Simultaneous Optimization of Deacetylation Degree and Molar Mass of Chitosan from Shrimp Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Investigative Methods
2.2. Chitin/Chitosan Extraction Technique Protocol
2.2.1. Chitin Extraction Process
2.2.2. Optimization of the Deacetylation Step
3. Results and Discussion
3.1. Experimental Design
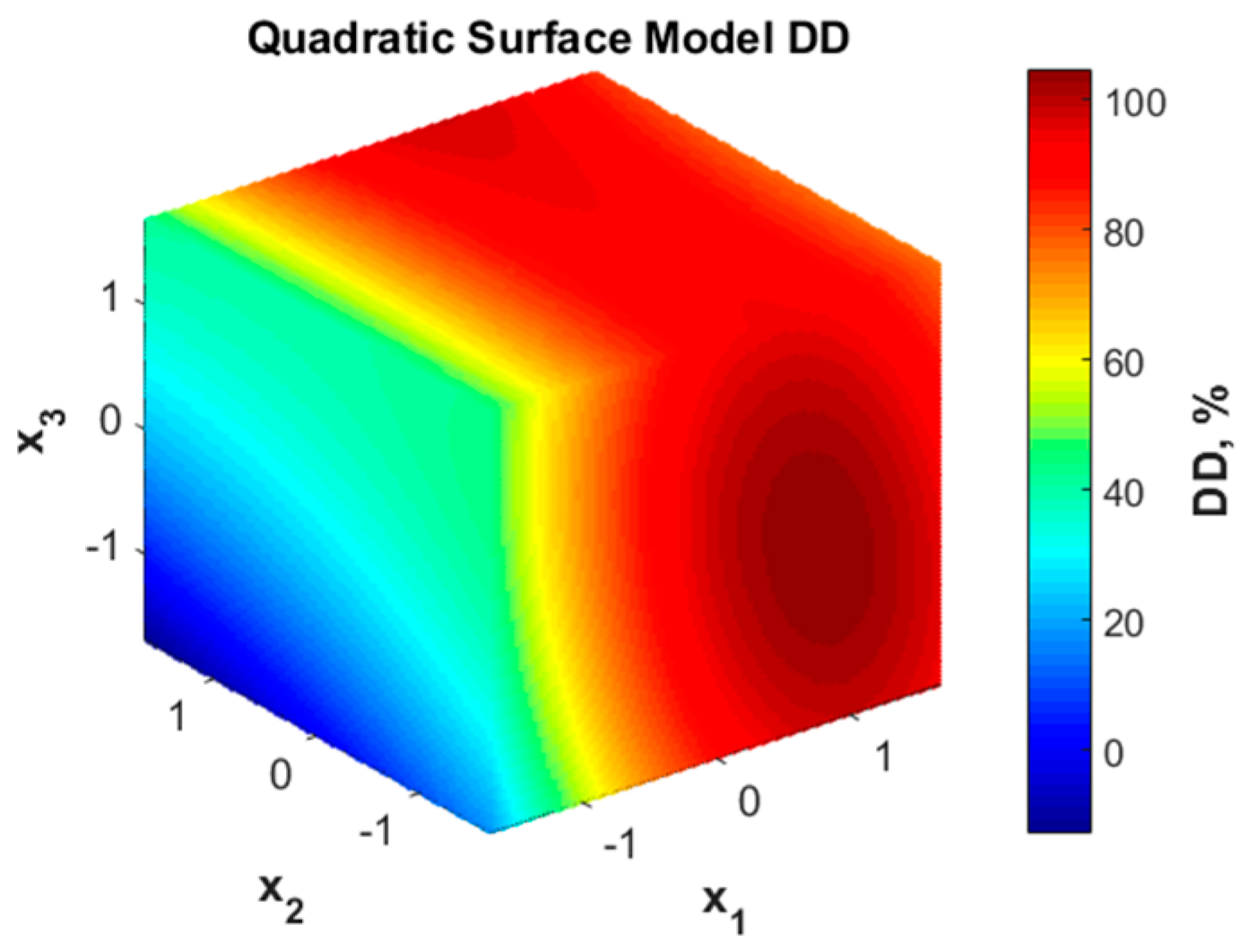
3.2. RSM Model for Deacetylation Degree (DD)
3.3. RSM Model for Molar Mass (MM)
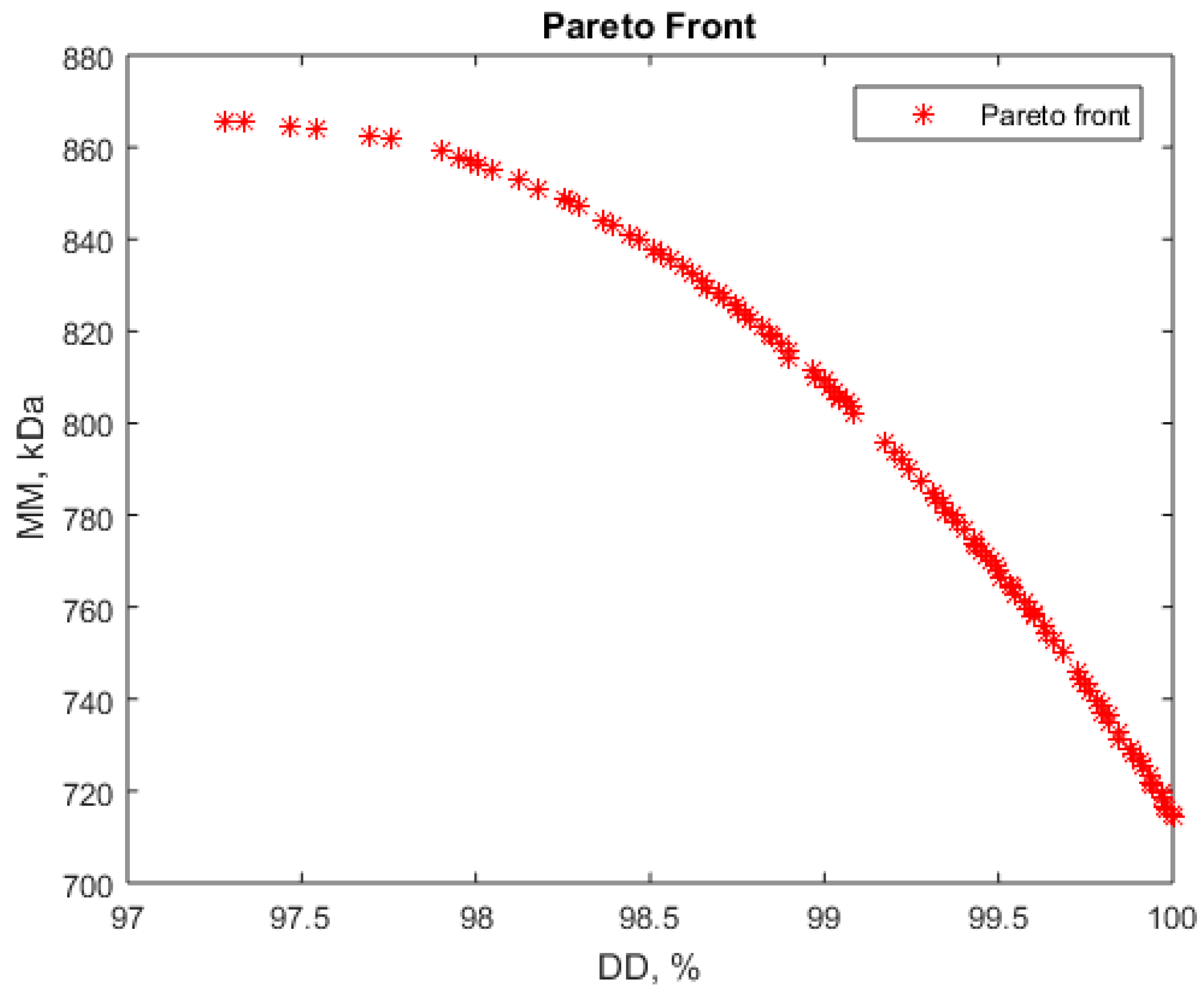
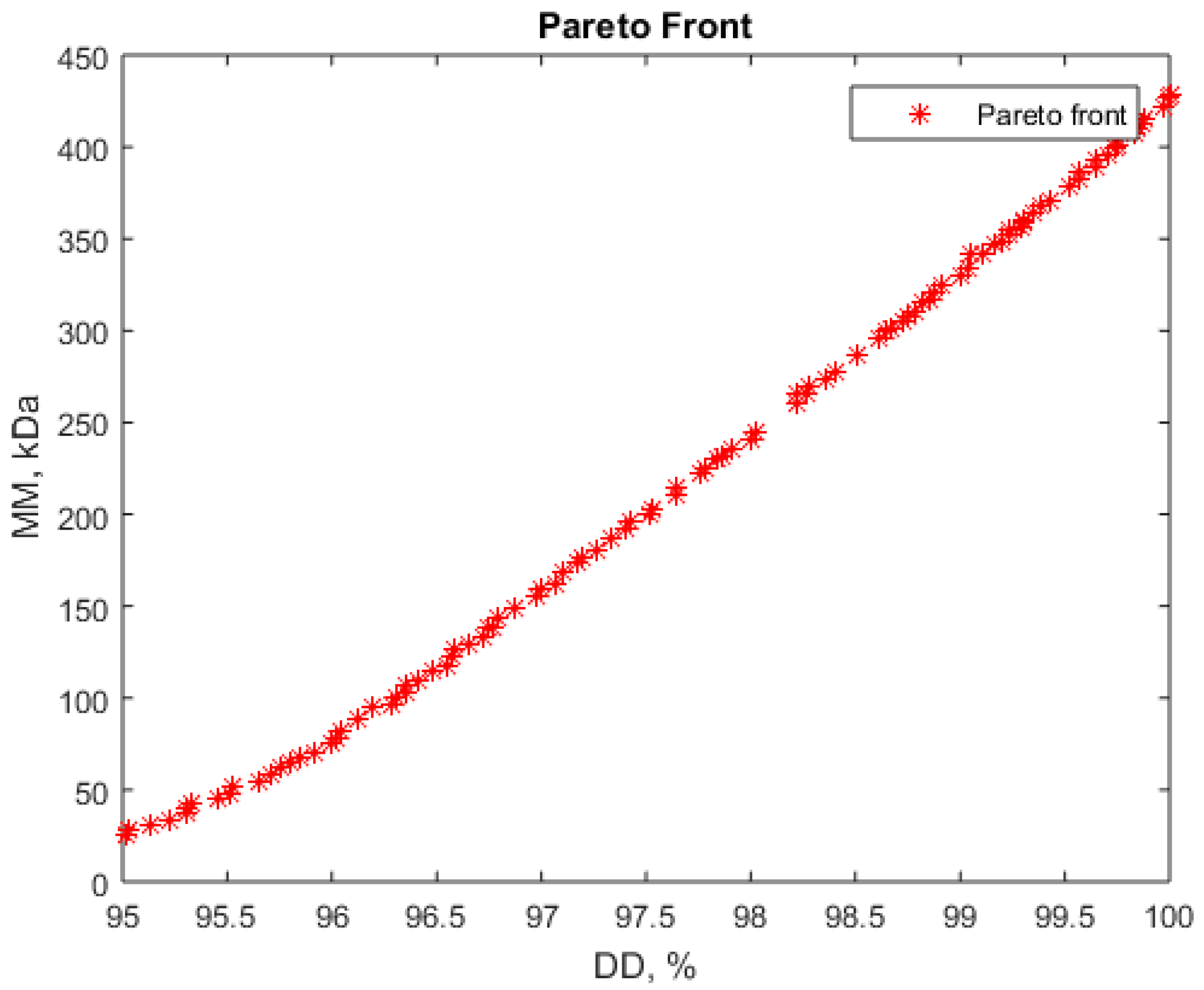
3.4. Solving Multiobjective Optimization for Chitosan Production from Shrimps
- (a)
- Maximizing deacetylation degree (DD) and maximizing molar mass (MM)
- (b)
- Maximizing deacetylation degree (DD) and minimizing molar mass (MM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ayisi, C.L.; Hua, X.; Apraku, A.; Afriyie, G.; Kyei, B.A. Recent Studies Toward the Development of Practical Diets for Shrimp and Their Nutritional Requirements. HAYATI J. Biosci. 2017, 24, 109–117. [Google Scholar] [CrossRef]
- Mesa, M.; Gil, F.; Olmedo, P.; Gil, A. Nutritional Importance of Selected Fresh Fishes, Shrimps and Mollusks to Meet Compliance with Nutritional Guidelines of n-3 LC-PUFA Intake in Spain. Nutrients 2021, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Mathew, G.M.; Mathew, D.C.; Sukumaran, R.K.; Sindhu, R.; Huang, C.C.; Binod, P.; Sirohi, R.; Kim, S.H.; Pandey, A. Sustainable and eco-friendly strategies for shrimp shell valorization. Environ. Pollut. 2020, 267, 115656. [Google Scholar] [CrossRef] [PubMed]
- Motta de Moura, C.; Motta de Moura, J.; Madeira Soares, N.; de Almeida Pinto, L.A. Evaluation of molar weight and deacetylation degree of chitosan during chitin deacetylation reaction: Used to produce biofilm. Chem. Eng. Process. Process Intensif. 2011, 50, 351–355. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Rajarajeswaran, J.; Divya, D.; Kumar, R.; Kandasamy, S.; Zhang, S.; Ganesan, A.R. β-Chitin and chitosan from waste shells of edible mollusks as a functional ingredient. Food Front. 2023, 1–27. [Google Scholar] [CrossRef]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Zairy, E.M.R. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; InTech: London, UK, 2015. [Google Scholar]
- Minh, N.C.; Hoa, N.V.; Trang, S.T. Handbook of Chitin and Chitosan; Preparation, Properties, and Application of Low-MolecularWeight Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 453–471. [Google Scholar]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Nunthanid, J.; Puttipipatkhachorn, S.; Yamamoto, K.; Peck, G.E. Physical properties, and molecular behavior of chitosan films. Drug Dev. Ind. Pharm. 2001, 27, 143–157. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Harish Prashanth, K.V.; Kittur, F.S.; Tharanathan, R.N. Solid state structure of chitosan prepared under different N-deacetylating conditions. Carbohydr. Polym. 2002, 50, 27–33. [Google Scholar] [CrossRef]
- Tsaih, M.L.; Chen, R.H. The effect of reaction time and temperature during heterogenous alkali deacetylation on degree of deacetylation and molecular weight of resulting chitosan. J. Appl. Polym. Sci. 2003, 88, 2917–2923. [Google Scholar] [CrossRef]
- Hosney, A.; Ullah, S.; Barčauskaitė, K. A Review of the Chemical Extraction of Chitosan from Shrimp Wastes and Prediction of Factors Affecting Chitosan Yield by Using an Artificial Neural Network. Mar. Drugs 2022, 20, 675. [Google Scholar] [CrossRef] [PubMed]
- Iber, B.T.; Torsabo, D.; Chick, C.E.N.C.E.; Wahab, F.; Abdullah, S.R.S.; Abu Hasan, H.; Kasan, N.A. Optimization of chitosan coagulant from dry legs of giant freshwater prawn, Macrobrachium rosenbergii in aquaculture wastewater treatment using response surface methodology (RSM). J. Environ. Chem. Eng. 2023, 11, 109761. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012, 47, 2032–2039. [Google Scholar] [CrossRef]
- Bajić, M.; Oberlintner, A.; Kõrge, K.; Likozar, B.; Novak, U. Formulation of active food packaging by design: Linking composition of the film-forming solution to properties of the chitosan-based film by response surface methodology (RSM) modelling. Int. J. Biol. Macromol. 2020, 160, 971–997. [Google Scholar] [CrossRef]
- Bello, V.E.; Olafadehan, O.A. Comparative investigation of RSM and ANN for multi-response modeling and optimization studies of derived chitosan from Archachatina marginata shell. Alex. Eng. J. 2021, 60, 3869–3899. [Google Scholar] [CrossRef]
- Pădurețu, C.C.; Isopescu, R.; Rău, I.; Apetroaei, M.R.; Schröder, V. Influence of the parameters of chitin deacetylation process on the chitosan obtained from crab shell waste. Korean J. Chem. Eng. 2019, 36, 1890–1899. [Google Scholar] [CrossRef]
- Pădurețu, C.C.; Apetroaei, M.R.; Rău, I.; Schröder, V. Characterization of chitosan extracted from different Romanian Black Sea Crustaceans. UPB Sci. Bull. B Chem. Mater. Sci. 2018, 80, 13–24. [Google Scholar]
- Apetroaei, M.R.; Manea, A.M.; Tihan, G.; Zgârian, R.; Schröder, V.; Rău, I. Improved method of chitosan extraction from different crustacean species of Romanian Black Sea coast. UPB Sci. Bull. B Chem. Mater. Sci. 2017, 79, 25–36. [Google Scholar]
- Dima, J.B.; Sequeiros, C.; Zaritzky, N. Chitosan from Marine Crustaceans: Production, Characterization and Applications. In Biological Activities and Application of Marine Polysaccharides, 1st ed.; Shalaby, E.A., Ed.; InTech Open: London, UK, 2017; pp. 39–56. [Google Scholar]
- Gîjiu, C.L.; Isopescu, R.; Dinculescu, D.; Memecică, M.; Apetroaei, M.R.; Anton, M.; Schröder, V.; Rău, I. Crabs Marine Waste—A Valuable Source of Chitosan: Tuning Chitosan Properties by Chitin Extraction Optimization. Polymers 2022, 14, 4492. [Google Scholar] [CrossRef] [PubMed]
- Pădurețu, C.C.; Isopescu, R.D.; Gîjiu, C.L.; Rău, I.; Apetroaei, M.R.; Schröder, V. Optimization of chitin extraction procedure from shrimp waste using Taguchi method and chitosan characterization. Mol. Cryst. Liq. Cryst. 2019, 695, 19–28. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Weska, R.F.; Moura, J.M.; Batista, L.M.; Rizzi, J.; Pinto, L.A.A. Optimization of deacetylation in the production of chitosan from shrimp wastes: Use of response surface methodology. J. Food Eng. 2007, 80, 749–753. [Google Scholar] [CrossRef]
- Amoo, K.O.; Olafadehan, O.A.; Ajayi, T.O. Optimization studies of chitin and chitosan production from Penaeus notialis shell waste. Afr. J. Biotechnol. 2019, 18, 670–688. [Google Scholar]
- Danarto, Y.C.; Distantina, S. Optimizing deacetylation process for chitosan production from green mussel (Perna viridis) shell. In 6th Nanoscience and Nanotechnology Symposium (NNS2015). AIP Conf. Proc. 2016, 1710, 010001. [Google Scholar]
- Hamdi, M.; Nasri, R.; Hajji, S.; Nigen, M.; Li, S.M.; Nasri, M. Acetylation degree, a key parameter modulating chitosan rheological, thermal, and film-forming properties. Food Hydrocoll. 2018, 87, 48–60. [Google Scholar] [CrossRef]
- Gamiz-González, M.A.; Correia, D.M.; Lanceros-Mendez, S.; Sencadas, V.; Gómez Ribelles, J.L.; Vidaurre, A. Kinetic study of thermal degradation of chitosan as a function of deacetylation degree. Carbohydr. Polym. 2017, 167, 52–58. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan and gelatin based biodegradable packaging films with UV-light protection. J. Photochem. Photobiol. B Biol. 2016, 163, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Li, J.; Yao, F.; Yin, Y. Chitosan Based Hydrogel: Functions and Appications, 1st ed.; CRC Taylor & Francis Group: Boca Raton, FL, USA, 2011; p. 3. [Google Scholar]
- Tokatli, K.; Demirdöven, A. Optimization of chitin and chitosan production from shrimp wastes and characterization. J. Food Proc. Preserv. 2017, 42, 13494. [Google Scholar] [CrossRef]



| Variables (Operating Parameters) | Coded Variables | Coded Levels and Actual Values | ||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||
| NaOH concentration, % | x1 | 28 | 35 | 45 | 55 | 62 |
| Liquid:solid ratio | x2 | 9.5 | 13 | 18 | 23 | 26.5 |
| Duration, min | x3 | 70 | 90 | 120 | 150 | 170 |
| Exp. Nr. | x1 | x2 | x3 | Chitosan Yield, % | DD, % | MM, kDa |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 7.36 | 62.0 | 69.83 |
| 2 | −1 | −1 | 1 | 9.63 | 75.5 | 26.39 |
| 3 | −1 | 1 | −1 | 8.41 | 52.0 | 66.43 |
| 4 | −1 | 1 | 1 | 10.09 | 62.5 | 67.04 |
| 5 | 1 | −1 | −1 | 7.76 | 100 | 363.62 |
| 6 | 1 | −1 | 1 | 8.79 | 88.5 | 663.81 |
| 7 | 1 | 1 | −1 | 7.62 | 82.95 | 541.53 |
| 8 | 1 | 1 | 1 | 8.7 | 91.5 | 825.00 |
| 9 | −1.68 | 0 | 0 | 11.57 | 27.5 | 34.10 |
| 10 | 1.68 | 0 | 0 | 9.56 | 92.0 | 612.14 |
| 11 | 0 | −1.68 | 0 | 10 | 88.5 | 475.43 |
| 12 | 0 | 1.68 | 0 | 9.32 | 93.77 | 413.22 |
| 13 | 0 | 0 | −1.68 | 11.22 | 63.33 | 155.52 |
| 14 | 0 | 0 | 1.68 | 11.5 | 96.95 | 170.50 |
| 15 | 0 | 0 | 0 | 8.88 | 96.27 | 819.99 |
| 16 | 0 | 0 | 0 | 9.43 | 86.65 | 804.33 |
| 17 | 0 | 0 | 0 | 10.94 | 88.25 | 669.64 |
| Coefficients | Standard Error | p-Value | |
|---|---|---|---|
| Intercept | 90.3432 | 4.9765 | 3.81·10−7 |
| x1 | 16.0728 | 2.3384 | 0.0002 |
| x2 | −2.0665 | 2.3384 | 0.4062 |
| x3 | 5.6821 | 2.3384 | 0.0454 |
| x1·x2 | 1.1188 | 3.0539 | 0.7249 |
| x1·x3 | −3.3688 | 3.0539 | 0.3065 |
| x2·x3 | 2.1313 | 3.0539 | 0.5078 |
| x12 | −10.6993 | 2.5761 | 0.0043 |
| x22 | 0.4207 | 2.5761 | 0.8749 |
| x32 | −3.4749 | 2.5761 | 0.2194 |
| Coefficients | Standard Error | p-Value | |
|---|---|---|---|
| Intercept | 761.5591 | 61.7843 | 5.31·10−6 |
| x1 | 229.7859 | 29.0314 | 9.76·10−5 |
| x2 | 19.9224 | 29.0314 | 0.5146 |
| x3 | 41.4797 | 29.0314 | 0.1961 |
| x1·x2 | 37.7305 | 37.9146 | 0.3528 |
| x1·x3 | 78.3116 | 37.9146 | 0.0777 |
| x2·x3 | 3.4153 | 37.9146 | 0.9307 |
| x12 | −146.0860 | 31.9832 | 0.0026 |
| x22 | −103.1420 | 31.9832 | 0.0146 |
| x32 | −202.8130 | 31.9832 | 0.0004 |
| Variables | Objective Functions | ||||||
|---|---|---|---|---|---|---|---|
| Coded Values | Real Values | DD, % | MM, kDa | ||||
| x1 | x2 | x3 | NaOH, % | Liquid/Solid Ratio | Duration, min | ||
| 0.80 | −0.10 | 0.31 | 52.97 | 17.52 | 129.33 | 97.60 | 863.66 |
| 0.86 | −0.01 | 0.25 | 53.61 | 17.96 | 127.54 | 97.18 | 865.66 |
| 0.75 | −0.29 | 0.34 | 52.53 | 16.55 | 130.18 | 98.10 | 853.78 |
| 0.78 | −0.20 | 0.29 | 52.75 | 17.02 | 128.76 | 97.83 | 860.45 |
| Variables | Objective Functions | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | Coded Values | Real Values | DD, % | MM, kDa | ||||
| x1 | x2 | x3 | NaOH, % | Liquid/Solid Ratio | Duration, min | |||
| 1 | 0.35 | −1.67 | 1.61 | 48.51 | 9.64 | 168.30 | 96.57 | 122.4 |
| 2 | 0.36 | −1.67 | 1.43 | 48.64 | 9.64 | 162.80 | 97.79 | 225.3 |
| 3 | 0.32 | −1.67 | 1.67 | 48.16 | 9.64 | 170.10 | 96.00 | 75.60 |
| 4 | 0.37 | −1.68 | 1.47 | 48.69 | 9.62 | 164.20 | 97.52 | 199.7 |
| 5 | 1.07 | −1.48 | −1.21 | 55.70 | 10.55 | 83.7 | 94.9 | 72.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinculescu, D.D.; Apetroaei, M.R.; Gîjiu, C.L.; Anton, M.; Enache, L.; Schröder, V.; Isopescu, R.; Rău, I. Simultaneous Optimization of Deacetylation Degree and Molar Mass of Chitosan from Shrimp Waste. Polymers 2024, 16, 170. https://doi.org/10.3390/polym16020170
Dinculescu DD, Apetroaei MR, Gîjiu CL, Anton M, Enache L, Schröder V, Isopescu R, Rău I. Simultaneous Optimization of Deacetylation Degree and Molar Mass of Chitosan from Shrimp Waste. Polymers. 2024; 16(2):170. https://doi.org/10.3390/polym16020170
Chicago/Turabian StyleDinculescu, Daniel Dumitru, Manuela Rossemary Apetroaei, Cristiana Luminița Gîjiu, Mirela Anton, Laura Enache, Verginica Schröder, Raluca Isopescu, and Ileana Rău. 2024. "Simultaneous Optimization of Deacetylation Degree and Molar Mass of Chitosan from Shrimp Waste" Polymers 16, no. 2: 170. https://doi.org/10.3390/polym16020170
APA StyleDinculescu, D. D., Apetroaei, M. R., Gîjiu, C. L., Anton, M., Enache, L., Schröder, V., Isopescu, R., & Rău, I. (2024). Simultaneous Optimization of Deacetylation Degree and Molar Mass of Chitosan from Shrimp Waste. Polymers, 16(2), 170. https://doi.org/10.3390/polym16020170









