Enhanced Lutetium Ion Sorption from Aqueous Solutions Using Activated Ion Exchangers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
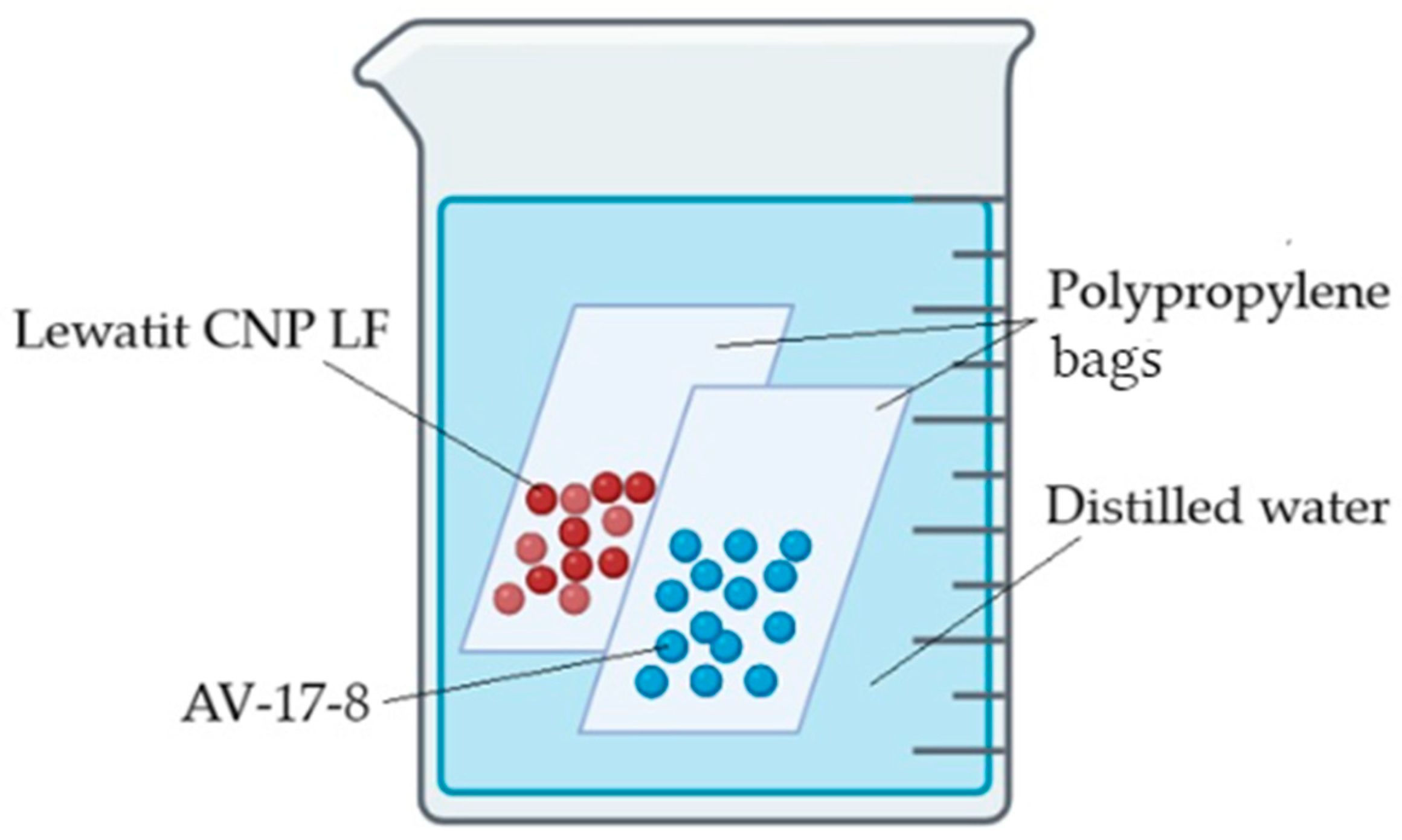
2.2. Preparation of the Interpolymer System “Lewatit CNP LF@AV-17-8” (X:Y)
2.3. Activation of the Interpolymer System “Lewatit CNP LF@AV-17-8” (X:Y)
- 1.
- The cation exchanger Lewatit CNP LF (in H+ form) dissociates in an aqueous solution according to the Scheme 1:
- 2.
- The anion exchanger AV-17-8 (in OH− form) dissociates in an aqueous solution according to the Scheme 2:
2.4. Determination of the Polymer Chain Binding Properties
2.5. Plotting a Calibration Curve
2.6. Determination of the Sorption Rate of the Lutetium Ions by the Interpolymer System “Lewatit CNP LF@AV-17-8” (X:Y)
2.7. Determination of the Desorption Rate of the Lutetium Ions from the Interpolymer System “Lewatit CNP LF@AV-17-8” (4:2)
2.8. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) Analysis
2.9. Freundlich Adsorption Isotherm Model
3. Results and Discussion
3.1. Impact of the Polymers Activation Mechanism in the Interpolymer System “Lewatit CNP LF@AV-17-8” (X:Y) on the Sorption of the Lutetium Ions
3.2. Sorption Characteristics of the Interpolymer System “Lewatit CNP LF@AV-17-8” (X:Y) in Relation to Lutetium Ions
3.3. Determination of the Polymer Chain Binding Properties
3.4. Determination of the Sorption Rate of Lutetium Ions
3.5. Kinetics of Lutetium Desorption from the Interpolymer System “Lewatit CNP LF@AV-17-8” (4:2)
3.6. ICP-OES Analysis of the Residual Concentration of Lutetium Ions
3.7. Freundlich Adsorption Model for the Interpolymer System “Lewatit CNP LF@AV-17-8” (4:2)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.-Z.; Meng, Q.; Zhang, L.; Lobont, O.-R.; Shen, Y. How do rare earth prices respond to economic and geopolitical factors? Resour. Policy 2023, 85, 103853. [Google Scholar] [CrossRef]
- Khalil, M.; Dakroury, G.A.R.S.; Borai, E.H. Efficient sorption and group separation of rare earth elements using modified CuO nanocomposite. Surf. Interfaces 2022, 33, 102233. [Google Scholar] [CrossRef]
- Majumder, A.; Pulhani, A.K.; Ghosh, A.; Singh, P.; Maiti, N. Need for enrichment of lutetium isotope and design of a laser-based separator module. Appl. Radiat. Isot. 2023, 202, 111038. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, M.; Wang, T.; Gao, X.; Shi, Y. Elaboration and luminescence of cerium-doped lutetium silicate glass-ceramics via in-situ growth from containerless processed lutetium silicate glass. J. Non-Cryst. Solids 2022, 577, 121317. [Google Scholar] [CrossRef]
- Jia, A.Y.; Kashani, R.; Zaorsky, N.G.; Baumann, B.C.; Michalski, J.; Zoberi, J.E.; Kiess, A.P.; Spratt, D.E. Lutetium-177 Prostate-Specific Membrane Antigen Therapy: A Practical Review. Pract. Radiat. Oncol. 2022, 12, 294–299. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Ponsard, B.; Sarilar, M.; Wolterbeek, B.; Denkova, A.; Serra-Crespo, P. Large-scale production of lutetium-177M for the 177m Lu/177 Lu radionuclide generator. Appl. Radiat. Isot. 2020, 156, 108986. [Google Scholar] [CrossRef]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F. Production of 177Lu for targeted radionuclide therapy: Available options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Kumar, V.; Lisok, A.; Chen, J.; Minn, I.; Brummet, M.; Boinapally, S.; Cole, M.; Ngen, E.; Wharram, B.; et al. 177Lu-labeled low-molecular-weight agents for PSMA-targeted radiopharmaceutical therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- Willowson, K.; Blanksby, A.; Emmett, L.; Lee, J.; Shin, J.; Violet, J. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar]
- Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C. Global Rare Earth Elements Projects: New Developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Jowitt, S.M.; Werner, T.T.; Weng, Z.; Mudd, G.M. Recycling of the rare earth elements. Curr. Opin. Green Sustain. Chem. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare earths and the balance problem: How to deal with changing markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Barros, Ó.; Costa, L.; Costa, F.; Lago, A.; Rocha, V.; Vipotnik, Z.; Silva, B.; Tavares, T. Recovery of rare earth elements from wastewater towards a circular economy. Molecules 2019, 24, 1005. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Erust, C.; Karacahan, M.K.; Uysal, T. Hydrometallurgical roadmaps and future strategies for recovery of rare earth elements. Miner. Process. Extr. Metall. Rev. 2023, 44, 436–450. [Google Scholar] [CrossRef]
- Jyothi, R.K.; Thenepalli, T.; Ahn, J.W.; Parhi, P.K.; Chung, K.W.; Lee, J.-Y. Review of rare earth elements recovery from sec-ondary resources for clean energy technologies: Grand Opportunities to create wealth from waste. J. Clean. Prod. 2020, 267, 122048. [Google Scholar] [CrossRef]
- Artiushenko, O.; da Silva, R.F.; Zaitsev, V. Recent advances in functional materials for rare earth recovery: A Review. Sustain. Mater. Technol. 2023, 37, e00681. [Google Scholar] [CrossRef]
- Ni, S.; Chen, Q.; Gao, Y.; Guo, X.; Sun, X. Recovery of rare earths from industrial wastewater using extraction-precipitation strategy for resource and environmental concerns. Miner. Eng. 2020, 151, 106315. [Google Scholar] [CrossRef]
- Akcil, A.; Ibrahim, Y.A.; Meshram, P.; Panda, S.; Abhilash. Hydrometallurgical recycling strategies for recovery of rare earth elements from consumer electronic scraps: A review. J. Chem. Technol. Biotechnol. 2021, 96, 1785–1797. [Google Scholar] [CrossRef]
- Dinh, T.; Dobo, Z.; Kovacs, H. Phyto mining of rare earth elements—A review. Chemosphere 2022, 297, 134259. [Google Scholar] [CrossRef]
- Chen, P.; Van Loon, L.R.; Fernandes, M.M.; Churakov, S. Sorption mechanism of Fe(II) on illite: Sorption and modelling. J. Appl. Geochem. 2022, 143, 105389. [Google Scholar] [CrossRef]
- Farrah, H.; Pickering, W.F. Extraction of heavy metal ions sorbed on clays. Water Air Soil Pollut. 1978, 9, 491–498. [Google Scholar] [CrossRef]
- Parisi, F.; Lazzara, G.; Merli, M.; Milioto, S.; Princivalle, F.; Sciascia, L. Simultaneous Removal and Recovery of Metal Ions and Dyes from Wastewater through Montmorillonite Clay Mineral. Nanomaterials 2019, 9, 1699. [Google Scholar] [CrossRef] [PubMed]
- Algamdi, M.; Alshahrani, A.; Alsuhybani, M. Chitosan grafted tetracarboxylic functionalized magnetic nanoparticles for removal of Pb(II) from an aqueous environment. Int. J. Biol. Macromol. 2023, 225, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Benettayeb, A.; Morsli, A.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Recovery of Heavy Metal Ions Using Magnetic Glycine-Modified Chitosan—Application to Aqueous Solutions and Tailing Leachate. Appl. Sci. 2021, 11, 8377. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Mafhouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E. Cysteine-Functionalized Chitosan Magnetic Nano-Based Particles for the Recovery of Light and Heavy Rare Earth Metals: Uptake Kinetics and Sorption Isotherms. Nanomaterials 2015, 5, 154–179. [Google Scholar] [CrossRef] [PubMed]
- Kerimkulova, A.R.; Azat, S.; Velasco, L.; Mansurov, Z.A.; Lodewyckx, P.; Tulepov, M.I.; Kerimkulova, M.R.; Berezovskaya, I.; Imangazy, A. Granular rice husk-based sorbents for sorption of vapors of organic and inorganic matters. J. Chem. Technol. Metall. 2019, 54, 578–584. Available online: https://journal.uctm.edu/node/j2019-3/16_18-55_p_578-584.pdf (accessed on 10 December 2023).
- Cardoso, C.E.; Almeida, J.C.; Lopes, C.B.; Trindade, T.; Vale, C.; Pereira, E. Recovery of rare earth elements by carbon-based nanomaterials—A review. Nanomaterials 2019, 9, 814. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Senthilkumar, P.; Thamaraiselvi, K.; Subburam, V. Activated carbon prepared from biomass as adsorbent: Elimination of Ni (II) from aqueous solution. Bioresour. Technol. 2002, 81, 87–90. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Ismailova, A.G.; Akanova, G.Z.; Tassibekov, K.S.; Kamysbayev, D.K.; Yermagambet, B.T.; Kazankapova, M.K.; Kassenova, Z.M. Sorption of neodymium from nitrate solutions by humic acids-based sorbent. Eurasian Chem. Technol. J. 2022, 24, 313–321. [Google Scholar] [CrossRef]
- Kammerlander, K.K.; Köhler, L.; Huittinen, N.; Bok, F.; Steudtner, R.; Oschatz, C.; Vogel, M.; Stumpf, T.; Brunner, E. Sorption of europium on diatom biosilica as model of a “green” sorbent for F-elements. Appl. Geochemistry 2021, 126, 104823. [Google Scholar] [CrossRef]
- Seidi, F.; Reza Saeb, M.; Huang, Y.; Akbari, A.; Xiao, H. Thiomers of chitosan and cellulose: Effective biosorbents for detection, removal and recovery of metal ions from aqueous medium. Chem. Rec. 2021, 21, 1876–1896. [Google Scholar] [CrossRef] [PubMed]
- Jumadilov, T.; Malimbayeva, Z.; Khimersen, K.; Saparbekova, I.; Imangazy, A.; Suberlyak, O. Specific features of praseodymium extraction by intergel system based on polyacrylic acid and poly-4-vinylpyridine hydrogels. Bull. Karaganda Univ. Chem. Ser. 2021, 103, 53–59. [Google Scholar] [CrossRef]
- Jumadilov, T.; Totkhuskyzy, B.; Imangazy, A.; Gražulevičius, J. Anomalous sorption of yttrium ions by the mutual activated hydrogels in the interpolymer system of poly(methacrylic acid) and poly(4-vinylpyridine). Chem. Chem. Technol. 2023, 17, 52–59. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z. Synthesis and characterization of poly (HEA/MALA) hydrogel and its application in removal of heavy metal ions from water. J. Chem. Eng. 2013, 215, 894–902. [Google Scholar] [CrossRef]
- Jumadilov, T.; Kondaurov, R.; Imangazy, A.; Myrzakhmetova, N.; Saparbekova, I. Phenomenon of remote interaction and sorption ability of rare cross-linked hydrogels of polymethacrylic acid and poly-4-vinylpyridine in relation to erbium ions. Chem. Chem. Technol. 2019, 13, 451–458. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, M.J. Removal of heavy metal ions by poly (vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze-thaw method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Miller, D.D.; Siriwardane, R.; McIntyre, D. Anion structural effects on interaction of rare earth element ions with Dowex 50W X8 cation exchange resin. J. Rare Earths 2018, 36, 879–890. [Google Scholar] [CrossRef]
- Jumadilov, T.; Yskak, L.; Imangazy, A.; Suberlyak, O. Ion Exchange Dynamics in Cerium Nitrate Solution Regulated by Remotely Activated Industrial Ion Exchangers. Materials 2021, 14, 3491. [Google Scholar] [CrossRef]
- Jumadilov, T.; Khimersen, K.; Haponiuk, J. Influence of Initial States on the Electrochemical Behavior of Industrial Ionites in the Interpolymer System Lewatit CNP LF-AB-17-8. In Advanced Polymer Structures: Chemistry for Engineering Applications, 1st ed.; Mukbaniani, O., Tatrishvili, T., Abadie, M.J., Eds.; Apple Academic Press: Goldenrod, FL, USA, 2023; 544p. [Google Scholar] [CrossRef]
- Kaith, B.S. Removal of hazardous metal ions from polluted water using biomaterial-based ion-exchangers: A review. Mater. Today Proc. 2022, 53, 174–178. [Google Scholar] [CrossRef]
- Vorobyev, P.; Mikhailovskaya, T.; Yugay, O.; Serebryanskaya, A.; Chukhno, N.; Imangazy, A. Catalytic Oxidation of 4-Methylpyridine on Modified Vanadium Oxide Catalysts. Iran. J. Chem. Chem. Eng. 2018, 37, 81–89. [Google Scholar] [CrossRef]
- Karami, Z.; Ganjali, M.R.; Zarghami Dehaghani, M.; Aghazadeh, M.; Jouyandeh, M.; Esmaeili, A.; Habibzadeh, S.; Mohaddespour, A.; Inamuddin; Formela, K.; et al. Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides. Polymers 2020, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Imangazy, A.; Smagulova, G.; Kaidar, B.; Mansurov, Z.; Kerimkulova, A.; Umbetkaliev, K.; Zakhidov, A.; Vorobyev, P.; Jumadilov, T. Compositional Fibers Based on Coal Tar Mesophase Pitch Obtained by Electrospinning Method. Chem. Chem. Technol. 2021, 15, 403–407. [Google Scholar] [CrossRef]
- Yelemessova, Z.; Imangazy, A.; Tulepov, M.; Mansurov, Z. Energetic Metal–Organic Frameworks: Thermal Behaviors and Combustion of Nickel Oxide (II) Based on Activated Carbon Compositions. J. Eng. Phys. Thermophys. 2021, 94, 804–811. [Google Scholar] [CrossRef]
- Kamunur, K.; Jandosov, J.M.; Abdulkarimova, R.G.; Hori, K.; Yelemessova, Z.K. Combustion study of different transitional metal oxide based on AN/MgAl composites gas generators. Eurasian Chem. Technol. J. 2017, 19, 341. [Google Scholar] [CrossRef]
- Yelemessova, Z.; Kydyrbekova, S.; Yerken, A. Thermal Characteristics Enhancement of AN/Mg/NC Composite Using Activated Carbon/Cobalt Oxide as Highly Effective Catalytic Additive. J. Compos. Sci. 2023, 7, 471. [Google Scholar] [CrossRef]
- Hermassi, M.; Granados, M.; Valderrama, C.; Skoglund, N.; Ayora, C.; Cortina, J.L. Impact of functional group types in ion exchange resins on rare earth element recovery from treated acid mine waters. J. Clean. Prod. 2022, 379, 134742. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Prabhakaran, D.; Subramanian, M.S. A new chelating sorbent for metal ion extraction under high saline conditions. Talanta 2003, 59, 1227–1236. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. An overview of rare-earth recovery by ion-exchange leaching from ion-adsorption clays of various origins. Mineral. Mag. 2016, 80, 63–76. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Traore, M.; Gong, A.; Wang, Y.; Qiu, L.; Bai, Y.; Zhao, W.; Chen, Y.; Liu, Y.; Wu, H.; Li, S. Research progress of rare earth separation methods and tech-nologies. J. Rare Earths 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Bekturganova, G.; Jumadilov, T.; Bekturov, E. Electroconductivity and viscosity of complexes of poly(vinylpyridines) with alkali metal salts in organic solvents. Macromol. Chem. Phys. 1996, 197, 105–111. [Google Scholar] [CrossRef]
- Bekturov, E.; Tolendina, A.; Shaikhutdinov, Y.; Dzhumadilov, T. Complexation of poly(ethylene glycol) with some salts of alkali-earth metals. Polym. Adv. Technol. 1993, 4, 564–566. [Google Scholar] [CrossRef]
- Jumadilov, T.K.; Yermukhambetova, B.; Panchenko, S.; Suleimenov, I. Long-distance electrochemical interactions and anomalous ion exchange phenomenon. AASRI Procedia 2012, 3, 553–558. [Google Scholar] [CrossRef]
- Imangazy, A.; Jumadilov, T.; Khimersen, K.; Bayshibekov, A. Enhanced sorption of europium and scandium ions from nitrate solutions by remotely activated Ion Exchangers. Polymers 2023, 15, 1194. [Google Scholar] [CrossRef]
- Haddad, P.R. Ion Exchange Overview. In Encyclopedia of Analytical Science, 2nd ed.; Elsevier Academic: San Diego, CA, USA, 2005; pp. 440–446. [Google Scholar] [CrossRef]
- Amid, P.K.; Shulman, A.G.; Lichtenstein, I.L.; Sostrin, S.; Young, J.; Hakakha, M. Experimental evaluation of a new composite mesh with the selective property of incorporation to the abdominal wall without adhering to the intestines. J. Biomed. Mater. Res. 1994, 28, 373–375. [Google Scholar] [CrossRef]
- Jumadilov, T.K.; Imangazy, A.M.; Khimersen, K.; Haponiuk, J.T. Remote interaction effect of industrial ion exchangers on the electrochemical and sorption equilibrium in scandium sulfate solution. Polym. Bull. 2023, (article in press). [Google Scholar] [CrossRef]
- Jumadilov, T.; Utesheva, A.; Grazulevicius, J.; Imangazy, A. Selective Sorption of Cerium Ions from Uranium-Containing Solutions by Remotely Activated Ion Exchangers. Polymers 2023, 15, 816. [Google Scholar] [CrossRef]
- Matharu, K.; Mittal, S.K.; Kumar, S.A.; Sahoo, S.K. Selectivity enhancement of Arsenazo (III) reagent towards heavier lan-tha-nides using polyaminocarboxylic acids: A spectrophotometric study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Anu, N.; Giri Nandagopal, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Ahmed Adam, O.E.-A.; Al-Dujaili, A.H. The Removal of Phenol and Its Derivatives from Aqueous Solutions by Adsorption on Petroleum Asphaltene. J. Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Vassileva, P.S.; Radoykova, T.H.; Detcheva, A.K.; Avramova, I.A.; Aleksieva, K.I.; Nenkova, S.K.; Valchev, I.V.; Mehandjiev, D.R. Adsorption of Ag+ ions on hydrolyzed lignocellulosic materials based on willow, paulownia, wheat straw and maize stalks. Int. J. Environ. Sci. Technol. 2016, 13, 1319–1328. [Google Scholar] [CrossRef]
- Alfarra, A.; Frackowiak, E.; Béguin, F. The HSAB concept as a means to interpret the adsorption of metal ions onto activated carbons. Appl. Surf. Sci. 2004, 228, 84–92. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Machlin, E.S.; Weinig, S. Le Chatelier’s principle and stress-induced displacive transformations. Acta Metall. 1953, 1, 480–482. [Google Scholar] [CrossRef]
- Joos, J.J.; Poelman, D.; Smet, P.F. Energy level modeling of lanthanide materials: Review and uncertainty analysis. Phys. Chem. Chem. Phys. 2015, 17, 19058–19078. [Google Scholar] [CrossRef]
- Stechynska, E.; Vasylechko, V.; Gryshchouk, G.; Patsay, I. Preconcentration of Lutetium from Aqueous Solution by Transcarpathian Clinoptilolite. Acta Chim. Slov. 2020, 67, 105–112. [Google Scholar] [CrossRef]
- Mohammedi, H.; Miloudi, H.; Tayeb, A.; Bertagnolli, C.; Boos, A. Study on the extraction of lanthanides by a mesoporous MCM-41 silica impregnated with Cyanex 272. Sep. Purif. Technol. 2019, 209, 359–367. [Google Scholar] [CrossRef]
- Awual, M.R.; Alharthi, N.H.; Okamoto, Y.; Karim, M.R.; Halim, M.E.; Hasan, M.M.; Rahman, M.M.; Islam, M.M.; Khaleque, M.A.; Sheikh, M.C. Ligand field effect for Dysprosium(III) and Lutetium(III) adsorption and EXAFS coordination with novel composite nanomaterials. Chem. Eng. J. 2017, 320, 427–435. [Google Scholar] [CrossRef]
- Hasan, M.N.; Salman, M.S.; Hasan, M.M.; Kubra, K.T.; Sheikh, M.C.; Rehan, A.I.; Rasee, A.I.; Awual, M.E.; Waliullah, R.M.; Hossain, M.S.; et al. Assessing sustainable Lutetium(III) ions adsorption and recovery using novel composite hybrid nanomaterials. J. Mol. Struct. 2023, 1276, 134795. [Google Scholar] [CrossRef]








| Molar Ratio of Lewatit CNP LF@AV-17-8 (X:Y) | Mass Ratio of Lewatit CNP LF@AV-17-8 (X:Y) a |
|---|---|
| 6:0 | 0.200 g:0.000 g |
| 5:1 | 0.170 g:0.025 g |
| 4:2 | 0.140 g:0.050 g |
| 3:3 | 0.100 g:0.075 g |
| 2:4 | 0.070 g:0.100 g |
| 1:5 | 0.035 g:0.125 g |
| 0:6 | 0.000 g:0.150 g |
| Molar Ratio of Lewatit CNP LF@AV-17-8 (X:Y) | Mass Ratio of Lewatit CNP LF@AV-17-8 (X:Y) a | θ after 1 h | θ after 6 h | θ after 24 h | θ after 48 h |
|---|---|---|---|---|---|
| 6:0 | 0.200 g:0.000 g | 0.08% | 0.08% | 1.54% | 2.23% |
| 5:1 | 0.170 g:0.025 g | 0.41% | 0.54% | 2.04% | 2.95% |
| 4:2 | 0.140 g:0.050 g | 0.15% | 1.18% | 2.60% | 3.91% |
| 3:3 | 0.100 g:0.075 g | 0.06% | 0.47% | 2.00% | 3.26% |
| 2:4 | 0.070 g:0.100 g | 0.16% | 0.95% | 1.74% | 3.32% |
| 1:5 | 0.035 g:0.125 g | 0.06% | 0.64% | 1.68% | 3.24% |
| 0:6 | 0.000 g:0.150 g | 0.01% | 0.17% | 0.91% | 2.12% |
| Sorbents | pH Range | Optimum pH | Maximum Sorption Capacity (mg/g) | References |
|---|---|---|---|---|
| Transcarpathian clinoptilolite | 4.0–13.0 | 10.0 | 9.37 | [71] |
| Mesoporous MCM-41 silica impregnated with Cyanex 272 | 1.0–3.0 | 2.5 | 44.00 | [72] |
| Alumina–silica-based composite | 1.0–4.0 | 4.0 | 129.77 | [73] |
| Organic ligand-based composite hybrid material | 4.0–5.0 | 4.0 | 171.76 | [74] |
| Interpolymer system “Lewatit CNP LF@AV-17-8” (4:2) | 4.0–5.0 | 4.7 | 221.05 | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumadilov, T.; Khimersen, K.; Haponiuk, J.; Totkhuskyzy, B. Enhanced Lutetium Ion Sorption from Aqueous Solutions Using Activated Ion Exchangers. Polymers 2024, 16, 220. https://doi.org/10.3390/polym16020220
Jumadilov T, Khimersen K, Haponiuk J, Totkhuskyzy B. Enhanced Lutetium Ion Sorption from Aqueous Solutions Using Activated Ion Exchangers. Polymers. 2024; 16(2):220. https://doi.org/10.3390/polym16020220
Chicago/Turabian StyleJumadilov, Talkybek, Khuangul Khimersen, Józef Haponiuk, and Bakytgul Totkhuskyzy. 2024. "Enhanced Lutetium Ion Sorption from Aqueous Solutions Using Activated Ion Exchangers" Polymers 16, no. 2: 220. https://doi.org/10.3390/polym16020220






