Comprehensive Development of a Cellulose Acetate and Soy Protein-Based Scaffold for Nerve Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol for the Elaboration of NGC Cellulose Acetate/Soy Protein Acid Hydrolysate
- In a sterile beaker, CA was dissolved in acetone, at concentrations of 9.08, 11.3, and 14.4 wt%; the solution was mixed for 10 min with a sterile magnetic stirrer at room temperature until a clear and homogeneous solution was achieved.
- In parallel, in a sterile beaker, the SPAH was dissolved in acetone at a concentration of 25 and 30 wt%; the mixture was stirred for 30 min at room temperature until a homogeneous solution was achieved under constant UV light irradiation.
- The SPAH solution was slowly poured into the CA solution, stirring constantly for 15 min at room temperature under UV light irradiation until a homogeneous and bubble-free mixture was achieved. The final concentrations were as follows: S1, CA: 4.1 and SPAH: 16.3 wt%; S2, CA: 5.1 and SPAH: 13.6 wt%; and S3, CA: 6.5 and SPAH: 13.6 wt%.
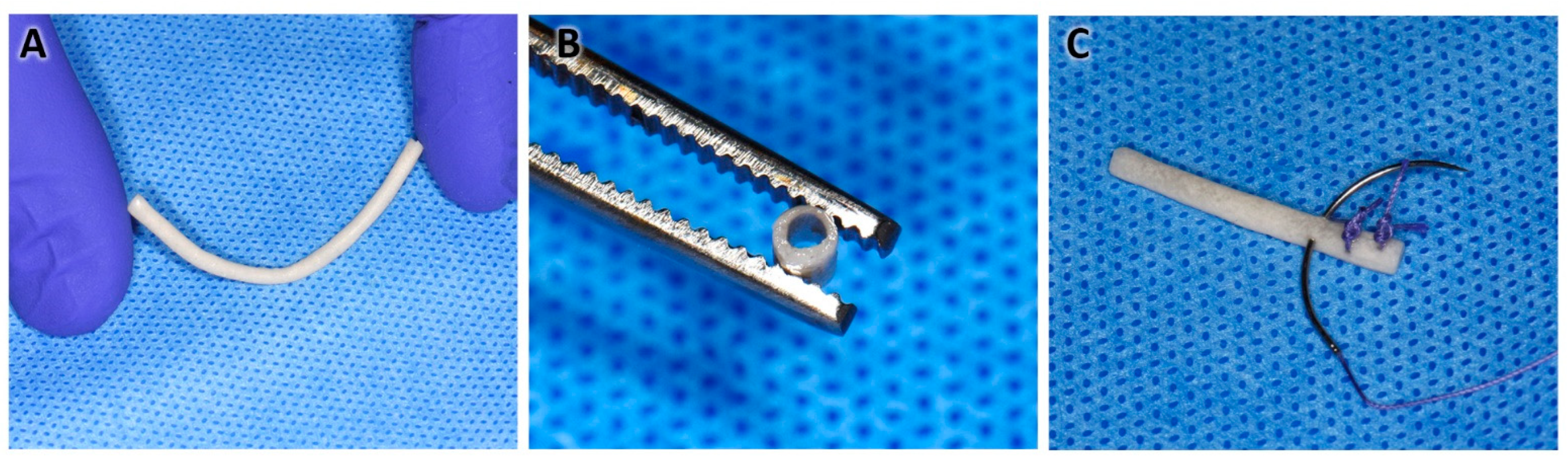
- The solution was deposited in 15 mL conical-bottom tubes/graduated cylinder (Corning, Corning, NY, USA), and before it settled, a 1.4 mm diameter galvanized rod/wire was introduced for 5 s and slowly withdrawn while making rotational movements so the polymer excess dropped off. In this way, the tubular shape of the NGC was obtained (Figure 1A).
- The above procedure was repeated 9 times, always letting it drain between each immersion. The new immersions were carried out 60 s apart to allow the complete evaporation of the acetone and integration of the layers.
- Finally, the rod with the impregnated polymers was placed in ultrapure distilled water for 5 min.
- With the help of scissors, the ends were cut, and the NGC was removed by pushing and rotating at the same time (Figure 1B,C).
- Disinfection protocol: the NGCs were submerged in 70° alcohol in 2 cycles of 30 min each (Figure 1D).
- Subsequently, the NGCs were preserved in a 1% penicillin/streptomycin antibiotic solution.
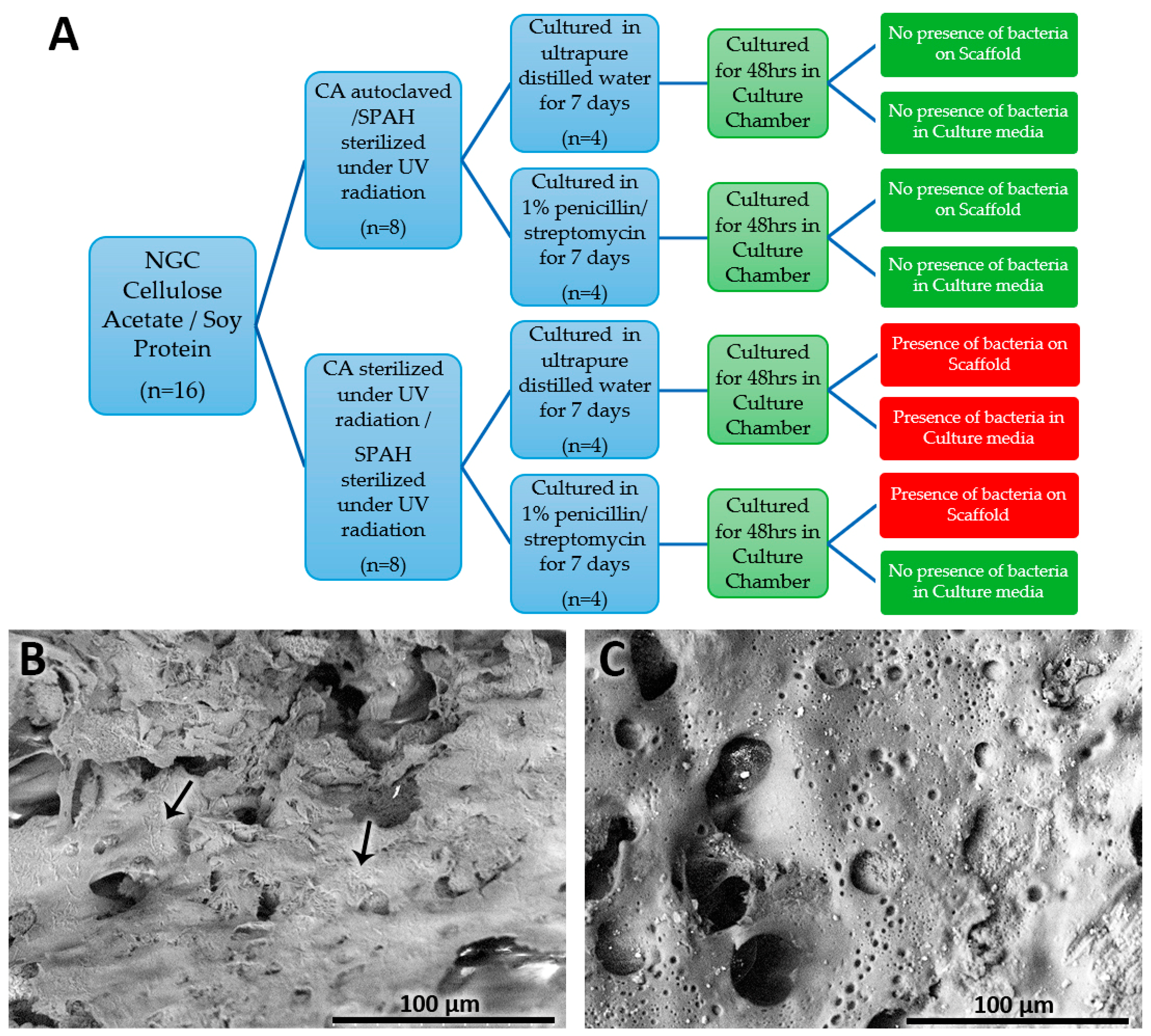
2.2. NGC Microbiological Growth Tests
2.3. NGC Structural Characterization—Macroscopic and Scanning Electron Microscopy Analyses
2.4. NGC Porosity and Surface Area—Nitrogen Adsorption and Degassing
2.5. Schwann Cell Viability Direct Biocompatibility—MTT Assay and Scanning Electron Microscopy
2.6. Statistical Analysis
3. Results
3.1. NGC Microbiological Growth Tests
3.2. NGC Structural Characterization
3.2.1. NGC Porosity and Surface Area—Liquid Nitrogen Adsorption and Degassing
3.2.2. Macroscopic Structural Characterization
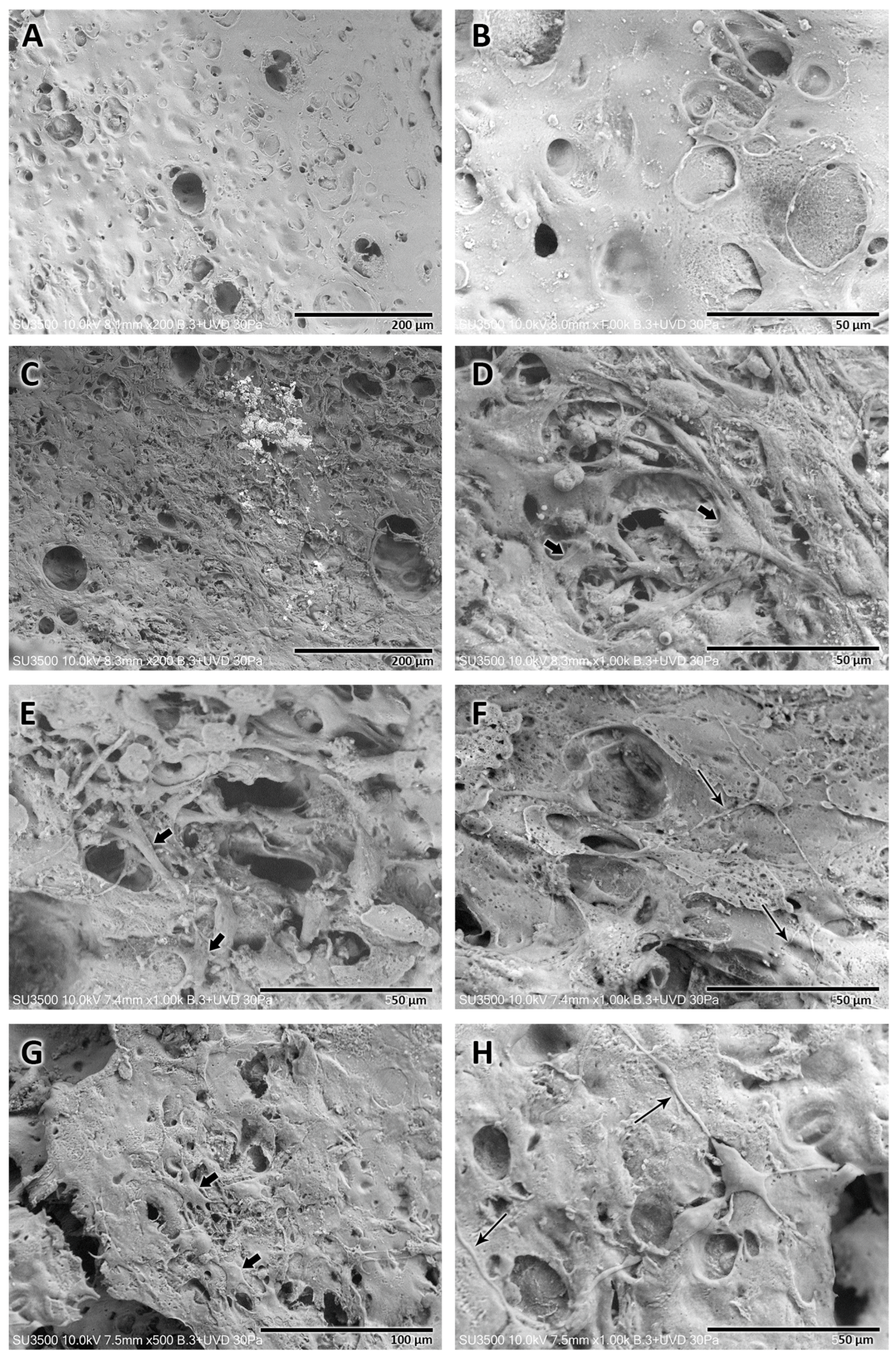
3.2.3. Microscopic Structural Characterization—Scanning Electron Microscopy
3.2.4. Microscopic NGC Morphometrics—Scanning Electron Microscopy
3.3. MTT Assay—Schwann Cell Viability/Biocompatibility
3.4. NGC Direct Cytocompatibility of Schwann Cells
4. Discussion
- I.
- Solvent casting (molding method) used cellulose/soy protein dissolved in urea/NaOH solution and poured into a polyurethane mold with a central glass rod; the coagulation was carried out with the application of acetic acid [23,31]. Another study used hydroxyethyl cellulose and isolated soy protein dissolved in NaOH solution, and the solution was poured into molds with 1.3 or 1.7 metal rods; through a freeze-drying process, the final tubular shape was achieved [28].
- II.
- Rolling layer method (coiled membrane, Figure 6B–D): This method was used before to manufacture cellulose/soy protein membranes [31]. To give it a tubular shape, the floating membrane in distilled water was taken with tweezers, and with the help of a metallic rod with a 1.4 mm diameter, it was rolled by pressing on a sterile gauze or gauze pad; the rolling method is complex, and three disadvantages were detected:
- 1.
- The membrane, still wet, is fragile, and therefore cracks and ruptures of the membrane occur, which are difficult to prevent during handling.
- 2.
- The thickness of the floating membrane cannot be estimated, so it is difficult to predict the thickness of the resulting NGC walls; in addition, water and acetone are trapped between each membrane twist.
- 3.
- As the soy protein is hydrophilic and disintegrates into islets it negatively affects the final morphology and porosity of the NGCs.
- III.
- Dip-coating method: After the failure of the aforementioned methods, the idea of granting the tubular shape by introducing a rod into the polymer solution [21] arose. When it was withdrawn intermittently from the solution, the evaporation of the solvent occurred, leaving the mixed polymers adhered to the rod; successively, in this way, the thickness of the NGC can be increased in a controlled manner. The process is practical and simple, allowing us to develop an NGC with a determined thickness and internal diameter; in addition, the resulting NGC presents relative flexibility, an ideal morphological characteristic that an NGC should possess [15]. Currently, the dip-coating manufacturing method has already been used in the manufacture of NGCs [21]; however, this process has not been reported in the literature when used with cellulose acetate functionalized with soy protein. A systematic review [21] reported that a disadvantage of the dip-coating method for the manufacture of NGCs is the impossibility of creating pores for the transport of nutrients. Our study, however, showed that the manufactured NGCs had pores on the inner face, in addition to a porous structure on the wall of the tubular structure.
4.1. Concentrations of Cellulose Acetate and Soy Protein for NGC Manufacturing
4.2. Bacterial Control of NGCs
4.3. Structural Characteristics of NGCs
4.4. Biocompatibility Evaluation of the Tubular Scaffold
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tong, Z.; Luo, L.; Zhao, Y.; Chen, F.; Li, Y.; Huselstein, C.; Ye, Q.; Ye, Q.; Chen, Y. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact. Mater. 2021, 6, 3515–3527. [Google Scholar] [CrossRef]
- Murphy, R.N.A.; de Schoulepnikoff, C.; Chen, J.H.C.; Columb, M.O.; Bedford, J.; Wong, J.K.; Reid, A.J. The incidence and management of peripheral nerve injury in England (2005–2020). J. Plast. Reconstr. Aesthet. Surg. 2023, 80, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kwon, Y.M.; Ahn, S.M.; Lee, J.H.; Lee, C.H. Epidemiology of upper extremity peripheral nerve injury in South Korea, 2008 to 2018. Medicine 2022, 101, e31655. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Z.; Pan, Y.; Chen, L.; Zhang, Z.; Lu, L. Peripheral Nerve Injuries Treatment: A Systematic Review. Cell Biochem. Biophys. 2014, 68, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Albornoz, P.; Delgado, P.J.; Forriol, F.; Maffulli, N. Non-surgical therapies for peripheral nerve injury. Br. Med. Bull. 2011, 100, 73–100. [Google Scholar] [CrossRef]
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef]
- Raimondo, S.; Fornaro, M.; Tos, P.; Battiston, B.; Giacobini-Robecchi, M.G.; Geuna, S. Perspectives in regeneration and tissue engineering of peripheral nerves. Ann. Anat.-Anat. Anz. 2011, 193, 334–340. [Google Scholar] [CrossRef]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef]
- Hasirci, V.; Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N. Peripheral nerve conduits: Technology update. Med. Devices Evid. Res. 2014, 7, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.A.; Son, Y.J. Extrinsic and intrinsic determinants of nerve regeneration. J. Tissue Eng. 2011, 2, 204173141141839. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lim, S.H.; Mao, H.Q.; Chew, S.Y. Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Apablaza, J.A.; Lezcano, M.F.; Lopez Marquez, A.; Godoy Sánchez, K.; Oporto, G.H.; Dias, F.J. Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration—A Scoping Review. Polymers 2021, 13, 2563. [Google Scholar] [CrossRef]
- Pfister, L.A.; Papaloïzos, M.; Merkle, H.P.; Gander, B. Nerve conduits and growth factor delivery in peripheral nerve repair. J. Peripher. Nerv. Syst. 2007, 12, 65–82. [Google Scholar] [CrossRef]
- Madduri, S.; Gander, B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J. Controlled Release 2012, 161, 274–282. [Google Scholar] [CrossRef]
- Lin, Y.C.; Marra, K.G. Injectable systems and implantable conduits for peripheral nerve repair. Biomed. Mater. 2012, 7, 024102. [Google Scholar] [CrossRef]
- Pierucci, A.; de Duek, E.A.R.; de Oliveira, A.L.R. Peripheral Nerve Regeneration through Biodegradable Conduits Prepared Using Solvent Evaporation. Tissue Eng. Part A 2008, 14, 595–606. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, J.R.; Kwon, G.B.; Namgung, U.; Song, K.S.; Lee, J.H. Effect of Surface Pore Structure of Nerve Guide Conduit on Peripheral Nerve Regeneration. Tissue Eng. Part C Methods 2013, 19, 233–243. [Google Scholar] [CrossRef]
- Kang, N.U.; Lee, S.J.; Gwak, S.J. Fabrication Techniques of Nerve Guidance Conduits for Nerve Regeneration. Yonsei Med. J. 2022, 63, 114. [Google Scholar] [CrossRef]
- Merle, M.; Dellon, A.L.; Campbell, J.N.; Chang, P.S. Complications from silicon-polymer intubulation of nerves. Microsurgery 1989, 10, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhao, L.; Zhao, Y.; Li, K.; Tong, Z.; Yi, L.; Wang, X.; Li, Y.; Tian, W.; He, X.; et al. Cellulose/soy protein composite-based nerve guidance conduits with designed microstructure for peripheral nerve regeneration. J. Neural Eng. 2016, 13, 056019. [Google Scholar] [CrossRef]
- Catrina, S.; Gander, B.; Madduri, S. Nerve conduit scaffolds for discrete delivery of two neurotrophic factors. Eur. J. Pharm. Biopharm. 2013, 85, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sathiyasree, M.; Rahim, S.B.A.; Renitta, R.E.; Kasipandian, K.; Krithika Shree, S.; Rajalakshmi, D.; Shobana, N.; Dhiva, S.; Abirami, S.; et al. Scaffold Using Chitosan, Agarose, Cellulose, Dextran and Protein for Tissue Engineering—A Review. Polymers 2023, 15, 1525. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Kurland, N.E.; Bano, S.; Patra, C.; Engel, F.B.; Yadavalli, V.K.; Kundu, S.C. Silk proteins for biomedical applications: Bioengineering perspectives. Prog. Polym. Sci. 2014, 39, 251–267. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Zhao, L.; Gan, L.; Yi, L.; Zhao, Y.; Xue, J.; Luo, L.; Du, Q.; Geng, R.; et al. Enhanced Peripheral Nerve Regeneration by a High Surface Area to Volume Ratio of Nerve Conduits Fabricated from Hydroxyethyl Cellulose/Soy Protein Composite Sponges. ACS Omega 2017, 2, 7471–7481. [Google Scholar] [CrossRef]
- Luo, L.H.; Zhang, Y.F.; Wang, X.M.; Wan, Y.; Chang, P.R.; Anderson, D.P.; Chen, Y. Preparation, Characterization, and In Vitro and In Vivo Evaluation of Cellulose/Soy Protein Isolate Composite Sponges. J. Biomater. Appl. 2010, 24, 503–526. [Google Scholar] [CrossRef]
- Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. [Google Scholar] [CrossRef]
- Luo, L.; Gong, W.; Zhou, Y.; Yang, L.; Li, D.; Huselstein, C.; Wang, X.; He, X.; Li, Y.; Chen, Y. Cellulose/soy protein isolate composite membranes: Evaluations of in vitro cytocompatibility with Schwann cells and in vivo toxicity to animals. Biomed. Mater. Eng. 2015, 25, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Netravali, A.N. Self-Healing Properties of Protein Resin with Soy Protein Isolate-Loaded Poly(D,L -lactide- co -glycolide) Microcapsules. Adv. Funct. Mater. 2016, 26, 4786–4796. [Google Scholar] [CrossRef]
- Sarhane, K.A.; Ibrahim, Z.; Martin, R.; Krick, K.; Cashman, C.R.; Tuffaha, S.H.; Broyles, J.M.; Prasad, N.; Yao, Z.C.; Cooney, D.S.; et al. Macroporous nanofiber wraps promote axonal regeneration and functional recovery in nerve repair by limiting fibrosis. Acta Biomater. 2019, 88, 332–345. [Google Scholar] [CrossRef]
- Romo-Valera, C.; Guerrero, P.; Arluzea, J.; Etxebarria, J.; de la Caba, K.; Andollo, N. Cytocompatibility and Suitability of Protein-Based Biomaterials as Potential Candidates for Corneal Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 3648. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, J.H.; Song, K.S.; Jeon, B.H.; Yoon, J.H.; Seo, T.B.; Namgung, U.; Lee, I.W.; Lee, J.H. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 2008, 29, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Mahto, S.K. Soy protein isolate supplemented silk fibroin nanofibers for skin tissue regeneration: Fabrication and characterization. Int. J. Biol. Macromol. 2020, 160, 112–127. [Google Scholar] [CrossRef]
- Luo, L.H.; Wang, X.M.; Zhang, Y.F.; Liu, Y.M.; Chang, P.R.; Wang, Y.; Chen, Y. Physical properties and biocompatibility of cellulose/soy protein isolate membranes coagulated from acetic aqueous solution. J. Biomater. Sci. Polym. Ed. 2008, 19, 479–496. [Google Scholar] [CrossRef]
- Zhao, Y.; He, M.; Zhao, L.; Wang, S.; Li, Y.; Gan, L.; Li, M.; Xu, L.; Chang, P.R.; Anderson, D.P.; et al. Epichlorohydrin-Cross-linked Hydroxyethyl Cellulose/Soy Protein Isolate Composite Films as Biocompatible and Biodegradable Implants for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 2781–2795. [Google Scholar] [CrossRef]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Li, C.; Gan, L.; He, M.; He, X.; Tian, W.; Li, M.; Xu, L.; Li, Y.; et al. Improvement in physical and biological properties of chitosan/soy protein films by surface grafted heparin. Int. J. Biol. Macromol. 2016, 83, 19–29. [Google Scholar] [CrossRef]



| S1. CA 4.1 and SPAH 16.3 wt% | S2. CA 5.1 and SPAH 13.6 wt% | S3. CA 6.5 and SPAH 13.6 wt% |
|---|---|---|
| Pore Volume = 0.041 cc/g | Pore Volume = 0.132 cc/g | Pore Volume = 0.074 cc/g |
| Pore Diameter Dv(d) = 3.251 nm | Pore Diameter Dv(d) = 3.249 nm | Pore Diameter Dv(d) = 3.28 nm |
| Surface Area = 40.63 m2/g | Surface Area = 146.4 m2/g | Surface Area = 76.2 m2/g |
| Material (Sterilization) | Storage Method | Wall Width (µm, Mean ± SD) | Range (µm) | |
|---|---|---|---|---|
| Min. | Max. | |||
| CA (UV rad.); SPAH (UV rad.) | ultrapure water | 256.4 ± 17.1 | 237.5 | 278.1 |
| CA (UV rad.); SPAH (UV rad.) | 1% penicillin/streptomycin | 267 ± 6.6 | 260.4 | 275.8 |
| CA (autoclave); SPAH (UV rad.) | ultrapure water | 248.5 ± 1.8 | 246.7 | 250.9 |
| CA (autoclave); SPAH (UV rad.) | 1% penicillin/streptomycin | 259.6 ± 12.1 | 249 | 273.6 |
| Material (Sterilization) | Storage Method | Major Pore Size (µm, Mean ± SD) | Range (µm) | |
|---|---|---|---|---|
| Min. | Max. | |||
| CA (UV rad.); SPAH (UV rad.) | ultrapure water | 61.1 ± 5.4 a | 54 | 66.6 |
| CA (UV rad.); SPAH (UV rad.) | 1% penicillin/streptomycin | 32.8 ± 3.7 b | 28.3 | 37.7 |
| CA (autoclave); SPAH (UV rad.) | ultrapure water | 42.1 ± 4.8 | 33.5 | 48.5 |
| CA (autoclave); SPAH (UV rad.) | 1% penicillin/streptomycin | 40.7 ± 10.1 | 27.4 | 60.1 |
| Material (Sterilization) | Storage Method | Minor Pore Size (µm, Mean ± SD) | Range (µm) | |
|---|---|---|---|---|
| Min. | Max. | |||
| CA (UV rad.); SPAH (UV rad.) | ultrapure water | 11 ± 4.4 | 5.2 | 22.1 |
| CA (UV rad.); SPAH (UV rad.) | 1% penicillin/streptomycin | 9.2 ± 5.5 | 1.9 | 23.5 |
| CA (autoclave); SPAH (UV rad.) | ultrapure water | 8.5 ± 4.3 | 2 | 23.3 |
| CA (autoclave); SPAH (UV rad.) | 1% penicillin/streptomycin | 9.2 ± 4.8 | 2.5 | 23.3 |
| Replicate 1 24–25 May 2023 | Observance 24 h (Arbitrary Units) | Observance 48 h (Arbitrary Units) | Viability (obs 24 h/obs 48 h) |
|---|---|---|---|
| Sample 1 | 0.0291 | 0.0216 | 74.23% |
| Sample 2 | 0.0062 | 0.0055 | 88.71% |
| Sample 3 | 0.0415 | 0.0257 | 61.93% |
| Mean | 0.026 | 0.018 | 68.75% |
| Replicate 2 31 May–1 June 2023 | Observance 24 h (Arbitrary Units) | Observance 48 h (Arbitrary Units) | Viability (obs 24 h/obs 48 h) |
|---|---|---|---|
| Sample 1 | 0.0479 | 0.0367 | 76.62% |
| Sample 2 | 0.0374 | 0.0304 | 81.28% |
| Sample 3 | 0.0912 | 0.0556 | 60.96% |
| Mean | 0.059 | 0.041 | 69.52% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, B.; González-Quijón, M.E.; Martínez-Rodríguez, P.; Alarcón-Apablaza, J.; Godoy, K.; Cury, D.P.; Lezcano, M.F.; Vargas-Chávez, D.; Dias, F.J. Comprehensive Development of a Cellulose Acetate and Soy Protein-Based Scaffold for Nerve Regeneration. Polymers 2024, 16, 216. https://doi.org/10.3390/polym16020216
Gutiérrez B, González-Quijón ME, Martínez-Rodríguez P, Alarcón-Apablaza J, Godoy K, Cury DP, Lezcano MF, Vargas-Chávez D, Dias FJ. Comprehensive Development of a Cellulose Acetate and Soy Protein-Based Scaffold for Nerve Regeneration. Polymers. 2024; 16(2):216. https://doi.org/10.3390/polym16020216
Chicago/Turabian StyleGutiérrez, Brandon, María Eugenia González-Quijón, Paulina Martínez-Rodríguez, Josefa Alarcón-Apablaza, Karina Godoy, Diego Pulzatto Cury, María Florencia Lezcano, Daniel Vargas-Chávez, and Fernando José Dias. 2024. "Comprehensive Development of a Cellulose Acetate and Soy Protein-Based Scaffold for Nerve Regeneration" Polymers 16, no. 2: 216. https://doi.org/10.3390/polym16020216
APA StyleGutiérrez, B., González-Quijón, M. E., Martínez-Rodríguez, P., Alarcón-Apablaza, J., Godoy, K., Cury, D. P., Lezcano, M. F., Vargas-Chávez, D., & Dias, F. J. (2024). Comprehensive Development of a Cellulose Acetate and Soy Protein-Based Scaffold for Nerve Regeneration. Polymers, 16(2), 216. https://doi.org/10.3390/polym16020216











