An Evaluation of the Wear Resistance of Electroplated Nickel Coatings Composited with 2,2,6,6-Tetramethylpiperidine 1-oxyl-Oxidized Cellulose Nanofibers
Abstract
:1. Introduction
- (1)
- Ni–silicon carbide (SiC) composite film—The production of SiC ingots is reported to consume 7000 kWh/ton of electricity [27], whereas, for example, cellulose nanofiber production using the 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) oxidation method [28,29,30] is reported to consume 100–500 kWh/ton [31]. Further, since plants, as raw materials, are a renewable resource, it can be said that Ni–cellulose nanofiber composite films have an advantage in terms of sustainability.
- (2)
- Ni–polytetrafluoroethylene (PTFE) composite film—Since PTFE has very strong water repellency, a strong surfactant is required to disperse it in the plating solution. Conventionally, perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) have been used for this function, but both are regulated by the Stockholm Convention on Persistent Organic Pollutants (POPs Convention) because they are refractory substances with strong toxicity [32,33]. In its original state, cellulose has hydroxy groups on its surface, making it a hydrophilic substance, and CNF disperses well in the plating solution without the use of special surfactants.
- (3)
- Ni electroless-plated film—Compared to a Ni electroplating solution, a Ni electroless plating solution has a shorter bath life, and it contains phosphate as a reducing agent and prevents bath decomposition, which makes waste solution treatment difficult [34,35,36]. Although the development of plating solutions without lead, which is restricted by the hazardous substances directive (RoHS), and waste solution recycling technologies are in progress, Ni electroplating has an advantage in terms of environmental protection [36]. In addition, in Ni electroless plating, it is more difficult to manage the bath, and it is more expensive to process. Ni electroplating is also easier to apply in developing countries, where facilities are not fully developed.
- (4)
- Hard chromium plating—Hard chromium (Cr) plating is an extremely hard and wear-resistant plated film obtained via electroplating. Its hardness reaches over 800 HV, and it is used for many sliding parts, such as the pistons and cylinder liners of automobile engines, steel-rolling rolls, shafts, and rods [37,38,39]. Hard Cr plating baths have conventionally contained hexavalent chromium, which is restricted by RoHS, and there is an urgent need to replace it. Decorative Cr plating with a trivalent chromium bath, which is not restricted, is widely used, but the hard Cr film obtained from this bath still has many problems in terms of adhesion, workability, wear resistance, etc. [38,39,40].
2. Experimental Procedure
2.1. Plating Process
- ;
- ;
- ;
- ;
- ;
- ).

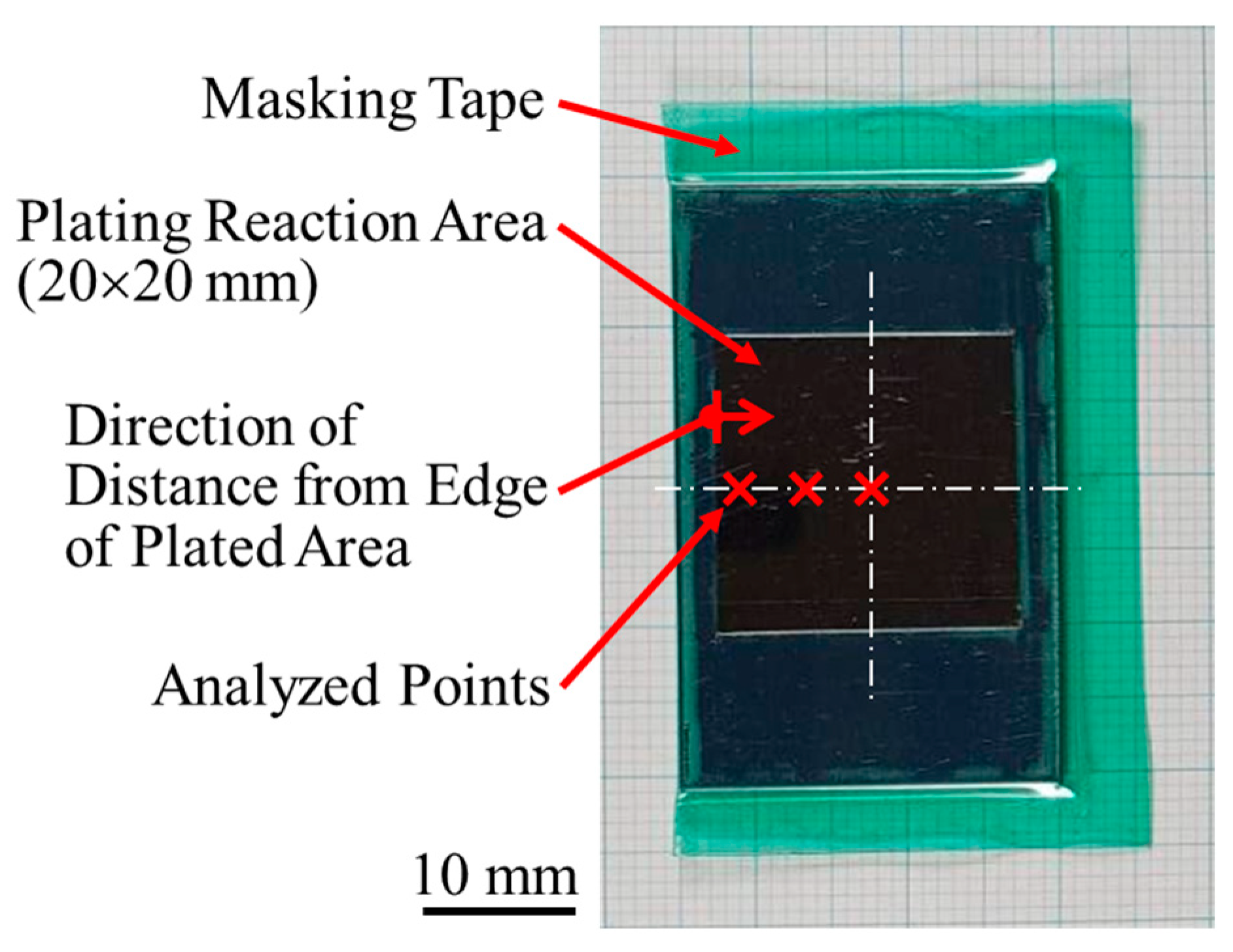
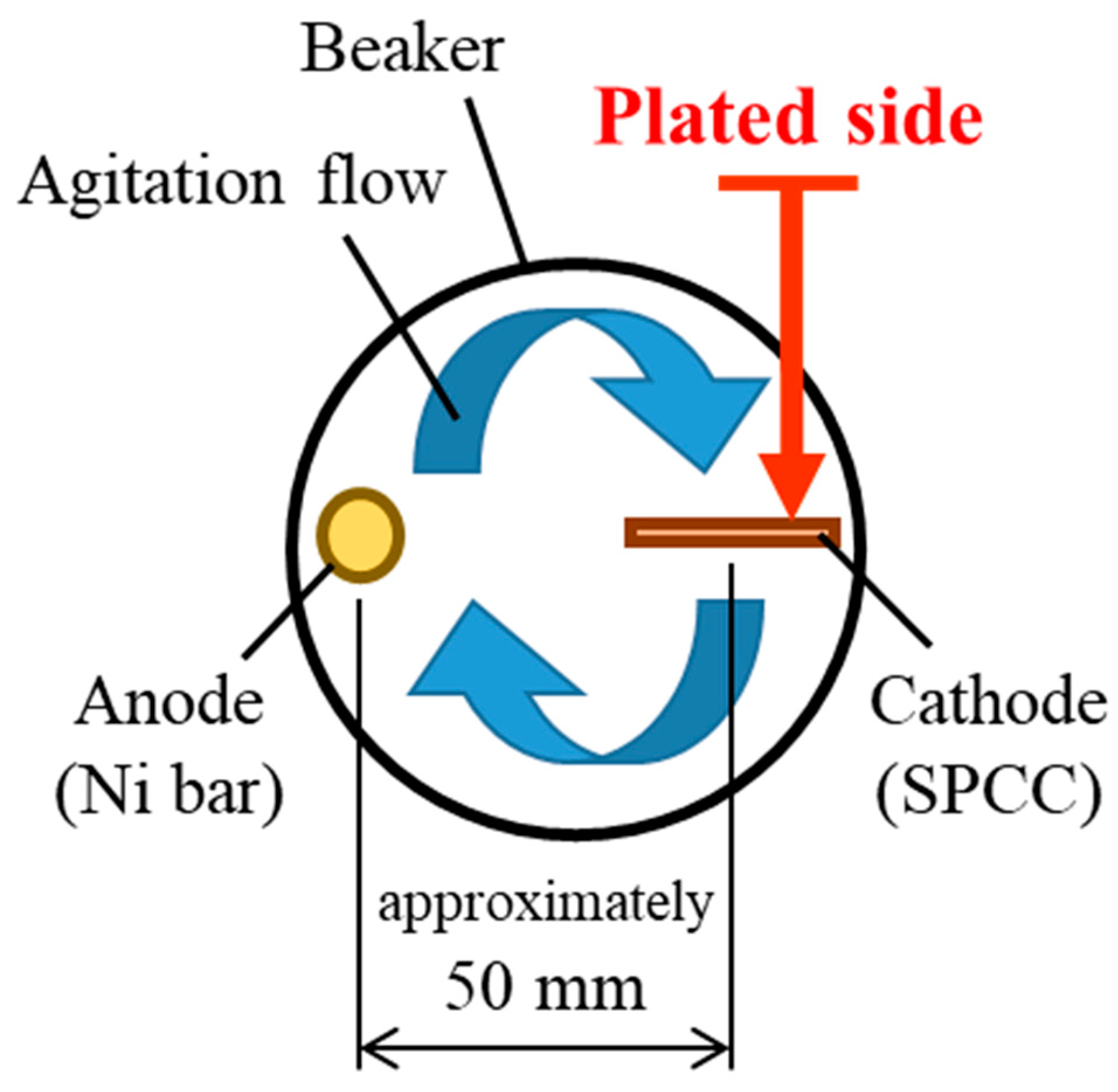
| Distilled Water | NiSO4·6H2O | NiCl2 | H3BO3 | CNF | |
|---|---|---|---|---|---|
| (g/L) | Bal. | 240 | 30 | 30 | 0–3 |
| Fe | C | Mn | P | S | |
|---|---|---|---|---|---|
| (mass%) | Bal. | 0–0.15 | 0–0.60 | 0–0.10 | 0–0.05 |
| Current Density (A/dm2) | Charge Density (C/dm2) | Plating Time (min) | Stirring Speed (rpm) |
|---|---|---|---|
| 2.5 | 12,000 | 80 | 300 |
2.2. Analysis Process of Plated Films
2.2.1. Observation of Plated Films
2.2.2. Surface Roughness Measurement
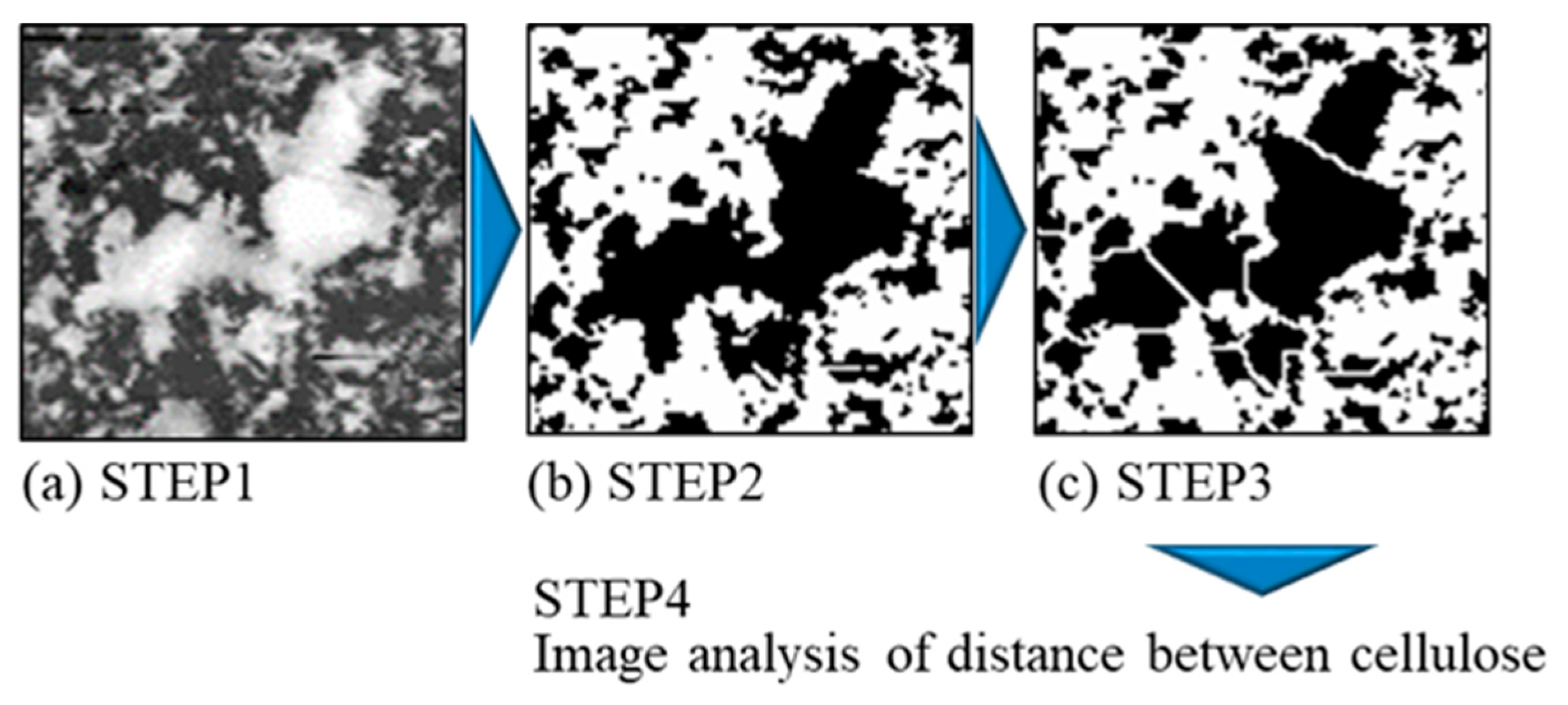
2.2.3. Evaluation of Dispersion of CNFs on Plated Films
2.2.4. Quantification Analysis
2.2.5. Vickers Hardness Test
2.2.6. X-ray Diffraction Analysis
- ;
- .
2.3. Abrasion Test
2.3.1. Abrasion Test Using Ball-on-Disk Method
2.3.2. Observation and Analysis of Abrasion Scars
Abrasion Scars of Plated Films
Abrasion Scars of Counterpart Balls
- 4.7625 mm).
3. Results and Discussion
3.1. Properties of Plated Films
3.1.1. Morphology and Microstructure
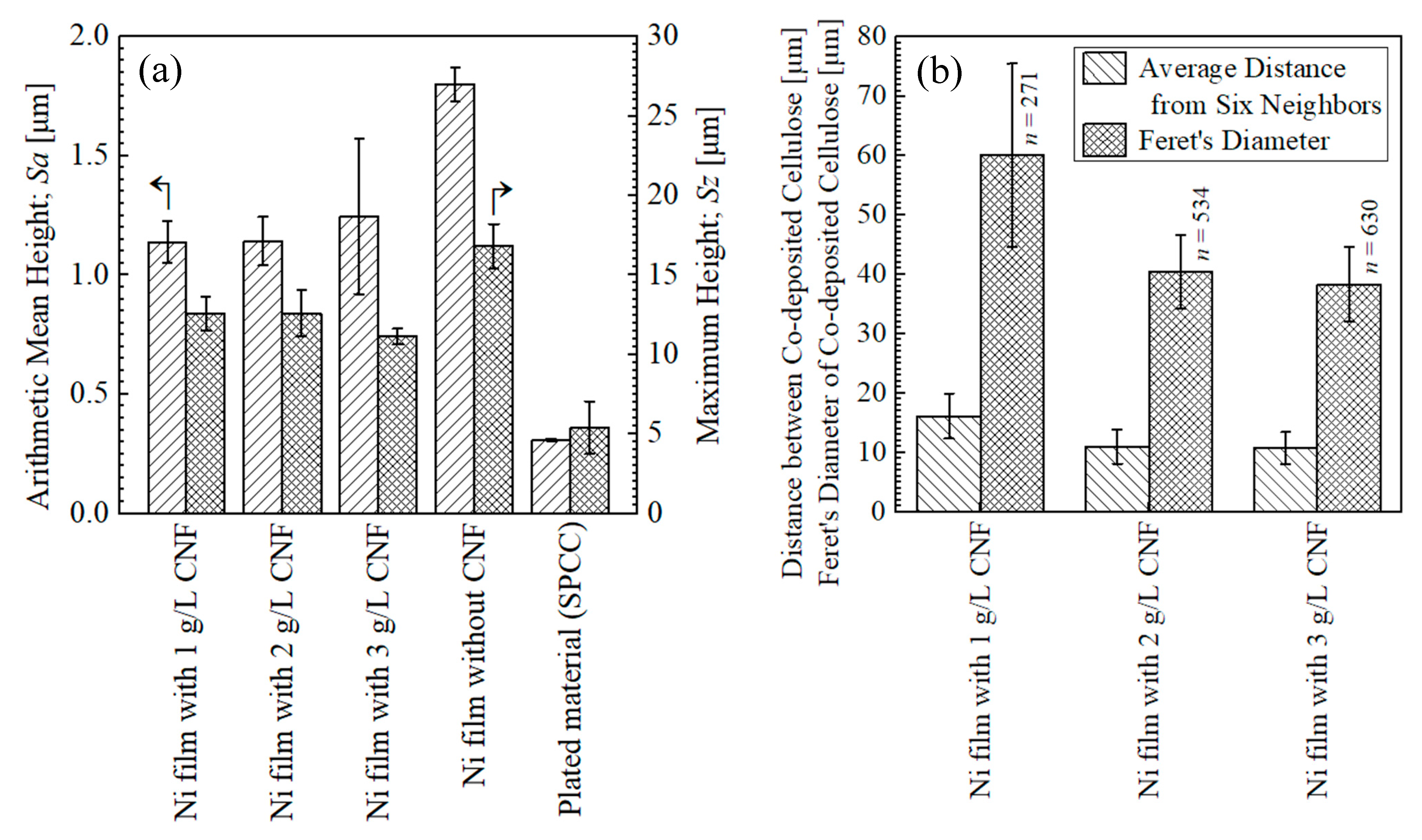
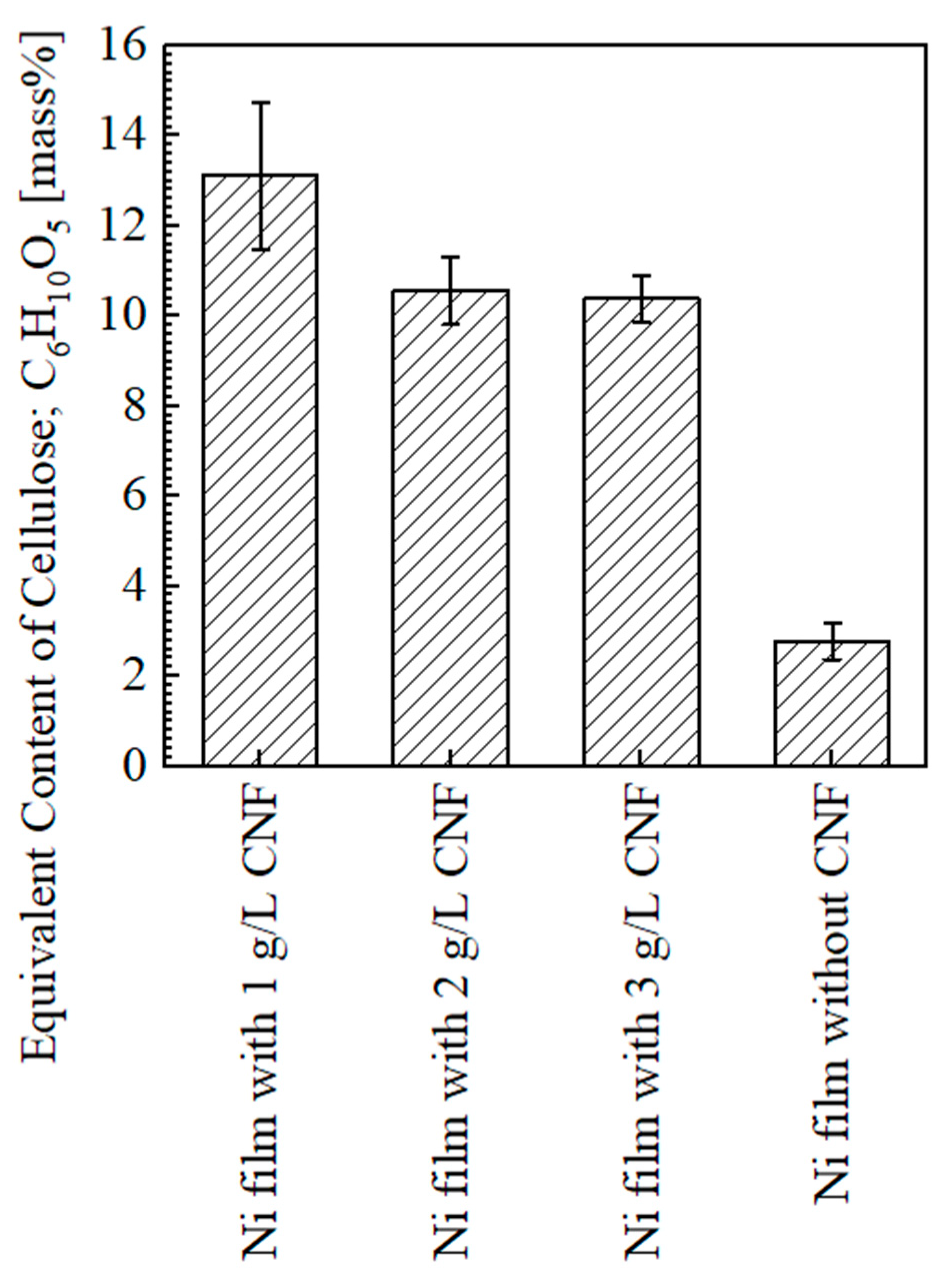
3.1.2. Content of Cellulose
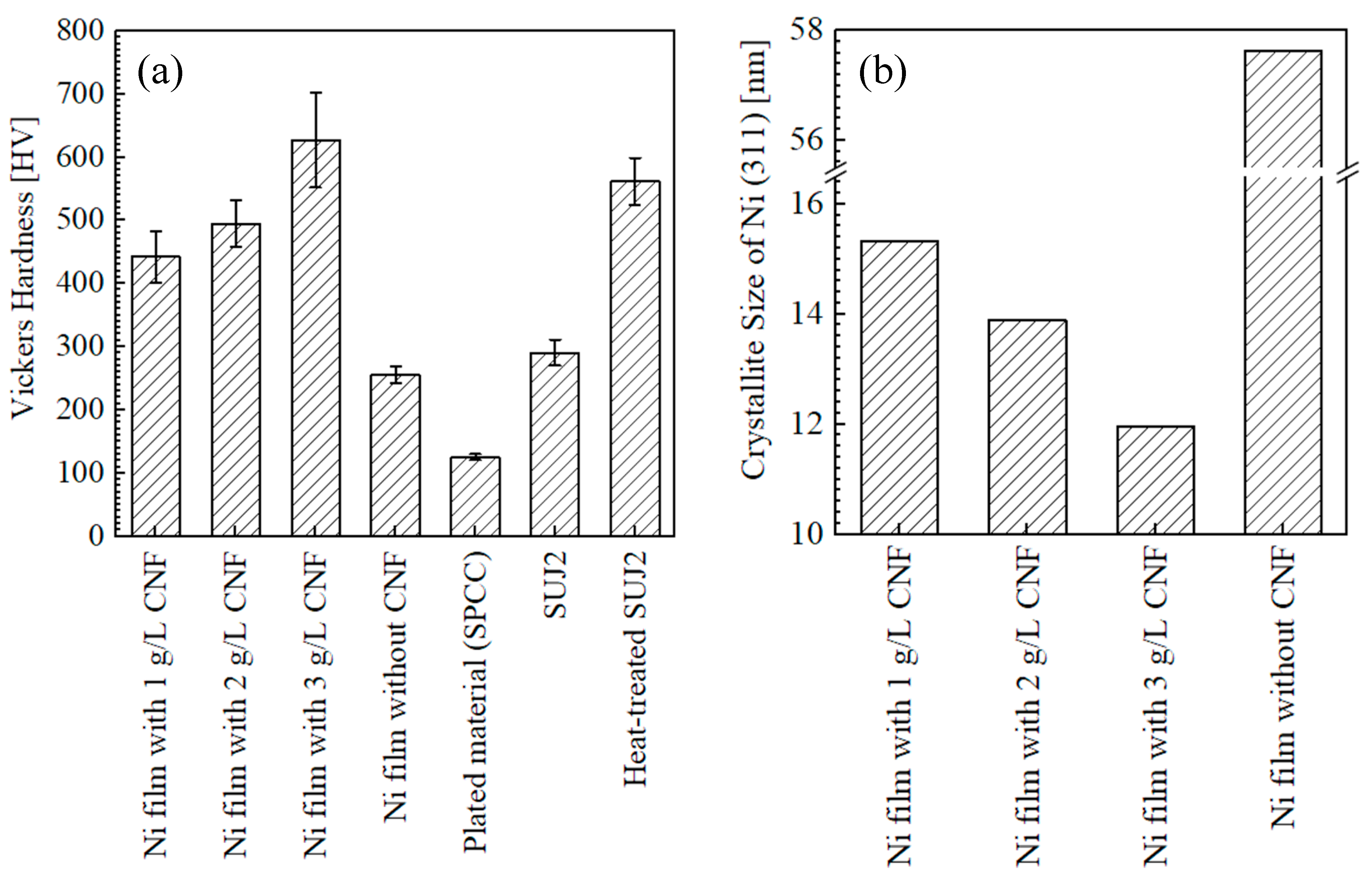
3.1.3. Hardness of Surfaces and Crystallite Size
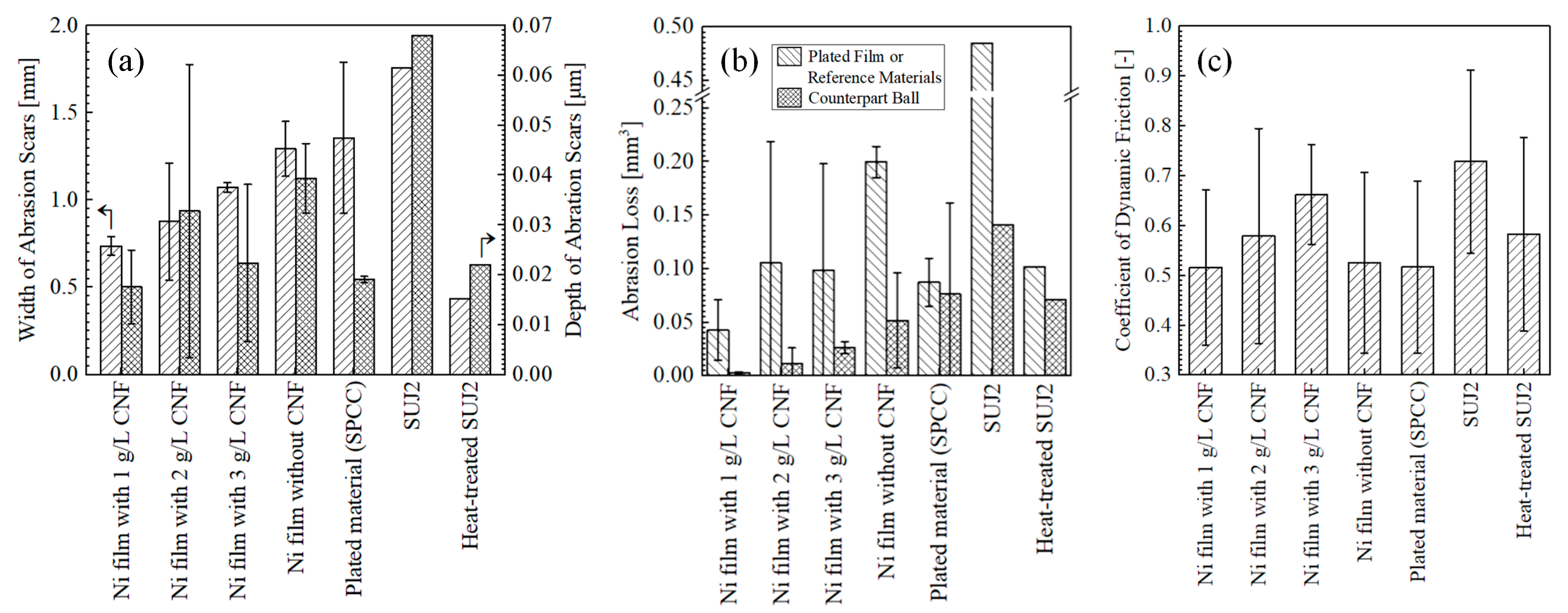
3.2. Wear Resistance of Plated Films
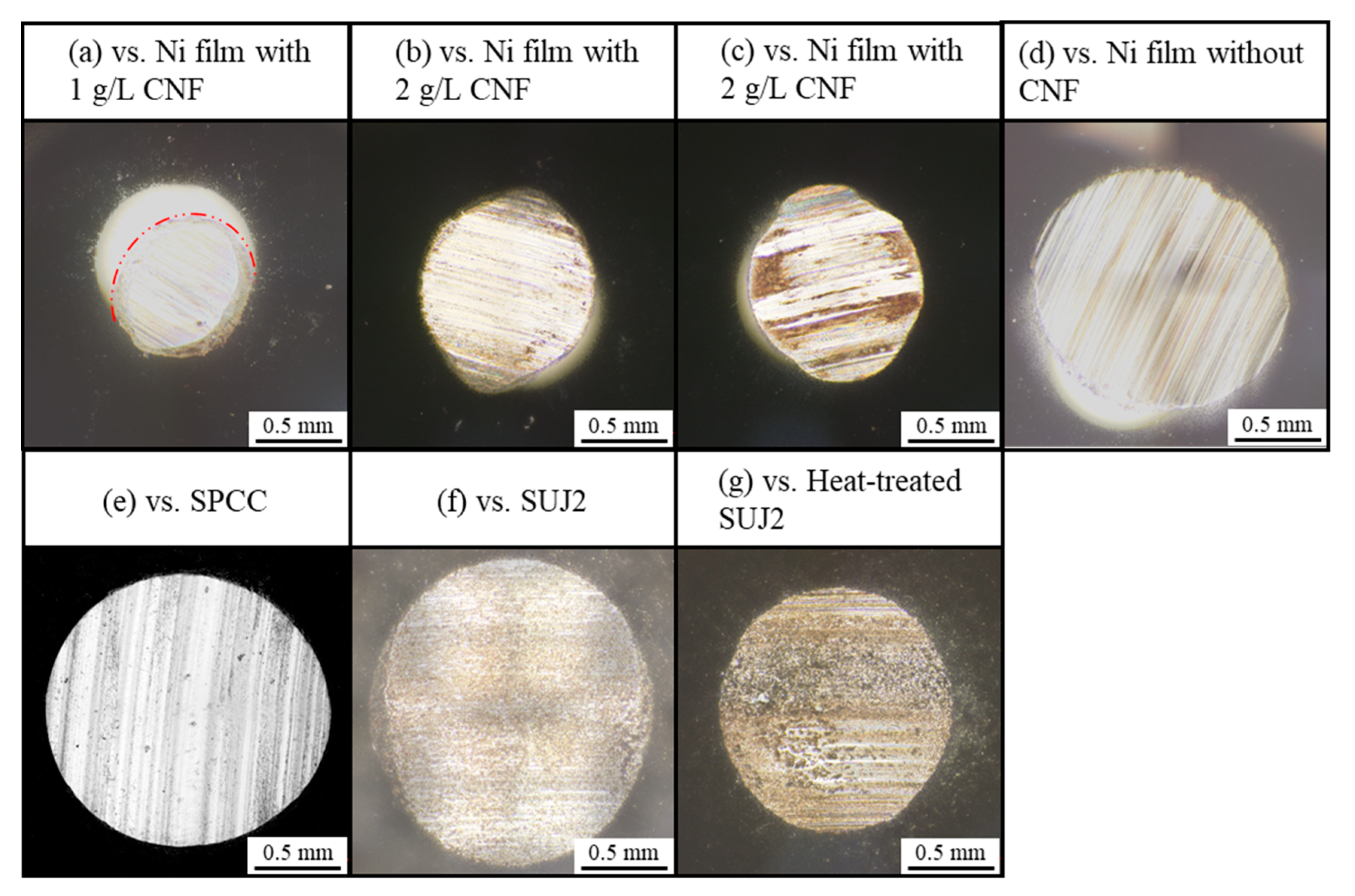
3.2.1. Macrostructure of Abrasion Scars
3.2.2. Microstructure of Abrasion Scars
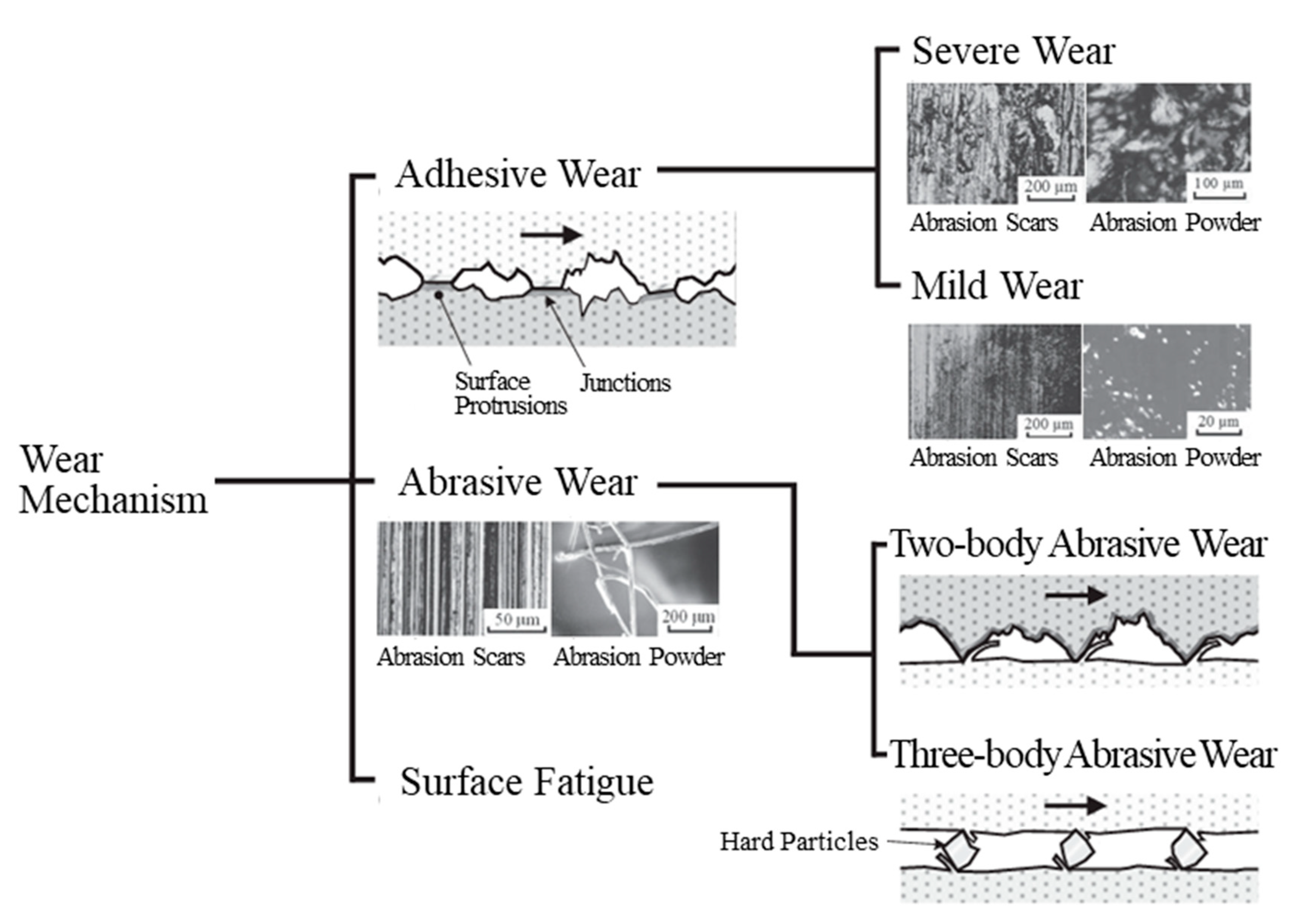
3.2.3. Abrasion Mechanism
Ni–CNF Composite Films
Ni Film and SPCC
3.3. Future Prospects
4. Limitations of This Study
5. Conclusions
- (1)
- The Ni–CNF plated films obtained with the addition of 1 g/L of CNF, a current density of 2.5 A/dm2, and a stirring speed of 300 rpm exhibited excellent wear resistance. Compared to the conventional Ni-plated film without CNF, the abrasion losses on the plated side and the ball side were reduced by approximately 79% and 94%, respectively;
- (2)
- The mechanism of abrasion reduction by the composite CNFs was considered to be the result of CNF aggregates intervening at the abrasion interface and the individual CNFs within the aggregates sliding;
- (3)
- The Vickers hardness of the surface of the plated films increased as the amount of CNF added to the plating solution increased in the range of 1–3 g/L. Moreover, the Ni (311) crystallite size was confirmed to become finer as the hardness increased;
- (4)
- In contrast to the results in our previous study [4], the content of cellulose in the plated films showed a decreasing trend with the addition of CNF in the range of 1–3 g/L to the plating solution. Insufficient agitation was considered to be the reason for this; however, it was inferred that even weak agitation was not a problem for transporting fine CNFs, leading to crystallite size refinement.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Iioka, M.; Kawanabe, W.; Shohji, I.; Kobayashi, T. An experimental study of fabrication of cellulose nano-fiber composited Ni film by electroplating. Mater. Trans. 2022, 63, 821–828. [Google Scholar] [CrossRef]
- Iioka, M.; Kawanabe, W.; Kobayashi, T.; Shohji, I.; Sakamoto, K. Fabrication of electroplated nickel composite films using cellulose nanofibers introduced with carboxy groups as co-deposited materials. Surfaces 2023, 6, 164–178. [Google Scholar] [CrossRef]
- Iioka, M.; Kawanabe, W.; Kobayashi, T.; Shohji, I. Application of metal sputtering treatment to cellulose materials used as co-deposited material for electrolytic nickel composite plating. Q. J. Jpn. Weld. Soc. 2023, 41, 337–347. [Google Scholar] [CrossRef]
- Iioka, M.; Kawanabe, W.; Kobayashi, T.; Shohji, I. Investigation of effects of electroplating conditions on TEMPO-oxidized cellulose nanofiber composited nickel electroplated films. Mater. Sci. Forum 2023, 1106, 55–62. [Google Scholar] [CrossRef]
- Yano, H. Cellulose nanofibers and their utilization. J. Imaging Soc. Jpn. 2016, 55, 356–360. [Google Scholar]
- Yano, H. III: Cellulosic nanocomposites. J. Soc. Mater. Sci. Jpn. 2008, 57, 310–315. [Google Scholar] [CrossRef]
- Lee, K.; Aitomäk, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Halim, N.A.; Shah, N.A.A.; Noor, S.A.M.; Jamal, S.H.; Ong, K.K.; Yunus, W.M.Z.W.; Farid, M.A.A.; et al. Performance evaluation of cellulose nanofiber reinforced polymer composites. Funct. Compos. Struct. 2021, 3, 024001. [Google Scholar] [CrossRef]
- Moon, D.; Tsukahara, K.; Sagisaka, M.; Tahara, K. Effect of cellulose nanofibers composites in automotive components on greenhouse gas emissions. J. Jpn. Inst. Energy 2016, 95, 648–652. [Google Scholar] [CrossRef]
- Savadekar, N.R.; Mhaske, S.T. Synthesis of nano cellulose fibers and effect on thermoplastics starch-based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef]
- Cherian, B.M.; Leão, A.L.; Souza, S.F.D.; Costa, L.M.M.; Olyveira, G.M.D.; Kottaisamy, M.; Nagarajan, E.R.; Thomas, S. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]
- Fujisaki, Y.; Koga, H.; Nakajima, Y.; Nakata, M.; Tsuji, H.; Yamamoto, T.; Kurita, T.; Nogi, M.; Shimidzu, N. Transparent nanopaper-based flexible organic thin-film transistor array. Adv. Funct. Mater. 2013, 24, 1657–1663. [Google Scholar] [CrossRef]
- Koga, H.; Nogi, M.; Isogai, A. Ionic liquid mediated dispersion and support of functional molecules on cellulose fibers for stimuli-responsive chromic paper devices. ACS Appl. Mater. Interfaces 2017, 9, 40914–40920. [Google Scholar] [CrossRef]
- Kang, Y.J.; Chun, S.J.; Lee, S.S.; Kim, B.Y.; Kim, J.H.; Chung, H.; Lee, S.Y.; Kim, W. All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and ion gels. ACS Nano 2012, 6, 6400–6406. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Rocha, J.H.A.; Farias, L.D.N.; Siqueira, T.P.L. Cellulose nanofibers (CNF) as reinforcement for cementitious matrices: A systematic literature review. Rev. ALCONPAT 2022, 12, 311–327. [Google Scholar]
- Subramanian, R.; Hiltunen, E.; Gane, P.A.C. Potential Use of Micro- and Nanofibrillated Cellulose Composites Exemplified by Paper. In Cellulose Fibers: Bio- and Nano-Polymer Composites; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 121–152. [Google Scholar]
- Kurita, H.; Kanno, T.; Narita, F. Progress on materials reinforcement using mechanically defibrillated cellulose nanofibers. J. Soc. Mater. Sci. Jpn. 2022, 71, 417–423. [Google Scholar] [CrossRef]
- Osada, T.; Kobayashi, S. Mechanical properties and deformation behavior of metal injection molded products with cellulose nanofiber. J. Jpn. Soc. Powder Powder Metall. 2023, 70, 168–172. [Google Scholar] [CrossRef]
- Kogure, A.; Shohji, I.; Kobayashi, T. Fabrication of lead-free solder by Sn–cellulose nanofiber composite plating. In Proceedings of the 28th Symposium on Microjoining and Assembly Technology in Electronics (Mate 2022), Online, 1–14 February 2022; pp. 257–258. [Google Scholar]
- Kobayashi, T.; Kogure, A.; Shohji, I. Development of Sn solder plating containing cellulose nanofiber. In Proceedings of the 2022 International Conference on Electronics Packaging (ICEP 2022), Sapporo, Japan, 11 May 2022; pp. 217–218. [Google Scholar]
- Kawanabe, W.; Iioka, M.; Shohji, I.; Kobayashi, T. Investigation of compositing method of cellulose nano-fiber into nickel plating film by electroless method. In Proceedings of the 29th Symposium on Microjoining and Assembly Technology in Electronics (Mate 2023), Yokohama, Japan, 25 January 2023; pp. 179–180. [Google Scholar]
- Kawanabe, W.; Iioka, M.; Kobayashi, T.; Shohji, I. Investigation of deposition conditions and basic properties of CNF composite Ni plated film by electroless plating method. Mater. Sci. Forum 2023, 1106, 69–74. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Gu, Y.; Xia, K.; Wu, D.; Mou, J.; Zheng, S. Technical characteristics and wear-resistant mechanism of nano coatings: A review. Coatings 2020, 10, 233. [Google Scholar] [CrossRef]
- Zaki, E.G.; Selim, M.S.; Hao, Z.; Elsaeed, S.M.; El-Saeed, A.M. Special issue: Recent trends in wear and erosion resistance of alloys. Coatings 2023, 13, 64. [Google Scholar] [CrossRef]
- Tanaka, H. Silicon carbide powder and sintered materials. J. Ceram. Soc. Jpn. 2011, 119, 218–233. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. Ion-exchange behavior of carboxylate groups in fibrous cellulose oxidized by the TEMPO-mediated system. Carbohydr. Polym. 2005, 61, 183–190. [Google Scholar] [CrossRef]
- Saito, T.; Okita, Y.; Nge, T.T.; Sugiyama, J.; Isogai, A. TEMPO-mediated oxidation of native cellulose. Microscopic analysis of fibrous fractions in the oxidized products. Carbohydr. Polym. 2006, 65, 435–440. [Google Scholar] [CrossRef]
- Petroudy, S.R.D.; Chabot, B.; Loranger, E.; Naebe, M.; Shojaeiarani, J.; Gharehkhani, S.; Ahvazi, B.; Hu, J.; Thomas, S. Recent advances in cellulose nanofibers preparation through energy-efficient approaches: A review. Energies 2021, 14, 6792. [Google Scholar] [CrossRef]
- Sato, M. Hydrophobic process by composite plating. J. Surf. Finish. Soc. Jpn. 2009, 60, 27–31. [Google Scholar] [CrossRef]
- Nishikawa, K. Electroless Ni–P/PTFE composite plating. J. Surf. Finish. Soc. Jpn. 2015, 66, 499–502. [Google Scholar] [CrossRef]
- Sugizaki, T. Environment conscious electroless Ni–P plating. J. Surf. Finish. Soc. Jpn. 2006, 57, 866–868. [Google Scholar] [CrossRef]
- Hashizume, K.; Naito, K.; Oka, H.; Okumura, H. The situation and future of electroless nickel plating. J. Surf. Finish. Soc. Jpn. 2007, 58, 87–91. [Google Scholar] [CrossRef]
- Okamura, Y. Environmental load reducing technologies in electroless plating process. J. Surf. Finish. Soc. Jpn. 2007, 68, 26–31. [Google Scholar] [CrossRef]
- Watanabe, K. Chromium plating. Hyomen Gijutsu 1999, 50, 149–154. [Google Scholar]
- Shimpo, R. Environmental corresponding of engineering hard plating. J. Jpn. Soc. Precis. Eng. 2012, 78, 1038–1042. [Google Scholar] [CrossRef]
- Shimpo, R. Properties of hard chrome plating. J. Surf. Finish. Soc. Jpn. 2014, 65, 123–128. [Google Scholar] [CrossRef]
- Onodera, H. Current status of trivalent chromium plating and its overseas trends. J. Surf. Finish. Soc. Jpn. 2018, 69, 234–236. [Google Scholar] [CrossRef]
- By courtesy of Nippon Paper Industries, Chiyoda-ku, Japan, 16 May 2023.
- ISO 25178-2:2012; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 2012.
- Rasband, W.S. ImageJ; U. S. National Institutes of Health: Bethesda, MA, USA, 1997–2020. Available online: https://imagej.nih.gov/ij/ (accessed on 8 November 2023).
- Haeri, M.; Haeri, M. ImageJ plugin for analysis of porous scaffolds used in tissue engineering. J. Open Res. Softw. 2015, 3, e1. [Google Scholar] [CrossRef]
- Tosaka, M. Basic knowledge of wide-angle X-ray diffraction. Nippon Gomu Kyokaishi 2021, 94, 130–136. [Google Scholar] [CrossRef]
- Doi, T. Acting mechanisms of citric acid on the properties of nickel citrate electroplated film. Bull. Tokyo Metrop. Ind. Technol. Res. Inst. 2015, 10, 18–21. [Google Scholar]
- Takeichi, Y. Tribology of polymer materials. J. Surf. Finish. Soc. Jpn. 2014, 65, 562–567. [Google Scholar] [CrossRef]
- Isogai, A.; Onabe, F. Cellulose: It’s higher-order structures and functionalities. Sen’i Kikai Gakkaishi (J. Text. Mach. Soc. Jpn.) 1997, 50, 181–185. [Google Scholar] [CrossRef]
- AI-Jawhari, I.F.H. Polymer Nanocomposite Matrix-Based Nanoproducts. In Handbook of Consumer Nanoproducts; Mallakpour, S., Hussain, C.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–14. [Google Scholar]
- Isogai, A. TEMPO-oxidized cellulose nanofibers. Kobunshi 2009, 58, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Hase, A. Research cases and trends of wear mechanism. J. Surf. Finish. Soc. Jpn. 2014, 65, 556–561. [Google Scholar] [CrossRef]
- Kikuchi, K.; Kamiya, O.; Saito, Y.; Kumagai, K. Running-in behavior of repeated dry wear on metals. J. Soc. Mater. Eng. Resour. Jpn. 1998, 11, 12–20. [Google Scholar]
- Mizumoto, M.; Usami, K. [Why wear occuers] Mamou ha naze okoru noka (in Japanese). Turbomachinery 1994, 24, 267–273. [Google Scholar]
- Tsujioka, M. The technological trend and application of environment-friendly hard coating. J. Surf. Finish. Soc. Jpn. 2012, 63, 134–139. [Google Scholar] [CrossRef]
- Mori, H. Latest environmental technologies in the automobile industry (2). J. Surf. Finish. Soc. Jpn. 2012, 63, 145–150. [Google Scholar] [CrossRef]
- Okano, T. Technical trends of lubricants for cold forging. Plastos (Bull. JSTP) 2019, 2, 106–110. [Google Scholar]




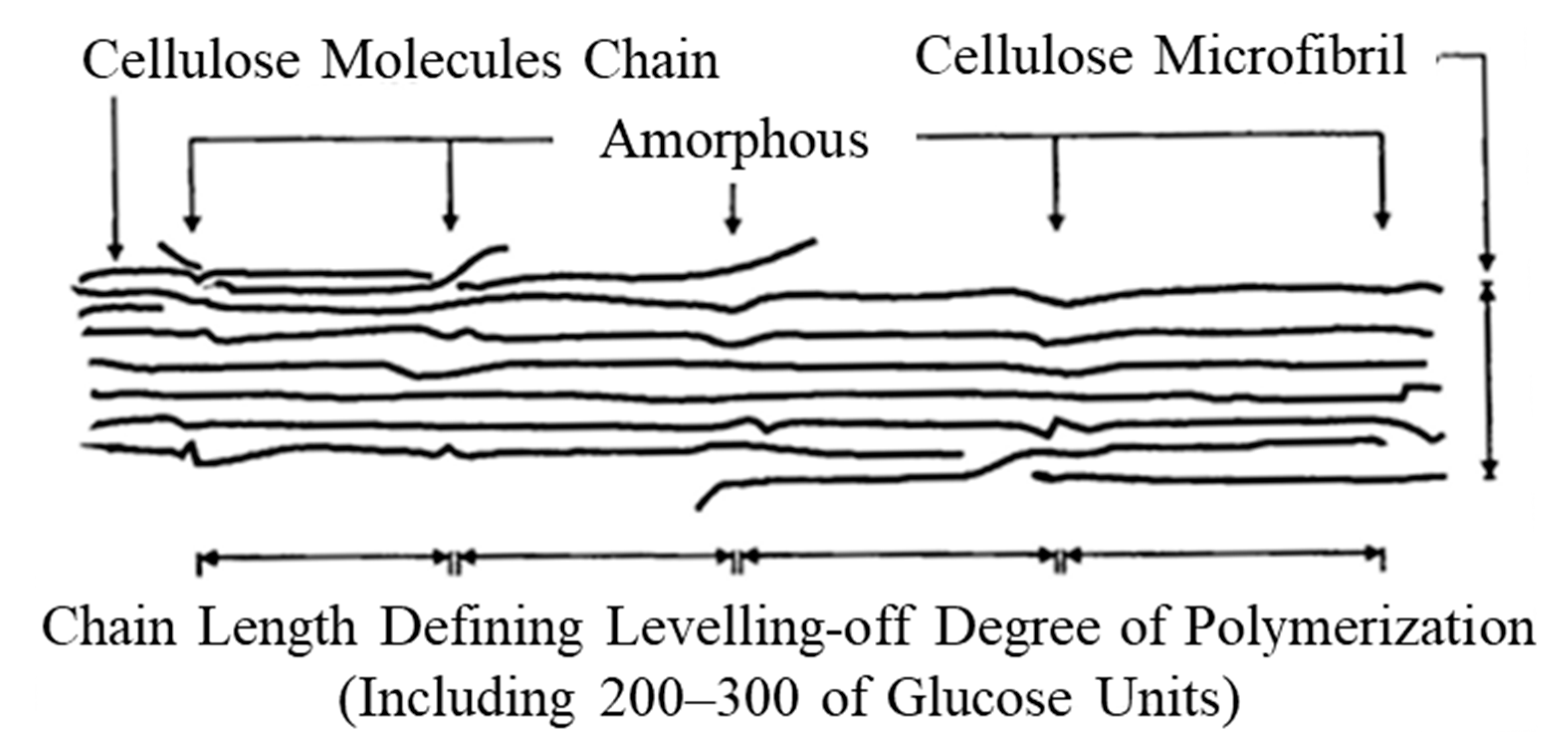
| Fe | C | Si | Mn | P | S | Cr | |
|---|---|---|---|---|---|---|---|
| (mass%) | Bal. | 0.95–1.10 | 0.15–0.35 | 0–0.50 | 0–0.025 | 0–0.025 | 1.30–1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iioka, M.; Kawanabe, W.; Tsujimura, S.; Kobayashi, T.; Shohji, I. An Evaluation of the Wear Resistance of Electroplated Nickel Coatings Composited with 2,2,6,6-Tetramethylpiperidine 1-oxyl-Oxidized Cellulose Nanofibers. Polymers 2024, 16, 224. https://doi.org/10.3390/polym16020224
Iioka M, Kawanabe W, Tsujimura S, Kobayashi T, Shohji I. An Evaluation of the Wear Resistance of Electroplated Nickel Coatings Composited with 2,2,6,6-Tetramethylpiperidine 1-oxyl-Oxidized Cellulose Nanofibers. Polymers. 2024; 16(2):224. https://doi.org/10.3390/polym16020224
Chicago/Turabian StyleIioka, Makoto, Wataru Kawanabe, Subaru Tsujimura, Tatsuya Kobayashi, and Ikuo Shohji. 2024. "An Evaluation of the Wear Resistance of Electroplated Nickel Coatings Composited with 2,2,6,6-Tetramethylpiperidine 1-oxyl-Oxidized Cellulose Nanofibers" Polymers 16, no. 2: 224. https://doi.org/10.3390/polym16020224







