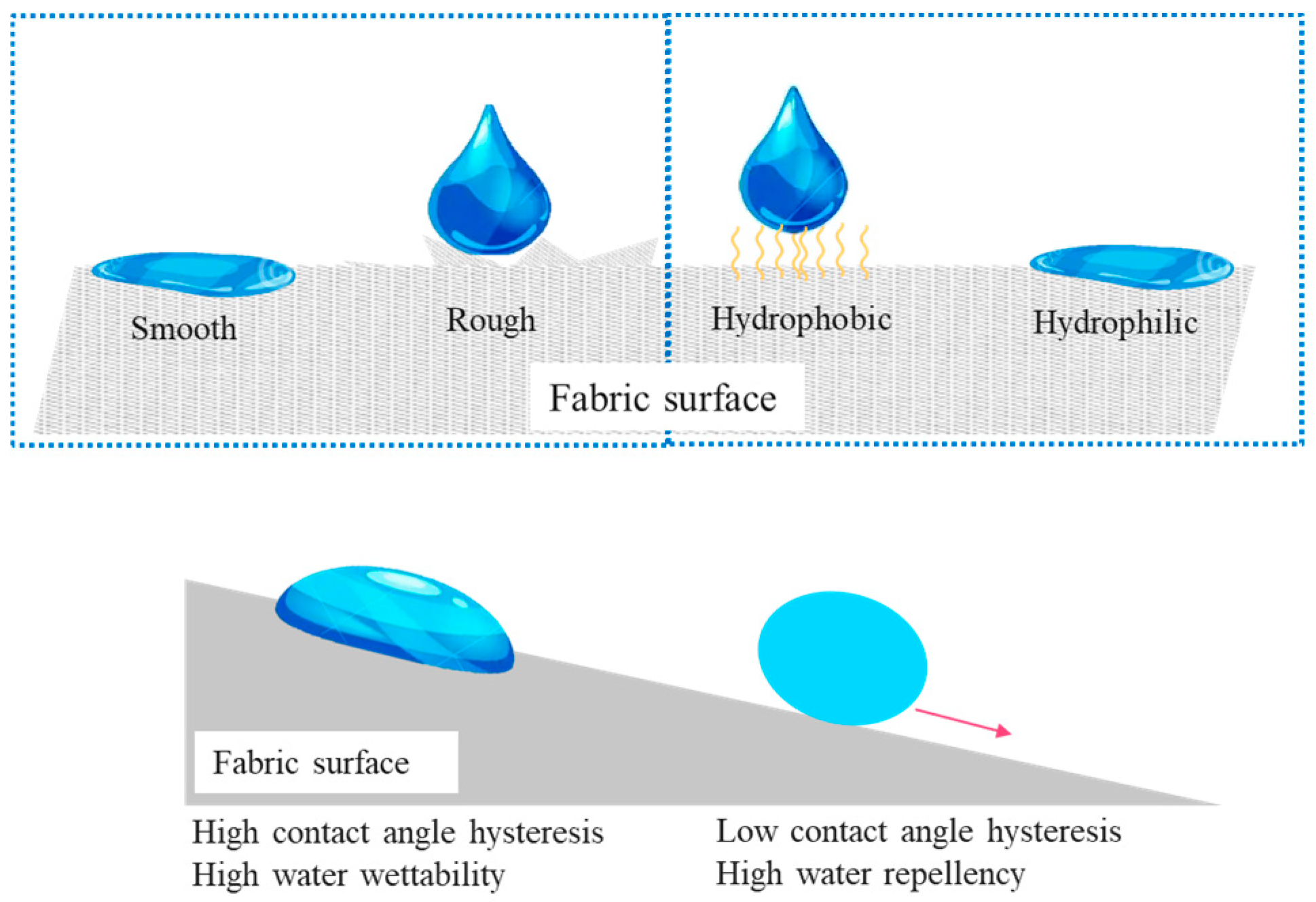
2. Water Repellent Coating in Textile
2.1. Application and Mechanism of Water Repellent in Textile
- Conventional Wet-Weather Clothing
- 2.
- Sports and Leisure Garments
- 3.
- Personal Protective Equipment
2.2. Water Repellent Coating Technology for Textiles
| Coating Compounds | Substrate Materials | Coating Method | Coating Solvent | Concentration of Coating Compounds in Solvent | Other Additives | Solvent Removal Method | Major Findings | Minor Finding | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Stearic acid | 100% cotton fabric, plain woven fabric | Padding method | Isopropyl alcohol | Citric acid, catalyst sodium hypophosphite (SHP) and enhance triethanolamine (TEA) | Drying at 100 °C for 3 min, and curing at 180 °C for 2 min. | - The fabric coated with stearic acid and citric acid with catalyst SHP and enhancer TEA had the highest water repellency rating of 4 and 5. - The coated fabric without catalyst is wet or readily absorbs the water. Moreover, it had a WCA value ranged from 138° to 145°. | - The interaction between stearic acid, citric acid, and cellulose occurred via ester bond, revealed in FT-IR spectra in 1712 and 1734 cm−1 attributed to C=O stretching. - SEM images showed a successful coating deposition onto cotton fabric at yarn and fiber level. | [4] | |
| PVA | Woven cotton fabric | Impregnation and padding method | Liquor | 5% | Ag NPs | Drying at 50 °C | - PVA coating causes a sticking between cotton fiber and more compact. While Ag NPs coating showed a fiber remains separated. - The WCA value of uncoated cotton is 0°, it is increased to 120–127° after coating with PVA and Ag NPs. While it is increased by increasing PVA coating cycles. | - The Ag NPs coated cotton causing a decrease in 2θ located at 14.9°, 16.7°, 22.8°, 34.6°. - Ag NPs-PVA coated fabric had lower UV transmittance than uncoated and PVA-coated fabric. - The Ag NPs coated fabric exhibited antimicrobial activity against Staphylococcus aureus up to 99.93% after 24 h of contact. | [5] |
| Ag NPs/ Polydi methyl siloxane (PDMS) | Twill weave fabric (100% cotton) | Dip-coating | Iso-propanol | PDMS (20 and 40 g/L) | Ag NPs (0.2, 0.4, 1, 2, and 4 g/L) | Scouring using non-ionic detergent (2 g/L) at 40 °C for 30 min, followed by drying at room temperature overnight. | - PDMS coating reduces surface roughness. - Increasing amounts of Ag NPs increases the surface roughness. - The WCA increases with increasing surface roughness at higher Ag NP concentrations. - Samples with higher superhydrophobicity exhibit lower contact angle hysteresis (CAH), which in turn leads to a lower sliding angle (SA), indicating better water repellency. - PDMS-40 coated cotton, the CAH increases with increasing Ag NP content. | - PDMS coating increased thermal stability of the fabrics, due to the protective role of PDMS as a heat barrier. - The antibacterial activity is more highly dependent on the concentration of Ag NPs than on superhydrophobicity. | [11] |
| Super hydrophobic precipitated calcium carbonate (SHPCC) | Polyester fabric | Dip-coating | Stearic acid (SA) and iso-propyl alcohol | 4% | Washed with iso-propyl alcohol and then air-dried. | - Higher saturation SHPCC coated fabric exhibited highest WCA 150° which categorized as superhydrophobic surface, while the uncoated fabric directly absorbed the water droplet. - The SA/SHPCC is successfully deposited on the fabric surface, it is intensified the FT-IR peak at 2929 and 2889 cm−1 attributed to C-H stretching and C-H bending, respectively. | - The coating causes a microrough on the fabric surface, where SA acts as a binder for SHPCC further enhance hydrophobicity. - The fabric coated with SHPCC showed a Td at 620–720 °C | [16] | |
| Hydrophobic SiO2 | Nubuck, denim, chenille, and nonwoven polyester | Spray-coating | Ethanol | 2% | Drying at room temperature for 1 h. | - The superhydrophobic surface was observed on coated textile with WCA 158–172°. Coated chenille and nonwoven exhibited the highest WCA while nubuck had the lowest WCA. - The sliding angle (SA) of coated textile was around 3°, while nubuck exhibited the highest SA (20°). Lower SA indicates better water repellence ability. - Superhydrophobic chenille and nonwoven fabric were resistant to water spray and water jet impact with value of 200 cycles and 600 s, respectively. While denim and nubuck are susceptible to water impact. Denim had water spray and water jet impact of 5 cycles and 10 s, respectively. | - SEM images showed a waving pattern of uncoated lining and denim, while chenille, nonwoven fabric, and nubuck had a random arrangement. - SiO2 deposition on the sample surface was detected in EDX, where O become stronger, and C become weaker after coating. | [17] | |
| Fluorine, silicone, and wax-based water-repellent | Cotton woven fabric | Spray-coating with 15 cm of distance | The water repellent agent is in a liquid state. | Curing at 80 °C for 10 min. | - Wax-based water repellent was the most effective with a water repellency rating of 4, compared to fluorine (1.35) and silicone (2.05)-based water repellent. - The water repellency of the fabric was increased by increasing the coating layer. | - The treated fabric had higher tensile elongation compared to untreated one, as it increased with increasing the coating layer. However, it started to decrease at 5 coating layers assembled. | [21] | ||
| Hexadecyltrimethoxysilane (HDTMS) alcosol | 80% calcium alginate + 20% polyester (PET) | Dippin | Ethanol | 0, 0.3, 1, 3, 5, 7% | Drying at 22 °C for 20 h | - The water droplet cannot stay, and it is permeated through the pristine/uncoated fabric, while 3% HDTMS treatment increased WCA and WSA to 158° and 9.3°, respectively. - Hydrolyzed and condensed HDTMS was successfully introduced on the fabric surface as revealed in FTIR peak. - The pristine fabric was completely wet and swelling deformed (As = 359.5%) after water immersion for 7 days, HDTMS treated fabric remained dry with As = 124.1%. | - The 3% HDTMS treatment reduces the breaking strength from 3.84 to 2.4 MPa. - The pristine alginate fabric was smooth and became rough after HDTMS treatment | [22] | |
| Polydimethylsiloxane (PDMS) | PET fabric | Dip-coating | Ethanol | 5 g/L | Drying in oven 70 °C for 30 min | - The Si-O-Si peaks at 1200–1000 cm−1 attributed to the crosslinking of PDMS on the PET fabric. - The good water repellency of PDMS coated PET fabric was observed by WCA and SA up to 152.2° and 8.4°, respectively. - The water repellency of PET fabric was observed by wetting behavior. PET fabric was totally wet, while PDMS coated PET fabric remained dry after contact with water. | - The uncoated PET fabric had a smooth surface. The PDMS coating creates a uniform thin with some wrinkles on the PET fabric. - The high breathability on pristine PET fabric indicates the presence of open holes which allow the air permeation. The air permeability of PET fabric increases after coating with PDMS, but the water vapor permeability decreases. | [24] | |
| 2,2,6,6-tetramethylpiperidine 1-oxyl radical (TEMPO)-oxidized cellulose nanofiber (TOCN), Amidation modified TOCN (AMT) + polyisocyanate cross-linking agent (PCA), Multimodified TOCN (MMT) MMT is the final product for coating material. | Cotton cloth | Spray coating | Tetrahydrofuran (THF) | 0.5 wt.% | Drying in vacuum oven at 70 °C for 2 h. | - The TOCN was successfully modified to MMT, revealed at FT-IR spectra at 2274 and 1700 cm−1 indication of presence of isocyanate group and carbamate linkage between isocyanate and hydroxyl group, respectively. - MMT coated cotton exhibited a superhydrophobic with WCA~151°, the value was close to commercial fluorine-based water repellence. - The combination of MMT coating and surface roughness plays an important role in the superhydrophobicity. - The WCA was slightly reduced to 125° after 10 washing cycles. The rolling angle was increased by increasing washing cycle. | - SEM images showed that AMT coating cannot adhere well on the cotton surface and showed an aggregate. The MMT coating had better coating ability due to carbamate linkage between isocyanate hydroxyl group. | [25] | |
| Long chain and short chain fluorochemical | Cotton fabric | Impregnation, padding and curing method | Long chain (3 and 5%), short chain (10 and 30%) | Drying at 90 °C for 20 min, followed by curing 1 and 5 min at 120 °C and 150 °C | - The cotton fabric coated with short and long chain fluorochemical had a similar WCA value (130°), indicates the short and long chain fluorochemical would have similar result by modifying its concentration. - The water repellence of the coated cotton fabric was evaluated by spray rating method. It is shown that all the coated cotton had high water repellence (up to 90°), which increase by increasing fluorochemical concentration. - The fluorochemical coating results in dense structure, causing less air permeability but increase shear stiffness. | - The overlapped peaks of F-C-F and C-O-C of the fluorochemical appeared between 1100 and 1250 cm−1. - ATR spectrum showed a new peak 1740 cm−1 in short-chain fluoropolymer. The peak is correspondent to ester moieties in polyacrylate. | [26] | ||
| 3-glycidyloxy(propyl)trimethoxysilane (G), hexadecyltrimethoxysilane (C16), triethoxy(octyl)silane (C8) and triethoxy(ethyl)silane (C2) | Plain weave PET fabric | Dip-Pad-Dry-Cure Method | Ethanol | - The highest WCA was achieved by the coated sample PL-G-C2-C16 and PL-G-C8-C16 with WCA value of 150.17° and 147.43°, respectively. While the PL-G (control) had WCA value of 140.32°. - The spray test water repellency showed that the longer alkyl chain of alkoxysilanes, the better ability to repel the water. PL-G had a rating number of 50, while long chain alkyl coated PL-G-C8-C8 and PL-G-C16-C16 exhibited rating numbers up to 100. | - Nano scale surface of treated fabric observed by SEM images, support the hydrophobicity. - The air permeability of the treated fabric was slightly reduced, compared to pristine fabric. | [27] | |||
| Hexamethyldisiloxane (HMDSO) | Cotton fabric | Plasma polymerization | Washing with ethanol and acetone | - The treated cotton fabric had WCA higher than 150° and water shedding angle were close to 10° - The ATR-FTIR showed a non-significant change between before and after 10 cycles of washing. However, the intensity was slightly decreased indicating a decreasing of coating polymer due to partial removal during washing. This also effects on decreasing the water repellency ability. | [29] | ||||
| Fluoropolymer | Toray-Toteron Cotton (TC) is made of 65% polyester and 35% cotton. | Spray coating | TiO2 as antimicrobial coating via immersion | Drying at room temperature, for 24 h. Finalization by heat treatment using iron. | - TiO2 coating results in rough surface. - The water repellency of unwashed coated fabric remained stable after 60 min of water contact. However, the stability was reduced after ~10 cycles of washing. On the other hand, the uncoated fabric had 0 WCA value which means no water repellency and instantly absorbs the water droplet. | - The uncoated fabric and fluoropolymer coated showed no inhibition effect, while TiO2 coated fabric showed an inhibition zone against Staphylococcus aureus and Klebsiella pneumoniae of 4.9 and 6.3 mm, respectively. - The coated fabric had higher mechanical properties, explained by the coating material fill the interstices at the fiber-fiber region of spun yarn. | [30] | ||
| Organosilane-modified cellulose nanofibers | Cotton fabric | Immersion | 0.25, 0.5, and 0.75% w/v | Drying and curing at 120 °C for 2 h, followed by immersion in acetone solution for 24 h to remove unreacted silane. Finally, fabric was dried at 60 °C | - Organosilane deposition results in rough surface of the cotton fabric. It also reduced air and water permeability. - Uncoated fabric had a hydrophilic property as the water absorbed instantly. - WCA and water absorption time increased as the concentration organosilane modified cellulose coating increase | - The coated cotton fabric exhibited antimicrobial activity against Staphylococcus aureus and Escherichia coli. The effectiveness was reduced after 15 cycles of washing. - A grafting of organosilane in the cellulose chain was revealed in FTIR spectra peak at 1000 and 1200 cm−1 attributed to Si-O-Si and Si-O-C, respectively. | [31] | ||
| Boric acid or boron-based coating | Cotton fabric | Sol-gel impregnation | Ethanol | Molar ratio of 0.1, 0.5, 1 and 2.5 | TiO2 dissolved in ethanol, hydrochloric acid, and water with molar ratios of 0.5, 60, 0.008, and 55, respectively. | Drying at 80 °C for 1 h, and curing at 120 °C for 1 h | - The FTIR peak located at 800 cm−1 attributed to Ti-O-Ti and Ti-C stretching vibration of TiO2 particle was increased, indicates a successful TiO2 deposition on the cotton fabric. - The uncoated cotton instantly absorbs the dropped water, while the coated cotton could hold the water on the fabric surface until 600 s after water dropping. - The water repellency was increased by increasing molar ratio of boric acid. | - A significant agglomeration on fabric surface was observed on the TiO2-boron-coated cotton fabric. - The coating increased char formation of cotton detected on the Tg = 600 °C. - The coating process reduced tensile strength of the cotton fabric due to clogging between yarn on the fabric. | [32] |
| rSiO2 and cross-linked amino long-chain alkyl polysiloxane (CAHPS) | Cotton fabric | Immersion and padding method | Deionized water | 20–120 g/L | Emulsifier: isomeric alcohol ethoxylates | Dry at 100 °C for 3 min and cure at 150–170 °C for 60–180 s. | - The coated cotton fabric had a good water repellency. - The contact angle of drops of salt water, tea, coffee, dying solution, milk and cola on the coated fabric were 147.8°, 148.5°, 148.3°, 150.2°, 145.9°, and 148.6°, respectively. - The uncoated cotton fiber is moistened after contact with water. While the coated cotton fiber could maintain its dryness. - The WCA still remained high at 144.2° and 140.9° after 15 and 30 cycles of washing. Result indicates the strong adhesive bonding between rSiO2-CAHPS coating and cotton fiber. - The dry rubbing test was used to evaluate the fabric surface abrasion that could affect on water repellency loss. - The WCA dropped to 137.6° and 112.2° after 5 and 20 rounds of dry rubbing. | - The rSiO2-CAHPS coated cotton fabric still have a good air permeability, thus it is considering as breathable fabric. | [33] |
| Polyurethane | Palm fiber/polyester fiber nonwoven composite | Impregnation | 5% by weight compared to established nonwoven | Sol-gel treatment using chloropropyl-triethoxylane (CPTS) and tetraethyl orthosilicate (TEOS) | Drying in 120 °C for 20 min | - A nonwoven treated with PU and sol-gel using CPTS (nonwoven-PU-CPTS) showed optimum water repellence using spray and rain test method. - This treatment further reduced water absorption. However, the PU coating alone did not mitigate the water absorption. - Aliphatic chain presence in CPTS creates a barrier against moisture. | - Sol-gel and PU coating reduced elongation at break and increased tensile strength. Indicates the stiffness of the nonwoven was increased after sol-gel treatment and PU coating. FTIR confirmed that condensation capacity of hydrolyzed CPTS is greater than hydrolyzed TEOS. | [34] |
2.3. Water Repellent Coating Material for Textile
2.3.1. Fluorochemical
2.3.2. Silane
2.3.3. Silicone
2.4. Standard Method for Water Repellency Testing
3. Water Repellent Coating in Paper
3.1. Paper Coating in Industry
3.2. Coating Technology for Paper
3.3. Coating Materials and Applications for Paper
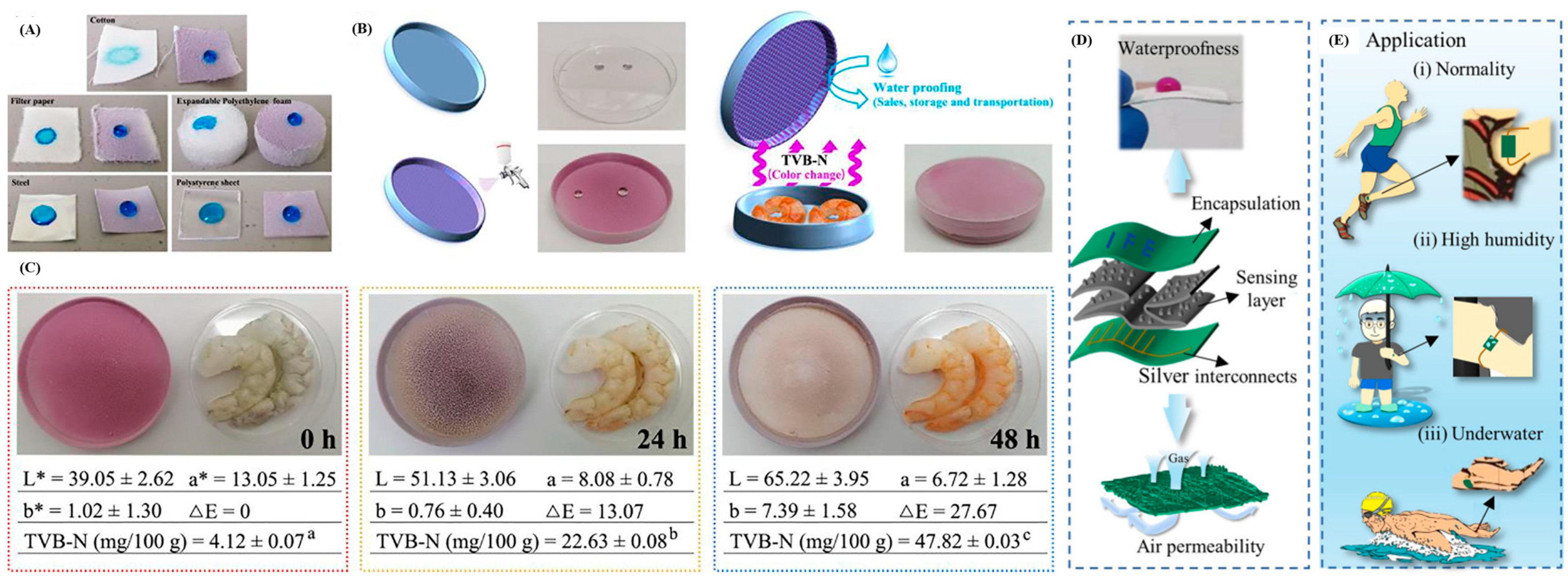
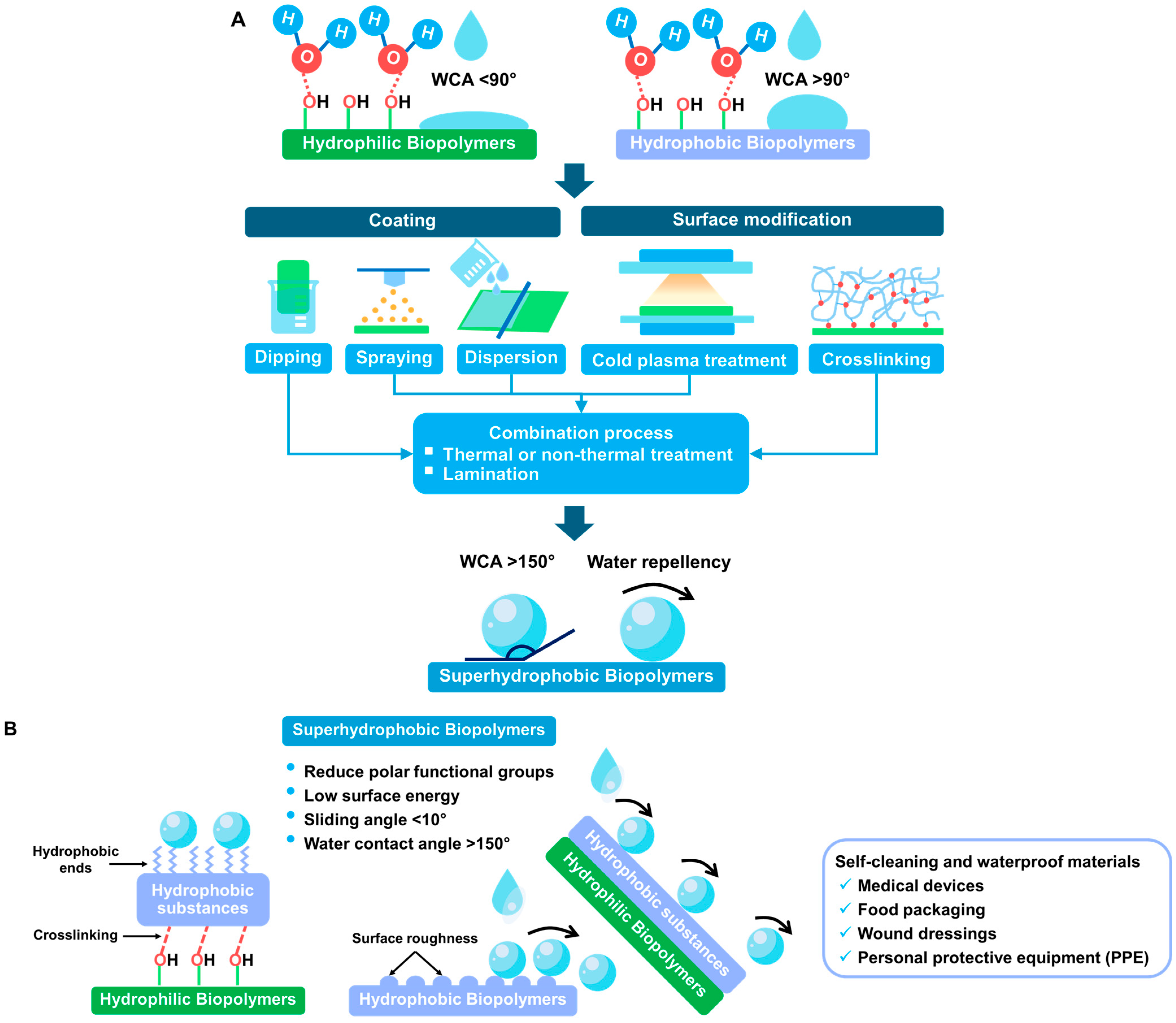
4. Water Repellent Coating in Biopolymers
4.1. Bioplastic Substrates
4.2. The Biopolymer Coating Materials and Technology
4.3. Superhydrophobic Coatings with Multifunctional Properties
5. Conclusions and Future Perspectives
- ▪
- Enhanced durability and performance; the development of coatings that are durable, even under harsh conditions, will be crucial.
- ▪
- Coatings that can withstand mechanical stress, such as friction and abrasion, will be essential for applications like outdoor textiles and industrial materials.
- ▪
- Coatings capable of resisting degradation from exposure to various chemicals, including acids, bases, and solvents, will expand their applicability in diverse environments.
- ▪
- The demand for sustainable materials will drive the development of water-repellent coatings derived from renewable resources and that are biodegradable or have minimal environmental impact.
- ▪
- Efforts to minimize the use of harmful chemicals in coating formulations will be a priority to promote sustainability and well-being.
- ▪
- Coatings that can actively repel dirt and contaminants, leading to self-cleaning surfaces, will be highly sought after in various applications, including textiles, building materials, and medical devices.
- ▪
- Incorporating antimicrobial agents into water-repellent coatings can improve antimicrobial properties, making them ideal for applications in healthcare, food packaging, and consumer products.
- ▪
- Coatings that can shield materials from harmful UV radiation will be valuable for applications exposed to sunlight, such as outdoor textiles and solar panels.
- ▪
- Water-repellent coatings can protect electronic components and sensors used in wearable devices from moisture and sweat, enhancing their reliability and durability.
- ▪
- Coatings can improve the performance and longevity of batteries and other energy storage devices by preventing moisture-related degradation.
- ▪
- Water-repellent coatings can enhance the biocompatibility and functionality of medical devices, such as catheters and implants.
- ▪
- The use of nanotechnology can enable the creation of coatings with tailored properties, such as superhydrophobicity and self-healing capabilities.
- ▪
- 3D printing techniques can be used to integrate water-repellent coatings directly into manufactured
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, A. A Panacea to Unsustainable Consumption? A Review of Resource Caps. Glob. Sustain. 2024, 7, 1–31. [Google Scholar] [CrossRef]
- Mishra, M.; Desul, S.; Santos, C.A.G.; Mishra, S.K.; Kamal, A.H.M.; Goswami, S.; Kalumba, A.M.; Biswal, R.; da Silva, R.M.; Dos Santos, C.A.C. A bibliometric analysis of sustainable development goals (SDGs): A review of progress, challenges, and opportunities. Environ. Dev. Sustain. 2024, 26, 11101–11143. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.F. Materials and Sustainable Development; Butterworth-Heinemann: Oxford, UK, 2022. [Google Scholar]
- Sharif, R.; Mohsin, M.; Ramzan, N.; Ahmad, S.W.; Qutab, H.G. Synthesis and application of fluorine-free environment-friendly stearic acid-based oil and water repellent for cotton fabric. J. Nat. Fibers 2022, 19, 1632–1647. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, S.; Peng, L.; Wang, Y.; Shang, S.; Miao, D.; Guo, R. AgNps-PVA-coated woven cotton fabric: Preparation, water repellency, shielding properties and antibacterial activity. J. Ind. Text. 2019, 48, 1545–1565. [Google Scholar] [CrossRef]
- Li, P.; Zhou, M.; Jian, B.; Lei, H.; Liu, R.; Zhou, X.; Li, X.; Wang, Y.; Zhou, B. Paper material coated with soybean residue nanocellulose waterproof agent and its application in food packaging. Ind. Crops Prod. 2023, 199, 116749. [Google Scholar] [CrossRef]
- Ren, F.; Guo, H.; Guo, Z.-Z.; Jin, Y.-L.; Duan, H.-J.; Ren, P.-G.; Yan, D.-X. Highly bendable and durable waterproof paper for ultra-high electromagnetic interference shielding. Polymers 2019, 11, 1486. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Saleh, R.I.; Kim, M.; Baek, S.Y.; Cha, C. Chitosan-functionalized silica nanoparticles as a multifunctional coating material for improved water repellency, antimicrobial activity and mechanical strength of degradable bioplastics. Cellulose 2022, 29, 7691–7701. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, L.; Tian, Y. Super anti-wetting colorimetric starch-based film modified with poly (dimethylsiloxane) and micro-/nano-starch for aquatic-product freshness monitoring. Biomacromolecules 2021, 22, 3769–3779. [Google Scholar] [CrossRef]
- Pakdel, E.; Kashi, S.; Sharp, J.; Wang, X. Superhydrophobic, antibacterial, and EMI shielding properties of Ag/PDMS-coated cotton fabrics. Cellulose 2024, 31, 3921–3946. [Google Scholar] [CrossRef]
- Wan, J.; Xu, J.; Zhu, S.; Li, J.; Wang, B.; Zeng, J.; Li, J.; Chen, K. Eco-friendly superhydrophobic composites with thermostability, UV resistance, and coating transparency. ACS Appl. Mater. Interfaces 2021, 13, 61681–61692. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, B.; Wang, X.; Yang, J.; Zhang, H. Preparation and characterization of paper-based high barrier material based on heterogeneous graft polymerization. Cellulose 2023, 30, 1811–1822. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, C.; Meng, L.; Tang, X. Preparation and characterization of carnauba wax-based particle with hierarchical structure and its use as hydrophobic coating for chitosan films. Carbohydr. Polym. 2023, 319, 121224. [Google Scholar] [CrossRef] [PubMed]
- Sundar, N.; Kumar, A.; Pavithra, A.; Ghosh, S. Studies on semi-crystalline poly lactic acid (PLA) as a hydrophobic coating material on kraft paper for imparting barrier properties in coated abrasive applications. Prog. Org. Coat. 2020, 145, 105682. [Google Scholar]
- Abeywardena, M.; Yashomala, M.; Elkaduwe, R.; Karunaratne, D.; Pitawala, H.; Rajapakse, R.; Manipura, A.; Mantilaka, M. Fabrication of water-repellent polyester textile via dip-coating of in-situ surface-modified superhydrophobic calcium carbonate from dolomite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127397. [Google Scholar] [CrossRef]
- Celik, N.; Altındal, S.; Gozutok, Z.; Ruzi, M.; Onses, M.S. Effect of fabric texture on the durability of fluorine-free superhydrophobic coatings. J. Coat. Technol. Res. 2020, 17, 785–796. [Google Scholar] [CrossRef]
- Liu, D.; Duan, Y.; Wang, S.; Gong, M.; Dai, H. Improvement of oil and water barrier properties of food packaging paper by coating with microcrystalline wax emulsion. Polymers 2022, 14, 1786. [Google Scholar] [CrossRef]
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for paper and textile coating: The importance of surface chemistry. ChemPlusChem 2022, 87, e202200204. [Google Scholar] [CrossRef]
- Loghin, C.; Ciobanu, L.; Ionesi, D.; Loghin, E.; Cristian, I. Introduction to waterproof and water repellent textiles. In Waterproof and Water Repellent Textiles and Clothing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–24. [Google Scholar]
- Kim, S.; Kim, J.-E.; Song, D.-E.; Cho, S.-Y.; Hwang, Y.; Chae, Y. Effects of household water-repellent agents and number of coating layers on the physical properties of cotton woven fabrics. PLoS ONE 2023, 18, e0283261. [Google Scholar] [CrossRef]
- Zheng, C.; Sun, Y.; Cui, Y.; Yang, W.; Lu, Z.; Shen, S.; Xia, Y.; Xiong, Z. Superhydrophobic and flame-retardant alginate fabrics prepared through a one-step dip-coating surface-treatment. Cellulose 2021, 28, 5973–5984. [Google Scholar] [CrossRef]
- Li, W.; Yang, L.; Huang, J.; Zheng, C.; Chen, Y.; Li, Y.; Yang, D.; Li, S.; Chen, Z.; Cai, W. Progress on fiber engineering for fabric innovation in ecological hydrophobic design and multifunctional applications. Ind. Chem. Mater. 2024, 2, 393–423. [Google Scholar] [CrossRef]
- Xu, L.; Xie, K.; Liu, Y.; Zhang, C. Stable super-hydrophobic and comfort PDMS-coated polyester fabric. e-Polymers 2021, 21, 654–661. [Google Scholar] [CrossRef]
- Ke, W.-T.; Chiu, H.-L.; Liao, Y.-C. Multifunctionalized cellulose nanofiber for water-repellent and wash-sustainable coatings on fabrics. Langmuir 2020, 36, 8144–8151. [Google Scholar] [CrossRef] [PubMed]
- Gargoubi, S.; Baffoun, A.; Harzallah, O.A.; Hamdi, M.; Boudokhane, C. Water repellent treatment for cotton fabrics with long-chain fluoropolymer and its short-chain eco-friendly alternative. J. Text. Inst. 2020, 111, 835–845. [Google Scholar] [CrossRef]
- Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Super-hydrophobicity of polyester fabrics driven by functional sustainable fluorine-free silane-based coatings. Gels 2023, 9, 109. [Google Scholar] [CrossRef]
- Yu, M.; Yang, L.; Yan, L.; Wang, T.; Wang, Y.; Qin, Y.; Xiong, L.; Shi, R.; Sun, Q. ZnO nanoparticles coated and stearic acid modified superhydrophobic chitosan film for self-cleaning and oil-water separation. Int. J. Biol. Macromol. 2023, 231, 123293. [Google Scholar] [CrossRef]
- Jebali, S.; Carneiro de Oliveira, J.; Airoudj, A.; Riahi, A.; Fioux, P.; Morlet-Savary, F.; Josien, L.; Ferreira, I.; Roucoules, V.; Bally-Le Gall, F. Fluorine-Free Plasma Polymers to Obtain Water-Repellent Cotton Fabrics: How to Control Their Durability? Coatings 2023, 13, 1827. [Google Scholar] [CrossRef]
- Jongprateep, O.; Mani-Lata, C.; Sakunrak, Y.; Audcharuk, K.; Narapong, T.; Janbooranapinij, K.; Pitiphattharabun, S.; Lertworasirikul, A.; Laobuthee, A.; Thengchaisri, N. Titanium dioxide and fluoropolymer-based coating for smart fabrics with antimicrobial and water-repellent properties. RSC Adv. 2022, 12, 588–594. [Google Scholar] [CrossRef]
- Hongrattanavichit, I.; Aht-Ong, D. Antibacterial and water-repellent cotton fabric coated with organosilane-modified cellulose nanofibers. Ind. Crops Prod. 2021, 171, 113858. [Google Scholar] [CrossRef]
- Bentis, A.; Boukhriss, A.; Gmouh, S. Flame-retardant and water-repellent coating on cotton fabric by titania-boron sol-gel method. J. Sol-Gel Sci. Technol. 2020, 94, 719–730. [Google Scholar] [CrossRef]
- Yu, C.; Shi, K.; Ning, J.; Zheng, Z.; Yu, H.; Yang, Z.; Liu, J. Preparation and application of fluorine-free finishing agent with excellent water repellency for cotton fabric. Polymers 2021, 13, 2980. [Google Scholar] [CrossRef] [PubMed]
- Azmami, O.; Sajid, L.; Boukhriss, A.; Majid, S.; El Ahmadi, Z.; Benayada, A.; Gmouh, S. Sol-gel and polyurethane based flame retardant and water repellent coating for Palm/PES nonwovens composite. J. Sol-Gel Sci. Technol. 2021, 97, 92–105. [Google Scholar] [CrossRef]
- Sharif, R.; Mohsin, M.; Qutab, H.G.; Saleem, F.; Bano, S.; Nasir, R.; Wahlah, A. Durable water and oil repellents along with green chemistries: An overview. Chem. Pap. 2023, 77, 3547–3560. [Google Scholar] [CrossRef]
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Cousins, I.T.; Vestergren, R.; Wang, Z.; Scheringer, M.; McLachlan, M.S. The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater. Environ. Int. 2016, 94, 331–340. [Google Scholar] [CrossRef]
- Milbrandt, A.; Zuboy, J.; Coney, K.; Badgett, A. Paper and cardboard waste in the United States: Geographic, market, and energy assessment. Waste Manag. Bull. 2024, 2, 21–28. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging; a review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Liu, S.; Chen, K.; Salim, A.; Li, J.; Bottone, D.; Seeger, S. Printable and versatile superhydrophobic paper via scalable nonsolvent armor strategy. ACS Nano 2022, 16, 9442–9451. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Q. Facile preparation of water-proof paper with tunable surface properties for water/oil separation. Appl. Surf. Sci. 2021, 567, 150738. [Google Scholar] [CrossRef]
- Yun, T.; Du, J.; Ji, X.; Tao, Y.; Cheng, Y.; Lv, Y.; Lu, J.; Wang, H. Waterproof and ultrasensitive paper-based wearable strain/pressure sensor from carbon black/multilayer graphene/carboxymethyl cellulose composite. Carbohydr. Polym. 2023, 313, 120898. [Google Scholar] [CrossRef]
- Chungsiriporn, J.; Khunthongkaew, P.; Wongnoipla, Y.; Sopajarn, A.; Karrila, S.; Iewkittayakorn, J. Fibrous packaging paper made of oil palm fiber with beeswax-chitosan solution to improve water resistance. Ind. Crops Prod. 2022, 177, 114541. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Y.; Cai, J.; Feng, Y.; Luo, B.; Liu, M. Facile fabrication of hydrophobic paper by HDTMS modified chitin nanocrystals coating for food packaging. Food Hydrocoll. 2022, 133, 107915. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Li, J.; Ying, G.; Chen, K. High-strength and super-hydrophobic multilayered paper based on nano-silica coating and micro-fibrillated cellulose. Carbohydr. Polym. 2022, 288, 119371. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Huang, S.; Wang, Y.; Ling, H.; Yang, X.; Jin, Y.; Wang, X.; Zhang, W. Robust, high-barrier, and fully recyclable cellulose-based plastic replacement enabled by a dynamic imine polymer. J. Mater. Chem. A 2020, 8, 14082–14090. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Wang, Q.; Wang, Y.; Ji, X.; Yang, G.; Chen, J.; Ni, Y. Superhydrophobic, strong and transparent paper made from cellulosic fibers. Cellulose 2022, 29, 1993–2003. [Google Scholar] [CrossRef]
- Jin, K.; Tang, Y.; Liu, J.; Wang, J.; Ye, C. Nanofibrillated cellulose as coating agent for food packaging paper. Int. J. Biol. Macromol. 2021, 168, 331–338. [Google Scholar] [CrossRef]
- Ye, M.; Tian, Z.; Wang, S.; Ji, X.; Wang, D.; Ci, X. Simple preparation of environmentally friendly and durable superhydrophobic antibacterial paper. Cellulose 2023, 30, 2427–2440. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, B.; Zhong, M.; Liu, W.; Yu, D.; Wang, Y.; Tam, K.C.; Zhou, G.; Zhang, Z. Sustainable and versatile superhydrophobic cellulose nanocrystals. ACS Sustain. Chem. Eng. 2022, 10, 5939–5948. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, P.; Yi, Y.; Gibril, M.E.; Wang, S.; Kong, F. Application of polyvinyl Acetate/Lignin copolymer as bio-based coating material and its effects on paper properties. Coatings 2021, 11, 192. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Q.; Li, X.; Meng, Y.; Ling, Z.; Ji, Z.; Chen, F. Preparation and characterization of degradable cellulose—Based paper with superhydrophobic, antibacterial, and barrier properties for food packaging. Int. J. Mol. Sci. 2022, 23, 11158. [Google Scholar] [CrossRef]
- Vasilev, S.; Vodyashkin, A.; Vasileva, D.; Zelenovskiy, P.; Chezganov, D.; Yuzhakov, V.; Shur, V.; O’Reilly, E.; Vinogradov, A. An investigative study on the effect of pre-coating polymer solutions on the fabrication of low cost anti-adhesive release paper. Nanomaterials 2020, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Liu, N.; Fu, Y.; Bian, H.; Zhang, Y.; Chen, X.; Gao, H.; Dai, H. Laccase-catalyzed chitosan-monophenol copolymer as a coating on paper enhances its hydrophobicity and strength. Prog. Org. Coat. 2021, 151, 106026. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Y.; Shi, B.; Chen, Y. Facile preparation of economical, eco-friendly superhydrophobic surface on paper substrate with excellent mechanical durability. Prog. Org. Coat. 2020, 147, 105877. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, X.; Yao, Z.; Zhi, J.; Hu, L.; Yan, R.; Shi, C.; Yu, H.-D.; Huang, W. Fully paper-integrated hydrophobic and air permeable piezoresistive sensors for high-humidity and underwater wearable motion monitoring. npj Flex. Electron. 2023, 7, 13. [Google Scholar] [CrossRef]
- Wang, F.; Ma, R.; Tian, Y. Facile fabrication of thermostable and colorimetric starch-based waterproof coating with edible organic materials. Food Chem. 2022, 382, 132269. [Google Scholar] [CrossRef]
- Daulay, I.R.S.; Ariyanta, H.A.; Karimah, A.; Fitria; Santoso, E.B.; Cahyana, A.H.; Bukhari, M.N.S.S.; Bakshi, M.I.; Dungani, R.; Hanifa, T.Z.; et al. Preparation of superhydrophobic biomedical pulp from rice straw coated with a stearic acid-cellulose composite. Bioresour. Technol. Rep. 2024, 25, 101781. [Google Scholar]
- Wang, X.; Chen, K.; Liu, Y.; He, R.; Wang, Q. Preparation and application of biodegradable and superhydrophobic polylactic acid/carnauba wax coating. Prog. Org. Coat. 2023, 177, 107434. [Google Scholar] [CrossRef]
- da Fonseca de Albuquerque, M.D.; Bastos, D.C.; Ţălu, Ş.; Matos, R.S.; Pires, M.A.; Salerno, M.; da Fonseca Filho, H.D.; Simão, R.A. Vapor barrier properties of cold plasma treated corn starch films. Coatings 2022, 12, 1006. [Google Scholar] [CrossRef]
- Wang, G.; Song, D.; Qiao, Y.; Cheng, J.; Liu, H.; Jiang, J.; Ma, A.; Ma, X. Developing super-hydrophobic and corrosion-resistant coating on magnesium-lithium alloy via one-step hydrothermal processing. J. Magnes. Alloys 2023, 11, 1422–1439. [Google Scholar] [CrossRef]
- Chen, Q.; Chang, C.; Zhang, L. Surface engineering of cellulose film with myristic acid for high strength, self-cleaning and biodegradable packaging materials. Carbohydr. Polym. 2021, 269, 118315. [Google Scholar] [CrossRef]
- Wang, F.; Ma, R.; Tian, Y. Superhydrophobic starch-based adsorbent with honeycomb coral-like surface fabricated via facile immersion process for removing oil from water. Int. J. Biol. Macromol. 2022, 207, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Mello, I.P.; Khalid, O.; Pires, J.R.A.; Rodrigues, C.; Alves, M.M.; Santos, C.; Fernando, A.L.; Coelhoso, I. Strategies to improve the barrier and mechanical properties of pectin films for food packaging: Comparing nanocomposites with bilayers. Coatings 2022, 12, 108. [Google Scholar] [CrossRef]
- Wu, X.H.; Then, Y.Y. Fabrication and characterization of superhydrophobic graphene/titanium dioxide nanoparticles composite. Polymers 2021, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.; Belgacem, M.N.; Gandini, A.; Carvalho, A.J.F. Low permeable hydrophobic nanofibrilated cellulose films modified by dipping and heating processing technique. Cellulose 2021, 28, 1617–1632. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Es-haghi, S.S.; Tajvidi, M.; Wang, J.; Gardner, D.J. Biodegradable and recyclable bio-based laminated films of poly (lactic acid) and cellulose nanocrystals for food barrier packaging. Food Packag. Shelf Life 2024, 42, 101244. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Guo, Z. Tomato-lotus inspired edible superhydrophobic artificial lotus leaf. Chem. Eng. J. 2020, 400, 125883. [Google Scholar] [CrossRef]
- Niu, S.; He, W.; Chang, Q.; Deng, X.; Xie, Y. Beeswax-Modified Starch-Cellulose Composites with Superhydrophobic and Self-Cleaning Capability. Starch-Stärke 2023, 75, 2200285. [Google Scholar] [CrossRef]
- Hasanah, N.F.; Revianashar, L.G.; Suhendar, D.; Abidin, K.Y.; Pradiva, M.I. Effect of chitosan coating concentration with glutaraldehyde on the characteristics of bioplastic for environmentally friendly personal protective equipment. Polym.-Plast. Technol. Mater. 2023, 62, 2095–2120. [Google Scholar] [CrossRef]
- Lin, Y.; Qiao, C.; Zhao, Z.; Xia, Y.; Zhao, G.; Xue, Z. Fabrication and Characterization of Carrageenan/ZnO/Chitosan Composite Films. Langmuir 2023, 39, 7930–7938. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, L.; Zhu, Z.; Xie, F.; Meng, L.; Yang, T.; Qian, J.-Y.; Chen, Y. Facile superhydrophobic modification on HPMC film using polydimethylsiloxane and starch granule coatings. Int. J. Biol. Macromol. 2024, 266, 131191. [Google Scholar] [CrossRef]
- Wang, S.; Sha, J.; Wang, W.; Qin, C.; Li, W.; Qin, C. Superhydrophobic surfaces generated by one-pot spray-coating of chitosan-based nanoparticles. Carbohydr. Polym. 2018, 195, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yin, M.; Liu, Y.; Li, L.; Ren, X.; Sun, Y.; Huang, T.-S. Biodegradable polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes modified by silica composite hydrol for super hydrophobic and outstanding antibacterial application. J. Ind. Eng. Chem. 2018, 63, 303–311. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable superhydrophobic and superamphiphobic polymeric surfaces and their applications: A review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Feng, Y.; Ye, X.; Zhou, Z. Tuning wettability and getting superhydrophobic surface by controlling surface roughness with well-designed microstructures. Sens. Actuators A Phys. 2006, 130, 595–600. [Google Scholar] [CrossRef]
- Cai, R.; Glinel, K.; De Smet, D.; Vanneste, M.; Mannu, N.; Kartheuser, B.; Nysten, B.; Jonas, A.M. Environmentally friendly super-water-repellent fabrics prepared from water-based suspensions. ACS Appl. Mater. Interfaces 2018, 10, 15346–15351. [Google Scholar] [CrossRef]
- Hossain, M.R.; Newby, S.; Ahmed, M.R. Optimised fluorine free polysiloxane based water repellent for cellulosic fabric. J. Text. Inst. 2024, 1–10. [Google Scholar] [CrossRef]


| Parameter | Coating Technology | |
|---|---|---|
| Padding Method | Spray Coating Method | |
| Coating material | Liquid coating, primarily based on polymer or resin. | Coating material in liquid, paste, or powder. |
| Surface Requirement | Generally flat surface. | Can accommodate both flat and uneven surfaces. |
| Major process | The fabric passes through the roller that contains coating solution. | Fine mist coating material is sprayed onto fabric surface. |
| Thickness control | Adjusted by controlling the gap between the rollers and using a doctor blade. | Controlled by spray times, spray duration, and nozzle-to-fabric distance. |
| Drying technology | Tension drying, oven drying, or hot air drying. | Forced air drying, infrared drying, or oven drying. |
| Advantages | High efficiency for large-scale production, lower equipment costs. | Applicable to a wider range of coating materials, easier control of coating thickness. |
| Disadvantages | Difficult to control shearing force on textile surface due to large number of process parameter. Consuming a great quantity of solution due to high porosity of the fabric. Longer production time for drying and curing Limited coating types. | Potential for overspray, environmental concerns, higher safety requirements. |
| Coating Compounds | Substrate Materials | Coating Method | Coating Solvent | Concentration of Coating Compounds in Solvent | Other Additives | Solvent Removal Method | Major Findings | Minor Finding | Reference |
|---|---|---|---|---|---|---|---|---|---|
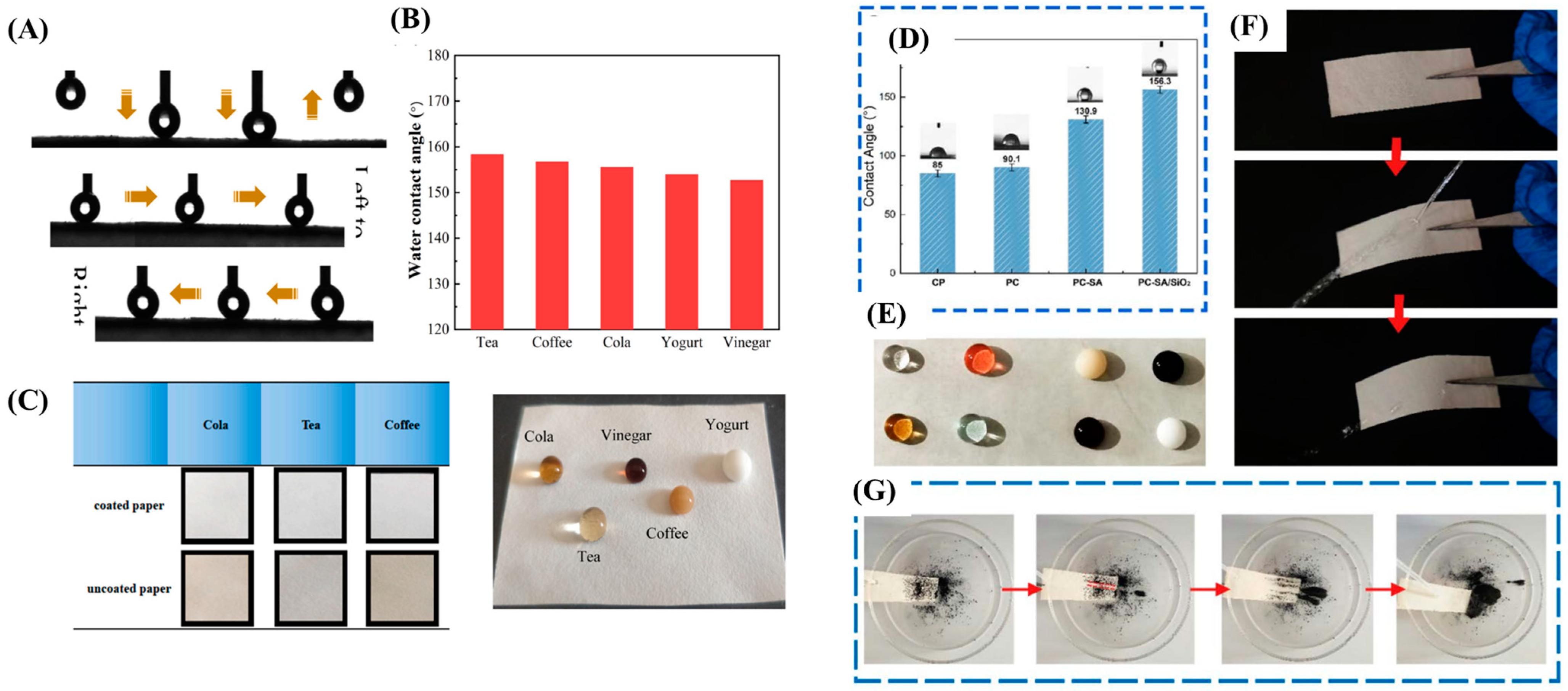
| Soybean residue nanocellulose (CNF) | Filter paper | Using the coater with iron coating rod | 1.5% Alkyl ketene dimer (AKD) lotion | 0.3 wt.% CNF and 0.1 wt.% AKD | 2 wt.% of precipitated calcium carbonate (PCC) as a binder | Drying | - CNF-AKD-PCC (CAP) coated paper showed excellent resistance to water droplet adhesion, leading to super hydrophobicity. - Contact angle over 150° for Cola, tea, coffee, vinegar and yogurt droplets, providing excellent liquid resistance. - Coated CAP paper maintained WCA beyond 150° for increasing the folding times, storage temperature, storage days and liquid pH, exhibiting great cold, heat, acid and alkali resistance and stability at storage days. - CAP coated paper reduced Cobb test value 8 times and water absorption rate by 88.2%. | - CAP coated paper improved surface roughness. - CAP coated paper improved tensile strength and elongation at break by 50.3% and 26.3% due to covalent bond and hydrogen bond force between CNF and filter paper. | [6] |
| Silver nanowires (AgNWs) and hydrophobic inorganic ceramic agent (H) | Filter paper | Dipping and Mayer-rod coating | Isopropyl alcohol | AgNW suspension (10 mg/m) | Drying | - H-AgNW/cellulose paper exhibits excellent hydrophobicity with the water droplet increased from 0° to 141°, indicating an excellent waterproofness. - H-AgNW/cellulose paper showed an antifouling function in dirty water. | - H-AgNW/cellulose paper maintained mechanical flexibility and resistance in comparison to conventional mechanics. - H-AgNW/cellulose paper maintained superior adhesion, even after the peeling off test for 1000 cycles. | [7] | |
| Beeswax and titanium dioxide (TiO2) | Cellulosic paper | Spraying | Anhydrous ethanol | 0.8 g TiO2, 5 g beeswax and 50 mL anhydrous ethanol | Drying and annealing | - The water contact angle of unannealed and annealed samples are all above 150°. - The coated paper showed rough structure and low surface Energy from beeswax. - The coated paper showed resistance to sandpaper-induced wear that showed water contact angle above 147° after four times of friction. | - Thermal annealing had a positive effect on improving the bonding strength between the coating and the paper substrate. - The coated paper showed excellent anti-adhesion properties for liquids (Cola, juice, milk, yogurt and lactobacillus beverage). | [12] | |
| Polydimethylsiloxane (PDMS) and polyvinyl alcohol (PVA) | Paper (70 g/m2) | Mayer coating stick and dipping | Water and acetone | 2, 2.5, 3, 3.5, 4% w/v PVA solution and 2 w/v% PDMS acetone | Hexamethylene diisocyanate trimer as a crosslinking agent | Drying | - PVA-coated paper slightly increased the contact angle of the paper surface from 97° to 105°. - PVA-PDMS-coated paper showed the contact angle value of 2, 3 and 4% PVA for 124.5°, 139° and 104°, respectively. - PVA-PDMS-coated paper decreased the Cobb values from 28.5 g/m2 to 11 g/m2, indicating excellent waterproof performance. | - PVA-PDMS had good thermal stability and improved barrier performance. | [13] |
| Poly lactic acid (PLA) | Kraft paper | Bar coater | Methylene dichloride | 5%, 10%, 15%, 20%, and 25% PLA | Drying | - PLA- treated Kraft papers reduced the Cobb value in water absorption by 3 to 8.5 times. - Contact angle values increased with increasing concentrations of PLA coatings from approximately 65° to 80°. | - PLA coated papers exhibited good compatibility possessed between PLA and cellulose fibers, affecting its penetration and adhesion. - PLA coated papers reduced surface roughness. - PLA coated papers enhanced the endurance of the paper material to impact load, depending on PLA concentration. - PLA coated papers increased tensile and bursting strength. | [15] | |
| Microcrystalline wax | Paper (grammage 70 g/m2, kit rating 0/12) | Manual coating | Deionized water | The solid content of the mixture at about 30% | Span-80 and Tween-80 | Drying | - The Cobb60 value of the coated paper reduced 11.3% and increasing coating load to 10 g/m2 the Cobb60 decreased for 54.3%. - Increased coating load increased the water contact angle to 106.1° due to microcrystalline wax caused waterproof performance, and the larger the coating load provided the better the water resistance capability of the paper. | - The coated paper reduced water vapor permeability by 54.1% and increasing coating load reduced water vapor permeability by 96.7%. | [18] |
| Polysilsesquioxane nanorods (PSNR) | Cellulosic paper | Scaping (PSNR) and using inkjet printer | Trichloroethylsilane | nonsolvent | - PSNR-paper exhibited water contact angle of 162° that was excellent water repellency due to PSNR-paper showed the low surface energy along with the nanoscale surface roughness. - PSNR-paper maintained superhydrophilicity with contact angle above 150° when tested with acid and alkali solution (HCL, NaOH and toluene). - PSNR-paper with both excellent ink adhesion and outstanding water repellency due to the rapid absorption and complete wetting (contact angle of 0°) of the ink on the paper surface. | - PSNR-paper demonstrated mechanical durability and flexibility due to PSNR-paper maintained its contact angle of above 150° after 20 abrasion cycles and did not affect the contact angle after despite 500 bending. - PSNR-paper showed excellent printability toward widely used inkjet printing techniques and showed water repellency after printing or writing. | [40] | ||
| Poly [1-cyanomethyl-3-vinylimidazolium] (PCMVIm) | Filter paper sheet | Dipping | Water | 0.2%, 0.4%, 0.6%, 0.8%, 1.2%, 1.6%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10% in weight. | Drying and ammonia vapor annealing | - 1.2 wt.% PCMVIm exhibited water resistance. - 0.6 wt.% PCMVIm was resistanct to high temperature and strong acid. - Water contact angle of PCMVIm maintained stable around 105–112° with increasing the amount of PCMVIm. | - PCMVIm gave smoother surface and filled the gaps between fibers, indicating dense networks. - Tensile strength of dry paper increases from 12.1 to 33.1 MPa and wet paper also increased from 0.6 to 20.7 MPa with increasing PCMVIm (0 wt.% to 30 wt.%). | [41] | |
| Multilayer graphene (MG), carbon black (CB), sodium carboxymethyl cellulose (CMC) and silicon dioxide (SiO2) | Filter paper | Dipping | Anhydrous ethanol and deionized water (1:1) | CB/MG/CMC is 15 mg/mL and the mass ratio of CB/MG/CMC is 6/4/5. | Drying | - The water contact angle of CB/MG/CMC increased from 12° to 133° and 156° after dipping for one and three times, respectively, indicating super hydrophobicity. - Superhydrophobic property of CB/MG/CMC/SiO2 paper showed hierarchal roughness as a result of nanoparticles. - CB/MG/CMC/SiO2 paper possessed excellent stability to water flow at different flow rates. - CB/MG/CMC/SiO2 paper showed excellent abrasion resistance due to water contact angle drops less than 5 (from 156° to 153°). - CB/MG/CMC/SiO2 paper exhibited conductive stability at water droplets interference. - CB/MG/CMC/SiO2 paper showed excellent superhydrophobicity, self-cleaning property, antifouling ability and mechanical durability. | - Surface color of composite paper changed from white to black and paper kept excellent flexibility after coating with CB/MG/CMC. | [42] | |
| Beeswax-chitosan | Paper sheet | Wire rod coater | Acetic acid | 1% chitosan, 4% glycerol and 30% beeswax | Tween 80 | Drying | - Beeswax-chitosan improved water repellency due to the strong hydrophobicity. | - Beeswax-chitosan gave a higher average tensile index than the uncoated base paper. | [43] |
| Chitin nanocrystals (ChNCs) and hexadecyltrimethoxysilane (HDTMS) | Filter paper | Dipping | Deionized Water and anhydrous ethanol | 0.5, 1, 2, 4 wt.% ChNCs and 1.25, 2.5 and 5 wt% HDTMS | Drying | - The paper surface became hydrophobic for 2% ChNCs and different amounts of HDTMS while water contact angle of 1.25% HDTMS was 128.4°. - ChNCs/HDTMS coating s increased water absorption (wetting behavior) which was up to 150.5% for immersing four days. - Water contact angle was stable above 130° after immersing in acid and alkali solution and explored to UV lamp. - The coated paper had excellent abrasion resistance after suffering from various mechanical forces, including bending, finger-wipe and sandpaper. | - ChNCs/HDTMS presented low-surface-energy compound to the filter paper. - ChNCs/HDTMS filled pores, giving a uniform and smooth surface which significantly decreased arithmetic average roughness from 84.3 nm to 38.0 nm. - The tensile strength of coated paper increased with the increase of ChNCs content. - The coated paper presented excellent self-cleaning and anti-fouling performance. - 2% ChNCs/HDTMS decreased water vapor permeability from 14.96 × 10−3 to 11.37 × 10−3 g⋅m/m2⋅Pa⋅h. - The coated paper enhanced thermal properties. - 2% ChNCs/HDTMS exhibited excellent antibacterial adhesion activity with lower numbers of both E. coli and S. aureus was over 98%. | [44] | |
| Hydrophobic n-SiO2 | Kraft paper | Spraying | Ethanol | 4% hydrophobic n-SiO2 | Methyltrimethoxysilane and ammonia | Drying | - n-SiO2 coated had a flatter surface, a higher contact angle (151.2°), and a higher roughness than the uncoated due to tight network structure and invisible boundaries. - Hydrophobic n-SiO2 exhibited water-resistant on the paper surface. | - The coated paper increased dry tensile strength for 56%. - The coated paper increased wet tensile strength for 2277% due to hydrogen bonding force among micro/nanoscale fibers increased exponentially with higher water retention value. - The water vapor transmission rate of coated paper decreased from 1996.60 to 378.24 g⋅m−2⋅day−1 due to dense structure on the surface. - Coated paper showed air permeability as low as 0.00317 μm⋅Pa−1⋅s−1. | [45] |
| Polyimine (PI) | Paper towel (PT), newspaper (NP), and printing paper (PP) | Dipping | Ethanol | 44%, 35%, and 16% of PI for PT, NP and PP, respectively. | Drying | - The water uptakes of NP and PP were significantly lower than the untreated NP which correspond to 6-fold and 2-fold decrease, respectively. - The coated paper showed good waterproof performance and resistance to organic solvents. | - Coated OT, NP and PP incresed tensile strength for 3.7, 8.1 and 1.6 times, respectively. - Coated OT, NP and PP maintained wet mechanical strength for 100, 87 and 14%, respectively. | [46] | |
| Trimethylsiloxy-terminated polydimethylsiloxane (PDMS) And hydrophobic fumed silica (Hf-SiO2) | Paper sheet | Dipping | Toluene | 0.5% PDMS and 3% Hf-SiO2 | Drying | - The coated paper significantly increased water contact angle by 155° and rapidly decreased sliding angle from 10° to 1°, providing super hydrophobicity. - The water gain after immersion in water for 24 h reduced from 31.9 g/m2 to 10.3 g/m2 due to superhydrophobic property and less porous structure. - Coated paper demonstrated antifouling and self-cleaning surfaces. | - The PDMS/Hf-SiO2 coated paper enhanced tensile stress from 1.6 MPa to 11.0 MPa due to the PDMS/Hf-SiO2 formed more condensed and compact fiber network. | [47] | |
| Nanofibrillated cellulose (NFC) | Base paper | Meyer rod coating | Styrene butadiene (SB) latex | 0.10, 0.20, 0.30, 0.40% NFC | Calcium carbonate, kaolin and calcined kaolin (45:40:15) and polyvinyl alcohol (PVA), | Drying | - The coated paper displayed increasing the NFC content (0.3–0.4%) decreased the Cobb value from 27.5 to 24.0 g/m2 which NFC reduced the gap size of the coated paper, decreasing the liquid penetration. | - The coated paper increased bursting strength, folding endurance and tensile strength due to NFC enhanced of the binding strength between the coating and base paper. - 0.10%, 0.20%, 0.30% and 0.40% NFC increased air resistance, indicating NFC was likely to reinforce the interaction. | [48] |
| Nanofibrillated cellulose (NFC) | Commercial paper for food packaging | Spraying | Anhydrous ethanol | 100 g anhydrous ethanol: 0.5 g NFC | Tetraethyl orthosilicate, ammonia and acetyl trimethoxysilane | Drying | - WCA of paper exceeded 150° and sliding angle was below 10°, indicating super hydrophobicity. - Waterproofing properties were enhanced by spray amount of 1.5 g/m2 due to the micro-nano rough structures and low surface energy on the superhydrophobic surface. - The micro nano rough structure and low surface energy of coated paper showed the self-cleaning properties. | - 2.5 g/m2 of spraying volume improved the paper strength and increased breakage resistance and tear indices by 12.94% and 10.17%, respectively. - The coating did not affect the water vapor permeability. - The coated paper provided excellent mechanical (rubbing and bending tests) and chemical (acid, alkali, temperature and UV irradiation) stability. - The coated paper influenced the antimicrobial adhesion performance Staphylococcus aureus and Escherichia coli which was reduced by 98.64% and 98.09%, respectively due to low surface energy prevented bacteria from stably adhering to the coated paper surface. | [49] |
| Cellulose nanocrystals (CNCs), octadecylamine (ODA) and polytannic acid (PTA) | Filter paper | Spraying | Ethanol | 2% ODA-PTA@CNC ethanol dispersion | Drying | - ODA-PTA@CNC coated paper displayed WCA for 158° with rougher surface from a nanoscale structure. - ODA-PTA@CNCs had low surface energy which came from octadecyl chain and the nanoscale structure of CNC rods. - ODA-PTA@CNC-coated paper showed water-repellent. | - ODA-PTA@CNC-coated paper exhibited tensile strength of about 22.7 MPa at the dry state and reduced to less than 2.5 MPa after being wetted state. - ODA-PTA@CNC coated papers used as semipermeable membranes of moisture-absorbing devices as a result of waterproof properties. - WCA of the ODA-PTA@CNC-coated filter paper slightly decreased with the friction distance (more than 100 cm) and remained stable and greater than 140° after sandpaper rub testing, indicating excellent wear resistance. | [50] | |
| Polyvinyl Acetate (PVC) and Lignin | Filter paper | Spraying | Acetone | 10 wt.% PVC and 5, 10, 15 and 20% lignin | Drying | - PVC and lignin enhanced the water-resistance and increased contact angle from 2.1° to 90° due to fact that lignin has a hydrophobic nature. | - Increasing the lignin content improved smoother surface and less pore structureresulted of good interpenetration and interaction between paper and copolymers. - Coated paper significantly increased tensile index and burst resistance with the increase of the lignin content. - Lignin increased adhesion of fiber, leading to inhibit sliding and stretching. - Coated paper decreased air permeability which filled the fiber gaps and reduced porosity. | [51] | |
| Polylactic acid (PLA), Stearic acid (SA), cinnamaldehyde (CIN) and nano-silica (SiO2) | Cellulose paper | Coating rod and spraying | Dichloromethane and 90% ethanol | 10%PLA, 1 g SA, 2 g SiO2 | Span80 | Drying | - Coated SA paper had contact angle of 130.9° which SA has more clusters, giving rougher surface, increasing the area of air trapping and enhancing hydrophobicity. - PC-SA/SiO2 enhanced the contact area with air and decreased the surface energy, indicating hydrophobicity with a contact angle of 156.3°. - PC-SA/SiO2 exhibited surface’s excellent self-cleaning capability. - the Cobb value of sample PC-SA/SiO2 decreased by 100% in 60–300 s, indicating superhydrophobic modified samples and excellent water resistance. | - SA/SiO2 increased degradation temperature from 355 to 360 °C, promoting its thermal stability. - Mechanical strength of PC-SA and PC-SA/SiO2 increased by 43.83% and 47.94%, respectively. - Coated PLA/CIN showed smooth surface and compact, indicating the excellent film-forming ability. - Oxygen and water vapor permeability of PC-SA/SiO2 was greatly reduced from 500,000 to 57.942 cm3/m2·day·0.1 MPa and 1802.35 to 206.95 g/m2 ·day·Pa, respectively. - PC-SA inhibited E. coli and S. aureus growth by 99.18 ± 0.23% and 99.31 ± 0.46%, respectively and PC-SA/SiO2 inhibited both bacteria reached up to 100%. | [52] |
| Polystyrene (PS), polyethylene vinyl acetate (PEVA), Carboxymethyl cellulose (CC) and Polyvinyl alcohol (PVOH) | Label paper | Commercia rotary printing press | Deionized water | 0.1–4.0% PS, 0.5–9.5% PEVA, 0.25–2% CC and 2–9.5% PVOH. | Epoxysilicone blend | Drying | - The WCA of all treatments slightly increased with increased coating compound which the coated paper required WCA achieve the 90° for anti-adhesive properties. - The PS-, PEVA-, PVOH and CC-coated paper showed WCA value for 100°, 110°, 94° and 94°, respectively. | - PS coated paper showed a rough surface while PEVA and PVOH had smoother surface than PS. - Adhesive tape test showed that PS occurred non-uniformly, PEVA showed very little paper residue remaining adhered on the surface, PVOH were no paper residues, indicating that the siliconization occurred evenly over the entire surface, resulting in low adhesion to the hydrophobic silicone structure. | [53] |
| Chitosan (CTS), laccase treated chitosan (Lac-CTS) and HP treated chitosan (HP-CTS) | Kraft paper | Brush | 2% v/v acetic acid solution | 0.1 mg, 0.2 mg, 0.3 mg, 0.4 mg, 0.5 mg and 0.6 mg in 100 mL acetic acid solution | Drying | - Lac/HP-CTS coated paper showed sizing degree for 331.7 s, indicating enhancement of water barrier property. - CTS and HP-CTS increased the Cobb values from 85.4 g/m2 to 110.06 g/m2 and 94.72 g/m2, respectively while Lac/HP-CTS coated paper was decreased to 48.66 g/m2 due to chitosan filled the gaps between cellulose fibers and formed a water-resistant layer. | - CTS, HP-CTS and Lac/ HP-CTS coated papers increased dry tensile index by 204.71%, 176.47% and 107.06%. - CTS, HP-CTS and Lac/ HP-CTS coated papers increased the tear index by 65.74%, 79.46% and 36.37%. - CTS, HP-CTS and Lac/ HP-CTS coated papers increased burst index by 117.69%, 110.20% and 42.86%. - SEM of coated paper presented good adhesion between the coating and cellulosic substrate. | [54] | |
| Polydimethylsiloxane (PDMS), γ-aminopropyltriethoxysilane and Titanium dioxide (TiO2) | Filter paper | laboratory roll coater | Absolute ethanol | 2 g of polydimethylsiloxane, 20 g of γ-aminopropyltriethoxysilane modified TiO2 in 100 g of absolute ethanol and | Drying | - The coated γ-aminopropyltriethoxysilane modified TiO2 paper showed hemispherical droplets and were slowly absorb. - Water contact angle of γ-aminopropyltriethoxysilane modified TiO2 increased from 39° to 87.9° due to the number of -OH decreased and the number of hydrophobic groups -CH3 increased. - Water contact angle of PMDS-γ-aminopropyltriethoxysilane modified TiO2 increased from 39° to 154.5°, indicating superhydrophobicity. - PMDS-γ-aminopropyltriethoxysilane modified TiO2 remained superhydrophobic surface after peeling for 40 cycles, indicating the abrasion performance. | - The roughness of the coated paper was reduced from 11.2 μm to 10.27 μm. - PMDS-γ-aminopropyltriethoxysilane modified TiO2 had good chemical stability in acidic and alkaline solutions and in long-term storage. - PMDS-γ-aminopropyltriethoxysilane modified TiO2 showed weaker adhesion between the water droplets, indicating good self-cleaning. | [55] |
| Coating Compounds | Substrate Materials | Form of Bioplastic | Coating Method | Coating Solvent | Concentration of Coating Compounds in Solvent | Solvent Removal Method | Major Findings | Minor Finding | Potential Application | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Chitosan functionalized silica nanoparticles (CS-SNP) | PLA and cellulose-reinforced polybutylene adipate terephthalate (rPBAT) | Film | Spray coating | Acetic acid | Silica nanoparticles powder mixed 20 mL chitosan solution (in 10% acetic acid) and coated film with varying MW of chitosan low MW (50,000–190,000 Da), medium MW (200,000–300,000 Da) and high MW (310,000–375,000 Da). | Dried at 80 °C overnight | - As the MW of chitosan increased, the WCA and water repellency of all films also increased. - The WCA was maximal at the intermediate MW of chitosan for all substrates. | - For both PLA and rPBAT films, the fracture strengths and tensile moduli were similarly enhanced by chitosan-functionalized silica nanoparticles. - The coating enhanced antibacterial activity against Escherichia coli, with up to 90% inhibition for both PLA and rPBAT. | Antimicrobial coating material | [9] |
| Poly (dimethyl siloxane) (PDMS) mixed with five common commercial nano-starch, potato starch, maize starch, rice starch, cassava starch, and wheat starch | Starch loaded anthocyanin | Film | Spray coating | Ethyl acetate | Mass ratios of 0/0.6, 0.1/0.5, 0.3/0.3, and 0.5/0.1 starch/PDMS composite coatings | Cured at 80 °C for 2 h | - The synergistic effect of the hierarchical micro/nanostructure formed by nano-starch aggregates and the low surface energy imparted by PDMS exhibited superhydrophobicity with extremely high WCA of 152.46° and low sliding angle of 8.15°. - The increase in the nano-starch content caused a notable increase in WVP, water solubility (at 100 and 25 °C) and moisture content. - Nano-starch/PDMS composite coating exhibited self-cleaning properties and repelled various liquid food products, including energy drinks, cola, tea, and honey. | - Tensile strength and elongation at break significantly increased with increasing nano-starch ratio in PDMS coating. - During freshness monitoring, film coated 0.3/0.3 starch/PDMS composite can be clearly distinguished without being disabled by water throughout freshness monitoring for 48 h. | Super anti-wetting colorimetric starch film to monitor the freshness of aquatic products | [10] |
| Polydimethylsiloxane (PDMS) and carnauba wax | Chitosan | Film | PDMS spread coating and then carnauba wax-based particles sieve covering the surface | - | 1 g carnauba wax in 60 mL TiO2 dispersion solution with varying the particle size of carnauba wax (23–48 µm and 48–70 µm) | Cured at 45 °C for 6 h | - The addition of carnauba wax particles creates a hierarchical structure on the film surface, resulting in a higher WCA (>135° at 0 s) and lower sliding angle compared to the uncoated film. - Film coated with a smaller size (23–48 µm) carnauba wax-based particles showed higher hydrophobicity than film coated film the larger size (48–70 µm). The smaller size (23–48 µm) carnauba wax-based particles resulted in higher WCA and lower sliding angle. - The coated film with smaller size (23–48 µm) carnauba wax-based particles exhibited excellent self-cleaning properties with low residue rates of liquid foods (yogurt 3.66% and honey 3.05%). | - Carnauba wax particles with a smaller size range (23–48 µm) created a flatter and more anisotropic surface with a higher density of small gaps and particles distributed on the surface. | Hydrophobic coating materials | [14] |
| ZnO nanoparticle mixed with stearic acid | Chitosan | Film | Dip-coating | Ethanol | 0.1%, 0.5%, and 1% of ZnO nanoparticle and 1% of stearic acid | Dried at 60 °C for 10 min | - At 1% concentration of ZnO nanoparticles and stearic acid exhibited the maximum roughness of the composite film (22.4 nm). - Chitosan film dip-coated with of 1% ZnO nanoparticles and 1% steric acid resulted in excellent superhydrophobicity, evidenced by a maximum WCA of 156°. - The WCA of coated chitosan film underwent little change at pH 4–12, and still retained its superhydrophobic properties. 1% concentration of ZnO nanoparticles and stearic acid coated film demonstrated superior self-cleaning and oil-water separation performance. | - The increase in ZnO concentration increased the film’s tensile strength. - The coating improved the thermal stability of the chitosan films. | Food packaging material, outdoor self-cleaning materials and in oil-water separations | [28] |
| Stearic acid/cellulose composite | Rice straw | Kidney tray (biomedical pulp product) | Resurfacing | Ethanol | Ethanolic stearic acid mixed with cellulose at a 2:3 (v/v) ratio | Dried at 90 °C for 24 h | - Coating treatment resulted in a superhydrophobic surface for the biomedical pulp, as evidenced by a WCA of 153° and perfectly spherical water droplets. - Coating treatment improved water resistance up to 7 days at 25 °C and 3 h at 100 °C. | - Coating treatment leads to an increase in the tensile strength, the tensile strain, and tensile energy absorption of the pulp product. | Biomedical applications | [58] |
| Hexamethyldisiloxane (HMDSO) | Corn starch | Film | He plasma modification | Cathode self-bias voltage −60 V for 20 min and 100 V for 10 min | - The combined He/HMDSO treatment resulted in a more homogeneous with the smoothest surface and smaller grains compared to treated with HMDSO only. - Films coated with HMDSO and He/HMDSO became hydrophobic, showing WCA exceeding 110° but does not achieve superhydrophobicity (WCA > 150°) due to a decrease in surface roughness. - The values of the reduction of absorbed water content were: 78.82 ± 3.91 (HMDSO) and 74.81 ± 3.25 (He/HMDSO), % by weight. | - WVP of untreated, treated HMDSO and HE/HMDSO were 8.64 ± 1.47, 8.49 ± 0.12 and 9.74 ± 1.16 (×10−10 g·Pa−1·s−2·m−1), respectively. | Packaging fresh vegetables | [60] | ||
| Myristic acid | Anisotropic cellulose | Film | Solvent-vaporized crystallization | Ethanol | 1, 3, 5, 10, and 20 g/100 mL | Hot-pressing plates | - Increasing the myristic acid concentration to 20% resulted in a hydrophobic surface with the highest WCA (132°) and low surface energy. - At 10% of myristic acid coating exhibited excellent water-repellent and self-cleaning property against various liquid foods including cola, mango juice, yogurt, milk, soy sauce, and vinegar. | - The coated film exhibited good tensile strength and toughness under both dry (188.7 MPa, 34.4 MJ m−3) and humid conditions (119.9 MPa, 28.7 MJ m−3). - Myristic coating also reduced low water uptake (by 35%) and WVP (by 3.5 × 10−5 ± 3.9 × 10−6 g·m−1·h−1·kPa−1). | Self-cleaning and waterproof packaging materials | [62] |
| Methyl trichlorosilane (MTS) | Starch | Porous starch-based nanofiber film and foam | Facile immersion process | Toluene | MTS concentration and reaction time (hours); 2%–4, 6%–2 and 10%–4) | Dried at 60 °C for 30 min | - The MTS coating provided superhydrophobic starch-based adsorbent with low-surface-energy, honeycomb coral-like micro/nanostructures (high WCA > 151.0°, low sliding angle < 15.0°). - The MSC coating exhibited excellent water repellency, self-cleaning properties against coffee powder, and antifouling properties against methylene blue-dyed water and muddy water. - The coated sample exhibited an oil adsorption capacity of 2.5–7.6 g/g for various organic liquids. | - Superhydrophobic starch-based adsorbent exhibits passable mechanical and chemical durability. | Heavy oil removal underwater and oil slick cleaning from the water surface | [63] |
| Chitosan | Pectin | Film | Solution casting method | Acetic acid | 1.5 wt.% in 1% v/v of acetic acid | Dried at 30 °C for 72 h | - The chitosan coating enhanced the surface hydrophobicity of pectin films that increased WCA of 89.5 ± 0.7°. - The coating increased both swelling degree, resistance to water solubility and WVP. - Bilayer pectin/chitosan films exhibited slightly higher WVP (2.69 ± 0.13 × 10−11 mol/m·s·Pa) compared to the neat pectin film (2.15 ± 0.28 × 10−11 mol/m·s·Pa). | - Adding layer of chitosan did not improve the mechanical and oxygen barrier properties of the pectin films. | An inner layer for multilayer packaging for high moisture content products | [64] |
| Graphene and titanium dioxide (TiO2) nanoparticles | PLA | Film | Dip-coating | Hexane | 0.1, 0.3, 0.5, 0.7, and 0.9% w/v of graphene/TiO2 coating solution | Cured at 50 °C for 1 h | - The graphene/TiO2 coating exhibited enhanced low surface wettability, retaining a WCA above 150° even after 24 h of water immersion testing. - Superhydrophobicity of the coated film was achieved with a WCA of 164.21 ± 1° at a graphene to TiO2 ratio of 1:9. | - The graphene/TiO2 coating exhibited enhanced surface roughness and excellent durability | Medical devices with self-cleaning properties | [65] |
| Blocked disocyanate solution | Nanofibrilated cellulose | Film | Dip-coating and then thermal treatment at 170 °C for 10 min. | Anhydrous butyl acetate | 6 g of blocked disocyanate solution mixed 0.25 g of zinc octanoate in 100 mL of xylene/butyl acetate (75/25). | Washing and dried at 60 °C for 1 h. | - Coated film increased in the average roughness, the mean square root roughness and the maximum profile height. - The treatment increased the WCA from 52° to 113°. | - Oxygen transmission rate (OTR) was decreased from 15 cm3/m2/day to 0.1 cm3/m2/day and WVTR was reduced from 153 g/m2·day to 40 g/m2·day for neat and treated film, respectively. | Flexible films for packaging | [66] |
| Cellulose nanocrystals (CNC) mixed with 15 wt.% PVA or kappa-carrageenan | PLA | Film | Solution casting method and laminating | Water | 6 and 10 wt. % | Dried at room temperature | - Laminating PLA as the outer layers significantly improves the water resistance of the CNC core layer, evidenced by 7 times decreased in WVP (<3000 g·µm·m−2 day−1·kPa−1) compared to neat CNC film (9603 g·µm·m−2 day−1·kPa−1). | - The CNC core layer provides excellent oxygen barrier properties achieving a reduction of more than 70 times compared to a pure PLA film. - The tensile strength of PLA film laminated with either CNC/PVA or CNC/CG coating does not significantly change. - The tear strength of the CNC film was improved when laminated with PLA films. | Recyclable food barrier packaging | [67] |
| Beeswax | Cutin/pectin membrane | Membranes | Spray coating | Ethanol | 2.5 mg/cm2 on one/both sides | Dried at 55 °C for 60 s | - Beeswax coated membrane exhibited superhydrophobicity with WCA at 152 °C and sliding angle at 3°. - Beeswax coated membrane showed anti-fouling against various liquid food products including water, honey, tea, energy drink, cola, yogurt, milk and coffee. | - Combination of coating and heat treatment showed no significant mechanical changes to the uncoated membrane. - Beeswax coated membrane could efficiently block the enzymatic browning of apple after placed at room temperature for 24 h. - The coated bag can be easily dissolved into boiled water, as the beeswax melt at temperature around 62 °C. | Recyclable functional packaging for underwater storage, oxidation blocking, selective release bags | [68] |
| Polyethyleneimine (PEI) and beeswax | Starch/cellulose composites | Film | Polyethyleneimine (PEI) immersion coating and then beeswax spray coating | Absolute ethanol for PEI and hexane and acetone for beeswax | 1.26 wt.% PEI solution and varying sprayed beeswax solution for 1, 3, 6, 9 and 12 times | Annealed at 55 °C for 1 min | - The beeswax-based creates a rough surface with low surface energy, resulting in WCA of 152° and sliding angle of 6°. - Water/moisture absorption of film reduced significantly (>30%) after beeswax functionalization. - The coated film showed excellent self-cleaning ability and anti-fouling performance against various liquid drinks including milk, cola, coffee and tea. | - Tensile strength of the beeswax treated substrate was slightly change (≈22 MPa) compared to untreated, while the elongation at break increased as the humidity increased. | Packaging materials | [69] |
| Chitosan crosslinked with glutaraldehyde | PLA, ZnO, and palm wax | Film | Spray coating | Acetic acid | 0.25%, 0.5%, 0.75%, 1.0%, and 1.25% (w/v) in 25 mL of 2% acetic acid solution | Dried at room temperature | - The chitosan-glutaraldehyde coated film exhibited significantly lower WVP (37% reduction) compared to the uncoated film that almost water repellent or waterproof. | - Increasing chitosan content resulted in increased tensile strength but decreased elongation at break, due to the enhanced Schiff base reaction between chitosan and glutaraldehyde. - At highest chitosan content (1.25% w/v) exhibited greater antibacterial activity against Escherichia coli (clear zone 3.2 mm) than Staphylococcus aureus (clear zone 2.2 mm). | Disposable waterproof aprons or personal protective equipment (PPE) material | [70] |
| Chitosan | ι-Carrageenan/ZnO | Film | Dispersion casting | Acetic acid | 1 g of chitosan in 100 mL of 1% wt. acetic acid | Dried at 50 °C for 7 h | - The WCA of the bilayer film (91.9°) was about 1.3 times higher than that of the neat carrageenan films (68.4°). - Bilayer film had the best swelling resistance (anti-expansion performance) in distilled water (1962.78%) and normal saline (830.30%). | - The tensile strength of bilayer film was 51.49 MPa, 1.5 times more than that of carrageenan/ZnO film (28.9 MPa). - WVP of bilayer film (11.75 × 10−11 g·m−1·s−2·Pa−1) was decreased compared to carrageenan/ZnO film (15.17 × 10−11 g·m−1·s−2·Pa−1). - The bilayer film exhibited greater antibacterial activity against Escherichia coli (clear zone 16.19 ± 1.04 mm) than Staphylococcus aureus (clear zone 15.73 ± 1.01 mm). | Food packaging materials and wound dressings | [71] |
| Polydimethylsiloxane (PDMS) mixed with ball-milled rice starch, corn starch, or potato starch | Hydroxypropyl methylcellulose (HPMC) | Film | Spray coating and immersion | Ethyl acetate | Varying ball-milling process (0, 2, 6, and 8) | Thermally curing at 105 °C for 2 h | - PDMS/rice starch and PDMS/corn starch coatings exhibited superhydrophobic behavior, while PDMS/potato starch coatings failed to achieve superhydrophobicity. - The synergistic combination of PDMS-coated HPMC with 2 h of ball-milled corn starch resulted in a highly superhydrophobic surface, exhibiting a high WCA of 170.5° and a minimal sliding angle of 5.2°. | - PDMS/ball-milled starch coatings exhibit significantly less Escherichia coli adhesion. - PDMS/ball-milled starch coatings also exhibit self-cleaning against various food liquids (water, milk, Coca-Cola, and honey). | Food packaging materials | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungruangkitkrai, N.; Phromphen, P.; Chartvivatpornchai, N.; Srisa, A.; Laorenza, Y.; Wongphan, P.; Harnkarnsujarit, N. Water Repellent Coating in Textile, Paper and Bioplastic Polymers: A Comprehensive Review. Polymers 2024, 16, 2790. https://doi.org/10.3390/polym16192790
Rungruangkitkrai N, Phromphen P, Chartvivatpornchai N, Srisa A, Laorenza Y, Wongphan P, Harnkarnsujarit N. Water Repellent Coating in Textile, Paper and Bioplastic Polymers: A Comprehensive Review. Polymers. 2024; 16(19):2790. https://doi.org/10.3390/polym16192790
Chicago/Turabian StyleRungruangkitkrai, Nattadon, Phannaphat Phromphen, Nawarat Chartvivatpornchai, Atcharawan Srisa, Yeyen Laorenza, Phanwipa Wongphan, and Nathdanai Harnkarnsujarit. 2024. "Water Repellent Coating in Textile, Paper and Bioplastic Polymers: A Comprehensive Review" Polymers 16, no. 19: 2790. https://doi.org/10.3390/polym16192790
APA StyleRungruangkitkrai, N., Phromphen, P., Chartvivatpornchai, N., Srisa, A., Laorenza, Y., Wongphan, P., & Harnkarnsujarit, N. (2024). Water Repellent Coating in Textile, Paper and Bioplastic Polymers: A Comprehensive Review. Polymers, 16(19), 2790. https://doi.org/10.3390/polym16192790






