Polymers Containing Diethylsiloxane Segment and Active Functional Group by Ring-Opening Polymerization of Hexaethylcyclotrisiloxane under the Catalysis of Linear Chlorinated Phosphazene Acid
Abstract
1. Introduction
2. Experiment Section
2.1. Raw Materials
2.2. Experimental Instruments
2.3. Preparation of Linear Chlorinated Phosphazene Acid
2.4. Synthesis of Linear PDES Oligomers and Copolymers
2.5. Preparation of Silicone Gels
3. Results and Discussion
3.1. PDES Oligomers
3.1.1. α, ω-bisdimethylsiloxyl-Terminated PDES Oligomers (PDES-H)
3.1.2. α, ω-bisdimethylvinylsiloxyl-Terminated PDES Oligomers (PDES-Vi)
3.2. Poly(methylhydrogen-diethyl)siloxane Copolymers (PMHS-co-PDES)
3.2.1. α, ω-bisdimethylsiloxyl-Terminated PMHS-co-PDES
3.2.2. α, ω-bistrimethylsiloxyl-Terminated PMHS-co-PDES
3.3. Poly(dimethyl-diethyl)siloxane Copolymer (PDMS-co-PDES)
3.4. Effect of Ethyl Content on Heat Performance of Silicone Gels
3.4.1. Structural Analysis of Silicone Gels
3.4.2. Thermal Properties of Silicone Gel Samples
DSC Curves
TGA and DTG Curves
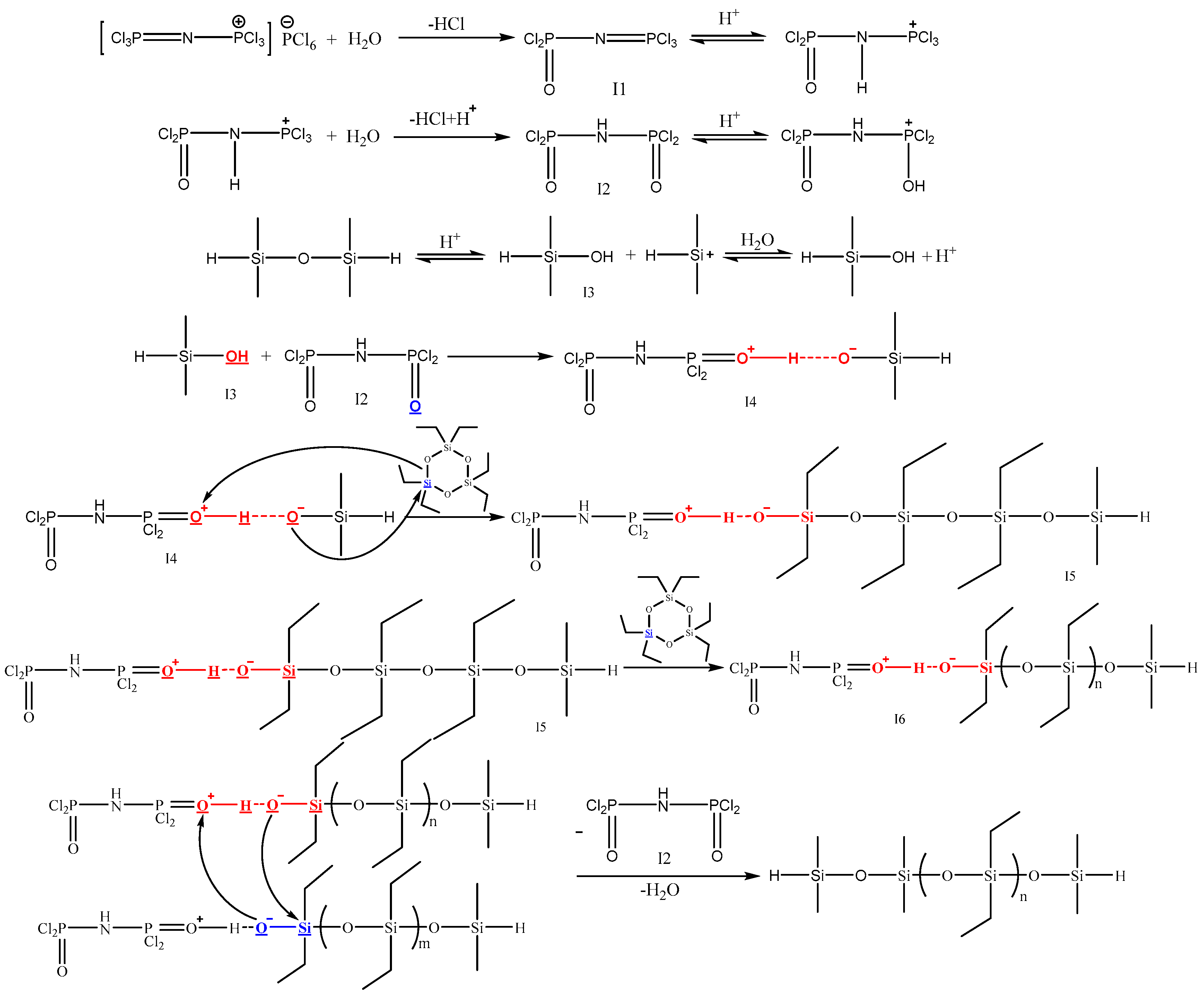
3.5. Possible Catalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Zalewski, K.; Chyłek, Z.; Trzciński, W.A. A Review of Polysiloxanes in Terms of Their Application in Explosives. Polymers 2021, 13, 1080. [Google Scholar] [CrossRef]
- Abdilla, A.; D’Ambra, C.A.; Geng, Z.; Shin, J.J.; Czuczola, M.; Goldfeld, D.J.; Biswas, S.; Mecca, J.M.; Swier, S.; Bekemeier, T.D.; et al. Silicone-based polymer blends: Enhancing properties through compatibilization. J. Polym. Sci. 2021, 59, 2114–2128. [Google Scholar] [CrossRef]
- Baceiredo, A.; Kato, T. Multiple bonds to silicon (recent advances in the chemistry of silicon containing multiple bonds). In Organosilicon Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 533–618. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Ji, C.; Hu, X.; He, Y.; Sun, Y.; Yun, Z. Synthesis and properties of MoO3/ZrO2 solid acid catalysts for the preparation of polydimethylsiloxane (PDMS) via octamethylcyclotetrasiloxane (D4) ring-opening. J. Macromol. Sci. A 2019, 56, 86–95. [Google Scholar] [CrossRef]
- Owen, M.J. Silicone Surface Fundamentals. Macromol. Rapid Commun. 2021, 42, e2000360. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, N.; Xia, S.; Liu, S.; Li, Z. Phosphazene superbase catalyzed ring-opening polymerization of cyclotetrasiloxane toward copolysiloxanes with high diphenyl siloxane content. Polym. Chem. 2019, 10, 2126–2133. [Google Scholar] [CrossRef]
- Li, C.; Zhang, D.; Wu, L.; Fan, H.; Wang, D.; Li, B.-G. Ring-Opening Copolymerization of Mixed Cyclic Monomers: A Facile, Versatile and Structure-Controllable Approach to Preparing Poly(methylphenylsiloxane) with Enhanced Thermal Stability. Ind. Eng. Chem. Res. 2017, 56, 7120–7130. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Kim, Y.; Kim, H.-Y.; Lim, Y.-W.; Bae, B.-S. Sol–gel synthesized linear oligosiloxane-based hybrid material for a thermally-resistant light emitting diode (LED) encapsulant. RSC Adv. 2013, 3, 8871–8877. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, Y.; Li, Y.; Li, G.; Wang, Y.; Han, Y.; Sun, X.; Tan, X. Fabrication of Siloxane Hybrid Material with High Adhesion and High Refractive Index for Light Emitting Diodes (LEDs) Encapsulation. J. Macromol. Sci. A 2014, 51, 653–658. [Google Scholar] [CrossRef]
- Kuo, C.-F.J.; Chen, J.-B. Study on the synthesis and application of silicone resin containing phenyl group. J. Sol-Gel Sci. Technol. 2015, 76, 66–73. [Google Scholar] [CrossRef]
- Fan, X.; Cao, X.; Shang, X.; Zhang, X.; Huang, C.; Zhang, J.; Zheng, K.; Ma, Y. A transparent cyclo-linear polyphenylsiloxane elastomer integrating high refractive index, thermal stability and flexibility. Polym. Chem. 2021, 12, 5149–5158. [Google Scholar] [CrossRef]
- Ke, Q.; Dai, Z.; Chen, X. Fluorosilicone resin as a high ultraviolet transmittance optical material. J. Appl. Polym. Sci. 2020, 137, 49102. [Google Scholar] [CrossRef]
- Indulekha, K.; Mathew, A.; Rajeev, R.; Ninan, K.; Gouri, C. A facile route to fluoroalkylsiloxane polymers having resistance to corrosive acidic conditions: Synthesis and characterization. Eur. Polym. J. 2017, 97, 94–103. [Google Scholar] [CrossRef]
- Fei, H.; Han, X.; Liu, B.; Gao, X.; Wang, Q.; Zhang, Z.; Xie, Z. Synthesis of gradient copolysiloxanes by simultaneous copolymerization of cyclotrisiloxanes and mechanism for kinetics inverse between anionic and cationic ring-opening polymerization. J. Macromol. Sci. A 2016, 54, 835–843. [Google Scholar] [CrossRef]
- Meng, Y.; Chu, J.; Xue, J.; Liu, C.; Wang, Z.; Zhang, L. Design and synthesis of non-crystallizable, low-Tg polysiloxane elastomers with functional epoxy groups through anionic copolymerization and subsequent epoxidation. RSC Adv. 2014, 4, 31249–31260. [Google Scholar] [CrossRef]
- Boehm, P.; Mondeshki, M.; Frey, H. Polysiloxane-Backbone Block Copolymers in a One-Pot Synthesis: A Silicone Platform for Facile Functionalization. Macromol. Rapid Commun. 2012, 33, 1861–1867. [Google Scholar] [CrossRef]
- Thomas, D. High temperature stability in fluorosilicone vulcanisates. Polymer 1972, 13, 479–484. [Google Scholar] [CrossRef]
- Zlatanic, A.; Radojcic, D.; Wan, X.; Messman, J.M.; Dvornic, P.R. Suppression of Crystallization in Polydimethylsiloxanes and Chain Branching in Their Phenyl-Containing Copolymers. Macromolecules 2017, 50, 3532–3543. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z.; Ma, D.; Zhai, Q.; Feng, S.; Zhang, J. Preparation and kinetic analysis of room-temperature vulcanized methylethylsilicone rubbers. J. Appl. Polym. Sci. 2015, 132, 3–7. [Google Scholar] [CrossRef]
- Ji, Y.; Huang, B.; Dai, L.; Zhao, Y.; Wang, Q.; Gao, X.; Fei, H.-F.; Zhang, Z. Ternary Siloxane Copolymers Based Tough Elastomer under Ultralow Temperatures Condition. ACS Appl. Polym. Mater. 2024, 6, 8918–8928. [Google Scholar] [CrossRef]
- Filimonova, L.V.; Makarova, L.I.; Voronina, A.A.; Strelkova, T.V.; Barakovskaya, I.G.; Zavin, B.G.; Pertsin, A.J.; Papkov, V.S. Polymerization of hexaethylcyclotrisiloxane during the synthesis of carbofunctional oligo(diethylsiloxane diols). Polym. Sci. Ser. B 2013, 55, 266–270. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y.; Hao, D.; Ji, Y.; Ouyang, D. Solvation structure and molecular interactions of ibuprofen with ethanol and water: A theoretical study. Fluid Phase Equilibria 2020, 510, 112454. [Google Scholar] [CrossRef]
- Zhi-Ming, D.; Yu-Gu, L. Effects of phosphoramidothiolate pesticides on rat erythrocyte membrane acetylcholinesterase. J. Tongji Med. Univ. 1987, 7, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Bezlepkina, K.A.; Milenin, S.A.; Vasilenko, N.G.; Muzafarov, A.M. Ring-Opening Polymerization (ROP) and Catalytic Rearrangement as a Way to Obtain Siloxane Mono- and Telechelics, as Well as Well-Organized Branching Centers: History and Prospects. Polymers 2022, 14, 2408. [Google Scholar] [CrossRef]
- Tsuchihara, K.; Fujishige, S. Living polymerization of hexaethylcyclotrisiloxane. Polym. Bull. 1999, 43, 129–134. [Google Scholar] [CrossRef]
- Brewer, J.; Tsuchihara, K.; Morita, R.; Jones, J.; Bloxsidge, J.; Fujishige, S. Poly(diethylsiloxane-co-ethylphenylsiloxane) and poly(diethylsiloxane-co-methylphenylsiloxane): Synthesis and characterization. Polymer 1994, 35, 5118–5123. [Google Scholar] [CrossRef]
- Obrezkova, M.A.; Selezneva, E.V.; Demchenko, N.V.; Möller, M.; Kotov, V.M. Polydiethylsiloxane Macroinitiators for the Synthesis of Block Copolymers. Nanostruct. Mater. 2020, 2, 176–181. [Google Scholar] [CrossRef]
- Hedden, R.C.; Cohen, C. Preparation of poly(diethylsiloxane) with the NaOH/12-crown-4 catalyst. Polymer 2000, 41, 6975–6979. [Google Scholar] [CrossRef]
- Out, G.; Turetskii, A.; Snijder, M.; Möller, M.; Papkov, V. Model polydiethylsiloxane networks: 1. Synthesis and phase behaviour. Polymer 1995, 36, 3213–3221. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Long, X.; Wang, Y.; Wu, C.; Qu, Z.; Pei, Z.; Shi, C.; Wang, T.; Dong, H. Preparation of Monotrimethoxylsilylethyl-Terminated Polysiloxane Fluids and Their Application in Thermal Interface Materials. Polymers 2023, 15, 3334. [Google Scholar] [CrossRef]
- Molenberg, A.; Siffrin, S.; Möller, M.; Boileau, S.; Teyssié, D. Well defined columnar liquid crystalline polydiethylsiloxane. Macromol. Symp. 1996, 102, 199–207. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, Y.; Xu, R.; Dai, L.; Zhang, Z.; Fei, H. Kinetics and thermal properties of polydiethylsiloxane initiated by potassium trimethylsilanolate. J. Appl. Polym. Sci. 2024, 141, e55147. [Google Scholar] [CrossRef]
- Zlatanic, A.; Radojcic, D.; Wan, X.; Messman, J.M.; Bowen, D.E.; Dvornic, P.R. Dimethyl-methylphenyl copolysiloxanes by dimethylsilanolate-initiated ring opening polymerization. Evidence for linearity of the resulting polymer structures. J. Polym. Sci. 2019, 57, 1122–1129. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Zhao, N.; Li, Z. Fast synthesis of high molecular weights polydiethylsiloxanes and random poly(dimethylsiloxane-co-diethylsiloxane) copolysiloxanes via cyclic trimeric phosphazene base catalyzed ring-opening (co)polymerization. Eur. Polym. J. 2022, 173, 111280. [Google Scholar] [CrossRef]
- Kimura, H.; Kanesaka, S.; Kuroki, S.; Ando, I.; Asano, A.; Kurosu, H. Structural characterization of poly(diethylsiloxane) in the crystalline, liquid crystalline and isotropic phases by solid-state17O NMR spectroscopy andab initio MO calculations. Magn. Reson. Chem. 2005, 43, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, Q.; Long, X.; Zhang, S.; Dong, H.; Song, Y.; Wu, C. Effect of segment structure of monotrimethoxysilylethyl-terminated asymmetric Polysiloxanes on the thermal management performance of AlN-filled silicone pastes. React. Funct. Polym. 2024, 198, 105889. [Google Scholar] [CrossRef]
- Hu, Q.; Qi, H.M.; Wang, F.; Zhu, Y.P. Thermal and Oxidative Stability Thermosets Derived from Hybrids of Cyclosilazanes and Silicon-Containing Polyarylacetylene. Adv. Mater. Res. 2014, 1004, 286–292. [Google Scholar] [CrossRef]
- Guo, K.K.; Liu, Y.B.; Qi, H.M.; Huang, F.R.; Du, L. Synthesis, Characterization and Oxidation Behavior of a Novel Curable Liquid Polysilane Precursors for C/C-SiC Composite. Adv. Mater. Res. 2011, 217, 1673–1678. [Google Scholar] [CrossRef]
- Singh, U.P.; Ray, B.C. Evaluation of Thermal Analysis, Morphology And FT-IR Study of Polypropylene Filled with Wollastonite and Silicon Rubber. Int. J. Eng. Res. Sci. Technol. 2012, 1, 1–13. [Google Scholar] [CrossRef]
- Li, Y.-S.; Wright, P.B.; Puritt, R.; Tran, T. Vibrational spectroscopic studies of vinyltriethoxysilane sol–gel and its coating. Spectrochim. Acta Part A 2004, 60, 2759–2766. [Google Scholar] [CrossRef]
- Johnson, L.M.; Gao, L.; Shields, C.W.; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, J.; Zhao, N.; Li, Z. Preparation of fluorine-containing polyethylsiloxane by ring-opening polymerization of cyclic siloxanes catalyzed by phosphazene base. J. Funct. Polym. 2022, 35, 417–424. [Google Scholar] [CrossRef]
- Chojnowski, J.; Fortuniak, W.; Habimana, J.; Taylor, R. Kinetics and mechanism of oligosiloxanol condensation and oligosiloxane rearrangement catalysed with model phosphonitrile chloride catalysts. J. Organomet. Chem. 1997, 534, 105–115. [Google Scholar] [CrossRef]
- Hurwitz, F.I.; Kacik, T.A.; Bu, X.-Y.; Masnovi, J.; Heimann, P.J.; Beyene, K. Pyrolytic conversion of methyl- and vinylsilane polymers to Si-C ceramics. J. Mater. Sci. 1995, 30, 3130–3136. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Planeix, J.M.; Vioux, A. Mechanism of pyrolysis of polycarbosilanes: Poly(silylethylene) and poly(dimethylsilylethylene). Organometallics 1993, 12, 454–462. [Google Scholar] [CrossRef]
- Lindemann, N.; Schawe, J.E.K.; Lacayo-Pineda, J. Kinetics of the glass transition of styrene-butadiene-rubber: Dielectric spectroscopy and fast differential scanning calorimetry. J. Appl. Polym. Sci. 2021, 138, 49769. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Zhang, Z.; Wang, Q.; Xie, Z. Synthesis and characterization of poly(diethylsiloxane) and its copolymers with different diorganosiloxane units. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2722–2730. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Song, Y.; Wang, W.; Ren, H.; Li, Q. Gas-Generation Mechanism of Silicone Gel in High-Temperature Thermal Decomposition for High-Voltage and High-Power Device Encapsulation. IEEE Trans. Dielectr. Electr. Insul. 2024, 1–10. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Z.; Zhao, N.; Liu, S.; Li, Z. Controlled Ring-Opening Polymerization of Hexamethylcyclotrisiloxane Catalyzed by Trisphosphazene Organobase to Well-Defined Poly(dimethylsiloxane)s. Macromolecules 2022, 55, 2844–2853. [Google Scholar] [CrossRef]
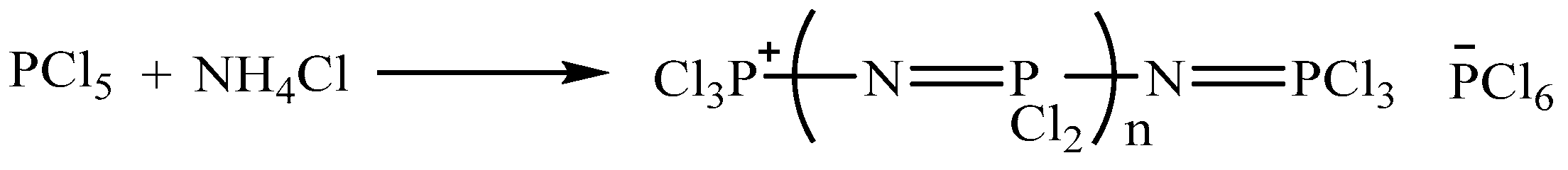

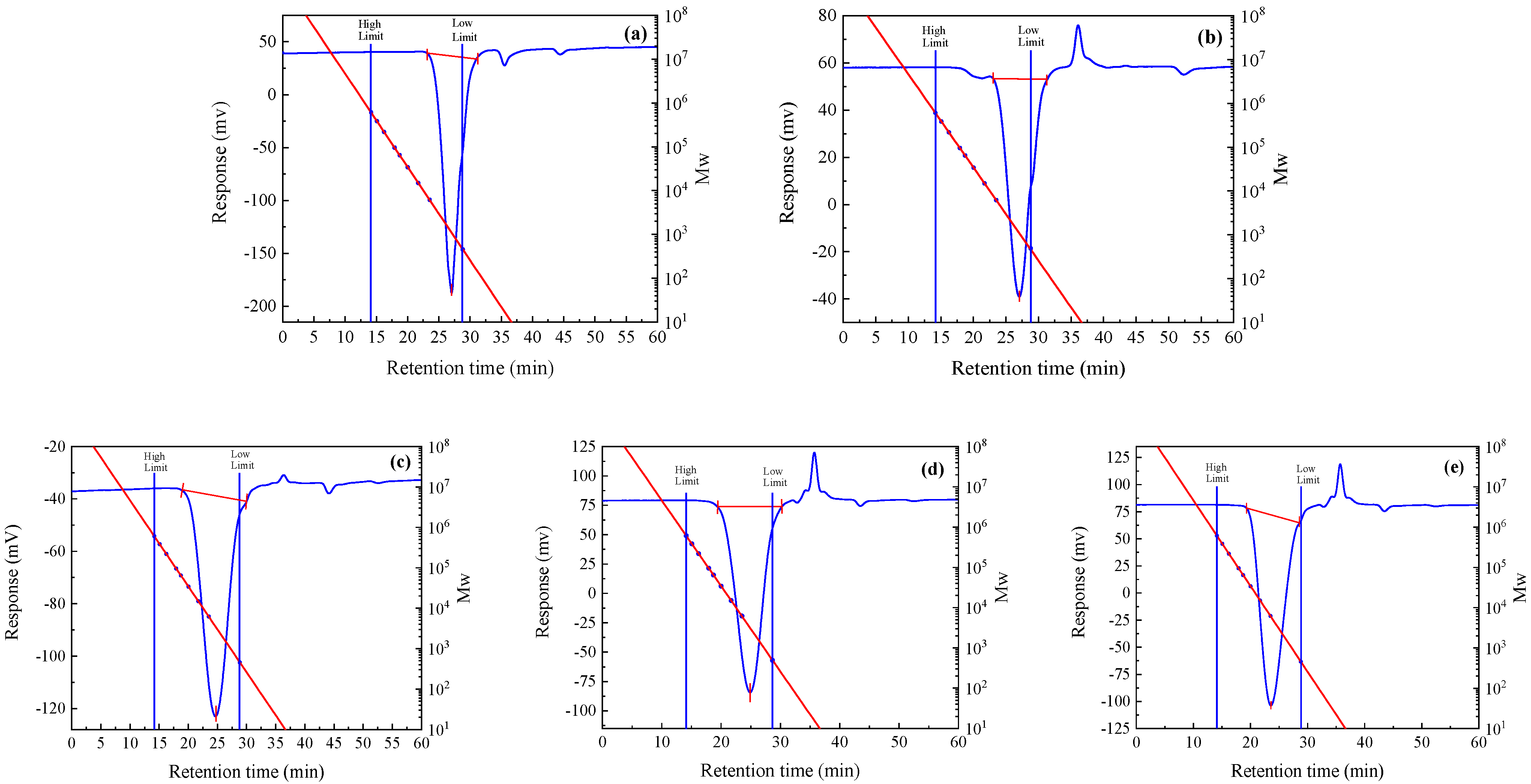


| Entry | mMHa/g | mD3Etb/g | mCatc/g | CCatd/ppm | RT/°C | t/h | mexp | η25/(mPa.s) | MNMR/(g·mol−1) | Mn/(g·mol−1) | PDI | nD25 | Yield/% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 3.13 | 21.46 | 0.4918 | 500 | 50 | 5 | 11 | 39.50 | 1256 | 900 | 1.46 | 1.4370 | 50 |
| 1B | 3.13 | 21.46 | 0.4918 | 500 | 50 | 7 | 12 | 41.00 | 1358 | 840 | 1.50 | 1.4371 | 93 |
| 1C | 3.00 | 20.54 | 0.4708 | 500 | 50 | 9 | 11 | 43.00 | 1256 | 900 | 1.46 | 1.4379 | 93 |
| 1D | 3.13 | 21.46 | 0.4918 | 500 | 50 | 11 | 11 | 45.00 | 1256 | 760 | 1.54 | 1.4371 | 86 |
| 1E | 3.09 | 21.16 | 0.1940 | 200 | 60 | 9 | 13 | 46.00 | 1460 | 1160 | 1.66 | 1.4389 | 50 |
| 1F | 3.09 | 21.16 | 0.2910 | 300 | 60 | 9 | 12 | 47.75 | 1358 | 800 | 1.42 | 1.4378 | 88 |
| 1G | 3.09 | 21.16 | 0.3880 | 400 | 60 | 9 | 12 | 44.50 | 1358 | 750 | 1.41 | 1.4371 | 66 |
| 1H | 3.09 | 21.16 | 0.4850 | 500 | 60 | 9 | 14 | 48.75 | 1562 | 920 | 1.69 | 1.4378 | 78 |
| 1I | 3.09 | 21.16 | 0.4850 | 500 | 70 | 9 | 13 | 42.75 | 1460 | 850 | 1.66 | 1.4371 | 85 |
| 1J | 3.09 | 21.16 | 0.4850 | 500 | 90 | 9 | 13 | 31.75 | 1562 | 820 | 1.63 | 1.4370 | 90 |
| Entry | mMHa/g | mD3Etb/g | mD4Hc/g | mCatd/g | CCate/ppm | RT/℃ | mexp | nexp | MNMR/(g.mol−1) | η25/(mPa.s) | Mn/(g.mol−1) | PDI | nD25 | Yield/% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2A | 2.42 | 16.56 | 8.66 | 0.5528 | 500 | 50 | 7.4 | 5.2 | 1201 | 42.00 | 3000 | 1.98 | 1.4248 | 58 |
| 2B | 2.42 | 16.56 | 8.66 | 0.5528 | 500 | 60 | 7.3 | 5.4 | 1203 | 26.00 | 1800 | 1.77 | 1.4231 | 68 |
| 2C | 2.42 | 16.56 | 8.66 | 0.4422 | 400 | 60 | 10.0 | 5.0 | 1454 | 30.25 | 2200 | 1.81 | 1.4231 | 69 |
| 2D | 2.42 | 16.56 | 8.66 | 0.3317 | 300 | 60 | 8.3 | 5.3 | 1300 | 34.00 | 2300 | 1.98 | 1.4239 | 61 |
| 2E | 2.42 | 16.56 | 8.66 | 0.2211 | 200 | 60 | 6.2 | 5.3 | 1084 | 27.25 | 1800 | 1.71 | 1.4232 | 37 |
| Entry | mMMa/g | mD3Etb/g | mD4Hc/g | mCatd/g | CCate/ppm | mexp | nexp | MNMR/(g.mol−1) | η25/(mPa.s) | Mn/(g.mol−1) | PDI | nD25 | Yield/% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3A | 3.00 | 16.43 | 8.59 | 0.5604 | 500 | 13.2 | 8.1 | 1994 | 38.75 | 2200 | 2.30 | 1.4241 | 81 |
| 3B | 2.52 | 13.80 | 7.22 | 0.3766 | 400 | 6.8 | 7.5 | 1306 | 30.25 | 1700 | 1.94 | 1.4233 | 69 |
| 3C | 2.52 | 13.80 | 7.22 | 0.2825 | 300 | 7.8 | 7.6 | 1414 | 29.25 | 1700 | 1.87 | 1.4230 | 68 |
| 3D | 2.52 | 13.80 | 7.22 | 0.1883 | 200 | 7.9 | 8.1 | 1454 | 36.00 | 2100 | 1.92 | 1.4238 | 61 |
| Entry | mMHa/g | mD3Etb/g | mD4c/g | mCatd/g | CCate/ppm | T/℃ | mexp | nexp | MNMR/(g.mol−1) | η25/(mPa.s) | Mn/(g.mol−1) | PDI | nD25 | Yield/% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4A | 2.42 | 16.56 | 10.68 | 0.5932 | 500 | 50 | 20.0 | 18.0 | 3506 | 169.4 | 3900 | 1.88 | 1.4278 | 83 |
| 4B | 2.42 | 16.56 | 10.68 | 0.5932 | 500 | 60 | 12.8 | 11.8 | 2313 | 57.75 | 1730 | 1.93 | 1.4258 | 69 |
| 4C | 2.42 | 16.56 | 10.68 | 0.4746 | 400 | 60 | 13.0 | 12.0 | 2348 | 57.00 | 1660 | 1.93 | 1.4269 | 67 |
| 4D | 2.42 | 16.56 | 10.68 | 0.3559 | 300 | 60 | 12.0 | 11.0 | 2172 | 57.00 | 1670 | 1.90 | 1.4270 | 69 |
| 4E | 2.42 | 16.56 | 10.68 | 0.2373 | 200 | 60 | 12.5 | 12.0 | 2297 | 59.75 | 1700 | 1.87 | 1.4271 | 65 |
| Entry | Si-Vi Polymera | Structure | mb/g | Si-H Polymerc | Structure | md/g | w(Si-Et)e/% | Tgf/°C | Tgg/°C | T5%h/°C |
|---|---|---|---|---|---|---|---|---|---|---|
| 5A | PDMS-273 | 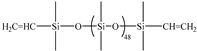 | 2.00 | UC-203-29 |  | 0.5167 | 0 | −124.5 | −122.1 | 347 |
| 5B | PDES-Vi |  | 2.00 | UC-203-29 | 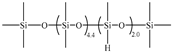 | 1.2771 | 30.43 | −131.9 | −124.2 | 341 |
| 5C | PDES-Vi | 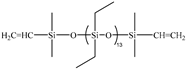 | 2.00 | UC-613-47 | 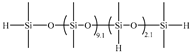 | 0.7881 | 35.77 | −132.9 | −125.3 | 326 |
| 5D | PDES-Vi | 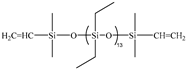 | 2.00 | Entry 2B |  | 0.6382 | 44.87 | −134.3 | −128.8 | 297 |
| 5E | PDES-Vi | 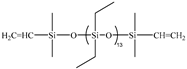 | 2.00 | Entry 3B | 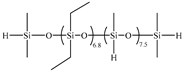 | 0.6024 | 46.44 | −138.6 | −126.9 | 312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Yang, H.; Zhang, Y.; Zhang, S.; Long, X.; Dong, H.; Song, Y.; Qu, Z.; Wu, C. Polymers Containing Diethylsiloxane Segment and Active Functional Group by Ring-Opening Polymerization of Hexaethylcyclotrisiloxane under the Catalysis of Linear Chlorinated Phosphazene Acid. Polymers 2024, 16, 2835. https://doi.org/10.3390/polym16192835
Jin C, Yang H, Zhang Y, Zhang S, Long X, Dong H, Song Y, Qu Z, Wu C. Polymers Containing Diethylsiloxane Segment and Active Functional Group by Ring-Opening Polymerization of Hexaethylcyclotrisiloxane under the Catalysis of Linear Chlorinated Phosphazene Acid. Polymers. 2024; 16(19):2835. https://doi.org/10.3390/polym16192835
Chicago/Turabian StyleJin, Chen, Hao Yang, Yang Zhang, Shuting Zhang, Xu Long, Hong Dong, Yanjiang Song, Zhirong Qu, and Chuan Wu. 2024. "Polymers Containing Diethylsiloxane Segment and Active Functional Group by Ring-Opening Polymerization of Hexaethylcyclotrisiloxane under the Catalysis of Linear Chlorinated Phosphazene Acid" Polymers 16, no. 19: 2835. https://doi.org/10.3390/polym16192835
APA StyleJin, C., Yang, H., Zhang, Y., Zhang, S., Long, X., Dong, H., Song, Y., Qu, Z., & Wu, C. (2024). Polymers Containing Diethylsiloxane Segment and Active Functional Group by Ring-Opening Polymerization of Hexaethylcyclotrisiloxane under the Catalysis of Linear Chlorinated Phosphazene Acid. Polymers, 16(19), 2835. https://doi.org/10.3390/polym16192835






