Synthesis and Performance Evaluation of Metallocene Polyalphaolefins (mPAO) Base Oil with Anti-Friction and Anti-Wear Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Metallocene PolyalphaOlefins (mPAO)
2.3. Alkylation of TPPT with mPAO
2.4. Physicochemical Properties
2.5. Structural Composition Analysis
2.6. Oxidation and Thermal Stability
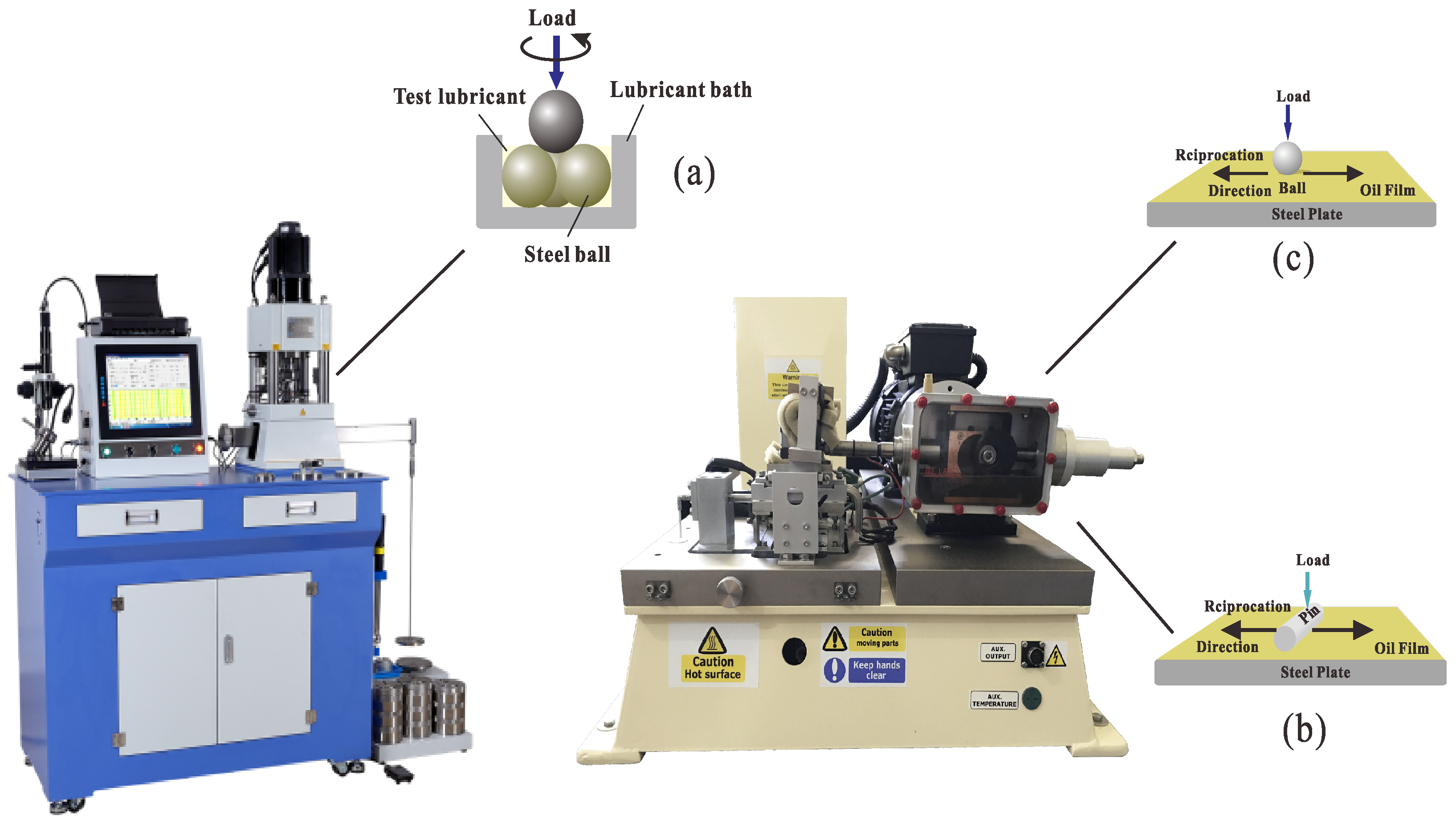
2.7. Friction and Wear Test
3. Results
3.1. Alkylation of Trithiophenyl Phosphate and mPAO
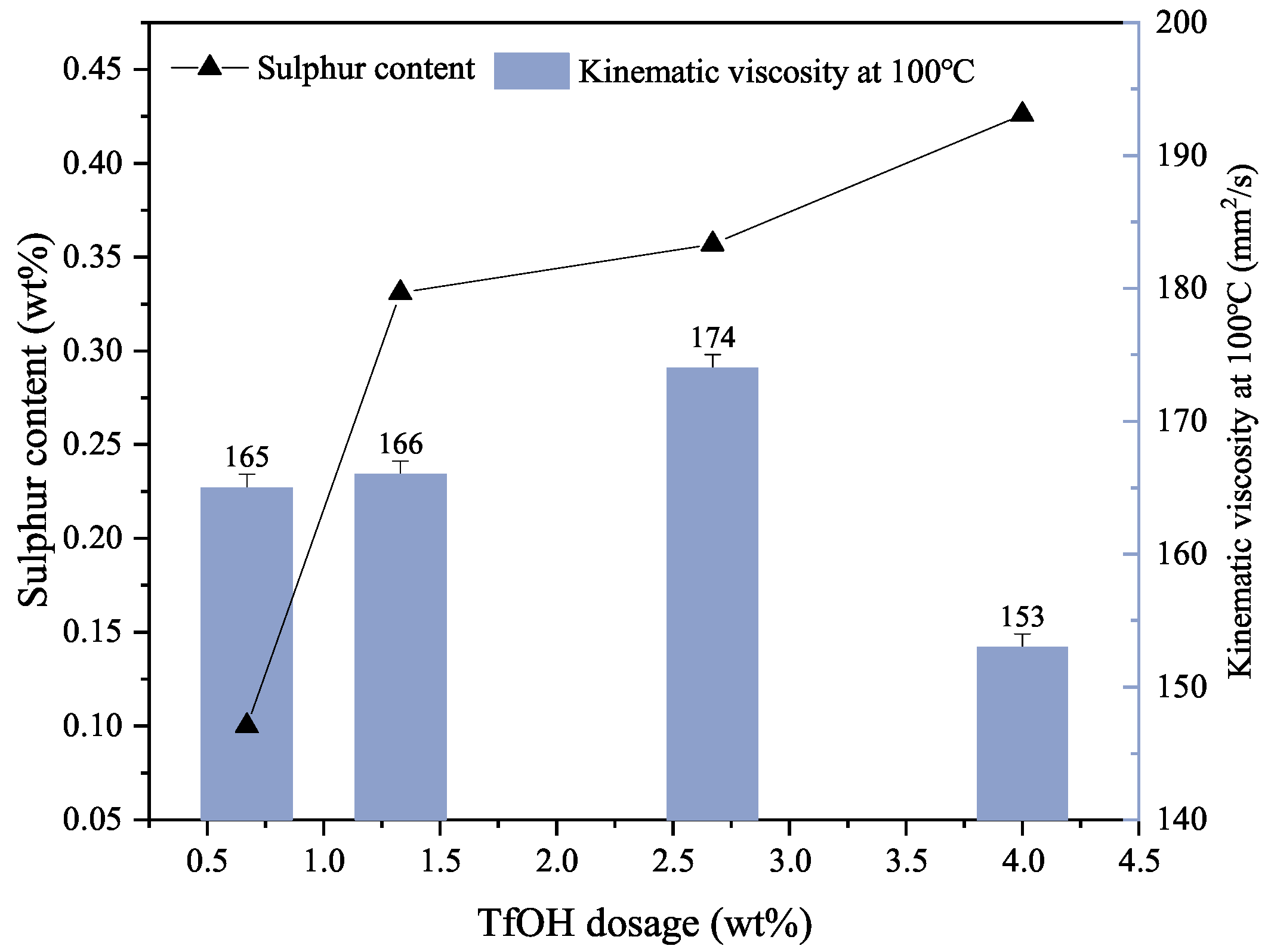
3.1.1. Catalyst Dosage
3.1.2. Reaction Temperature
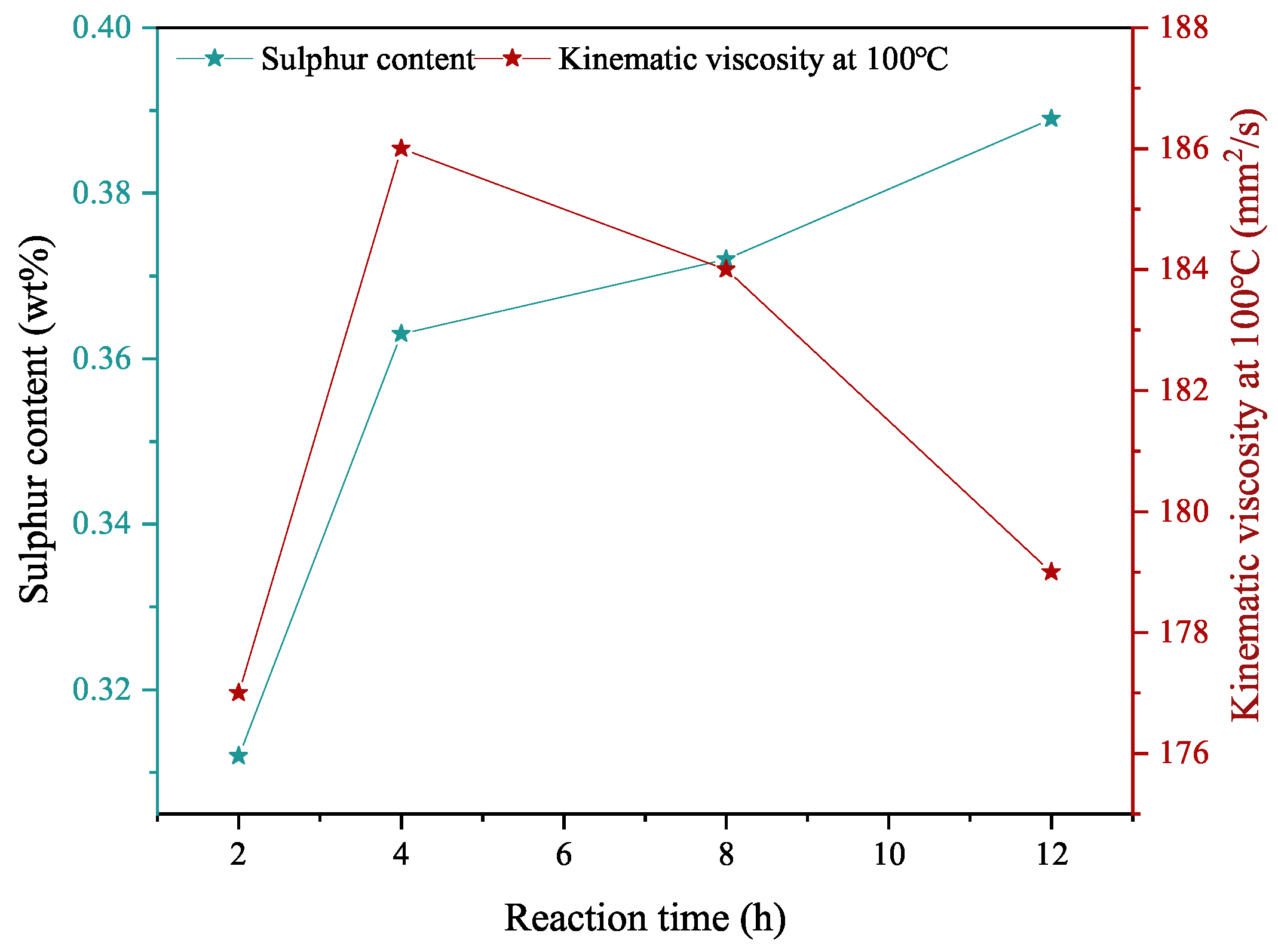
3.1.3. Reaction Time
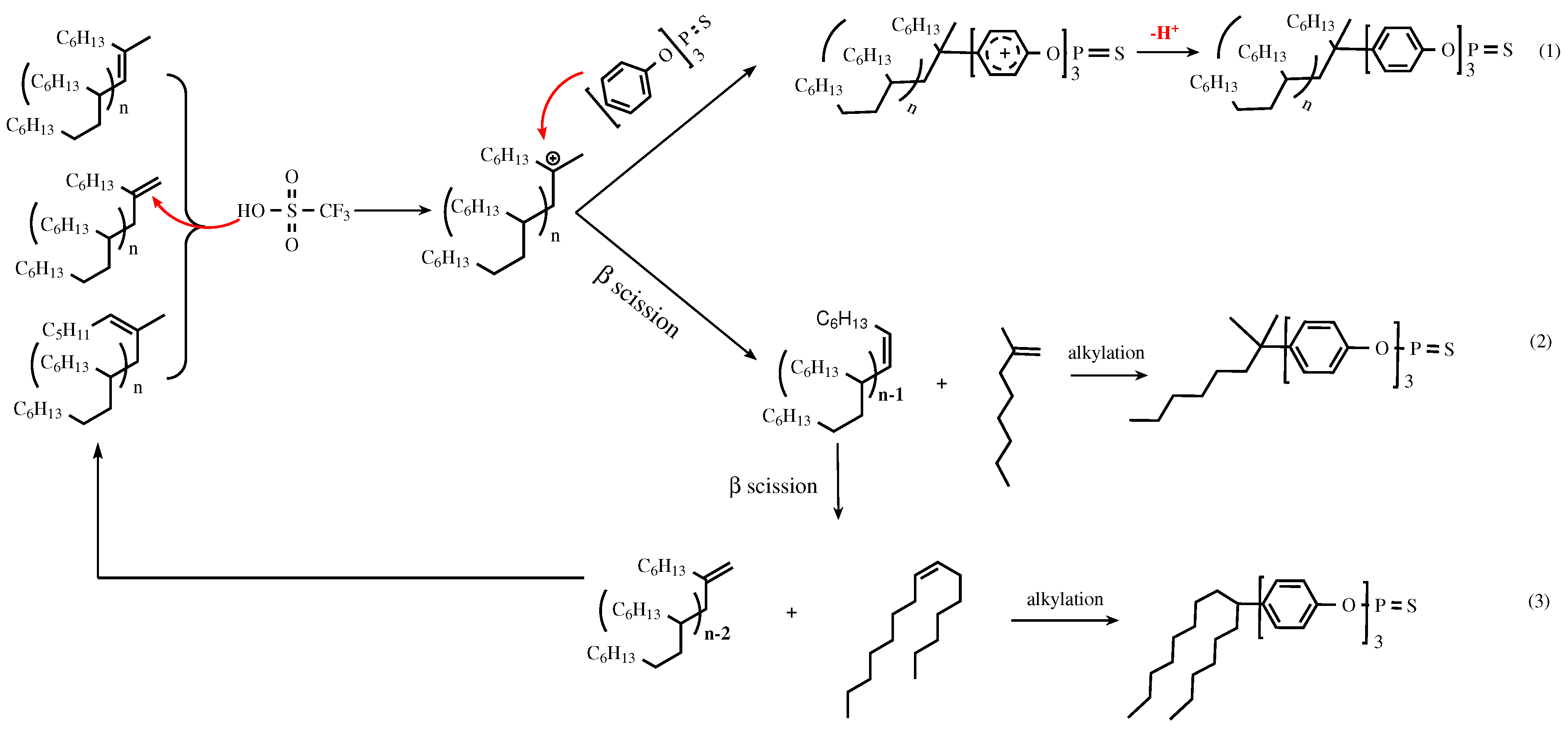
3.2. Catalytic Mechanism
3.3. Physicochemical Characterization
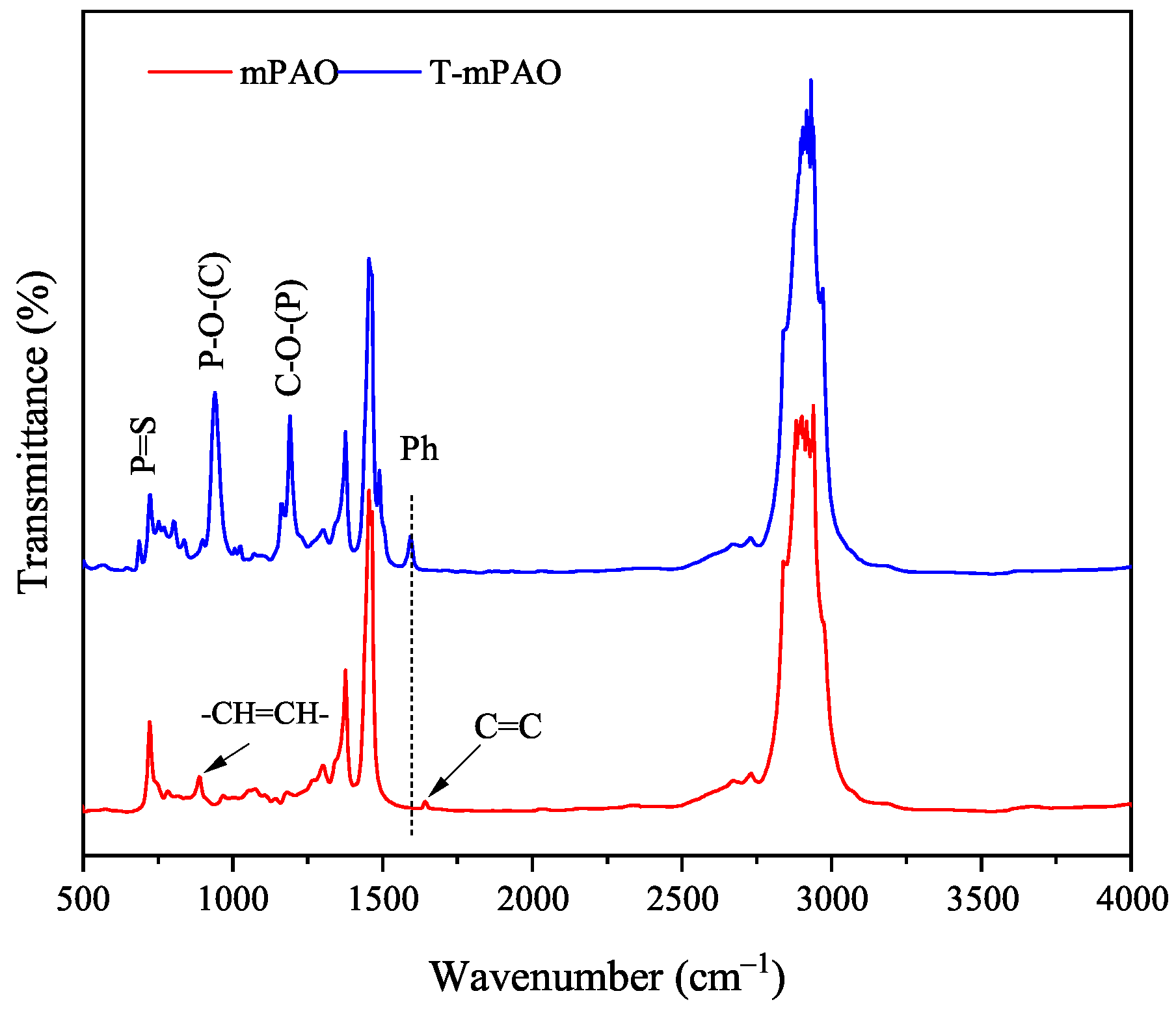
3.4. Fourier Transform Infrared Spectra of mPAO and T-mPAO
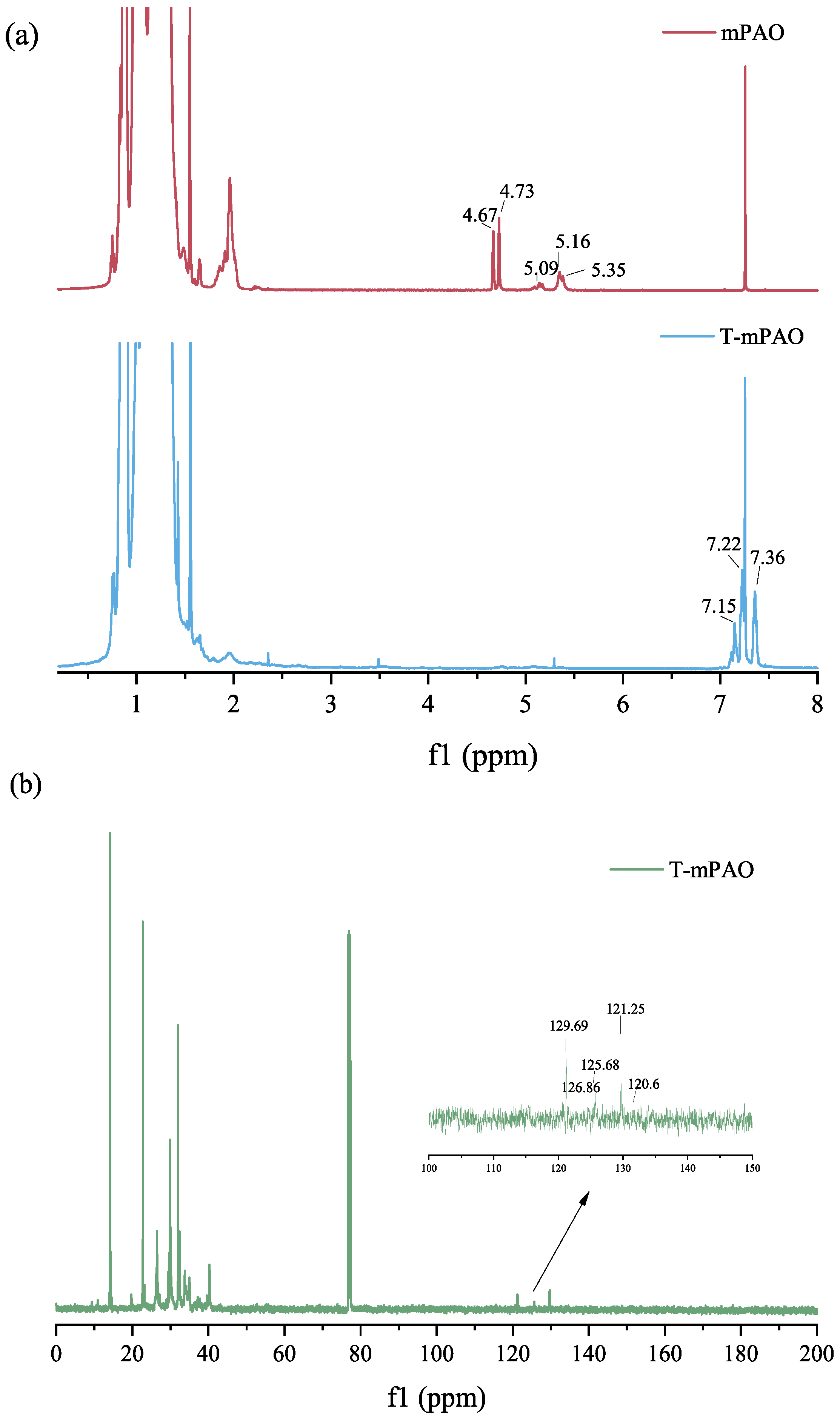
3.5. Nuclear Magnetic Resonance Spectra of mPAO and T-mPAO
3.6. Molecular Weight of mPAO and Its Derivatives
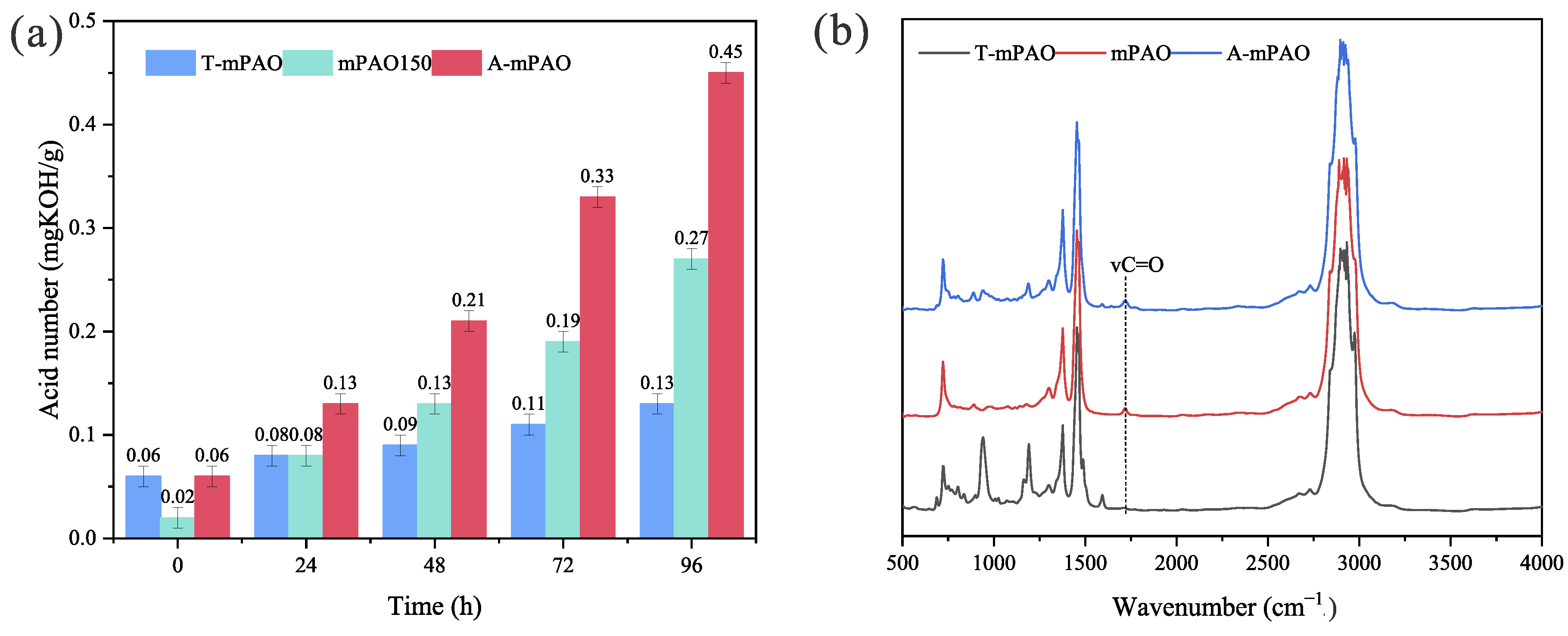
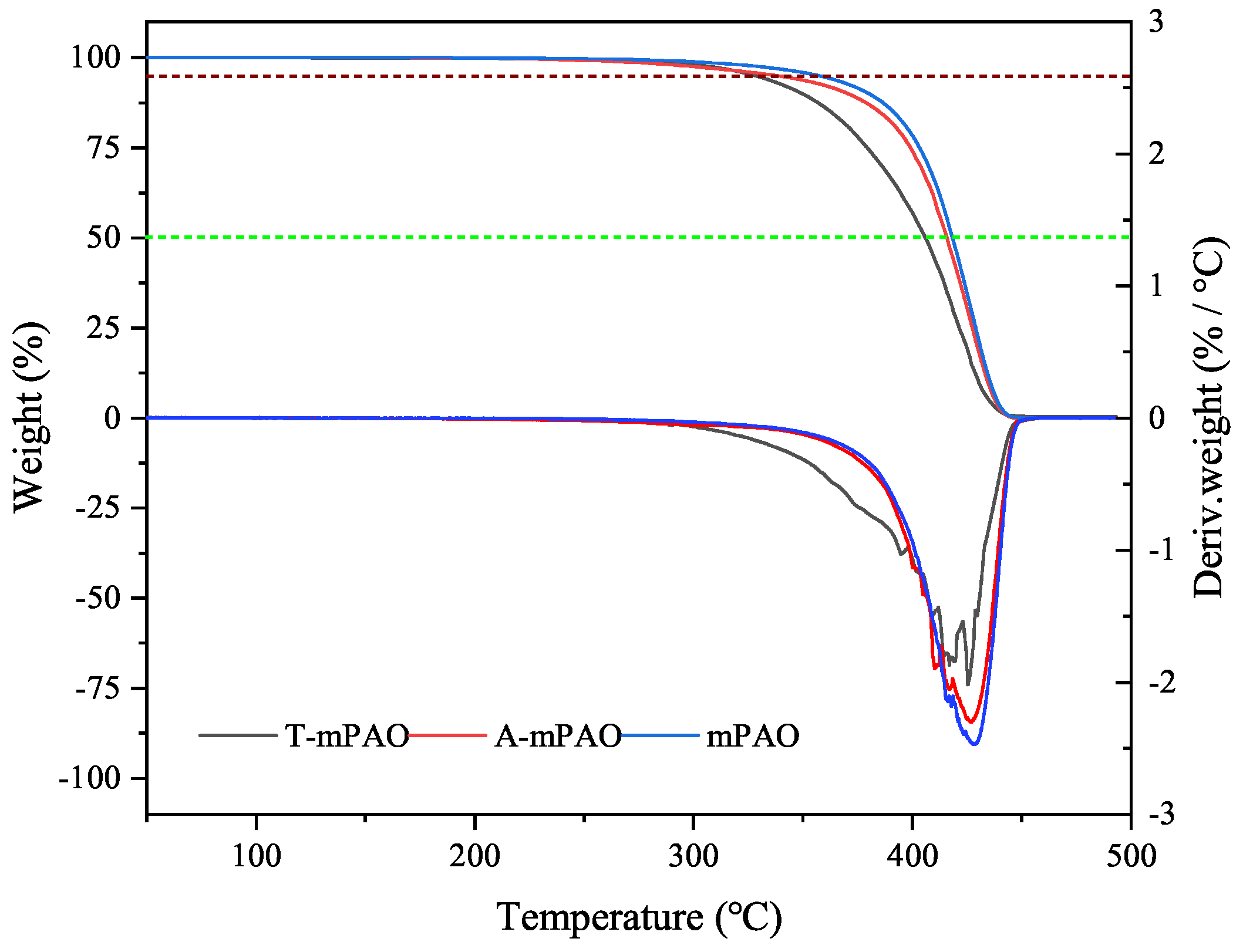
3.7. Thermal Oxidative Stability
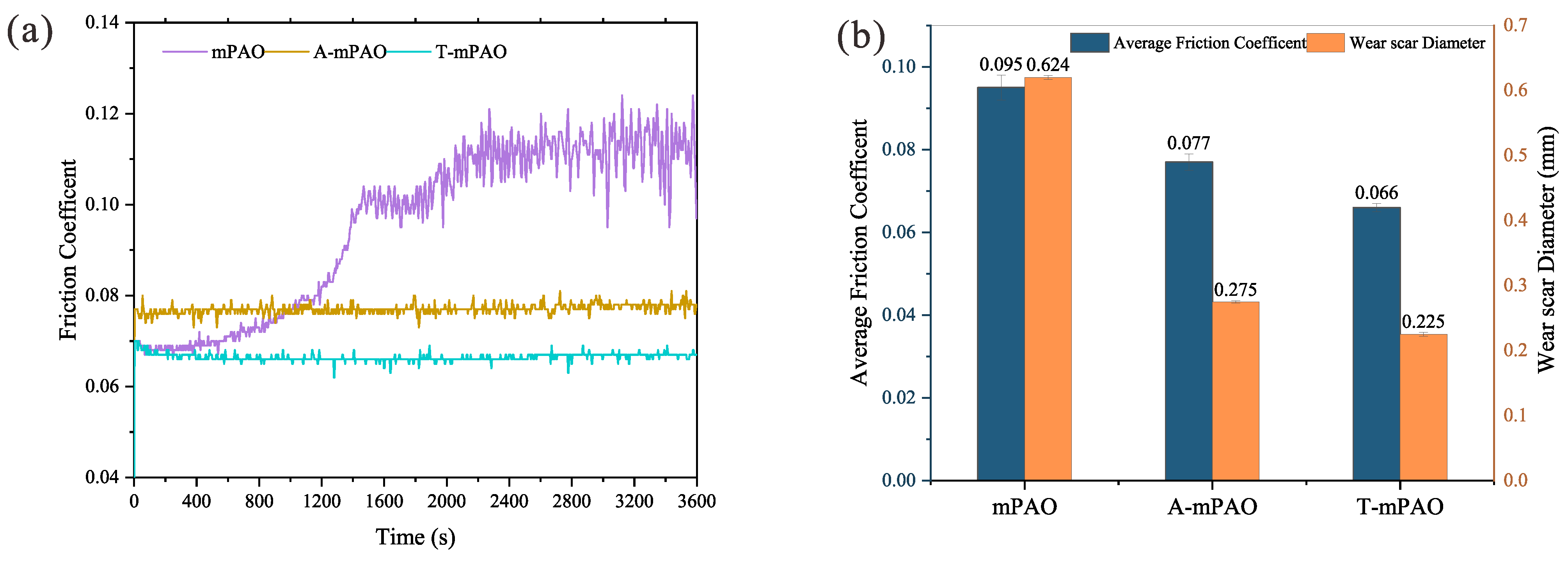
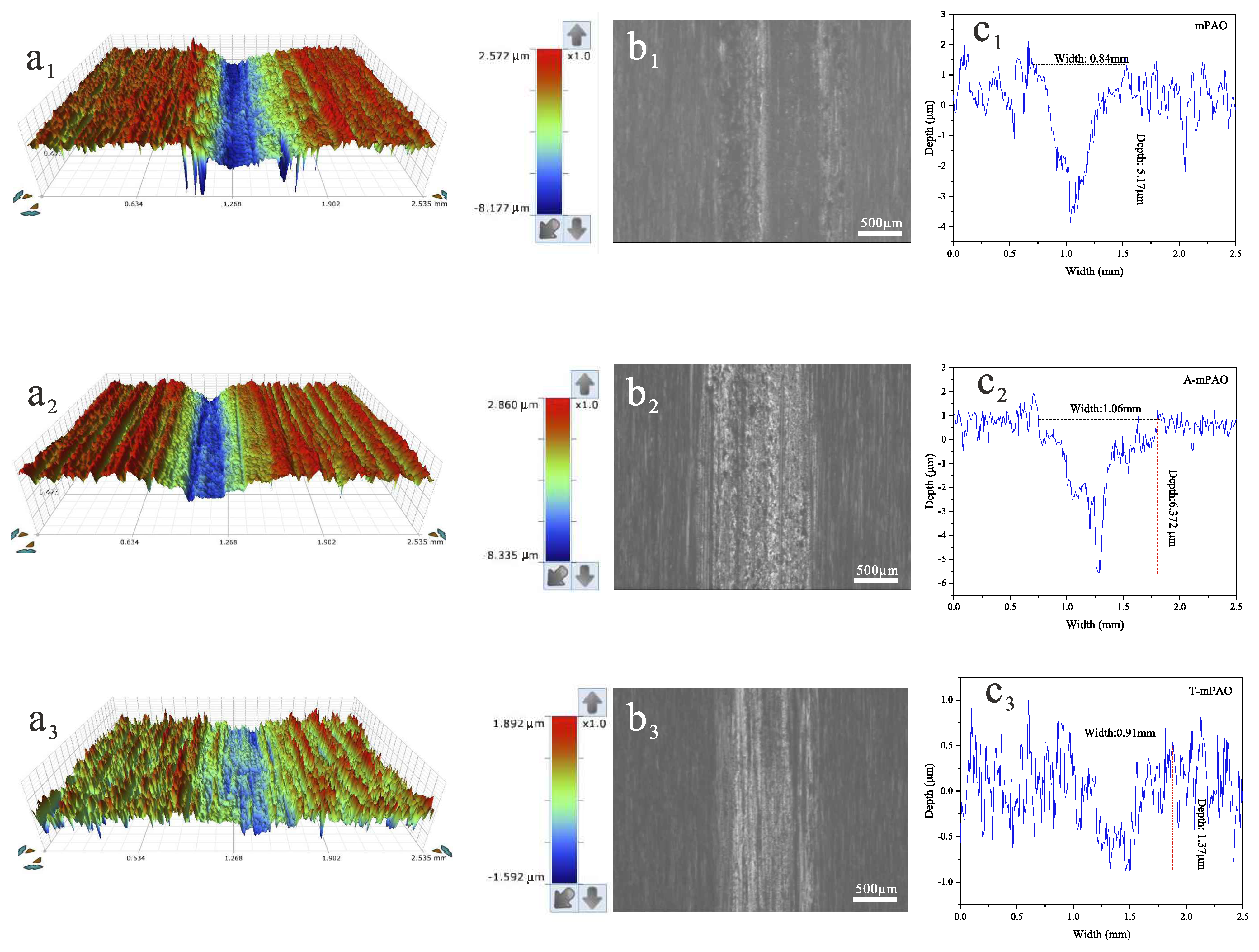
3.8. Tribological Results
3.8.1. Tribological Performance of Point-to-Point Contact on a Four-Ball Friction Tester
3.8.2. Tribological Performance of Point-on-Flat and Line-on-Flat Contact on a TE77 Reciprocating Friction Tester
3.8.3. Extreme Pressure Performance of mPAO, A-mPAO, and T-mPAO
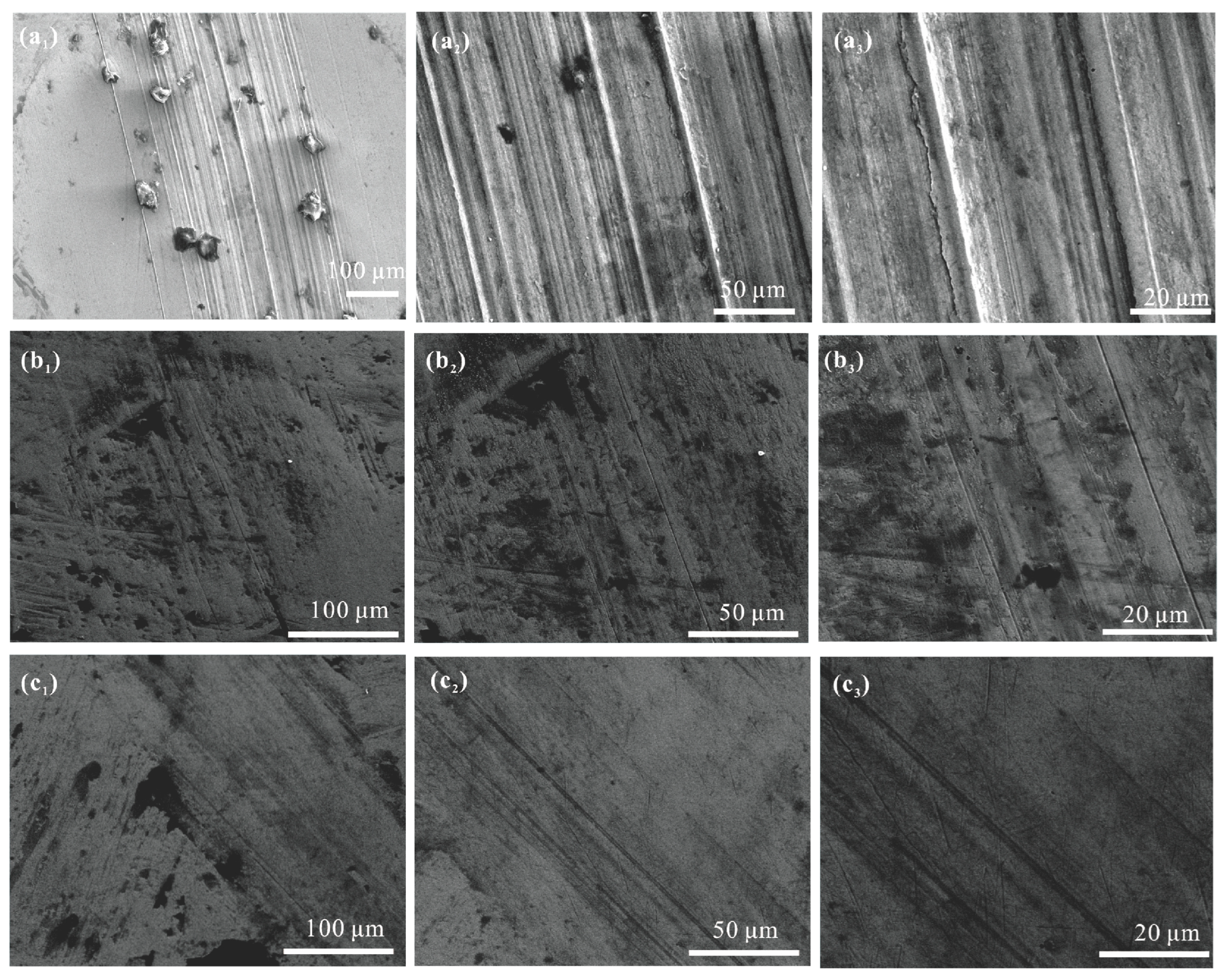
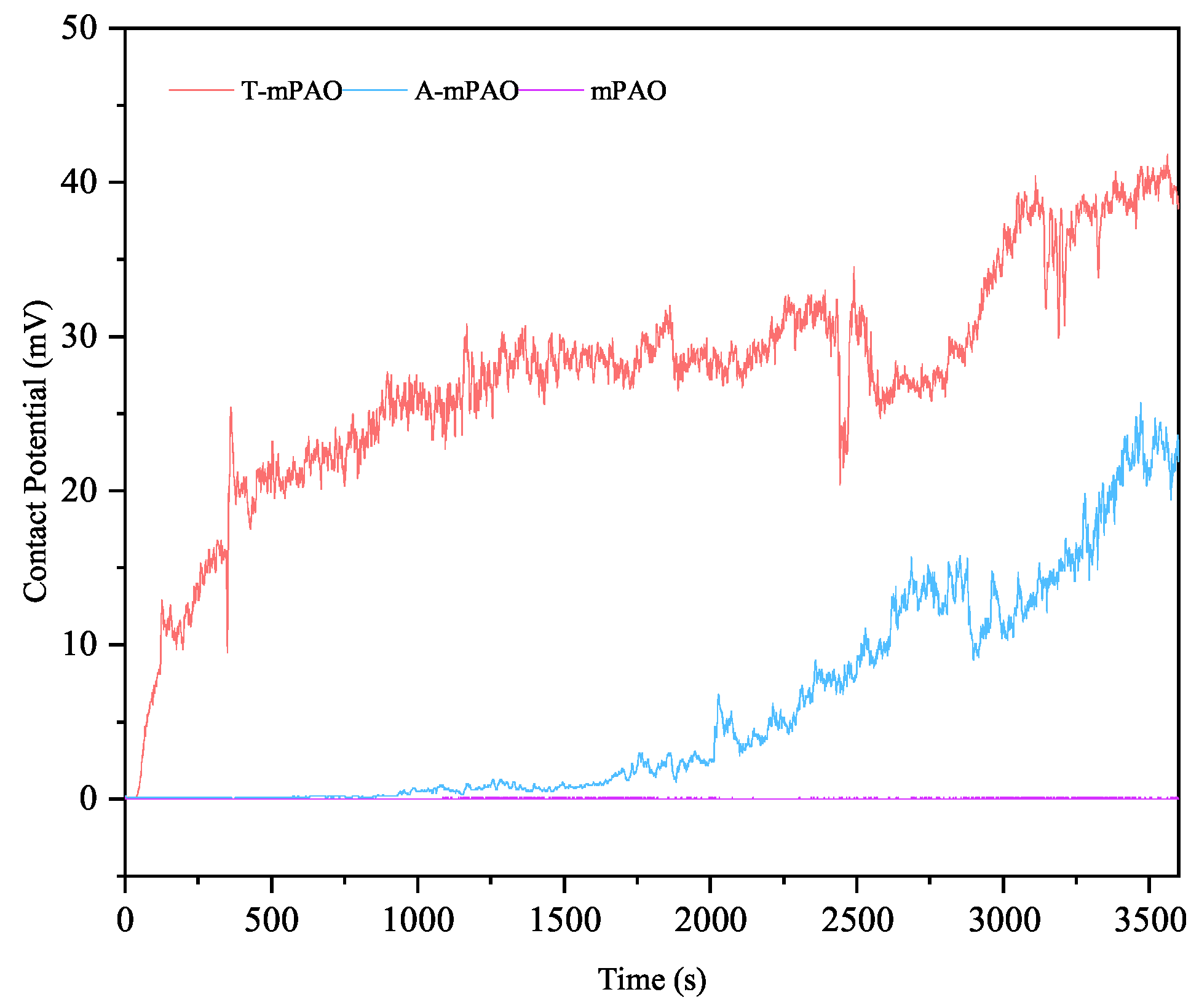
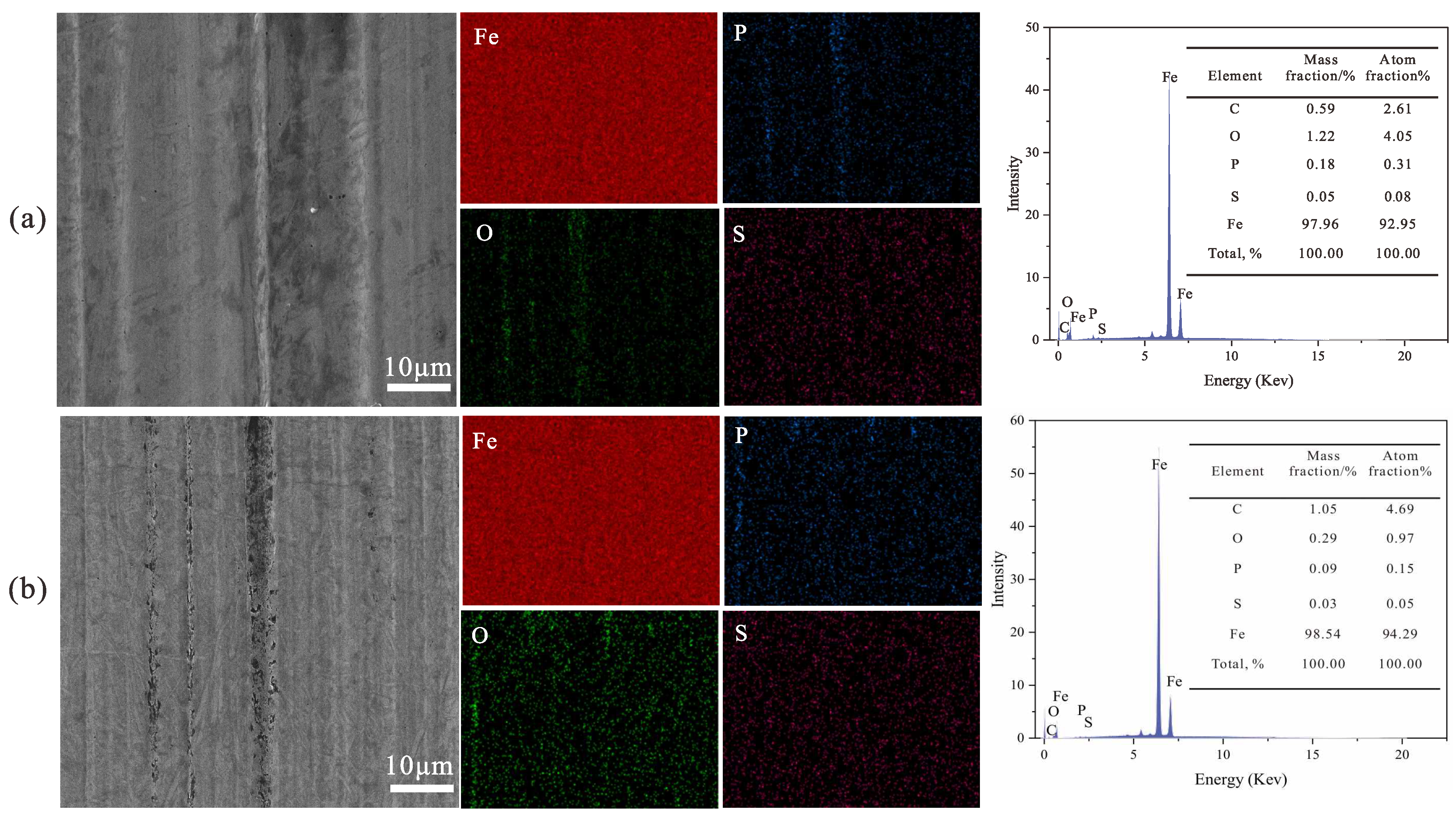
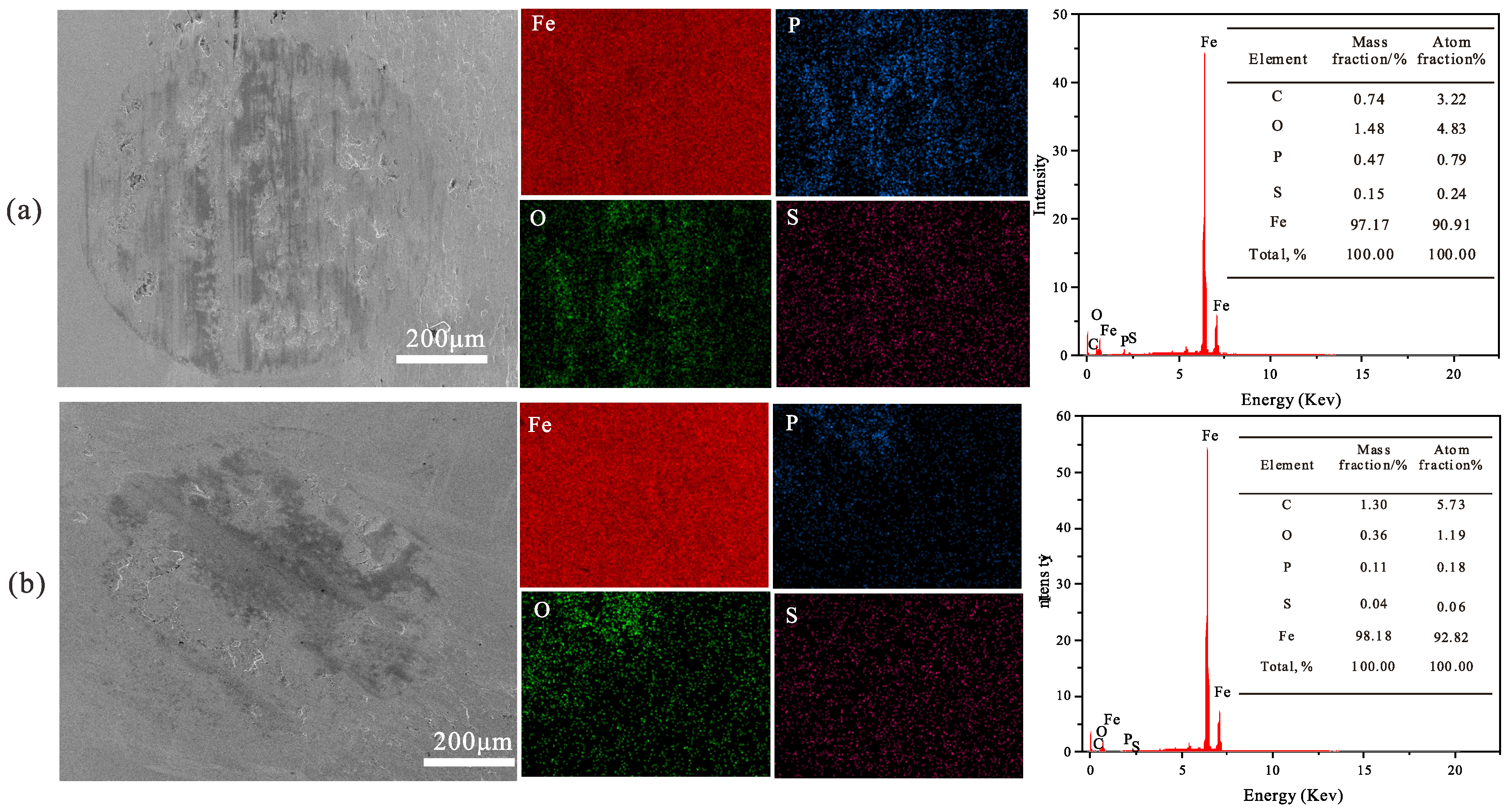
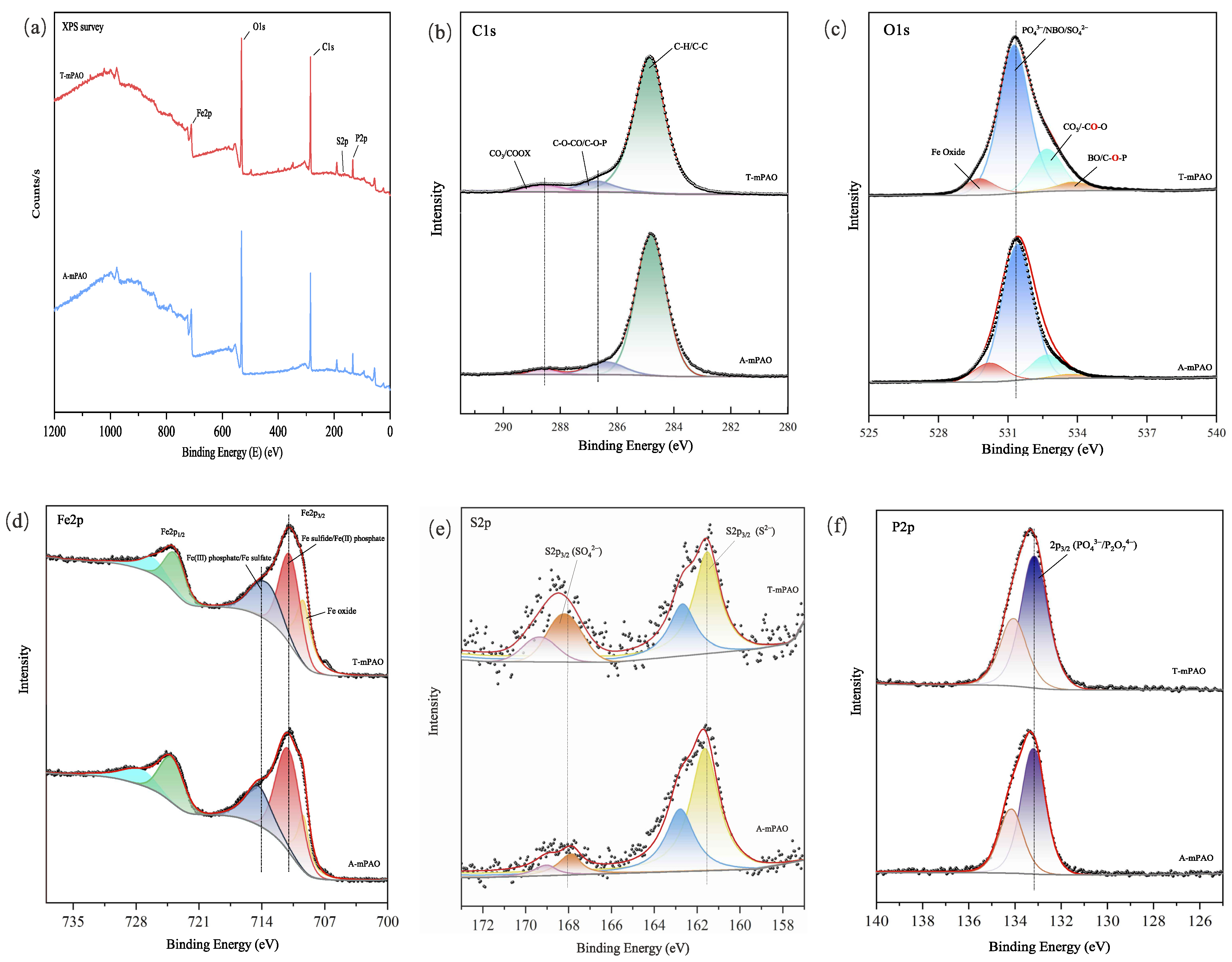
3.8.4. Elemental Analysis of Worn Surfaces
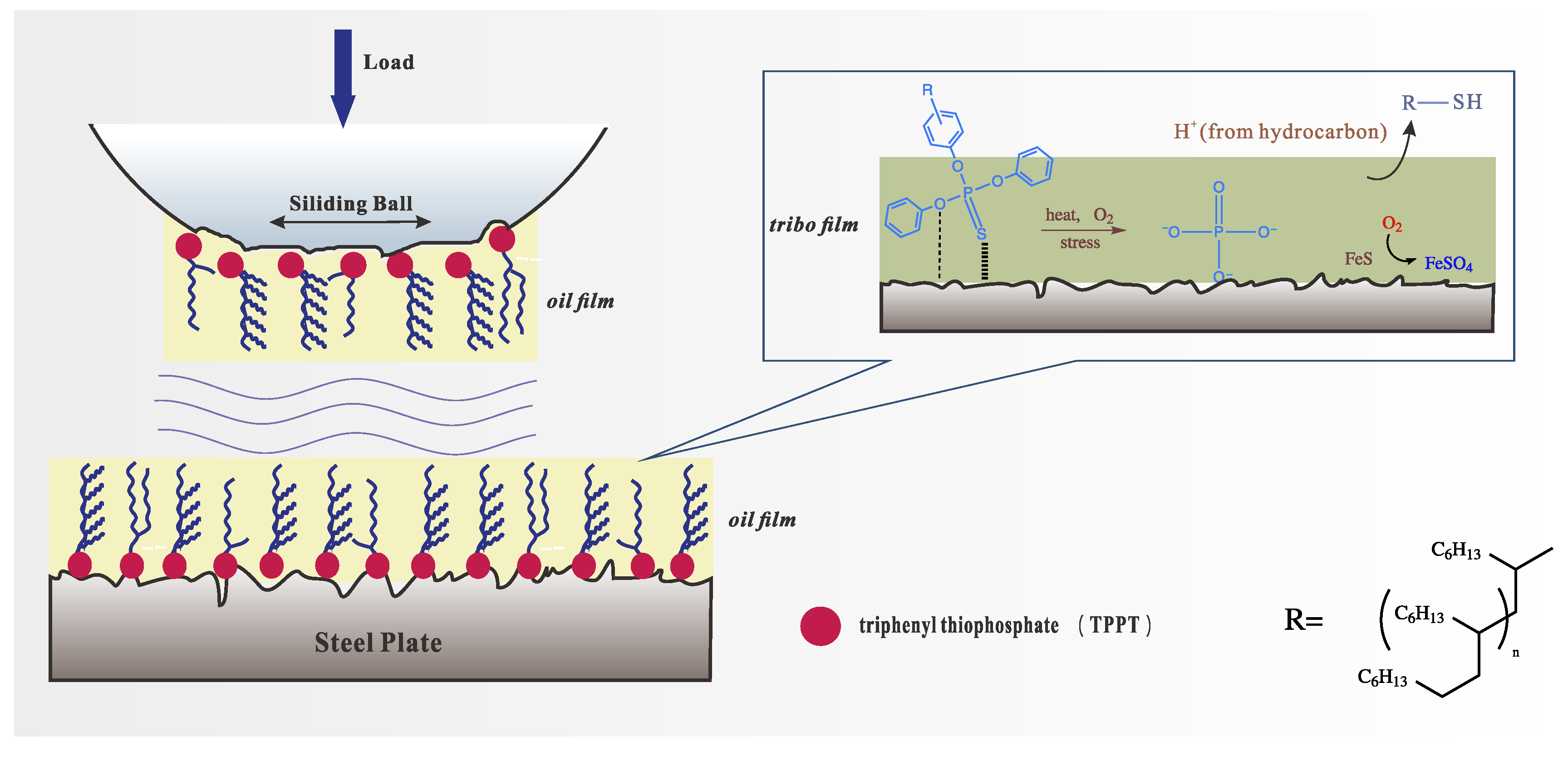
3.8.5. Lubrication Mechanism
4. Conclusions
- (1)
- TfOH is an efficient alkylating modification catalyst that can enhance the content of sulfur and phosphorus in the product by increasing the reaction temperature and catalyst dosage. During the reaction process, alkylation and -cleavage reactions occurred simultaneously. The success of the alkylation reaction was confirmed by NMR and FTIR analyses, indicating that almost all the double bonds in the starting material were successfully converted into saturated molecules.
- (2)
- The PDSC and oven oxidation experiments confirmed that the TPPT-modified mPAO had significantly improved oxidation resistance compared to mPAO.
- (3)
- In the four-ball and TE77 reciprocating friction tests, TPPT-modified mPAO demonstrated superior wear resistance. SEM, EDS, and XPS analyses indicated that during the friction test, a chemical film of wear-resistant ferro-sulfate and ferro-phosphate was generated on the friction surfaces.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poletto, J.; Fernandes, C.; Barros, L.; Neis, P.; Pondicherry, K.; Fauconnier, D.; Seabra, J.; De Baets, P.; Ferreira, N. Identification of gear wear damage using topography analysis. Wear 2023, 522, 204837. [Google Scholar] [CrossRef]
- Stepanova, T.Y.; Kuvaeva, E.Y. Wear of the Main Threads of a Weaving Machine. J. Frict. Wear 2020, 41, 459–462. [Google Scholar] [CrossRef]
- Li, M.; Shi, W.; Shi, J.; Wang, T.; Shi, L.; Wang, X. Regulation and control of wet friction of soft materials using surface texturing: A review. Friction 2023, 11, 333–353. [Google Scholar] [CrossRef]
- Hu, W.; Li, J. Effect of Molecular Weight on Tribological Properties of Polyether Amine Derivatives under Different Contact Modes. Lubricants 2022, 10, 105. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, C.; Huang, Q.; Yu, Q.; Yang, Z.; Zhou, C.; Yan, X.; Wang, R.; Yu, B.; Cai, M. Novel Phosphate Organic Guanidine Salt Water-Based Additive with Integrated Anti-Friction, Anti-Wear and Anti-Corrosion Properties. Tribol. Lett. 2022, 70, 33. [Google Scholar] [CrossRef]
- Vinogradov, G.; Arkharova, V.; Petrov, A. Anti-wear and anti-friction properties of hydrocarbons under heavy loads. Wear 1961, 4, 274–291. [Google Scholar] [CrossRef]
- Cañellas, G.; Emeric, A.; Combarros, M.; Navarro, A.; Beltran, L.; Vilaseca, M.; Vives, J. Tribological Performance of Esters, Friction Modifier and Antiwear Additives for Electric Vehicle Applications. Lubricants 2023, 11, 109. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, C.; Zhou, Z.; Nie, X.; Chen, Y.; Cao, H.; Liu, B.; Zhang, N.; Said, Z.; et al. Extreme pressure and antiwear additives for lubricant: Academic insights and perspectives. Int. J. Adv. Manuf. Technol. 2022, 120, 1–27. [Google Scholar] [CrossRef]
- Jaiswal, V.; Kalyani, K.; Rastogi, R.B.; Kumar, R. Tribological studies of some SAPS-free Schiff bases derived from 4-aminoantipyrine and aromatic aldehydes and their synergistic interaction with borate ester. J. Mater. Chem. A 2014, 2, 10424–10434. [Google Scholar] [CrossRef]
- Mangolini, F.; Rossi, A.; Spencer, N.D. Tribochemistry of Triphenyl Phosphorothionate (TPPT) by In Situ Attenuated Total Reflection (ATR/FT-IR) Tribometry. J. Phys. Chem. C 2012, 116, 5614–5627. [Google Scholar] [CrossRef]
- Mangolini, F.; Rossi, A.; Spencer, N.D. Chemical Reactivity of Triphenyl Phosphorothionate (TPPT) with Iron: An ATR/FT-IR and XPS Investigation. J. Phys. Chem. C 2011, 115, 1339–1354. [Google Scholar] [CrossRef]
- Mangolini, F.; Rossi, A.; Spencer, N.D. Substituent Effect on the Reactivity of Alkylated Triphenyl Phosphorothionates in Oil Solution in the Presence of Iron Particles. Tribol. Lett. 2010, 40, 375–394. [Google Scholar] [CrossRef]
- Hope, K. PAO Contributions to Energy Efficiency in 0W-20 Passenger Car Engine Oils. Lubricants 2018, 6, 73. [Google Scholar] [CrossRef]
- Coelho de Sousa Marques, M.A.; Guimarey, M.J.; Domínguez-Arca, V.; Amigo, A.; Fernández, J. Heat capacity, density, surface tension, and contact angle for polyalphaolefins and ester lubricants. Thermochim. Acta 2021, 703, 178994. [Google Scholar] [CrossRef]
- Salah, H.; Elkatory, M.R.; Abdel Fattah, M. Novel zinc-polymer complex with antioxidant activity for industrial lubricating oil. Fuel 2021, 305, 121536. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Q.; Li, J. Performance of Aromatic Amine-Modified Metallocene Polyalphaolefin Lubricant Base Oil. Lubricants 2024, 12, 255. [Google Scholar] [CrossRef]
- Abou, H.H.; Naga, E.; El Ashry, E.S.H.; Khattab, M.A.; Salem, A.E.; Boghdady, Y.M. Manufacture of a transformer oil from egyptian paraffinic base stocks via a hydrogenation process. Lubr. Sci. 2001, 13, 251–271. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bagrov, V.V.; Kiselev, A.V.; Minyaev, M.E.; Samurganova, T.I.; Ivchenko, P.V. Heterocene Catalysts and Reaction Temperature Gradient in Dec-1-ene Oligomerization for the Production of Low Viscosity PAO Base Stocks. Ind. Eng. Chem. Res. 2023, 62, 6347–6353. [Google Scholar] [CrossRef]
- ASTM D445-24; D02.07, Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2024; p. 16.
- ASTM D2270-10R16; D02.07, Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 °C and 100 °C. ASTM International: West Conshohocken, PA, USA, 2016; p. 5.
- ASTM D92-18; D02.08, Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester. ASTM International: West Conshohocken, PA, USA, 2018; p. 11.
- ASTM D5950-02; D02.07, Standard Test Method for Pour Point of Petroleum Products (Automatic Tilt Method). ASTM International: West Conshohocken, PA, USA, 2002; p. 5.
- ASTM D1159-07; D02.06, Standard Test Method for Bromine Numbers of Petroleum Distillates and Commercial Aliphatic Olefins by Electrometric Titration. ASTM International: West Conshohocken, PA, USA, 2018; p. 10.
- ASTM D664-24; D02.06, Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2024; p. 12.
- ASTM D611-12; D02.04.0D, Standard Test Methods for Aniline Point and Mixed Aniline Point of Petroleum Products and Hydrocarbon Solvents. ASTM International: West Conshohocken, PA, USA, 2016; p. 7.
- ASTM D2622-24; D02.03, Standard Test Method for Sulfur in Petroleum Products by Wavelength Dispersive X-ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2024; p. 12.
- ASTM D6481-14; D02.03, Standard Test Method for Determination of Phosphorus, Sulfur, Calcium, and Zinc in Lubrication Oils by Energy Dispersive X-ray Fluorescence Spectroscopy. ASTM International: West Conshohocken, PA, USA, 2019; p. 5.
- Corma, A.; Orchillés, A. Current views on the mechanism of catalytic cracking. Microporous Mesoporous Mater. 2000, 35–36, 21–30. [Google Scholar] [CrossRef]
- Fakhroleslam, M.; Sadrameli, S.M. Thermal/catalytic cracking of hydrocarbons for the production of olefins; a state-of-the-art review III: Process modeling and simulation. Fuel 2019, 252, 553–566. [Google Scholar] [CrossRef]
- Sadrameli, S. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Catalytic cracking review. Fuel 2016, 173, 285–297. [Google Scholar] [CrossRef]
- Hanifpour, A.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Poater, A. Coordinative chain transfer polymerization of 1-decene in the presence of a Ti-based diamine bis(phenolate) catalyst: A sustainable approach to produce low viscosity PAOs. Green Chem. 2020, 22, 4617–4626. [Google Scholar] [CrossRef]
- Mangolini, F.; Rossi, A.; Spencer, N.D. Reactivity of Triphenyl Phosphorothionate in Lubricant Oil Solution. Tribol. Lett. 2009, 35, 31–43. [Google Scholar] [CrossRef]
- Fox, N.; Tyrer, B.; Stachowiak, G. Boundary Lubrication Performance of Free Fatty Acids in Sunflower Oil. Tribol. Lett. 2004, 16, 275–281. [Google Scholar] [CrossRef]
- Najman, M.; Kasrai, M.; Bancroft, G. Chemistry of Antiwear Films from Ashless Thiophosphate Oil Additives. Tribol. Lett. 2004, 17, 217–229. [Google Scholar] [CrossRef]
- Gao, F.; Kotvis, P.; Tysoe, W. The surface and tribological chemistry of chlorine- and sulfur-containing lubricant additives. Tribol. Int. 2004, 37, 87–92. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, W.M.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Beamson, G.; Briggs, D.R. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: Chichester, UK, 1992. [Google Scholar]
- Eglin, M.; Rossi, A.; Spencer, N.D. X-ray Photoelectron Spectroscopy Analysis of Tribostressed Samples in the Presence of ZnDTP: A Combinatorial Approach. Tribol. Lett. 2003, 15, 199–209. [Google Scholar] [CrossRef]
- Wang, Y.; Asunskis, D.J.; Sherwood, P.M.A. Iron (II) Phosphate (Fe3(PO4)2 by XPS. Surf. Sci. Spectra 2002, 9, 91–98. [Google Scholar] [CrossRef]
- Heuberger, R.; Rossi, A.; Spencer, N.D. Reactivity of alkylated phosphorothionates with steel: A tribological and surface-analytical study. Lubr. Sci. 2008, 20, 79–102. [Google Scholar] [CrossRef]




| Pramater | mPAO | T-mPAO 1 | A-mPAO |
|---|---|---|---|
| Kinematic viscosity, mm2/s | |||
| 40 °C | 1622 | 2283 | 1618 |
| 100 °C | 150.2 | 186.6 | 149.2 |
| VI | 205 | 203 | 204 |
| Flash point/°C | 285 | 280 | 285 |
| Pour point/°C | −33 | −27 | −33 |
| Acid number, mg KOH/g | 0.02 | 0.06 | 0.06 |
| Bromine number, gBr/100 g | 1.908 | / | / |
| Sulphur content, wt% | 0 | 0.389 | 0.150 |
| Aniline point, °C | >170 | >170 | >170 |
| Sample | Mz (Daltons) | Mn (Daltons) | Mw (Daltons) | Mw/Mn |
|---|---|---|---|---|
| mPAO | 11,147 | 3439 | 6578 | 1.89 |
| 110-mPAO | 11,189 | 3254 | 6680 | 2.05 |
| 90-mPAO | 11,175 | 3530 | 6920 | 1.96 |
| T-mPAO | 11,772 | 3798 | 7316 | 1.92 |
| Parameter | mPAO | A-mPAO | T-mPAO |
|---|---|---|---|
| IOT, °C | 178 | 183 | 210 |
| Sample | PB/kg | PD/kg |
|---|---|---|
| mPAO | 94 | 126 |
| A-mPAO | 171 | 200 |
| T-mPAO | 238 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Zeng, K.; Han, S.; Xu, J.; Hu, W.; Li, J. Synthesis and Performance Evaluation of Metallocene Polyalphaolefins (mPAO) Base Oil with Anti-Friction and Anti-Wear Properties. Polymers 2024, 16, 2828. https://doi.org/10.3390/polym16192828
Hu Q, Zeng K, Han S, Xu J, Hu W, Li J. Synthesis and Performance Evaluation of Metallocene Polyalphaolefins (mPAO) Base Oil with Anti-Friction and Anti-Wear Properties. Polymers. 2024; 16(19):2828. https://doi.org/10.3390/polym16192828
Chicago/Turabian StyleHu, Qidi, Kai Zeng, Sheng Han, Jian Xu, Wenjing Hu, and Jiusheng Li. 2024. "Synthesis and Performance Evaluation of Metallocene Polyalphaolefins (mPAO) Base Oil with Anti-Friction and Anti-Wear Properties" Polymers 16, no. 19: 2828. https://doi.org/10.3390/polym16192828
APA StyleHu, Q., Zeng, K., Han, S., Xu, J., Hu, W., & Li, J. (2024). Synthesis and Performance Evaluation of Metallocene Polyalphaolefins (mPAO) Base Oil with Anti-Friction and Anti-Wear Properties. Polymers, 16(19), 2828. https://doi.org/10.3390/polym16192828








