Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review
Abstract
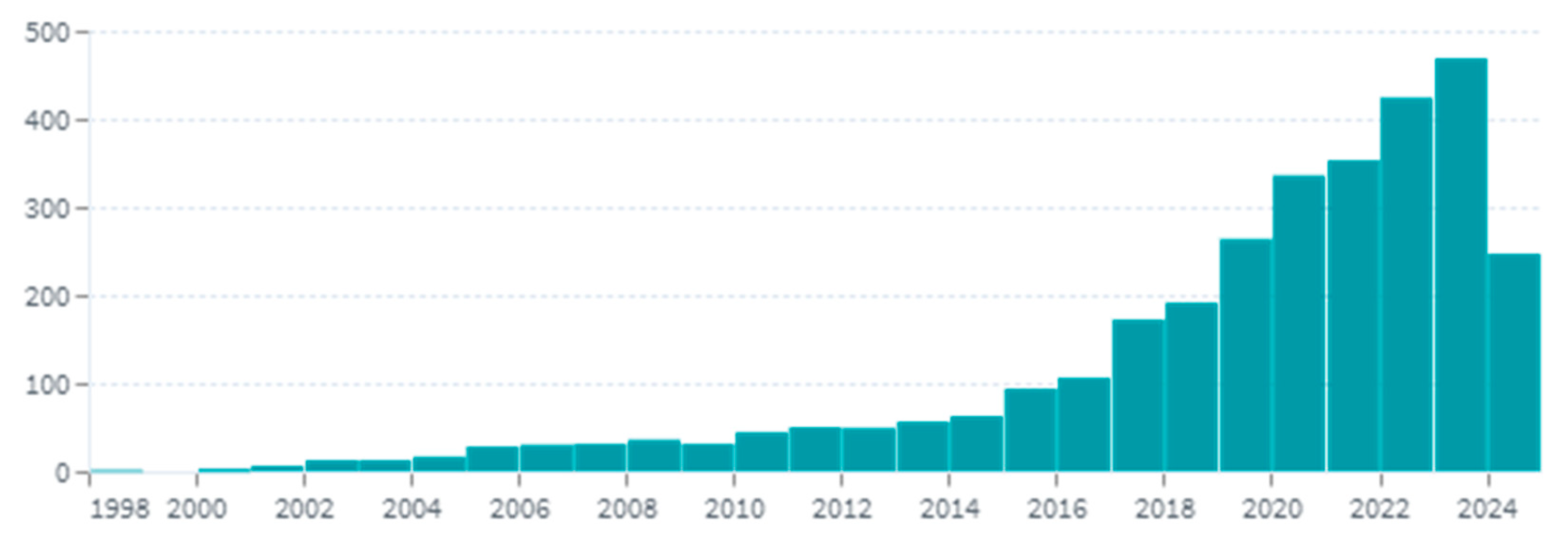
:1. Introduction
2. 3D Printing in Dentistry
2.1. Classification of 3D Printing Techniques
2.2. Importance of 3D Printing in Dentistry
2.3. Materials and Required Properties
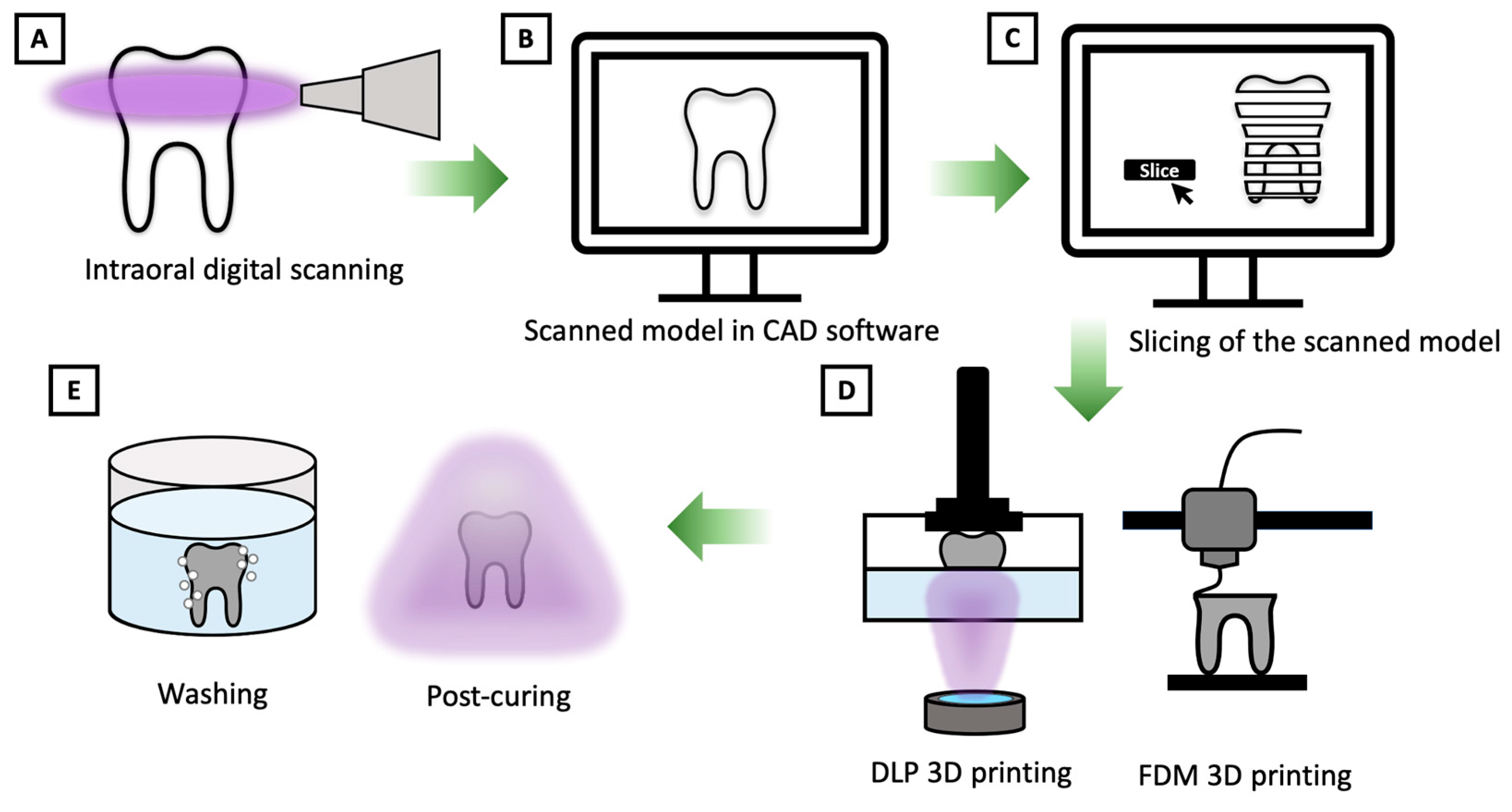
2.4. Workflow of 3D Printing Technology in Dental Applications
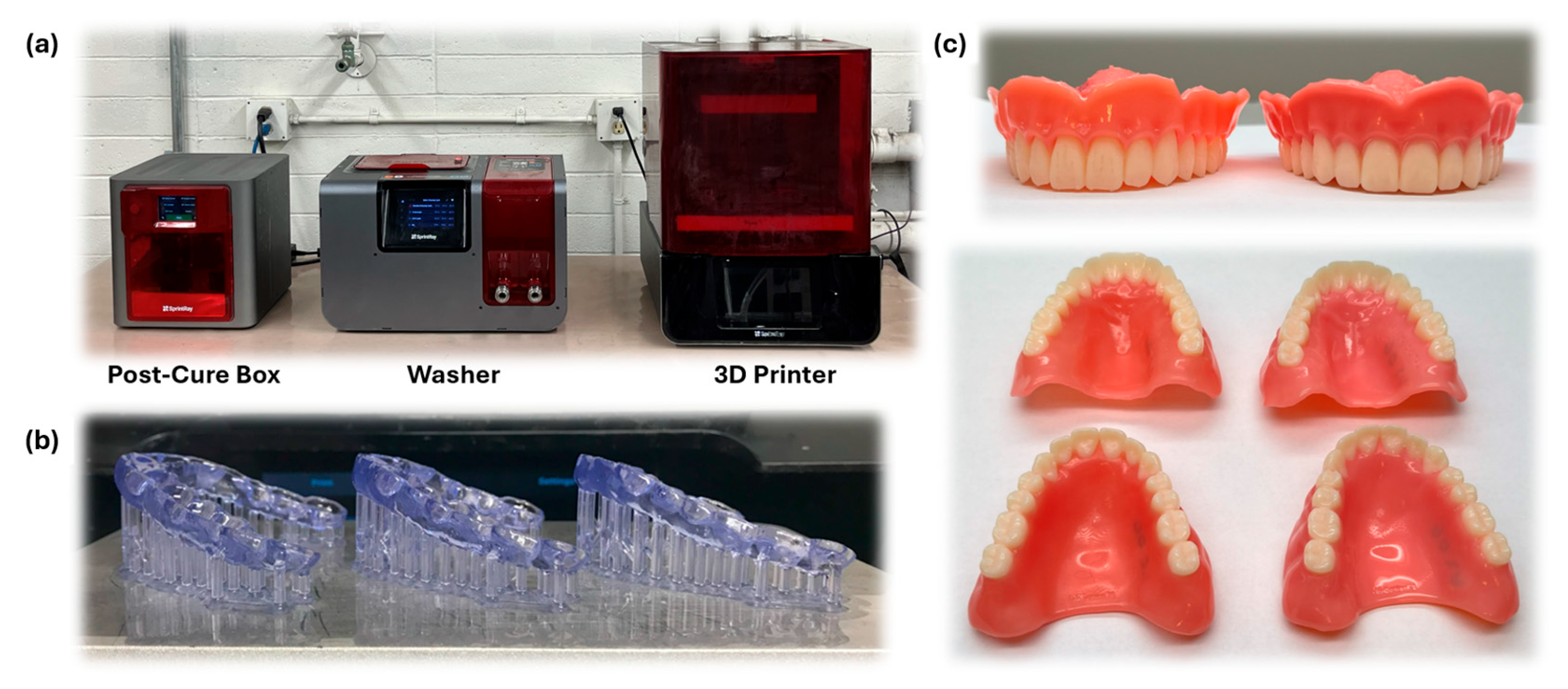
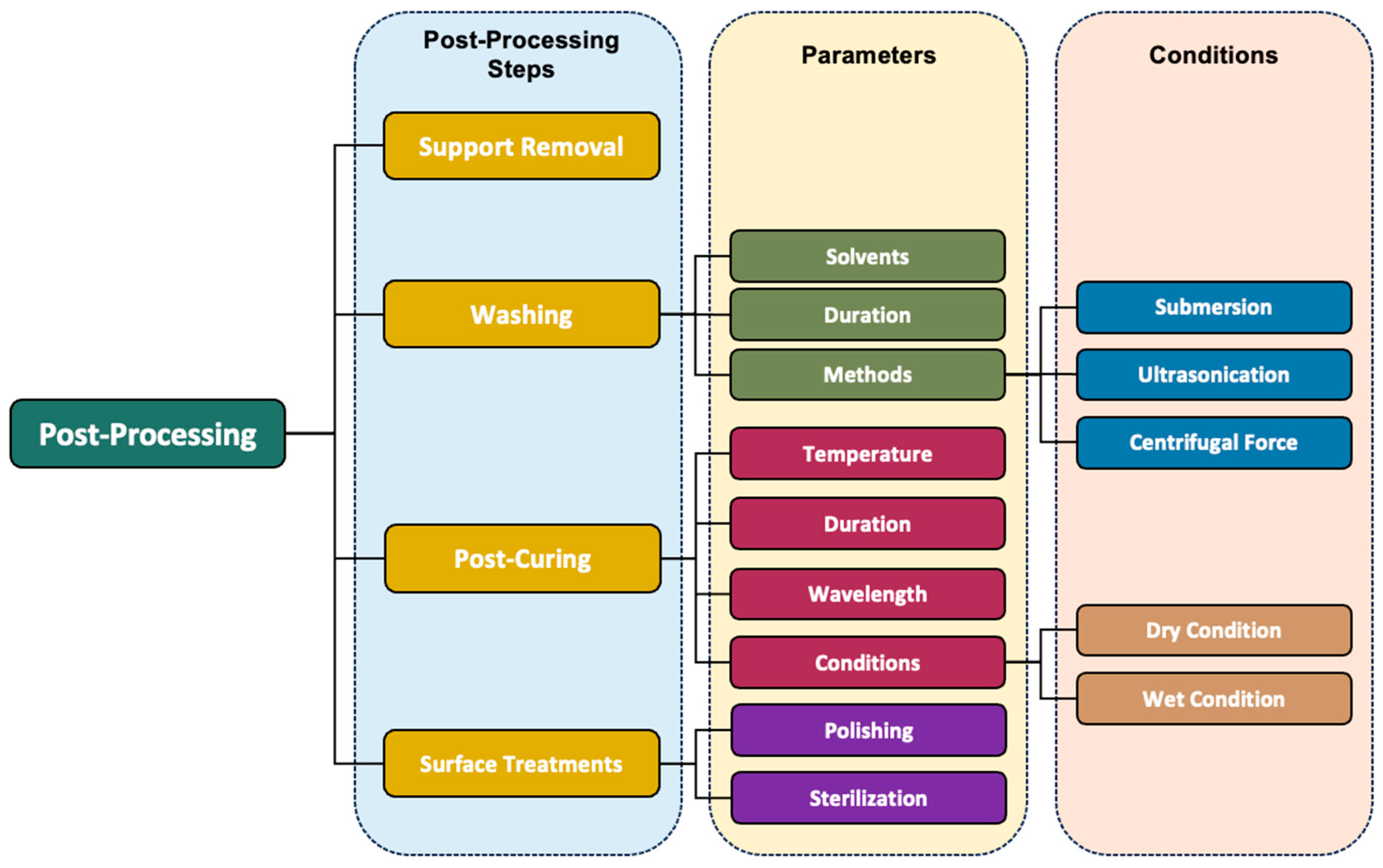
3. Post-Processing
3.1. Importance of Post-Processing and Considering Factors
3.2. Post-Processing Techniques
4. Post-Processing Steps
4.1. Removal of the Support Structure
4.2. Cleaning/Washing
4.2.1. Solvent Selection for Post-Processing of 3D-Printed Parts
4.2.2. Influence of Washing Time
4.2.3. Influence of Washing Method
4.3. Post-Polymerization (Secondary Curing Steps)
4.3.1. Importance of Post-Curing
4.3.2. Effects of Post-Curing Temperature
4.3.3. Effects of Post-Curing Duration
4.3.4. Effects of Wavelength/UV Intensity of the Cure-Box
4.3.5. Effects of Post-Curing Conditions
4.4. Polishing and Surface Treatment
5. Problems, Challenges, and Future Directions in Dental 3D Printing Post-Processing
6. Conclusions
Funding
Conflicts of Interest
References
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D Printing—A Review of Processes, Materials and Applications in Industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L.V. 3D-Printed vs. Heat-Polymerizing and Autopolymerizing Denture Base Acrylic Resins. Materials 2021, 14, 5781. [Google Scholar] [CrossRef] [PubMed]
- Nesic, D.; Schaefer, B.M.; Sun, Y.; Saulacic, N.; Sailer, I. 3D Printing Approach in Dentistry: The Future for Personalized Oral Soft Tissue Regeneration. J. Clin. Med. 2020, 9, 2238. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.-S.; Jiang, H.B. A Review of 3D Printing in Dentistry: Technologies, Affecting Factors, and Applications. Scanning 2021, 2021, 9950131. [Google Scholar] [CrossRef]
- Dimitrova, M.; Corsalini, M.; Kazakova, R.; Vlahova, A.; Chuchulska, B.; Barile, G.; Capodiferro, S.; Kazakov, S. Comparison Between Conventional PMMA and 3D Printed Resins for Denture Bases: A Narrative Review. J. Compos. Sci. 2022, 6, 87. [Google Scholar] [CrossRef]
- da Costa, L.P.G.; Zamalloa, S.I.D.; Alves, F.A.M.; Spigolon, R.; Mano, L.Y.; Costa, C.; Mazzo, A. 3D Printers in Dentistry: A Review of Additive Manufacturing Techniques and Materials. Clin. Lab. Res. Dent. 2021. preprint. [Google Scholar] [CrossRef]
- Hwangbo, N.-K.; Nam, N.-E.; Choi, J.-H.; Kim, J.-E. Effects of the Washing Time and Washing Solution on the Biocompatibility and Mechanical Properties of 3D Printed Dental Resin Materials. Polymers 2021, 13, 4410. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Dong, X.; Zhang, Y.; Shen, W. Recent progress in additive manufacturing of ceramic dental restorations. J. Mater. Res. Technol. 2023, 26, 1028–1049. [Google Scholar] [CrossRef]
- Karakurt, I.; Lin, L. 3D Printing Technologies: Techniques, Materials, and Post-Processing. Curr. Opin. Chem. Eng. 2020, 28, 134–143. [Google Scholar] [CrossRef]
- Piedra-Cascón, W.; Krishnamurthy, V.R.; Att, W.; Revilla-León, M. 3D Printing Parameters, Supporting Structures, Slicing, and Post-Processing Procedures of VAT-Polymerization Additive Manufacturing Technologies: A Narrative Review. J. Dent. 2021, 109, 103630. [Google Scholar] [CrossRef]
- Alsandi, Q.; Ikeda, M.; Arisaka, Y.; Nikaido, T.; Tsuchida, Y.; Sadr, A.; Yui, N.; Tagami, J. Evaluation of Mechanical and Physical Properties of Light and Heat Polymerized UDMA for DLP 3D Printer. Sensors 2021, 21, 3331. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-Curing 3D Printing Technique and Its Challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, R.; Cosma, M.P.; Marsavina, L.; Spagnoli, A.; Terzano, M. Laser-based additively manufactured polymers: A review on processes and mechanical models. J. Mater. Sci. 2021, 56, 961–998. [Google Scholar] [CrossRef]
- Uzcategui, A.C.; Muralidharan, A.; Ferguson, V.L.; Bryant, S.J.; McLeod, R.R. Understanding and Improving Mechanical Properties in 3D Printed Parts Using a Dual-Cure Acrylate-Based Resin for Stereolithography. Adv. Eng. Mater. 2018, 20, 1800876. [Google Scholar] [CrossRef]
- Schittecatte, L.; Geertsen, V.; Bonamy, D.; Nguyen, T.; Guenoun, P. From resin formulation and process parameters to the final mechanical properties of 3D printed acrylate materials. MRS Commun. 2023, 13, 357–377. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Z.; Zhang, Q.; Qi, H.J.; Fang, D. Mechanics of Shape Distortion of DLP 3D Printed Structures During UV Post-Curing. Soft Matter 2019, 15, 6151–6159. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, Y.M.; Lai, Y.L.; Lee, S.Y. Mechanical Properties, Accuracy, and Cytotoxicity of UV-Polymerized 3D Printing Resins Composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2020, 123, 349–354. [Google Scholar] [CrossRef]
- Chaudhary, R.; Fabbri, P.; Leoni, E.; Mazzanti, F.; Akbari, R.; Antonini, C. Additive Manufacturing by Digital Light Processing: A Review. Prog. Addit. Manuf. 2023, 8, 331–351. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Mapelli, A.; Maspero, C.; Santaniello, T.; Serafin, M.; Farronato, M.; Caprioglio, A. Direct 3D Printing of Clear Orthodontic Aligners: Current State and Future Possibilities. Materials 2021, 14, 1799. [Google Scholar] [CrossRef]
- Riccio, C.; Civera, M.; Ruiz, O.G.; Pedullà, P.; Reinoso, M.R.; Tommasi, G.; Vollaro, M.; Burgio, V.; Surace, C. Effects of Curing on Photosensitive Resins in SLA Additive Manufacturing. Appl. Mech. 2021, 2, 942–955. [Google Scholar] [CrossRef]
- Nakano, H.; Kato, R.; Kakami, C.; Okamoto, H.; Mamada, K.; Maki, K. Development of Biocompatible Resins for 3D Printing of Direct Aligners. J. Photopolym. Sci. Technol. 2019, 32, 209–216. [Google Scholar] [CrossRef]
- Alshamrani, A.; Alhotan, A.; Kelly, E.; Ellakwa, A. Mechanical and biocompatibility properties of 3D-printed dental resin reinforced with glass silica and zirconia nanoparticles: In vitro study. Polymers 2023, 15, 2523. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, T.; Kreuter, P.; Kismarczi-Antalffy, A.A.; Demeter, T.; Banyai, D.; Vegh, A.; Geczi, Z.; Hermann, P.; Payer, M.; Zsembery, A.; et al. User Experience and Sustainability of 3D Printing in Dentistry. Int. J. Environ. Res. Public Health 2022, 19, 1921. [Google Scholar] [CrossRef]
- Schweiger, J.; Beuer, F.; Stimmelmayr, M.; Edelhoff, D.; Magne, P.; Güth, J.F. Histo-anatomic 3D printing of dental structures. Br. Dent. J. 2016, 221, 555–560. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing—Encompassing the Facets of Dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef]
- Kessler, A.; Reichl, F.X.; Folwaczny, M.; Högg, C. Monomer release from surgical guide resins manufactured with different 3D printing devices. Dent. Mater. 2020, 36, 1486–1492. [Google Scholar] [CrossRef]
- Jung, S.K.; Kim, T.W. New approach for the diagnosis of extractions with neural network machine learning. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 127–133. [Google Scholar] [CrossRef]
- Goh, G.D.; Sing, S.L.; Yeong, W.Y. A review on machine learning in 3D printing: Applications, potential, and challenges. Artif. Intell. Rev. 2021, 54, 63–94. [Google Scholar] [CrossRef]
- Fatima, A.; Shafi, I.; Afzal, H.; Díez, I.D.L.T.; Lourdes, D.R.-S.M.; Breñosa, J.; Espinosa, J.C.M.; Ashraf, I. Advancements in Dentistry with Artificial Intelligence: Current Clinical Applications and Future Perspectives. Healthcare 2022, 10, 2188. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Kim, J.-E.; Jeong, S.-H.; Choi, Y.-J.; Ryu, J.-J. Printing Accuracy, Mechanical Properties, Surface Characteristics, and Microbial Adhesion of 3D-Printed Resins with Various Printing Orientations. J. Prosthet. Dent. 2020, 124, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Felipe, C.; Patrocinio, D.; Laza, J.M.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Evaluation of Postcuring Process on the Thermal and Mechanical Properties of the Clear02™ Resin Used in Stereolithography. Polym. Test. 2018, 72, 115–121. [Google Scholar] [CrossRef]
- Jacobs, P.F. Rapid Prototyping & Manufacturing: Fundamentals of Stereolithography, 1st ed.; Society of Manufacturing Engineers: Dearborn, MI, USA, 1992. [Google Scholar]
- Aktas, N.; Bankoglu Güngör, M. Effects of 3D-Printing Technology and Cement Type on the Fracture Resistance of Permanent Resin Crowns for Primary Teeth. Int. J. Prosthodont. 2024, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- González, G.; Baruffaldi, D.; Martinengo, C.; Angelini, A.; Chiappone, A.; Roppolo, I.; Pirri, C.F.; Frascella, F. Materials Testing for the Development of Biocompatible Devices Through VAT-Polymerization 3D Printing. Nanomaterials 2020, 10, 1788. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D Printing of Polymer Nanocomposites via Stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Tzeng, J.-J.; Yang, T.-S.; Lee, W.-F.; Chen, H.; Chang, H.-M. Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers 2021, 13, 822. [Google Scholar] [CrossRef]
- Falahchai, M.; Ghavami-Lahiji, M.; Rasaie, V.; Amin, M.; Asli, H.N. Comparison of mechanical properties, surface roughness, and color stability of 3D-printed and conventional heat-polymerizing denture base materials. J. Prosthet. Dent. 2023, 130, 266.e1–266.e8. [Google Scholar] [CrossRef]
- Alharbi, N.; Osman, R.; Wismeijer, D. Effects of Build Direction on the Mechanical Properties of 3D-Printed Complete Coverage Interim Dental Restorations. J. Prosthet. Dent. 2016, 115, 760–767. [Google Scholar] [CrossRef]
- Kim, D.; Shim, J.S.; Lee, D.; Shin, S.H.; Nam, N.E.; Park, K.H.; Shim, J.-S.; Kim, J.E. Effects of Post-Curing Time on the Mechanical and Color Properties of Three-Dimensional Printed Crown and Bridge Materials. Polymers 2020, 12, 2762. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Bui, P.H.-B.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects Build Orientation, Positioning, and Curing Influence Dimensional Accuracy and Flexural Properties of Stereolithographically Printed Resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.N.; Thompson, G.A.; Cho, S.H.; Berzins, D.W.; Ahn, K.W. Die Spacer Thickness Reproduction for Central Incisor Crown Fabrication with Combined Computer-Aided Design and 3D Printing Technology: An In Vitro Study. J. Prosthet. Dent. 2015, 113, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Narongdej, P.; Hassanpour, M.; Alterman, N.; Rawlins-Buchanan, F.; Barjasteh, E. Advancements in Clear Aligner Fabrication: A Comprehensive Review of Direct-3D Printing Technologies. Polymers 2024, 16, 371. [Google Scholar] [CrossRef] [PubMed]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D Printed versus Conventionally Cured Provisional Crown and Bridge Dental Materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef]
- Vara, R.; Lin, W.; Low, J.K.; Smith, D.; Grimm, A.; Calvert, G.; Tadakamadla, S.K.; Alifui-Segbaya, F.; Ahmed, K.E. Assessing the Impact of Resin Type, Post-Processing Technique, and Arch Location on the Trueness and Precision of 3D-Printed Full-Arch Implant Surgical Guides. Appl. Sci. 2023, 13, 2491. [Google Scholar] [CrossRef]
- Dawood, A.; Marti Marti, B.; Sauret-Jackson, V.; Darwood, A. 3D Printing in Dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Soto-Montero, J.; de Castro, E.F.; Romano, B.D.C.; Nima, G.; Shimokawa, C.A.; Giannini, M. Color alterations, flexural strength, and microhardness of 3D printed resins for fixed provisional restoration using different post-curing times. Dent. Mater. 2022, 38, 1271–1282. [Google Scholar] [CrossRef]
- Guttridge, C.; Shannon, A.; O’Sullivan, A.; O’Sullivan, K.J.; O’Sullivan, L.W. Biocompatible 3D Printing Resins for Medical Applications: A Review of Marketed Intended Use, Biocompatibility Certification, and Post-Processing Guidance. Ann. 3D Print. Med. 2022, 5, 100044. [Google Scholar] [CrossRef]
- Prakash, J.; Shenoy, M.; Alhasmi, A.; Al Saleh, A.A.; Shivakumar, G.C.; Shivakumar, S. Biocompatibility of 3D-printed dental resins: A systematic review. Cureus 2024, 16, e51721. [Google Scholar] [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. VAT Photopolymerization of Polymers and Polymer Composites: Processes and Applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Gache, C.C.L.; Cascolan, H.M.S.; Cancino, L.T.; Advincula, R.C. Post-Processing of 3D-Printed Polymers. Technologies 2021, 9, 61. [Google Scholar] [CrossRef]
- Loriot, B.; Ralph, S.; Gorria, P. Non-Model Based Method for an Automation of 3D Acquisition and Post-Processing. ELCVIA Electron. Lett. Comput. Vis. Image Anal. 2009, 7, 67. [Google Scholar] [CrossRef]
- Cao, J.; Liu, X.; Cameron, A.; Aarts, J.; Choi, J.J. Influence of Different Post-Processing Methods on the Dimensional Accuracy of 3D-Printed Photopolymers for Dental Crown Applications—A Systematic Review. J. Mech. Behav. Biomed. Mater. 2023, 150, 106314. [Google Scholar] [CrossRef] [PubMed]
- Short, D.B.; Sirinterlikci, A.; Badger, P.; Artieri, B. Environmental, Health, and Safety Issues in Rapid Prototyping. Rapid Prototyp. J. 2015, 21, 105–110. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Ayatollahi, M.R.; Reinicke, T. Effects of Post-Processing Techniques on the Mechanical Characterization of Additively Manufactured Parts. J. Manuf. Process. 2023, 107, 98–114. [Google Scholar] [CrossRef]
- Kumbhar, N.N.; Mulay, A.V. Post Processing Methods Used to Improve Surface Finish of Products Which Are Manufactured by Additive Manufacturing Technologies: A Review. J. Inst. Eng. (India) Ser. C 2016, 99, 481–487. [Google Scholar] [CrossRef]
- Linares-Alvelais, J.A.R.; Figueroa-Cavazos, J.O.; Chuck-Hernandez, C.; Siller, H.R.; Rodríguez, C.A.; Martínez-López, J.I. Hydrostatic High-Pressure Post-Processing of Specimens Fabricated by DLP, SLA, and FDM: An Alternative for the Sterilization of Polymer-Based Biomedical Devices. Materials 2018, 11, 2540. [Google Scholar] [CrossRef]
- Dahl, J.E.; Stenhagen, I.S.R. Optimizing Quality and Safety of Dental Materials. Eur. J. Oral Sci. 2018, 126, 102–105. [Google Scholar] [CrossRef]
- FayyazAhamed, S.; Kumar, S.M.; Vijayakumar, R.K.; AprosKanna, A.S.; Indrapriyadharshini, K. Cytotoxic Evaluation of Directly 3D Printed Aligners and Invisalign. Eur. J. Mol. Clin. Med. 2020, 7, 1129–1140. [Google Scholar]
- Šimunović, L.; Jurela, A.; Sudarević, K.; Bačić, I.; Haramina, T.; Meštrović, S. Influence of Post-Processing on the Degree of Conversion and Mechanical Properties of 3D-Printed Polyurethane Aligners. Polymers 2023, 16, 17. [Google Scholar] [CrossRef]
- Tiba, A.; Zeller, G.; Estrich, C.G.; Hong, A. A Laboratory Evaluation of Bulk-Fill versus Traditional Multi-Increment–Fill Resin-Based Composites. J. Am. Dent. Assoc. 2013, 144, 1182–1183. [Google Scholar] [CrossRef] [PubMed]
- Snowwhite, P.; Snowwhite, C.; Smith, T. Additive Manufacturing Post Process; 7D Innovators, LLC: Dexter, MI, USA, 2022. [Google Scholar]
- Bardelcik, A.; Yang, S.; Alderson, F.; Gadsden, A. The Effect of Wash Treatment on the Mechanical Properties and Energy Absorption Potential of a 3D Printed Polymethyl Methacrylate (PMMA). Mater. Today Commun. 2021, 26, 101728. [Google Scholar] [CrossRef]
- Scherer, M.D.; Husain, N.A.-H.; Barmak, A.B.; Kois, J.C.; Özcan, M.; Revilla-León, M. Influence of Postprocessing Rinsing Solutions and Duration on Flexural Strength of Aged and Nonaged Additively Manufactured Interim Dental Material. J. Prosthet. Dent. 2022, 131, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Jindal, P.; Juneja, M.; Bajaj, D.; Siena, F.L.; Breedon, P. Effects of Post-Curing Conditions on Mechanical Properties of 3D Printed Clear Dental Aligners. Rapid Prototyp. J. 2020, 26, 1337–1344. [Google Scholar] [CrossRef]
- Mayer, J.; Reymus, M.; Mayinger, F.; Edelhoff, D.; Hickel, R.; Stawarczyk, B. Temporary 3D-Printed Fixed Dental Prosthesis Materials: Impact of Postprinting Cleaning Methods on Degree of Conversion and Surface and Mechanical Properties. Int. J. Prosthodont. 2021, 34, 784–795. [Google Scholar] [CrossRef]
- Mayer, J.; Stawarczyk, B.; Vogt, K.; Hickel, R.; Edelhoff, D.; Reymus, M. Influence of Cleaning Methods after 3D Printing on Two-Body Wear and Fracture Load of Resin-Based Temporary Crown and Bridge Material. Clin. Oral Investig. 2021, 25, 5987–5996. [Google Scholar] [CrossRef]
- Jang, W.; Kook, G.-S.; Kang, J.-H.; Kim, Y.; Yun, Y.; Lee, S.-K.; Park, S.-W.; Lim, H.-P.; Yun, K.-D.; Park, C. Effect of Washing Condition on the Fracture Strength, and the Degree of Conversion of 3D Printing Resin. Appl. Sci. 2021, 11, 11676. [Google Scholar] [CrossRef]
- Xu, Y.; Xepapadeas, A.B.; Koos, B.; Geis-Gerstorfer, J.; Li, P.; Spintzyk, S. Effect of Post-Rinsing Time on the Mechanical Strength and Cytotoxicity of a 3D Printed Orthodontic Splint Material. Dent. Mater. 2021, 37, e314–e327. [Google Scholar] [CrossRef]
- Nowacki, B.; Kowol, P.; Kozioł, M.; Olesik, P.; Wieczorek, J.; Wacławiak, K. Effect of Post-Process Curing and Washing Time on Mechanical Properties of MSLA Printouts. Materials 2021, 14, 4856. [Google Scholar] [CrossRef]
- Oh, R.; Lim, J.H.; Lee, C.G.; Lee, K.W.; Kim, S.Y.; Kim, J.E. Effects of washing solution temperature on the biocompatibility and mechanical properties of 3D-Printed dental resin material. J. Mech. Behav. Biomed. Mater. 2023, 143, 105906. [Google Scholar] [CrossRef]
- Lambart, A.-L.; Xepapadeas, A.B.; Koos, B.; Li, P.; Spintzyk, S. Rinsing Postprocessing Procedure of a 3D-Printed Orthodontic Appliance Material: Impact of Alternative Post-Rinsing Solutions on the Roughness, Flexural Strength and Cytotoxicity. Dent. Mater. 2022, 38, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Gu, H.; Jang, M.; Bayarsaikhan, E.; Lim, J.H.; Shim, J.S.; Lee, K.-W.; Kim, J.E. Influence of postwashing process on the elution of residual monomers, degree of conversion, and mechanical properties of a 3D printed crown and bridge materials. Dent. Mater. 2022, 38, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Virinthorn, R.N.V.C.; Chandrasekaran, M.; Wang, K.; Goh, K.L. Post-Process Optimization of 3D Printed Poly(lactic-co-glycolic acid) Dental Implant Scaffold for Enhanced Structure and Mechanical Properties: Effects of Sonication Duration and Power. J. Mater. Sci. Mater. Med. 2021, 32, 91. [Google Scholar] [CrossRef] [PubMed]
- Galvão, M.R.; Caldas, S.G.F.R.; Bagnato, V.S.; de Souza Rastelli, A.N.; de Andrade, M.F. Evaluation of Degree of Conversion and Hardness of Dental Composites Photo-Activated with Different Light Guide Tips. Eur. J. Dent. 2013, 7, 86. [Google Scholar]
- Bayarsaikhan, E.; Lim, J.-H.; Shin, S.-H.; Park, K.-H.; Park, Y.-B.; Lee, J.-H.; Kim, J.-E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13, 1180. [Google Scholar] [CrossRef]
- Par, M.; Gamulin, O.; Marovic, D.; Klaric, E.; Tarle, Z. Effect of temperature on post-cure polymerization of bulk-fill composites. J. Dent. 2014, 42, 1255–1260. [Google Scholar] [CrossRef]
- Katheng, A.; Kanazawa, M.; Iwaki, M.; Minakuchi, S. Evaluation of Dimensional Accuracy and Degree of Polymerization of Stereolithography Photopolymer Resin under Different Postpolymerization Conditions: An In Vitro Study. J. Prosthet. Dent. 2021, 125, 695–702. [Google Scholar] [CrossRef]
- Yeung, K.C.; Chow, T.W.; Clark, R.K.F. Temperature and Dimensional Changes in the Two-Stage Processing Technique for Complete Dentures. J. Dent. 1995, 23, 245–253. [Google Scholar] [CrossRef]
- Hague, R.; Mansour, S.; Saleh, N.; Harris, R. Materials Analysis of Stereolithography Resins for Use in Rapid Manufacturing. J. Mater. Sci. 2004, 39, 2457–2464. [Google Scholar] [CrossRef]
- Bağis, Y.H.; Rueggeberg, F.A. Effect of Post-Cure Temperature and Heat Duration on Monomer Conversion of Photo-Activated Dental Resin Composite. Dent. Mater. 1997, 13, 228–232. [Google Scholar] [CrossRef]
- Miedzińska, D.; Gieleta, R.; Popławski, A. Experimental Study on Influence of Curing Time on Strength Behavior of SLA-Printed Samples Loaded with Different Strain Rates. Materials 2020, 13, 5825. [Google Scholar] [CrossRef] [PubMed]
- Reymus, M.; Fabritius, R.; Keßler, A.; Hickel, R.; Edelhoff, D.; Stawarczyk, B. Fracture Load of 3D-Printed Fixed Dental Prostheses Compared with Milled and Conventionally Fabricated Ones: The Impact of Resin Material, Build Direction, Post-Curing, and Artificial Aging—An In Vitro Study. Clin. Oral Investig. 2019, 24, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Monzón, M.; Ortega, Z.; Hernández, A.; Paz, R.; Ortega, F. Anisotropy of Photopolymer Parts Made by Digital Light Processing. Materials 2017, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.A.; Ayranci, C.; Qureshi, A.J. Material Property-Manufacturing Process Optimization for Form 2 VAT-Photo Polymerization 3D Printers. J. Manuf. Mater. Process. 2020, 4, 12. [Google Scholar] [CrossRef]
- Kim, R.J.Y.; Kim, D.H.; Seo, D.G. Post-polymerization of three-dimensional printing resin using a dental light curing unit. J. Dent. Sci. 2024, 19, 945–951. [Google Scholar] [CrossRef]
- Zguris, Z. How Mechanical Properties of Stereolithography 3D Prints Are Affected by UV Curing; Formlabs White Paper; Formlabs: Somerville, MA, USA, 2016; pp. 1–11. [Google Scholar]
- Kim, J.H.; Kwon, J.S.; Park, J.M.; Russo, L.L.; Shim, J.S. Effects of postpolymerization conditions on the physical properties, cytotoxicity, and dimensional accuracy of a 3D-printed dental restorative material. J. Prosthet. Dent. 2022, 132, 241–250. [Google Scholar] [CrossRef]
- Kim, D.S.; Suriboot, J.; Shih, C.-C.; Cwiklik, A.; Grunlan, M.A.; Tai, B.L. Mechanical Isotropy and Postcure Shrinkage of Polydimethylsiloxane Printed with Digital Light Processing. Rapid Prototyp. J. 2020, 26, 1447–1452. [Google Scholar] [CrossRef]
- Scherer, M.D.; Barmak, A.B.; Özcan, M.; Revilla-León, M. Influence of Postpolymerization Methods and Artificial Aging Procedures on the Fracture Resistance and Flexural Strength of a Vat-Polymerized Interim Dental Material. J. Prosthet. Dent. 2022, 128, 1085–1093. [Google Scholar] [CrossRef]
- Zhao, Z.; Mu, X.; Wu, J.; Qi, H.J.; Fang, D. Effects of Oxygen on Interfacial Strength of Incremental Forming of Materials by Photopolymerization. Extrem. Mech. Lett. 2016, 9, 108–118. [Google Scholar] [CrossRef]
- Jariwala, A.S.; Ding, F.; Boddapati, A.; Breedveld, V.; Grover, M.A.; Henderson, C.L.; Rosen, D.W. Modeling Effects of Oxygen Inhibition in Mask-Based Stereolithography. Rapid Prototyp. J. 2011, 17, 168–175. [Google Scholar] [CrossRef]
- Chang, J.; Choi, Y.; Moon, W.; Chung, S.H. Impact of postpolymerization devices and locations on the color, translucency, and mechanical properties of 3D-printed interim resin materials. J. Prosthet. Dent. 2022, 132, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.; Sánchez-Romate, X.F.; Jiménez-Suárez, A.; Campo, M.; Ureña, A.; Prolongo, S.G. Mechanical and Strain-Sensing Capabilities of Carbon Nanotube Reinforced Composites by Digital Light Processing 3D Printing Technology. Polymers 2020, 12, 975. [Google Scholar] [CrossRef] [PubMed]
- Kirby, S.; Pesun, I.; Nowakowski, A.; França, R. Effect of different post-curing methods on the degree of conversion of 3D-printed resin for models in dentistry. Polymers 2024, 16, 549. [Google Scholar] [CrossRef] [PubMed]
- Cingesar, I.K.; Marković, M.P.; Vrsaljko, D. Effect of post-processing conditions on polyacrylate materials used in stereolithography. Addit. Manuf. 2022, 55, 102813. [Google Scholar] [CrossRef]
- Štaffová, M.; Ondreáš, F.; Svatik, J.; Zbončák, M.; Jančář, J.; Lepcio, P. 3D Printing and Post-Curing Optimization of Photopolymerized Structures: Basic Concepts and Effective Tools for Improved Thermomechanical Properties. Polym. Test. 2022, 108, 107499. [Google Scholar] [CrossRef]
- Klein, T.R.; Kirillova, A.; Gall, K.; Becker, M.L. Influence of post-processing on the properties of 3D-printed poly(propylene fumarate) star polymer hydroxyapatite nanocomposites. RSC Appl. Polym. 2023, 1, 73–81. [Google Scholar] [CrossRef]
- Vasques, M.T.; Mulder, J.N.; Machado, D.S.; Lagana, D.C. The influence of the post-processing method on Knoop hardness of photosensitive resins for 3D SLA printer used in Dentistry. Clin. Lab. Res. Dent. 2019, 33. [Google Scholar] [CrossRef]
- Reymus, M.; Lümkemann, N.; Stawarczyk, B. 3D-Printed Material for Temporary Restorations: Impact of Print Layer Thickness and Post-Curing Method on Degree of Conversion. Int. J. Comput. Dent. 2019, 22, 231–237. [Google Scholar]
- Bayarsaikhan, E.; Gu, H.; Hwangbo, N.K.; Lim, J.H.; Shim, J.S.; Lee, K.W.; Kim, J.E. Influence of Different Postcuring Parameters on Mechanical Properties and Biocompatibility of 3D Printed Crown and Bridge Resin for Temporary Restorations. J. Mech. Behav. Biomed. Mater. 2022, 128, 105127. [Google Scholar] [CrossRef]
- Lassila, L.; Mangoush, E.; He, J.; Vallittu, P.K.; Garoushi, S. Effect of Post-Printing Conditions on the Mechanical and Optical Properties of 3D-Printed Dental Resin. Polymers 2024, 16, 1713. [Google Scholar] [CrossRef]
- Abed, Y.A.; Sabry, H.A.; Alrobeigy, N.A. Degree of Conversion and Surface Hardness of Bulk-Fill Composite versus Incremental-Fill Composite. Tanta Dent. J. 2015, 12, 71–80. [Google Scholar] [CrossRef]
- Urízar, G.V.; Huerta, J.H.A.; Cruz, N. Color stability in 3D printed provisional dental restorations. Polymers 2024, 16, 9876. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Shrestha, B.; Patel, J.; Shih, B.; Shearston, K.; Ngo, H.; Fawzy, A. Effect of post-curing light exposure time on the physico–mechanical properties and cytotoxicity of 3D-printed denture base material. Dent. Mater. 2022, 38, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Tangpothitham, S.; Pongprueksa, P.; Inokoshi, M.; Mitrirattanakul, S. Effect of post-polymerization with autoclaving treatment on monomer elution and mechanical properties of 3D-printing acrylic resin for splint fabrication. J. Mech. Behav. Biomed. Mater. 2022, 126, 105015. [Google Scholar] [CrossRef]
| Input Variables | Critical Attributes | Non-Critical Attributes |
|---|---|---|
| Solvent Selection [7,9,14,21,36,63,64,65,66,67,68,69] |
|
|
| Washing Duration [7,36,65,69,70,71,72] |
|
|
| Washing Method [14,46,67,68,73,74,75] |
|
|
| Post-Curing Temperature [2,9,11,14,21,66,76,77,78,79,80,81,82,83,84] |
|
|
| Post-Curing Duration [2,14,21,38,41,48,49,66,70,71,79,82,83,84,85,86,87,88] |
|
|
| Post-Curing Wavelength [16,48,84,88,89] |
|
|
| Post-Curing Condition [46,65,71,83,90,91,92,93,94] |
|
|
| No. | Authors | Year | Type of Material and 3D-Printing Technique | Varied Factors | Tested Factors | Post-Processing Results |
|---|---|---|---|---|---|---|
| 1 | Perea-Lowery et al. [2] | 2021 |
|
Assessing the effect of
|
| Flexural strength and elastic modulus were higher after post-curing but decreased after 30 days in water storage. Water sorption and solubility were better-managed post-curing. |
| 2 | Uzcategui et al. [14] | 2018 |
|
|
| Post-curing improved compressive modulus, while the degree of conversion increased, reducing plasticization. |
| 3 | Jindal et al. [66] | 2020 |
|
|
| Post-curing at higher temperatures and longer times improved the compression strength significantly. |
| 4 | Jang et al. [69] | 2021 |
|
|
| Longer washing times reduced residual monomers, enhancing the degree of conversion and flexural strength. |
| 5 | Scherer et al. [65] | 2022 |
|
|
| Dry post-polymerization improved flexural strength and fracture resistance compared to wet conditions. Aged groups showed lower performance. |
| 6 | Snowwhite et al. [63] | 2022 |
|
|
| Water-based washing solutions enhanced flexural modulus and maximum stress, while improper solutions reduced surface quality. |
| 7 | Bardelcik et al. [64] | 2021 |
|
|
| Certain washing solutions improved tensile properties, while others had adverse effects on stress–strain behavior. |
| 8 | Alsandi et al. [11] | 2021 |
|
|
| Additional heat during post-curing significantly improved the tensile strength and degree of conversion. |
| 9 | Reymus et al. [84] | 2019 |
|
|
| Post-curing and optimal build direction improved fracture load, while aging reduced it. |
| 10 | Tzeng et al. [38] | 2021 |
|
|
| Extended post-curing duration led to improved surface characteristics and higher degree of conversion. |
| 11 | Bagis et al. [82] | 1997 |
|
|
| Higher post-curing time and temperature increased the degree of conversion, improving the final properties of the composite. |
| 12 | Scherer et al. [65] | 2022 |
|
|
| Increased washing duration and solution concentration improved flexural strength, though aged groups showed lower overall performance. |
| 13 | Lambart et al. [73] | 2022 |
|
|
| Proper washing solutions reduced roughness and enhanced flexural strength while maintaining low cytotoxicity levels. |
| 14 | Wu et al. [16] | 2019 |
|
|
| Higher UV intensity during post-curing improved accuracy and degree of conversion but led to increased distortion. |
| 15 | Mayer et al. [67] | 2021 |
|
|
| Centrifugal washing improved the degree of conversion and surface roughness, leading to better mechanical properties. |
| 16 | Xu et al. [70] | 2021 |
|
|
| Optimal washing time improved flexural strength and reduced cytotoxicity, with no significant effect on water sorption and solubility. |
| 17 | Garcia et al. [86] | 2020 |
|
|
| Enhanced washing and extended post-curing improved tensile strength and surface quality while ensuring biocompatibility. |
| 18 | Monzón et al. [85] | 2017 |
|
|
| Post-processing reduced anisotropy, improving uniformity and mechanical performance across different orientations. |
| 19 | Nowacki et al. [71] | 2021 |
|
|
| Extended washing adversely affected tensile strength due to void formation, while flexural strength remained unaffected. |
| 20 | Katheng et al. [79] | 2020 |
|
|
| Higher post-curing temperature led to dimensional distortion, while an optimal temperature of 60 °C maximized polymerization. |
| 21 | Miedzinska et al. [83] | 2020 |
|
|
| Higher post-curing temperatures improved mechanical properties in shorter times, with static and dynamic conditions showing significant changes. |
| 22 | Mayer et al. [68] | 2021 |
|
|
| Centrifugal washing improved fracture load and wear resistance while washing solutions had varying effects on mechanical properties. |
| 23 | Kim et al. [90] | 2020 |
|
|
| Optimized post-curing reduced shrinkage, improving dimensional accuracy and overall part integrity. |
| 24 | Riccio et al. [20] | 2021 |
|
|
| Extended post-curing improved tensile strength and modulus but increased brittleness. |
| 25 | Cortés et al. [95] | 2020 |
|
|
| Post-curing enhanced Young’s modulus and bending strength, optimizing the mechanical properties of the composites. |
| 26 | Zachary Zguris [88] | 2016 |
|
|
| Specific wavelengths during post-curing significantly increased tensile modulus and strength. |
| 27 | Hague et al. [81] | 2004 |
|
|
| Higher post-curing levels improved all mechanical properties, with thermal post-curing providing the best results. |
| 28 | Xu et al. [70] | 2021 |
|
|
| Extended post-cure times improved surface characteristics and flexural strength but did not significantly affect cytotoxicity. |
| 29 | Oh et al. [72] | 2023 |
|
|
| Higher washing temperatures increased degree of conversion and cell viability but reduced flexural strength and hardness. |
| 30 | Kirby et al. [96] | 2024 |
|
|
| Alternative curing units provided a similar degree of conversion, emphasizing the importance of curing time over unit type. |
| 31 | Cingesar et al. [97] | 2022 |
|
|
| Variations in post-processing conditions and printing angles affected mechanical and thermal properties. |
| 32 | Vara et al. [46] | 2023 |
|
|
| Nitrogen post-curing significantly improved dimensional accuracy compared to standard post-curing methods. |
| 33 | Prakash et al. [50] | 2024 |
|
|
| Post-processing optimized biocompatibility and mechanical properties while reducing cytotoxicity. |
| 34 | Šimunović et al. [61] | 2024 |
|
|
| Nitrogen post-curing and specific rinsing protocols enhanced the degree of conversion, flexural modulus, and hardness. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanpour, M.; Narongdej, P.; Alterman, N.; Moghtadernejad, S.; Barjasteh, E. Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review. Polymers 2024, 16, 2795. https://doi.org/10.3390/polym16192795
Hassanpour M, Narongdej P, Alterman N, Moghtadernejad S, Barjasteh E. Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review. Polymers. 2024; 16(19):2795. https://doi.org/10.3390/polym16192795
Chicago/Turabian StyleHassanpour, Mana, Poom Narongdej, Nicolas Alterman, Sara Moghtadernejad, and Ehsan Barjasteh. 2024. "Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review" Polymers 16, no. 19: 2795. https://doi.org/10.3390/polym16192795
APA StyleHassanpour, M., Narongdej, P., Alterman, N., Moghtadernejad, S., & Barjasteh, E. (2024). Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review. Polymers, 16(19), 2795. https://doi.org/10.3390/polym16192795








