Melt-Processed Polybutylene-Succinate Biocomposites with Chitosan: Development and Characterization of Rheological, Thermal, Mechanical and Antimicrobial Properties
Abstract
:1. Introduction
2. Materials
Bio-Based Polymer Composite Film Preparation
3. Testing Methods
4. Results and Discussion
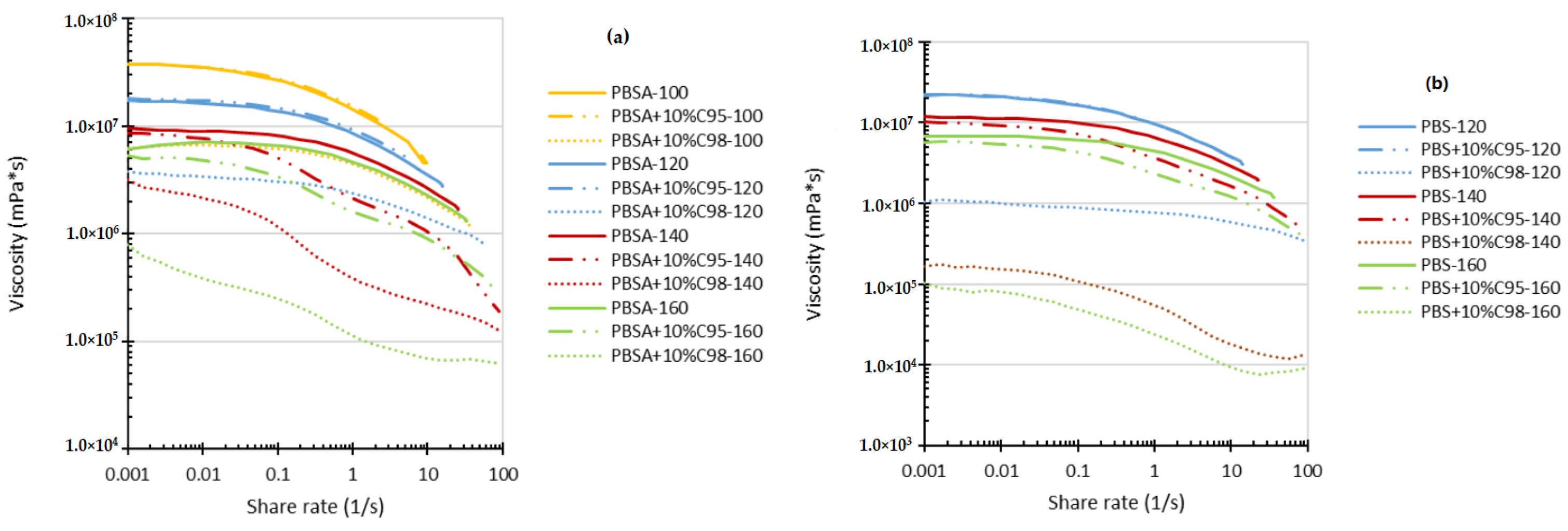
4.1. Rheology
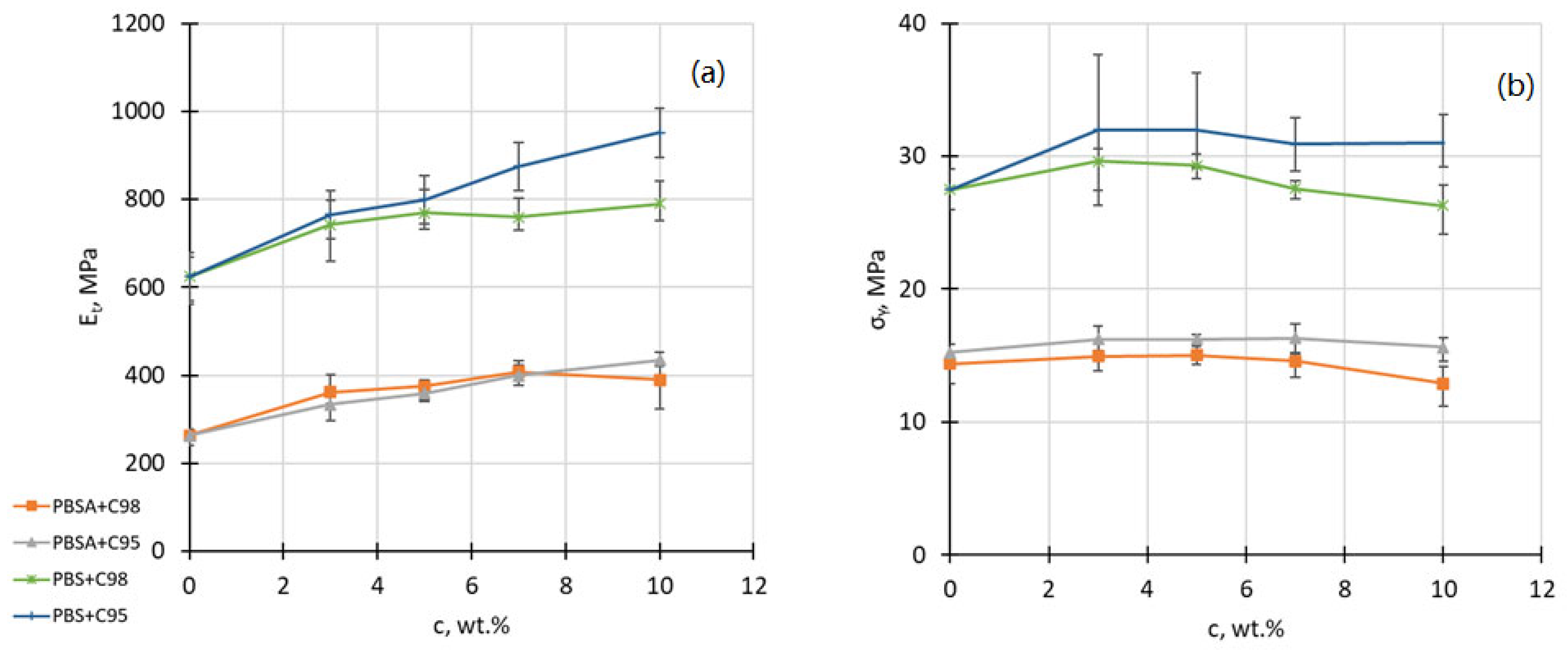
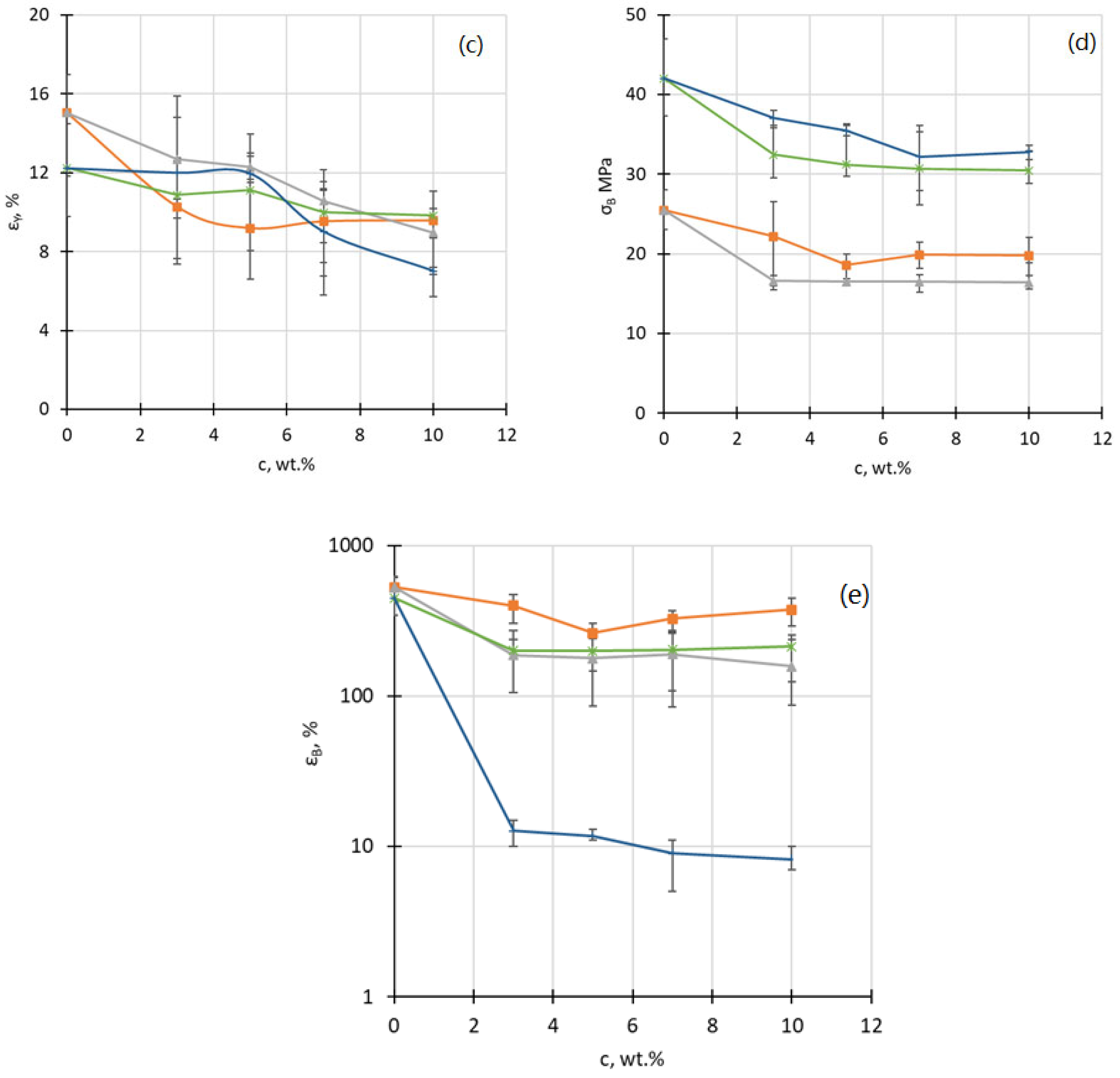
4.2. Tensile Properties
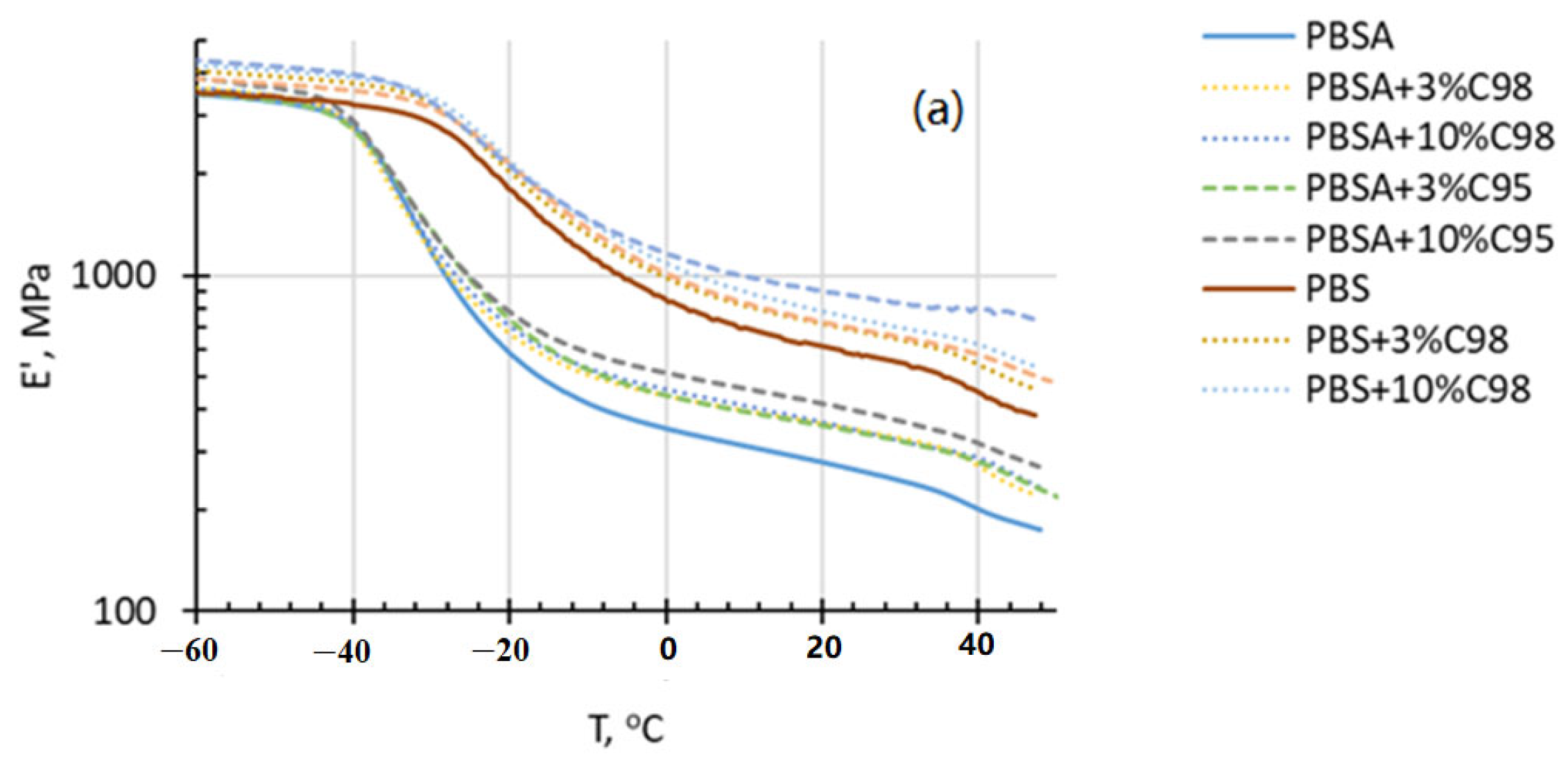
4.3. Dynamic Mechanical Analysis
4.4. Differential Scanning Calorimetry
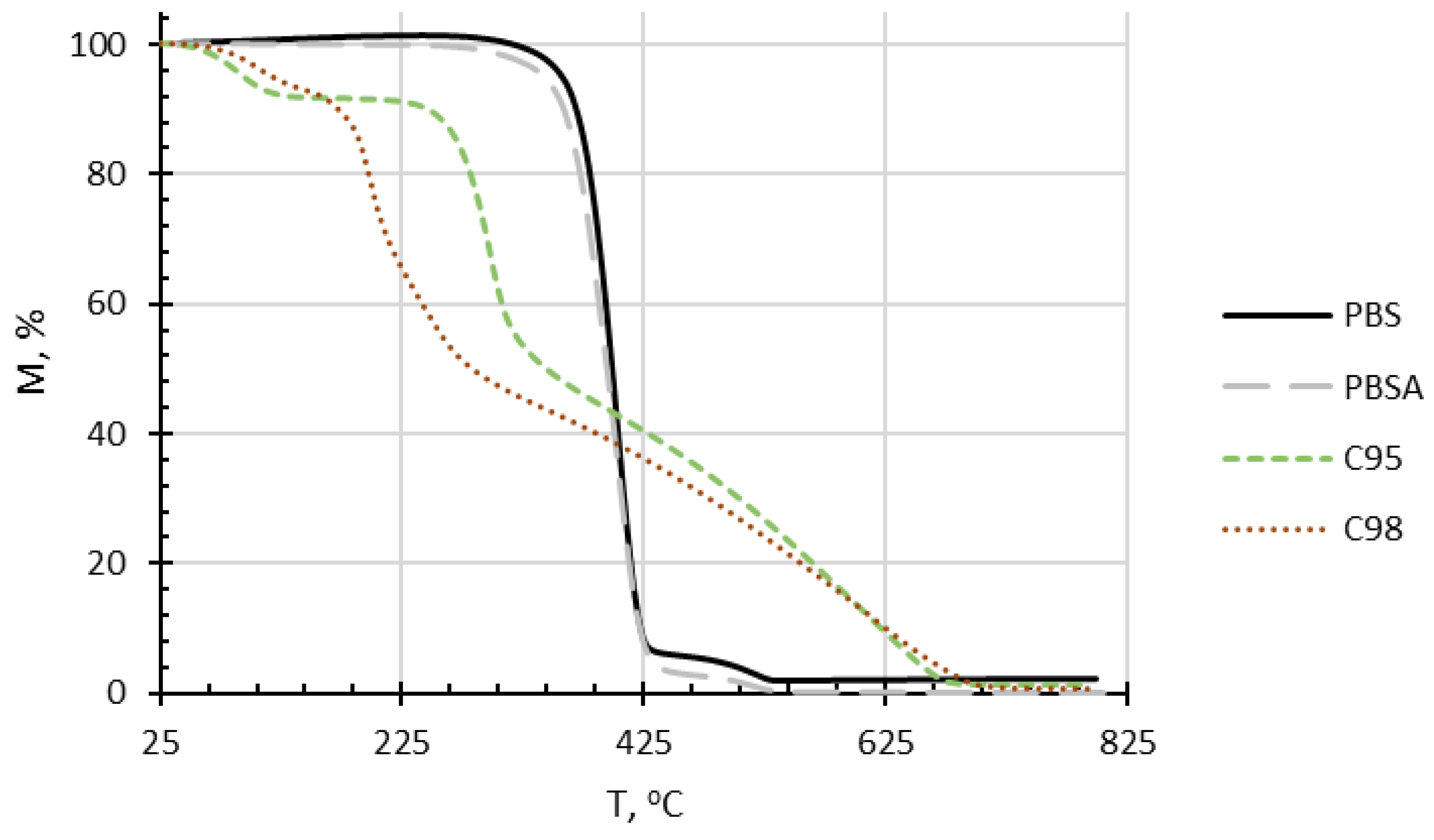
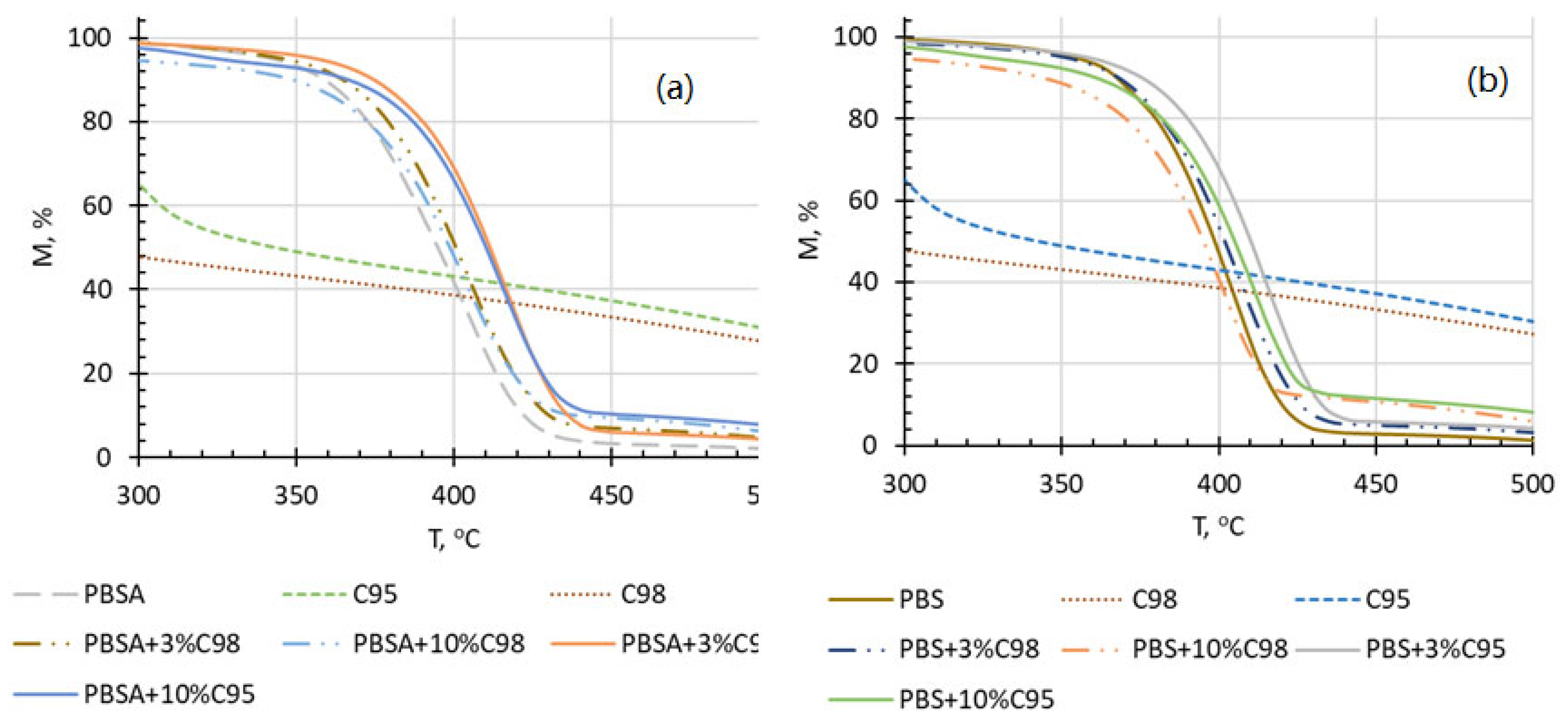
4.5. Thermogravimetric Analysis
4.6. Fourier Transform Infrared Spectroscopy
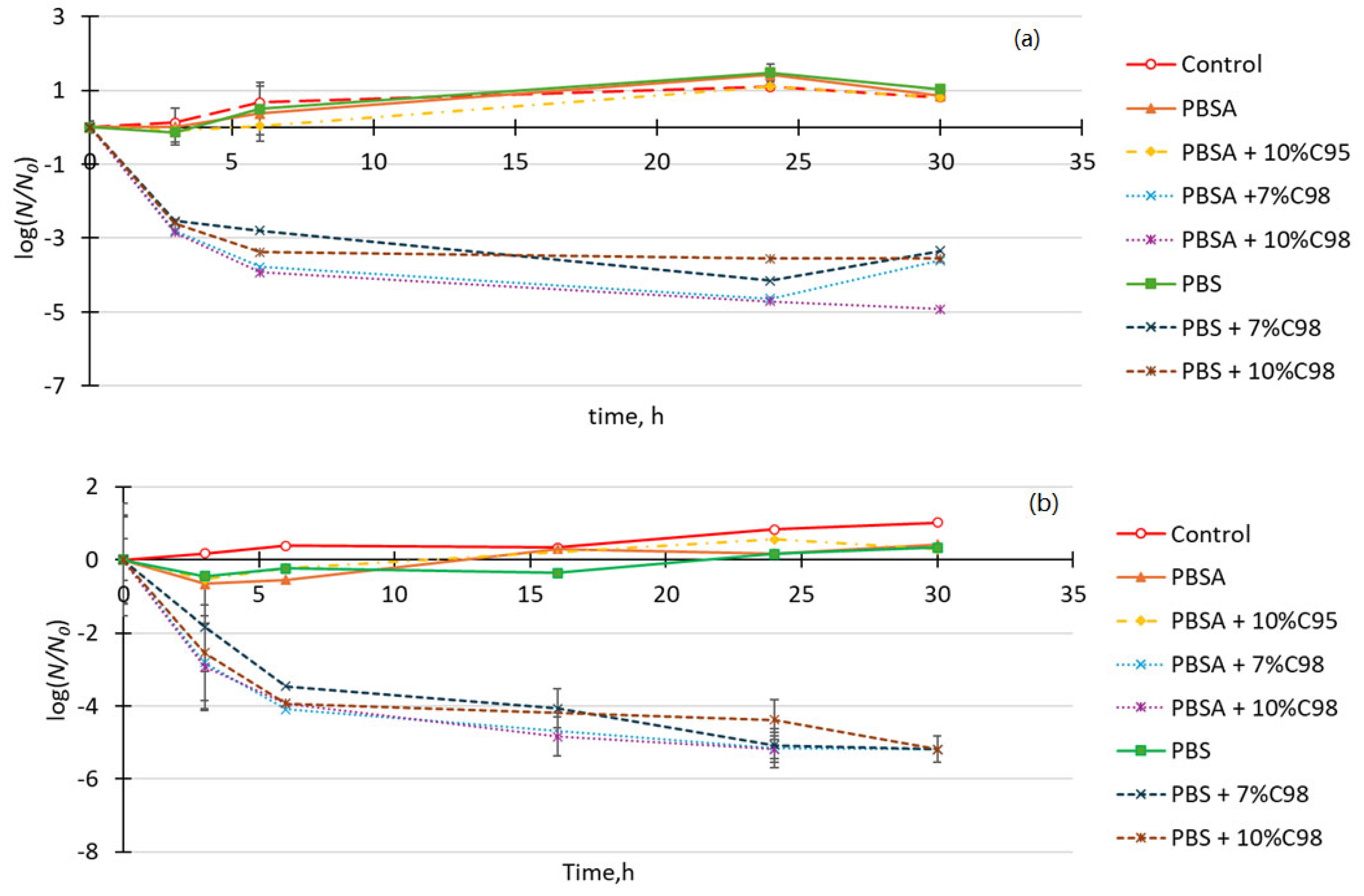
4.7. Antimicrobial Properties
4.8. Moisture Vapor Sorption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tajeddin, B.; Arabkhedri, M. Polymers and food packaging. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–543. [Google Scholar]
- Eurostat Statistics Explained, Packaging Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Packaging_waste_statistics (accessed on 1 July 2024.).
- Santhosh Kumar, B.M.; Keerthana, P.; Ravi, G.; Pant, K.; Sudhakar, M. Bio polymer based food packaging using composite materials. Mater. Today Proc. 2022, 66, 834–837. [Google Scholar]
- Zhang, Q.; Song, M.; Xu, Y.; Wang, W.; Wang, Z.; Zhang, L. Bio-based polyesters: Recent progress and future prospects. Prog. Polym. Sci. 2021, 120, 101430. [Google Scholar] [CrossRef]
- Jo, S.Y.; Lim, S.H.; Lee, J.Y.; Son, J.; Choi, J.I.; Park, S.J. Microbial production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate), from lab to the shelf: A review. Int. J. Biol. Macromol. 2024, 274 Pt 1, 133157. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Shekhar, N.; Mondal, A. Synthesis, properties, environmental degradation, processing, and applications of Polylactic Acid (PLA): An overview. Polym. Bull. 2024, 81, 11421–11457. [Google Scholar] [CrossRef]
- Nature Plast Biopolyesters. Available online: https://natureplast.eu/en/matiere/biopolyesters/ (accessed on 1 July 2024.).
- Chiu, F.-C. Halloysite nanotube- and organoclay-filled biodegradable poly(butylene succinate-co-adipate)/maleated polyethylene blendbased nanocomposites with enhanced rigidity. Compos. B Eng. 2017, 110, 193–203. [Google Scholar] [CrossRef]
- Taleb, K.; Saidi-Besbes, S.; Pillin, I.; Grohens, Y. Biodegradable Poly(Butylene Succinate) Nanocomposites Based on Dimeric Surfactant Organomodified Clays with Enhanced Water Vapor Barrier and Mechanical Properties. ACS Omega 2022, 7, 43254–43264. [Google Scholar] [CrossRef]
- Taleb, K.; Pillin, I.; Grohens, Y.; Saidi-Besbes, S. Polylactic acid/Gemini surfactant modified clay bio-nanocomposites: Morphological, thermal, mechanical and barrier properties. Int. J. Biol. Macromol. 2021, 177, 505–516. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R.; Pollet, E.; Avérous, L. Morphology and properties of thermoplastic starch blended with biodegradable polyester and filled with halloysite nanoclay. Carbohydr. Polym. 2020, 242, 116392. [Google Scholar] [CrossRef]
- Sathish Kumar, R.K.; Dhilipkumar, T.; Jessie, J.A.; Gaayathri, K.K.; Arumugam, S. Advancements in bio-polymeric composite materials for active and intelligent food packaging: A comprehensive review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Threepopnatkul, P.; Preedanorawut, R. Poly(lactic acid) and polybutylene succinate films incorporated with modified zeolite. Mat. Today Proc. 2022, 65, 2309–2314. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of PLA-PBSA based biodegradable active film and its application to salmon slices. Food Packag. Shelf Life. 2019, 22, 100393. [Google Scholar]
- Feijoo, P.; Mohanty, A.K.; Rodriguez-Uribe, A.; G´amez-P´erez, J.; Cabedo, L.; Misra, M. Biodegradable blends from bacterial biopolyester PHBV and bio-based PBSA: Study of the effect of chain extender on the thermal, mechanical and morphological properties. Int. J. Biol. Macromol. 2023, 225, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yu, W.; Zhang, J.; Liu, W.; Zhu, F.; Ye, Y.; Zheng, Q. Enhanced antibacterial properties of poly(butylenes succinate-co-terephthalate)/Ag@MgO nanocomposite films for food packaging. Polym. Test. 2023, 128, 108230. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Roy, S.; Ghosh, T.; Biswas, D.; Rhim, J.-W. Antimicrobial nanofillers reinforced biopolymer composite films for active food packaging applications-A review. Sustain. Mater. Techno. 2022, 32, e00353. [Google Scholar]
- Packialakshmi, J.S.; Kang, J.; Jayakumar, A.; Park, S.; Chang, Y.; Kim, J.T. Insights into the antibacterial and antiviral mechanisms of metal oxide nanoparticles used in food packaging. Food Packag. Shelf Life 2023, 40, 101213. [Google Scholar] [CrossRef]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, A.; Puri, V.; Aggarwal, G.; Maman, P.; Huanbutta, K.; Nagpal, M.; Sangnim, T. Chitosan-Based Polymer Blends for Drug Delivery Systems. Polymers 2023, 15, 2028. [Google Scholar] [CrossRef]
- Smagina, V.; Yudaev, P.; Kuskov, A.; Chistyakov, E. Polymeric Gel Systems Cytotoxicity and Drug Release as Key Features for their Effective Application in Various Fields of Addressed Pharmaceuticals Delivery. Pharmaceutics 2023, 15, 830. [Google Scholar] [CrossRef]
- Silva, I.M.V.; Machado, F.; Moreno, M.J.; Nunes, C.; Coimbra, M.A.; Coreta-Gomes, F. Polysaccharide Structures and Their Hypocholesterolemic Potential. Molecules 2021, 26, 4559. [Google Scholar] [CrossRef]
- Fatima, M.; Mir, S.; Ali, M.; Hassan, S.; Khan, Z.U.H.; Waqar, K. Synthesis and applications of chitosan derivatives in food preservation-A review. Eur. Polym. J. 2024, 216, 113242. [Google Scholar] [CrossRef]
- Maroulas, K.N.; Trikkaliotis, D.G.; Metaxa, Z.S.; AbdelAll, N.; Alodhayb, A.; Khouqeer, G.A.; Kyzas, G.Z. Super-hydrophobic chitosan/graphene-based aerogels for oil absorption. J. Mol. Liq. 2023, 390 Pt A, 123071. [Google Scholar] [CrossRef]
- Yudaev, P.; Semenova, A.; Chistyakov, E. Gel based on modified chitosan for oil spill cleanup. J. Appl. Polym. Sci. 2024, 141, e54838. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ariffin, F.; Jeevani Osadee Wijekoon, M.M.; Al-Hassan, A.A.; Dheyab, M.A.; Ghasemlou, M. Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 2022, 277, 118876. [Google Scholar] [CrossRef] [PubMed]
- Lia, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Celayeta Frias, J.M. Extraction, quantification, characterization, and application in food packaging of chitin and chitosan from mushrooms: A review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. New Insights into Sources, Bioavailability, Health-Promoting Effects, and Applications of Chitin and Chitosan. J. Agric. Food Chem. 2024, 72, 17138–17152. [Google Scholar] [CrossRef]
- Sarfraz, M.H.; Hayat, S.; Siddique, M.H.; Aslam, B.; Ashraf, A.; Saqalein, M.; Khurshid, M.; Sarfraz, M.F.; Afzal, M.; Muzammil, S. Chitosan based coatings and films: A perspective on antimicrobial, antioxidant, and intelligent food packaging. Prog. Org. Coat. 2024, 188, 108235. [Google Scholar] [CrossRef]
- Kusumastuti, Y.; Putri, N.R.E.; Timotius, D.; Syabani, M.W. Rochmadi Effect of chitosan addition on the properties of low-density polyethylene blend as potential bioplastic. Heliyon 2020, 6, e05280. [Google Scholar] [CrossRef]
- Reesha, K.V.; Panda, S.K.; Bindu, J.; Varghese, T.O. Development and characterization of an LDPE/chitosan compositeantimicrobial film for chilled fish storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Baikousi, M.; Moschovas, D.; Asimakopoulos, G.; Zafeiropoulos, N.E.; Avgeropoulos, A. Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life. Molecules 2021, 26, 1585. [Google Scholar] [CrossRef] [PubMed]
- Monika; Katiyar, V.; Mulchandani, N. Generalized kinetics for thermal degradation and melt rheology for poly (lactic acid)/poly (butylene succinate)/functionalized chitosan based reactive nanobiocomposite. Int. J. Biol. Macromol. 2019, 141, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.M.; Knoop Rutger, J.I.; Kappen Frans, H.J.; Boeriu Carmen, G. Chitosan films and blends for packaging material. Carbohyd. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Aisyah, H.A.; Nordin, A.H.; Ngadi, N.; Zuhri, M.Y.M.; Asyraf, M.R.M.; Sapuan, S.M.; Zainudin, E.S.; Sharma, S.; Abral, H.; et al. Natural-Fiber-Reinforced Chitosan, Chitosan Blends and Their Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 874. [Google Scholar] [CrossRef] [PubMed]
- Signori, F.; Pelagaggi, M.; Bronco, S.; Righetti, M.C. Amorphous/crystal and polymer/filler interphases in biocomposites from poly(butylene succinate). Thermochim. Acta 2012, 543, 74–81. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Chapter 5-Calorimetric Properties, Properties of Polymers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 109–127. [Google Scholar]
- Debuissy, T.; Pollet, E.; Avérous, L. Synthesis and characterization of biobased poly(butylene succinate-ran-butylene adipate). Analysis of the composition-dependent physicochemical properties. Eur. Polym. J. 2017, 87, 84–98. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Li, L. Multiple melting behavior of poly(butylene succinate). Eur. Polym. J. 2007, 43, 3163–3170. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential Scanning Calorimetry Techniques: Applications in Biology and Nanoscience. J. Biomol. Technol. 2010, 21, 167–193. [Google Scholar]
- Eulalio, H.Y.C.; Rodrigues, J.F.B.; Santos, K.O.; Peniche, C.; LiaFook, M.V. Characterization and thermal properties of chitosan films prepared with different acid solvents. Rev. Cuba. Quím. 2019, 31, 309–323. [Google Scholar]
- Malucelli, G. Flame-Retardant Systems Based on Chitosan and Its Derivatives: State of the Art and Perspectives. Molecules 2020, 25, 4046. [Google Scholar] [CrossRef] [PubMed]




| Concentration of The Chitosan Additive | Glass Transition Temperature | 1st Local Maxima of the 1st Melting Peak | 2nd Local Maxima of the 1st Melting Peak | Crystallinity Degree of the 1st Melting Peak | Maxima of the 2nd Melting Peak | Crystallinity Degree of the 2nd Melting Peak | Total Crystallinity Degree |
|---|---|---|---|---|---|---|---|
| C % | Tg °C | Tm1.1 °C | Tm1.2 °C | X1.2 % | Tm2 °C | X2 % | Xt % |
| PBSA + C98 | |||||||
| 0 | −50 | 50 | 67 | One Multi maxima Peak Observed | 87 | One Multi maxima Peak Observed | 26 |
| 3 | −49 | 39 | 60 | 5 | 87 | 21 | 26 |
| 5 | −47 | 41 | 61 | 5 | 88 | 20 | 25 |
| 7 | −47 | 42 | 59 | 5 | 88 | 20 | 25 |
| 10 | −47 | 39 | 59 | 5 | 88 | 22 | 27 |
| PBSA + C95 | |||||||
| 0 | −50 | 50 | 67 | One multi maxima peak observed | 87 | One multi maxima peak observed | 26 |
| 3 | −48 | 45 | 72 | 88 | 33 | ||
| 5 | −48 | 44 | 71 | 87 | 27 | ||
| 7 | −49 | 43 | 71 | 87 | 29 | ||
| 10 | −49 | 44 | 71 | 87 | 29 | ||
| Concentration of The Chitosan Additive | Glass Transition Temperature | Maxima of the 1st Melting Peak | Crystallinity Degree of the 1st Melting Peak | Maxima of the Cold Crystallization Peak | Crystallinity Degree of Cold Crystallization Peak | Maxima of the 2nd Melting Peak | Crystallinity Degree of the 2nd Melting Peak | Maxima of the 3rd Melting Peak | Crystallinity Degree of the 3rd Melting Peak | Initial Crystallinity Degree |
|---|---|---|---|---|---|---|---|---|---|---|
| C % | Tg °C | Tm1 °C | X1 % | TCC °C | XCC % | Tm2 °C | X2 % | Tm3 °C | X3 % | Xi* % |
| PBS + C98 | ||||||||||
| 0 | −35 | 49 | 1 | 94 | 2 | Not observed | 117 | 31 | 31 | |
| 3 | −36 | 40 | 1 | 91 | 3 | Not observed | 115 | 30 | 30 | |
| 5 | −37 | 41 | 1 | 90 | 3 | Not observed | 115 | 33 | 30 | |
| 7 | −37 | 41 | 1 | 91 | 3 | Not observed | 115 | 32 | 30 | |
| 10 | −38 | 41 | 1 | 91 | 3 | Not observed | 115 | 33 | 30 | |
| PBS + C95 | ||||||||||
| 0 | −35 | 49 | 1 | 94 | 2 | Not observed | 117 | 31 | 31 | |
| 3 | −37 | 45 | One multi maxima peak observed | No cold crystallization observed | 102 | One multi maxima peak observed | 115 | One multi maxima peak observed | 41 | |
| 5 | −39 | 43 | 102 | 114 | 46 | |||||
| 7 | −39 | 45 | 101 | 114 | 45 | |||||
| 10 | −39 | 45 | 102 | 114 | 47 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merijs-Meri, R.; Zicans, J.; Ivanova, T.; Mezule, L.; Ivanickins, A.; Bockovs, I.; Bitenieks, J.; Berzina, R.; Lebedeva, A. Melt-Processed Polybutylene-Succinate Biocomposites with Chitosan: Development and Characterization of Rheological, Thermal, Mechanical and Antimicrobial Properties. Polymers 2024, 16, 2808. https://doi.org/10.3390/polym16192808
Merijs-Meri R, Zicans J, Ivanova T, Mezule L, Ivanickins A, Bockovs I, Bitenieks J, Berzina R, Lebedeva A. Melt-Processed Polybutylene-Succinate Biocomposites with Chitosan: Development and Characterization of Rheological, Thermal, Mechanical and Antimicrobial Properties. Polymers. 2024; 16(19):2808. https://doi.org/10.3390/polym16192808
Chicago/Turabian StyleMerijs-Meri, Remo, Janis Zicans, Tatjana Ivanova, Linda Mezule, Aleksandrs Ivanickins, Ivan Bockovs, Juris Bitenieks, Rita Berzina, and Alina Lebedeva. 2024. "Melt-Processed Polybutylene-Succinate Biocomposites with Chitosan: Development and Characterization of Rheological, Thermal, Mechanical and Antimicrobial Properties" Polymers 16, no. 19: 2808. https://doi.org/10.3390/polym16192808
APA StyleMerijs-Meri, R., Zicans, J., Ivanova, T., Mezule, L., Ivanickins, A., Bockovs, I., Bitenieks, J., Berzina, R., & Lebedeva, A. (2024). Melt-Processed Polybutylene-Succinate Biocomposites with Chitosan: Development and Characterization of Rheological, Thermal, Mechanical and Antimicrobial Properties. Polymers, 16(19), 2808. https://doi.org/10.3390/polym16192808






