Evaluating the Effectiveness of Cellulose-Based Surfactants in Expandable Graphite Wood Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Wood
2.1.2. Expandable Graphite (EG)
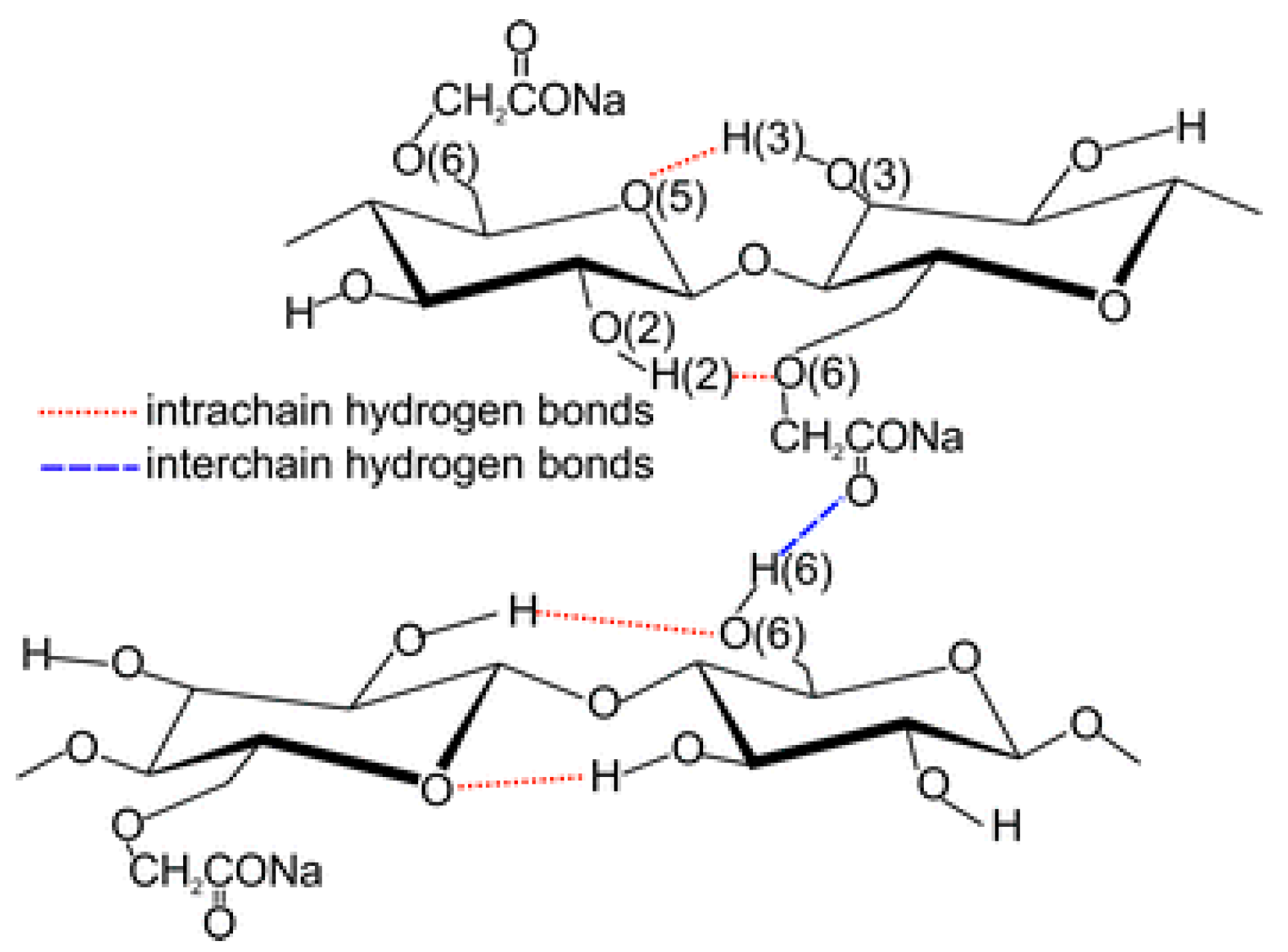
2.1.3. Cellulose-Based Surfactants
Cellulose Ethers
- Lovose TS 20 (LOV)
- Tylose MH 300 (TYL)
- Klucel H (KLU)
Nanocrystaline Cellulose (CNC)
2.2. Methods
2.2.1. Recipe Design of EG Fire-Retardant Coatings with Individual Surfactants
2.2.2. Application on Wood
2.2.3. Radiant Heat Source Test
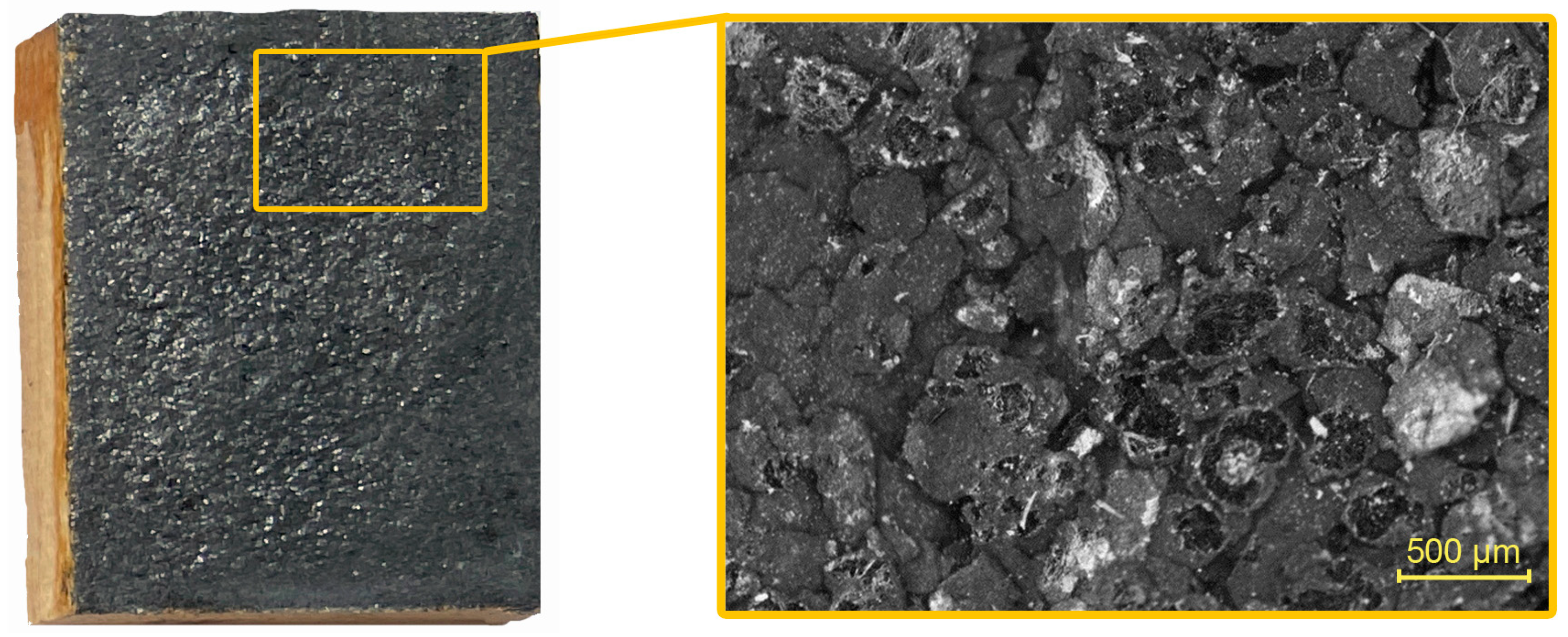
2.2.4. Microscopy Analysis
3. Results and Discussion
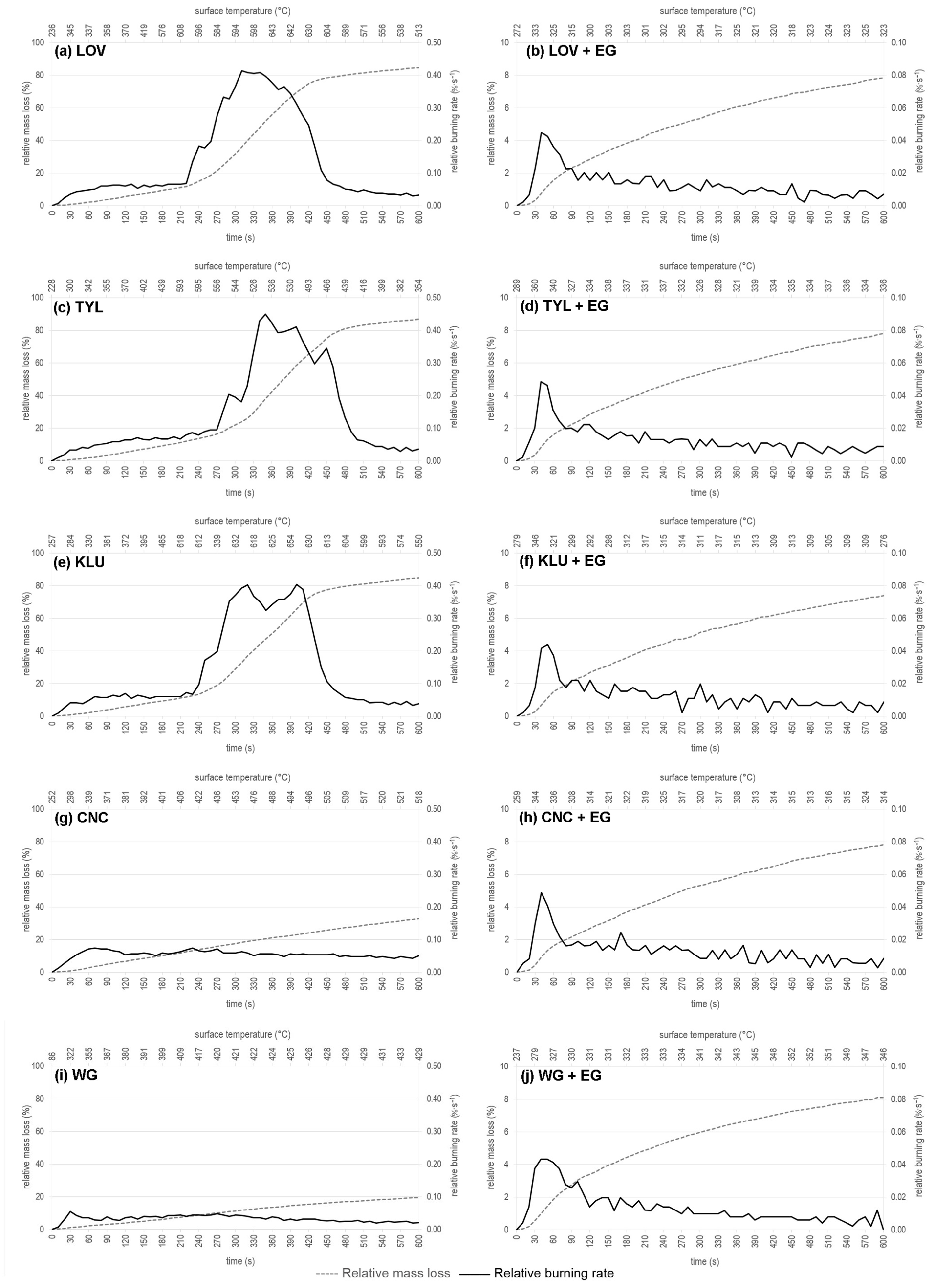
3.1. Relative Mass Loss
3.2. Evolution of Relative Mass Loss and Relative Burning Rate in Time and Sample Surface Temperature Course during a Radiant Heat Source Exposure
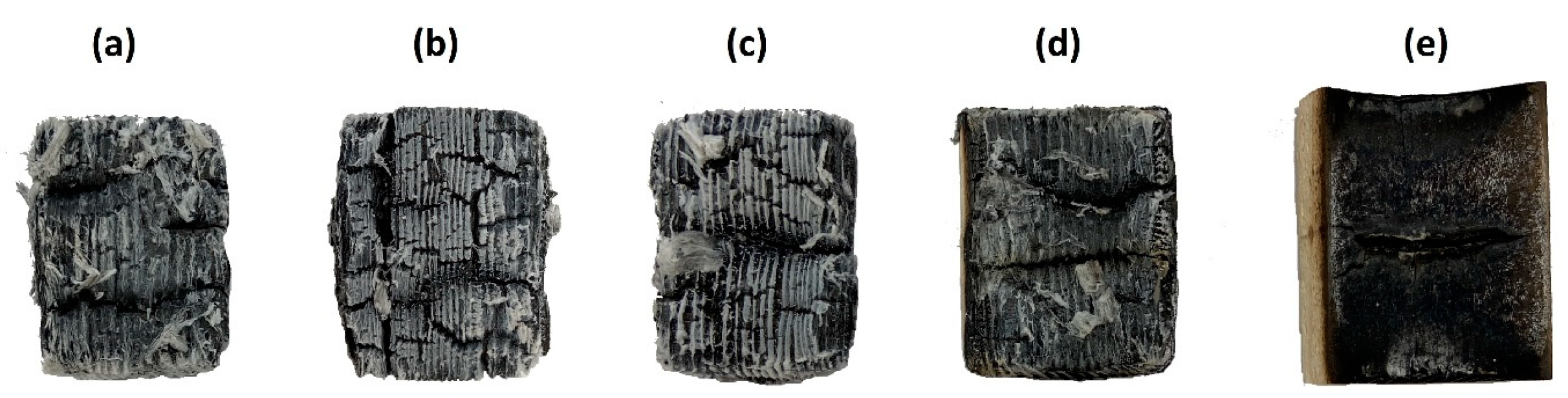
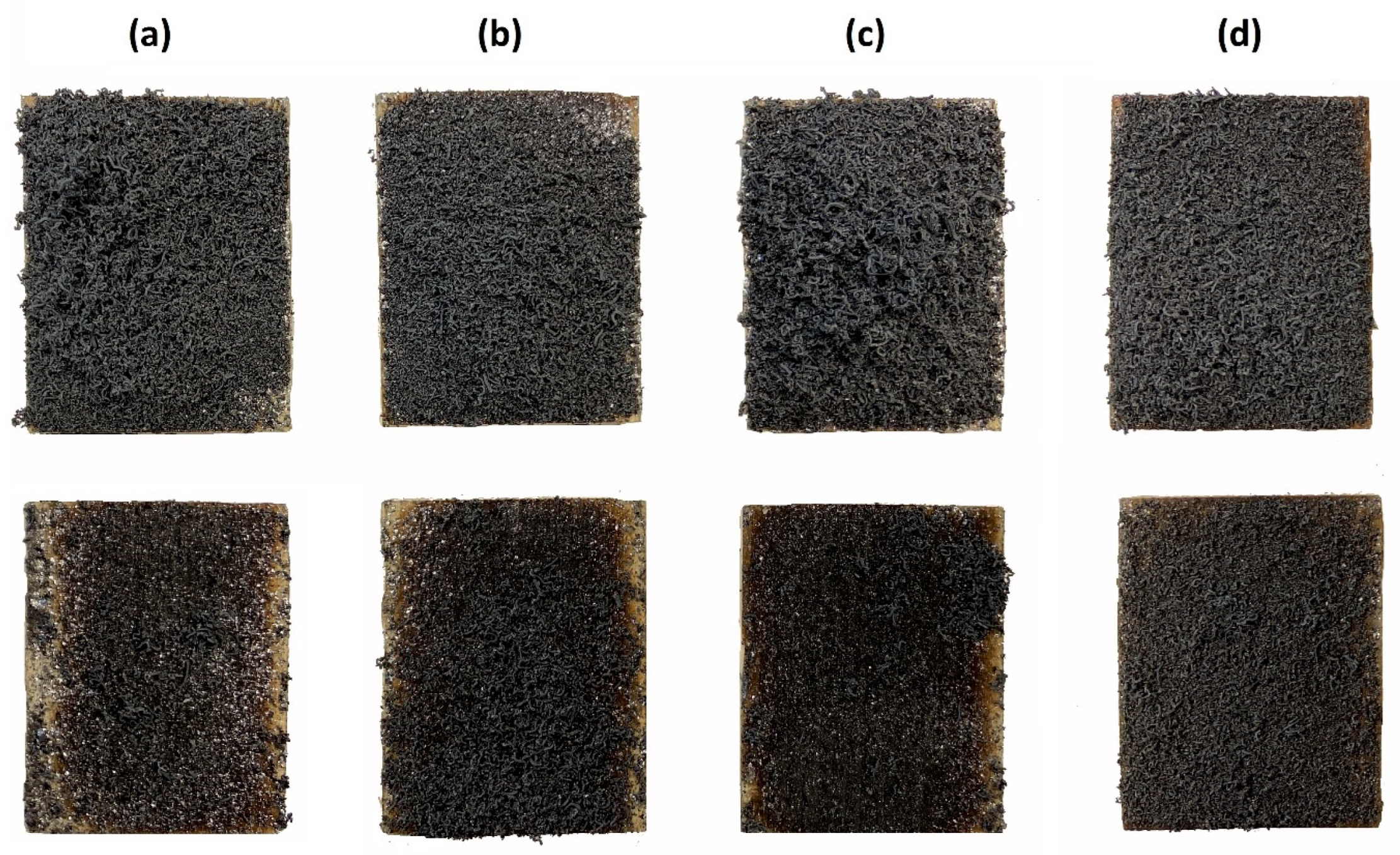
3.3. Evaluation of Expanded Layer Cohesion
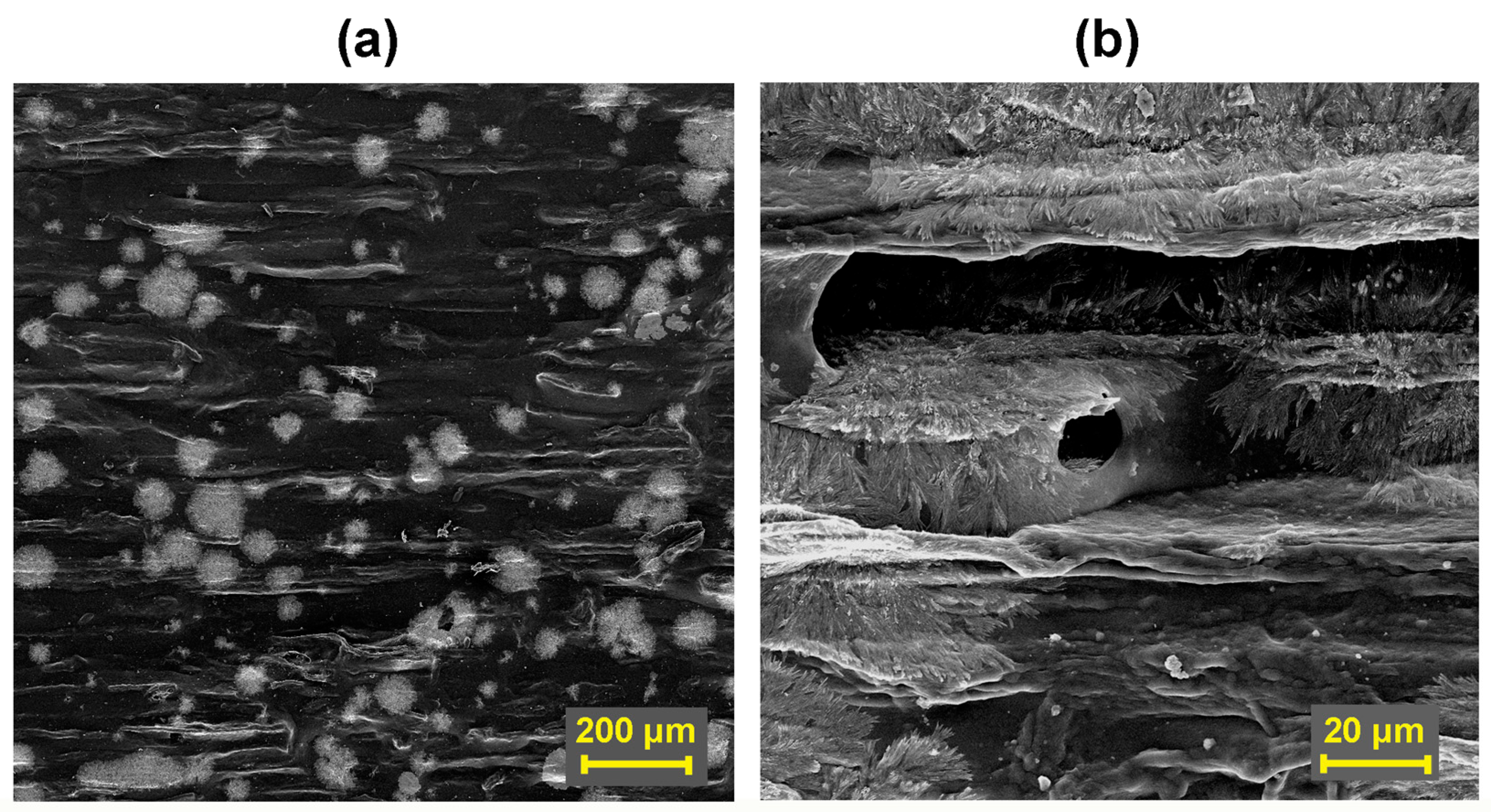
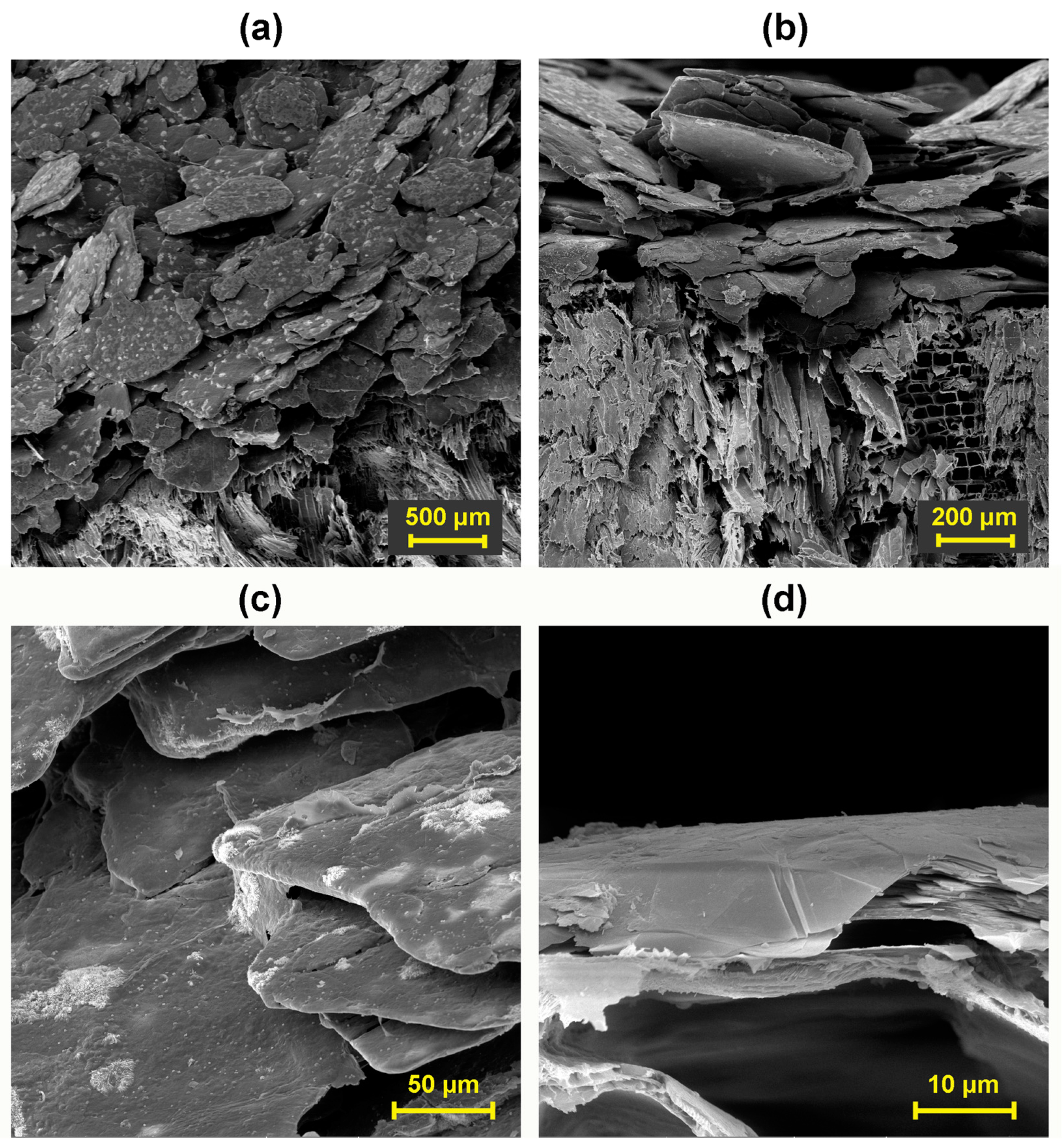
3.4. Evaluation of Coating Adhesion to Wood by SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, R.A.; Jiang, L.; Renner, J.S.; Xu, Q. Characterisation of the fire behaviour of wood: From pyrolysis to fire retardant mechanisms. J. Therm. Anal. Calorim. 2023, 148, 1407–1422. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Verlag Kessel: Remagen, Germany, 2003; pp. 26–65. ISBN 3935638396. [Google Scholar]
- Wessels, C.B.; Malan, F.S.; Rypstra, T. A review of measurement methods used on standing trees for the prediction of some mechanical properties of timber. Eur. J. For. Res. 2011, 130, 88193. [Google Scholar] [CrossRef]
- Östman, B.A.-L. Fire performance of wood products and timber structures. Int. Wood Prod. J. 2017, 8, 74–79. [Google Scholar] [CrossRef]
- Branca, C.; Giudicianni, P.; Di Blasi, C. GC/MS characterization of liquids generated from low-temperature pyrolysis of wood. Ind. Eng. Chem. Res. 2003, 42, 3190–3202. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Flemings, M.C.; Kramer, E.J.; Veyssière, P.; Cahn, R.W.; Ilschner, B.; Mahajan, S. (Eds.) Encyclopedia of Materials: Science and Technology; Elsevier: New York, NY, USA, 2001; p. 474. ISBN 978-0-08-043152-9. [Google Scholar]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef]
- Rani, A.; Singh, J.; Singh, T. An experimental characterization of physical properties of timber woods. Mater. Sci. Nanotechnol. 2017, 1, 4–45. [Google Scholar] [CrossRef]
- Kasymov, D.P.; Agafontsev, M.V.; Perminov, V.V. Estimation of the influence of wood-fire retardants on fire behavior of some types of wood construction materials. J. Phys. Conf. Series 2018, 1105, 012039. [Google Scholar] [CrossRef]
- Reszka, P.; Torero, J.L. In-depth temperature measurements in wood exposed to intense radiant energy. Exp. Therm. Fluid Sci. 2008, 32, 1405–1411. [Google Scholar] [CrossRef]
- Frangi, A.; Fontana, M.; Hugi, E.; Jübstl, R. Experimental analysis of cross-laminated timber panels in fire. Fire Saf. J. 2009, 44, 1078–1087. [Google Scholar] [CrossRef]
- Babrauskas, V. Charring rate of wood as a tool for fire investigations. Fire Saf. J. 2005, 40, 528–554. [Google Scholar] [CrossRef]
- Grishin, A.M.; Filkov, A.I.; Loboda, E.L.; Reyno, V.V.; Kozlov, A.V.; Kuznetsov, V.T.; Kasymov, D.P.; Andreyuk, S.M.; Ivanov, A.I.; Stolyarchuk, N.D. A field experiment on grass fire effects on wooden constructions and peat layer ignition. IJWF 2014, 23, 445–449. [Google Scholar] [CrossRef]
- LeVan, S.L.; Winandy, J.E. Effect of fire retardant treatments of wood strength: A review. Wood Fiber Sci. 1990, 22, 112–131. [Google Scholar]
- Östman, B.; Tsantaridis, L.; Mikkola, E.; Hakkarainen, T.; Belloni, K.; Brumer, H.; Piispanen, P. Innovative eco-efficient high fire performance wood products for demanding applications. In Final Report for Vinnova-Tekes Project InnoFireWood; SP Rapport No. 30; Springer: Berlin, Germany, 2006; p. 98. ISBN 91-85533-15-7. [Google Scholar]
- Eaton, R.A.; Hale, M.D.C. Wood: Decay, Pests, and Protection, 1st ed.; Chapman & Hall: New York, NY, USA, 1993; p. 546. ISBN 9780412531200. [Google Scholar]
- Richardson, B.A. Wood Preservation; Construction Press: London, UK, 1993; p. 240. ISBN 9780419174905. [Google Scholar]
- Ibach, R.E. Wood preservation. In Wood Handbook: Wood as an Engineering Material: General Technical Report FPL; GTR-113; USA Forest Service, Forest Products Laboratory: Madison, WI, USA, 1999; pp. 14.1–14.27. [Google Scholar]
- Tribulová, T.; Kačík, F.; Evtuguin, D.V. Impacts of inorganic chemicals used for wood protection: A review. AFXZ 2017, 59, 5–22. [Google Scholar] [CrossRef]
- Lowden, L.; Hull, T. Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Wang, Q.W.; Wang, W.H.; Winandy, J.E. Effects of a new GUP-B fire-retardant on mechanical properties of Korean pine when exposed to elevated temperature. Forest Prod. J. 2005, 55, 214–220. [Google Scholar]
- Pondelak, A.; Škapin, A.S.; Knez, N.; Knez, F.; Pazlar, T. Improving the flame retardancy of wood using an eco-friendly mineralisation process. Green Chem. 2021, 23, 1130–1135. [Google Scholar] [CrossRef]
- Lawson, J.R. A history of fire testing: Past, present, and future. J. ASTM Int. 2009, 6, 1–39. [Google Scholar] [CrossRef]
- Zhao, S.; Song, G.Y.; Liu, B.C.; Zhou, Y.L. Study of flame retardant of guanidine phosphate to wood. Chem. Eng. 1994, 3, 13–14. [Google Scholar]
- Wang, F.; Wang, Q.; Wang, X. Progress in research on fire retardant–treated wood and wood-based composites: A Chinese perspective. For. Prod. J. 2010, 60, 668–678. [Google Scholar] [CrossRef]
- Fu, F.; Lin, L.; Xu, E. Functional pretreatments of natural raw materials. In Advanced High Strength Natural Fibre Composites in Construction, 1st ed.; Fan, M., Fu, F., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 87–114. ISBN 978-0-08-100411-1. [Google Scholar]
- Sauerbier, P.; Mayer, A.K.; Emmerich, L.; Militz, H. Fire retardant treatment of wood–state of the art and future perspectives. In Wood & Fire Safety: Proceedings of the 9th International Conference on Wood & Fire Safety, 1st ed.; Makovicka Osvaldova, L., Markert, F., Zelinka, S.L., Eds.; Springer: New York, NY, USA, 2020; pp. 97–102. ISBN 978-3-030-41234-0. [Google Scholar]
- Pabeliña, K.G.; Lumban, C.O.; Ramos, H.J. Plasma impregnation of wood with fire retardants. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. Atoms 2012, 272, 365–369. [Google Scholar] [CrossRef]
- Sun, Q.F.; Lu, Y.; Zhang, H.M.; Yang, D.J.; Xu, J.S.; Li, J.; Liu, Y.X.; Shi, J.T. Flame retardancy of wood coated by ZnO nanorod arrays via a hydrothermal method. Mater. Res. Innov. 2012, 16, 326–331. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Kmeťová, E.; Kačíková, D.; Kačík, F. The Effect of Intumescent Coating Containing Expandable Graphite onto Spruce Wood. Coatings 2024, 14, 490. [Google Scholar] [CrossRef]
- Kmeťová, E.; Kačíková, D.; Jurczyková, T.; Kačík, F. The Influence of Different Types of Expandable Graphite on the Thermal Resistance of Spruce Wood. Coatings 2023, 13, 1181. [Google Scholar] [CrossRef]
- Kmeťová, E.; Kačík, F.; Kubovský, I.; Kačíková, D. Effect of expandable graphite flakes on the flame resistance of oak wood. Coatings 2022, 12, 1908. [Google Scholar] [CrossRef]
- Jurczyková, T.; Kmeťová, E.; Kačík, F.; Lexa, M.; Ťoupal, J. An Innovative Wood Fire-Retardant Coating Based on Biocompatible Nanocellulose Surfactant and Expandable Graphite. Coatings 2024, 14, 1036. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Camino, G. Expandable graphite as an intumescent flame retardant in polyisocyanurate–polyurethane foams. Polym. Degrad. Stab. 2002, 77, 195–202. [Google Scholar] [CrossRef]
- Duquesne, S.; Bras, M.L.; Bourbigot, S.; Delobel, R.; Vezin, H.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T. Expandable graphite: A fire retardant additive for polyurethane coatings. Fire Mater. 2003, 27, 103–117. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.; Kim, H.; Kim, B.; Jeon, K.J.; Yoon, S.H. Expanding characteristics of graphite in microwave-assisted exfoliation. IJAME 2015, 1, 34–38. [Google Scholar]
- Wang, Z.; Han, E.; Ke, W. Influence of expandable graphite on fire resistance and water resistance of flame-retardant coatings. Corros. Sci. 2007, 49, 2237–2253. [Google Scholar] [CrossRef]
- Kumar, S.P.; Takamori, S.; Araki, H.; Kuroda, S. Flame retardancy of clay–sodium silicate composite coatings on wood for construction purposes. RSC Adv. 2015, 5, 34109–34116. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, G.; Zhao, J.; Han, Y. Effect of flaky graphite with different particle sizes on flame resistance of intumescent flame retardant coating. Results Mater. 2020, 5, 100061. [Google Scholar] [CrossRef]
- Çalın, Ö.; Kurt, A.; Çelik, Y. Influence of expansion conditions and precursor flake size on porous structure of expanded graphite. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 611–620. [Google Scholar] [CrossRef]
- Tohamy, H.A.S.; Elnasharty, M.M.; Abdel-Aziz, M.S.; El-Sakhawy, M.; Turky, G.; Kamel, S. Antibacterial activity and dielectric properties of the PVA/cellulose nanocrystal composite using the synergistic effect of rGO@ CuNPs. Int. J. Biol. Macromol. 2024, 261, 129801. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.N.; Sahai, R.S.N.; Biswas, D.; Samui, A.B. Comparative Study of Mechanical Properties of Multiwall Carbon Nanotubes and Functionalized Multiwall Carbon Nanotubes/Poly Aryl Ether Ketone Nanocomposites. Iran. Mater. Sci. Eng. 2023, 20, 119–219. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in sensing and biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly efficient and sustainable preparation of carboxylic and thermostable cellulose nanocrystals via FeCl3-catalyzed innocuous citric acid hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700. [Google Scholar] [CrossRef]
- Wei, D.; Wei, H.; Gauthier, A.; Song, J.; Jin, Y.; Xiao, H. Superhydrophobic modification of cellulose and cotton textiles: Methodologies and applications. J. Bioresour. Bioprod. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Gardner, K.H.; Blackwell, J. The structure of native cellulose. Biopolym 1974, 13, 1975–2001. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Jesionowski, T.; Kuznowicz, M.; Jędrzak, A.; Rębiś, T. Sensing materials: Biopolymeric nanostructures. Enc. Sens. Biosens. 2023, 2, 286–304. [Google Scholar] [CrossRef]
- Liu, H.; Du, H.; Zheng, T.; Liu, K.; Ji, X.; Xu, T.; Zhang, X.; Si, C. Cellulose based composite foams and aerogels for advanced energy storage devices. J. Chem. Eng. 2021, 426, 130817. [Google Scholar] [CrossRef]
- Jedverta, K.; Heinze, T. Cellulose modification and shaping-a review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohyd. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Hon, D.N.S.; Shiraishi, N. Wood and Cellulosic Chemistry; Marcel Dekker, Inc.: New York, NY, USA, 1991; p. 928. ISBN 9780824700249. [Google Scholar]
- Niaounakis, M. Biopolymers: Applications and Trends; William Andrew: San Diego, CA, USA, 2015; p. 587. ISBN 9780323353991. [Google Scholar]
- Ebnesajjad, S.; Landrock, A.H. Adhesives Technology Handbook; William Andrew: San Diego, CA, USA, 2014; p. 432. ISBN 9780323356022. [Google Scholar]
- Heinze, T.; Koschella, A.; Liebert, T.; Harabagiu, V.; Coseri, S. Cellulose: Chemistry of cellulose derivatization. In The European Polysaccharide Network of Excellence (EPNOE); Research Initiatives and Results; Springer Nature: Berlin, Germany, 2012; pp. 283–327. [Google Scholar]
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Santo Pereira, A.E.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Lebrun, L.; Khelifi, B.; Marais, S.; Dufresne, A. Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrils films. Carbohydr Polym. 2011, 83, 1740–1748. [Google Scholar] [CrossRef]
- Nielsen, L.J.; Eyley, S.; Thielemans, W.; Aylott, J.W. Dual fluorescent labelling of cellulose nanocrystals for pH sensing. Chem. Commun. 2010, 46, 8929–8931. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules 2011, 13, 267–275. [Google Scholar] [CrossRef]
- Taheri, A.; Mohammadi, M. The Use of Cellulose Nanocrystals for Potential Application in Topical Delivery of Hydroquinone. Chem. Biol. Drug Des. 2015, 86, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D. Changing the Reactive Coatings Market with Graphite. PCI 2023, 3, 1–4. [Google Scholar]
- Charmeau, J.Y.; Kientz, E.; Holl, Y. Adhesion of latex films; influence of surfactants. Prog. Org. Coat. 1996, 27, 87–93. [Google Scholar] [CrossRef]
- Andersson, C.; Järnström, L.; Hellgren, A.C. Effects of carboxylation of latex on polymer interdiffusion and water vapor permeability of latex films. NPPRJ 2022, 17, 20–28. [Google Scholar] [CrossRef]
- Roulstone, B.J.; Wilkinson, M.C.; Hearn, J. Studies on polymer latex films: II Effect of surfactants on the water vapour permeability of polymer latex films. Polym. Int. 1992, 27, 43–50. [Google Scholar] [CrossRef]
- Dányádi, L.; Móczó, J.; Pukánszky, B. Effect of various surface modifications of wood flour on the properties of PP/wood composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 199–206. [Google Scholar] [CrossRef]
- Ekstedt, J. Studies on the Barrier Properties of Exterior Wood Coatings. Doctoral Dissertation, KTH-Royal Institute of Technology, Stockholm, Sweden, 2002; p. 75. [Google Scholar]
- Hellgren, A.-C.; Weissenborn, P.; Holmberg, K. Surfactants in water-borne paints. Prog. Org. Coat. 1999, 35, 79–87. [Google Scholar] [CrossRef]
- Heilen, W.; Klocker, O.; Adams, J. Influence of Defoamers on the Efficiency of Waterborne Coating System. JCTR 1994, 66, 47–53. [Google Scholar]
- Feller, R.L.; Wilt, M.H. Evaluation of Cellulose Ethers for Conservation; Getty Publications: Los Angeles, CA, USA, 1991; p. 173. ISBN 0-89236-099-2. [Google Scholar]
- Burchard, W. Solubility and solution structure of cellulose derivatives. Cellulose 2003, 10, 213–225. [Google Scholar] [CrossRef]
- Schröder, S.; Ter-Akopow, A. Deriváty celulosy pro restaurátorské účely; X. seminář historiků a restaurátorů: Litomyšl, Czech Republic, 1997; pp. 242–247. (In Czech) [Google Scholar]
- Lukešová, I. Aplikace uměleckých děl na papíru na novou podložku. Bachelor Thesis, Univerzita Pardubice, Fakulta restaurování, Litomyšl, Czech Republic, 2009; p. 88. (In Czech). [Google Scholar]
- Kubička, R.; Zelinger, J. Výkladový Slovník; Grada: Praha, Czech Republic, 2004; p. 344. ISBN 978-80-247-9046-6. (In Czech) [Google Scholar]
- Ďurovič, M. Restaurování a Konzervování Archiválií a Knih; Paseka: Litomyšl, Czech Republic, 2002; p. 536. ISBN 80-7185-383-6. (In Czech) [Google Scholar]
- Kim, H.; Park, J.; Minn, K.S.; Youn, J.R.; Song, Y.S. Eco-friendly nanocellulose embedded polymer composite foam for flame retardancy improvement. Macromol. Res. 2020, 28, 165–171. [Google Scholar] [CrossRef]
- Luo, J.; Wang, H. Preparation, thermal insulation and flame retardance of cellulose nanocrystal aerogel modified by TiO2. Int. J. Heat Technol. 2018, 36, 614–618. [Google Scholar] [CrossRef]
- Santos, L.P.; da Silva, D.S.; Bertacchi, J.P.F.; Moreira, K.S.; Burgo, T.A.L.; Batista, B.C.; dos Santos, J.; de Paula, P.A.; Galembeck, F. Multifunctional coatings of exfoliated and reassembled graphite on cellulosic substrates. Faraday Discuss. 2021, 227, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, D.S.; Rehovsky, C.; Shojaeiarani, J.; Stark, N.; Bajwa, S.; Dietenberger, M.A. Functionalized cellulose nanocrystals: A potential fire retardant for polymer composites. Polymers 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Mou, K.; Jiang, X.; Cha, R. High-value applications of nanocellulose. Pap. Biomater. 2017, 2, 58–64. [Google Scholar] [CrossRef]
- Farooq, M.; Sipponen, M.H.; Seppälä, A.; Österberg, M. Eco-friendly flame-retardant cellulose nanofibril aerogels by incorporating sodium bicarbonate. ACS Appl. Mater. Interfaces 2018, 10, 27407–27415. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Dollimore, D. A thermal analysis investigation of partially hydrolyzed starch. Thermochim. Acta 1998, 319, 17–25. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; Lu, H. Flame retardancy and thermal degradation of intumescent flame retardant poly (lactic acid)/starch biocomposites. Ind. Eng. Chem. Res. 2011, 50, 713–720. [Google Scholar] [CrossRef]
- Zhan, W.; Gu, Z.; Jiang, J.; Chen, L. Influences of surface area of graphene on fire protection of waterborne intumescent fire resistive coating. PSEP 2020, 139, 106–113. [Google Scholar] [CrossRef]
- Li, W.; Sun, B.; Wu, P. Study on hydrogen bond of carboxymethyl cellulose sodium film with two-dimensional correlation infrared spectroscopy. Carbohydr. Polym. 2009, 78, 454–461. [Google Scholar] [CrossRef]
- Bulichen, D.; Kainz, J.; Plank, J. Working mechanism of methyl hydroxyethyl cellulose (MHEC) as water retention agent. Cem. Concr. Res. 2012, 42, 953–959. [Google Scholar] [CrossRef]
- Różańska, S.; Różański, J. Extensional Flow of Carboxymethylcellulose Sodium Salt Measured on the Opposed-Nozzle Device. Soft Mater. 2017, 15, 302–314. [Google Scholar] [CrossRef]
- Channab, B.-E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, Modification, Biodegradation and Applications in Agriculture as Slow/Controlled Release Fertilizer, Superabsorbent, and Crop Protection: A Review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T. The relationship between intramolecular hydrogen bonds and certain physical properties of regioselectively substituted cellulose derivatives. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 717–723. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Korzhikova-Vlakh, E. Modification of Cellulose Micro- and Nanomaterials to Improve Properties of Aliphatic Polyesters/Cellulose Composites: A Review. Polymers 2022, 14, 1477. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, J.; Wang, W.; Pan, C. Characterizations of expanded graphite/polymer composites prepared by in situ polymerization. Carbon 2004, 42, 2839–2847. [Google Scholar] [CrossRef]




| Surfactant Type/ Mixture Components | Lovose TS 20 | Tylose MH 300 | Klucel H | CNC |
|---|---|---|---|---|
| Surfactant dry matter (g) | 1 | 1 | 1 | 2 |
| Solvent type | Distilled water | Distilled water | Distilled water | 5% (wt/wt) NaOH |
| Solvent volume (mL) | 100 | 100 | 100 | 100 |
| EG content in formulation (g) | 60 | 60 | 60 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurczyková, T.; Kmeťová, E.; Kačík, F.; Lexa, M.; Dědič, D. Evaluating the Effectiveness of Cellulose-Based Surfactants in Expandable Graphite Wood Coatings. Polymers 2024, 16, 2832. https://doi.org/10.3390/polym16192832
Jurczyková T, Kmeťová E, Kačík F, Lexa M, Dědič D. Evaluating the Effectiveness of Cellulose-Based Surfactants in Expandable Graphite Wood Coatings. Polymers. 2024; 16(19):2832. https://doi.org/10.3390/polym16192832
Chicago/Turabian StyleJurczyková, Tereza, Elena Kmeťová, František Kačík, Martin Lexa, and Daniel Dědič. 2024. "Evaluating the Effectiveness of Cellulose-Based Surfactants in Expandable Graphite Wood Coatings" Polymers 16, no. 19: 2832. https://doi.org/10.3390/polym16192832
APA StyleJurczyková, T., Kmeťová, E., Kačík, F., Lexa, M., & Dědič, D. (2024). Evaluating the Effectiveness of Cellulose-Based Surfactants in Expandable Graphite Wood Coatings. Polymers, 16(19), 2832. https://doi.org/10.3390/polym16192832






