Structure–Performance Correlation Inspired Platinum-Assisted Anode with a Homogeneous Ionomer Layer for Proton Exchange Membrane Water Electrolysis
Abstract
:1. Introduction
2. Experimental Section
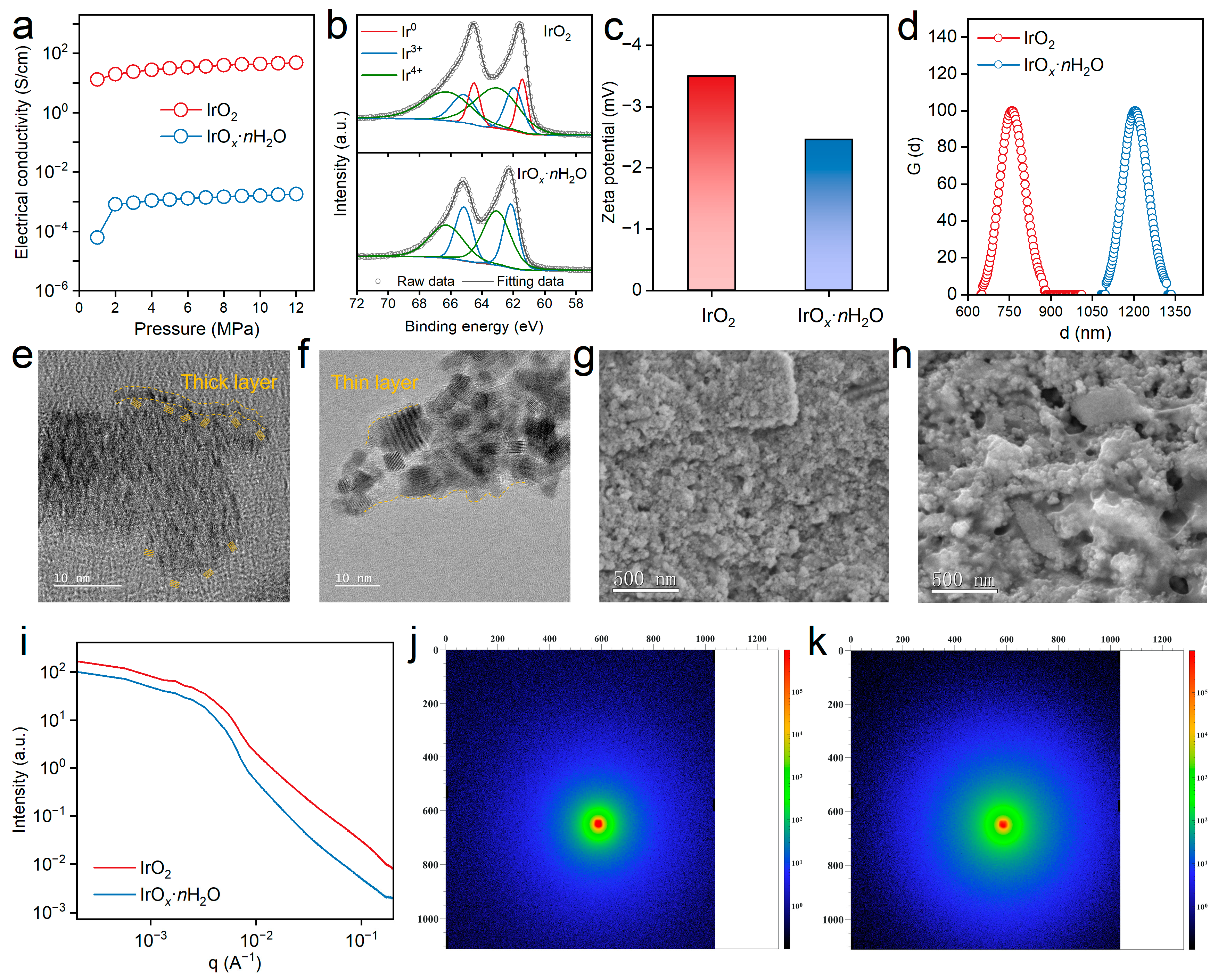
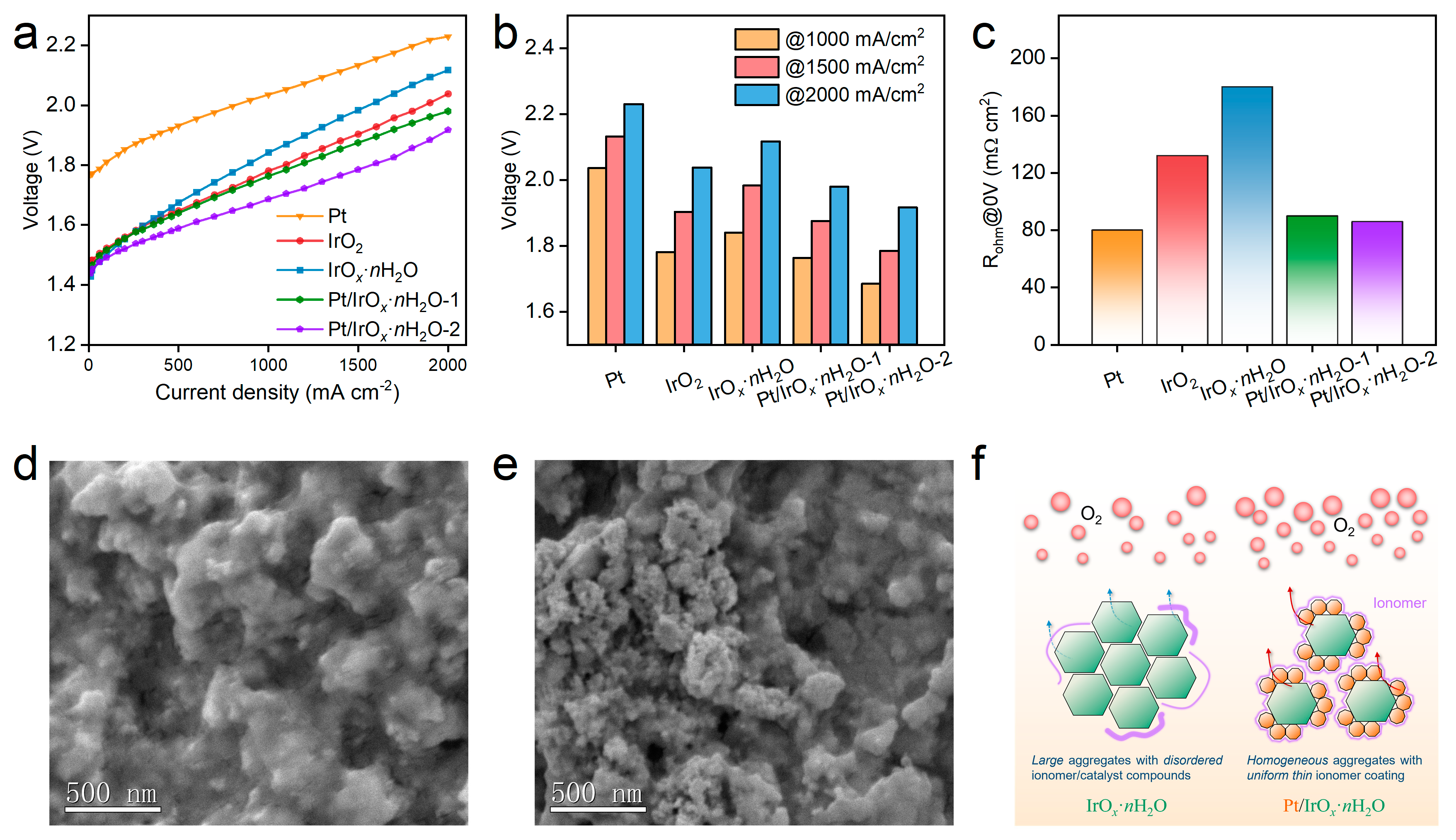
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Squadrito, G.; Maggio, G.; Nicita, A. The green hydrogen revolution. Renew. Energy 2023, 216, 119041. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.; Lv, Z.; Liu, J.; Zhou, H.; Xu, C. Green hydrogen: A promising way to the carbon-free society. Chin. J. Chem. Eng. 2022, 43, 2–13. [Google Scholar] [CrossRef]
- Mahato, N.; Jang, H.; Dhyani, A.; Cho, S. Recent Progress in Conducting Polymers for Hydrogen Storage and Fuel Cell Applications. Polymers 2020, 12, 2480. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrog. Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Libowitz, G. The role of Materials Science in the development of Hydrogen Energy systems. In Materials Science in Energy Technology; Academic Press: Cambridge, MA, USA, 1979; pp. 427–454. [Google Scholar]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Liu, H.; Dong, T.D.; Deng, Y.J.; Li, Y.X.; Lu, C.R.; Jia, W.D.; Meng, Z.H.; Zhou, M.Z.; Tang, H.L. Phosphonate poly(vinylbenzyl chloride)-Modified Sulfonated poly(aryl ether nitrile) for Blend Proton Exchange Membranes: Enhanced Mechanical and Electrochemical Properties. Polymers 2023, 15, 3203. [Google Scholar] [CrossRef]
- Yeo, K.-R.; Lee, K.-S.; Kim, H.; Lee, J.; Kim, S.-K. A highly active and stable 3D dandelion spore-structured self-supporting Ir-based electrocatalyst for proton exchange membrane water electrolysis fabricated using structural reconstruction. Energy Environ. Sci. 2022, 15, 3449–3461. [Google Scholar] [CrossRef]
- Huynh, T.N.; Song, J.; Bae, H.E.; Kim, Y.; Dickey, M.D.; Sung, Y.E.; Kim, M.J.; Kwon, O.J. Ir–Ru Electrocatalysts Embedded in N-Doped Carbon Matrix for Proton Exchange Membrane Water Electrolysis. Adv. Funct. Mater. 2023, 23, 01999. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Li, Q. Designing active and stable Ir-based catalysts for the acidic oxygen evolution reaction. Ind. Chem. Mater. 2023, 1, 299–311. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, F.; Wang, G.; Lai, D.; Zou, L.; Cheng, Q.; Li, J.; Zou, Z.; Yang, H. CO induced phase-segregation to construct robust and efficient IrRux@ Ir core-shell electrocatalyst towards acidic oxygen evolution. J. Power Sources 2022, 528, 231189. [Google Scholar] [CrossRef]
- So, S.; Oh, K.-H. Effect of dispersant on catalyst ink properties and catalyst layer structure for high performance polymer electrolyte membrane fuel cells. J. Power Sources 2023, 561, 232664. [Google Scholar] [CrossRef]
- Kang, Z.; Alia, S.M.; Young, J.L.; Bender, G. Effects of various parameters of different porous transport layers in proton exchange membrane water electrolysis. Electrochim. Acta 2020, 354, 136641. [Google Scholar] [CrossRef]
- Rheinländer, P.J.; Durst, J. Transformation of the OER-active IrOx species under transient operation conditions in PEM water electrolysis. J. Electrochem. Soc. 2021, 168, 024511. [Google Scholar] [CrossRef]
- Yu, H.; Bonville, L.; Jankovic, J.; Maric, R. Microscopic insights on the degradation of a PEM water electrolyzer with ultra-low catalyst loading. Appl. Catal. B Environ. 2020, 260, 118194. [Google Scholar] [CrossRef]
- Suermann, M.; Bensmann, B.; Hanke-Rauschenbach, R. Degradation of proton exchange membrane (PEM) water electrolysis cells: Looking beyond the cell voltage increase. J. Electrochem. Soc. 2019, 166, F645. [Google Scholar] [CrossRef]
- Lyu, X.; Foster, J.; Rice, R.; Padgett, E.; Creel, E.B.; Li, J.; Yu, H.; Cullen, D.A.; Kariuki, N.N.; Park, J.H. Aging gracefully? Investigating iridium oxide ink’s impact on microstructure, catalyst/ionomer interface, and PEMWE performance. J. Power Sources 2023, 581, 233503. [Google Scholar] [CrossRef]
- Song, C.; Tsay, K.; Fisher, E.; Sheibley, N.; Shaigan, N.; Malek, A.; Fatih, K. A Study on Effect of Ionomer Content on Catalyst Ink Property and PEM Water Electrolyzer Performance; Electrochemical Society Meeting Abstracts 243; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2023; p. 2110. [Google Scholar]
- Xu, J.; Jin, H.; Lu, T.; Li, J.; Liu, Y.; Davey, K.; Zheng, Y.; Qiao, S.-Z. IrO x · n H2O with lattice water–assisted oxygen exchange for high-performance proton exchange membrane water electrolyzers. Sci. Adv. 2023, 9, eadh1718. [Google Scholar] [CrossRef]
- Shi, G.; Tano, T.; Tryk, D.A.; Uchiyama, T.; Iiyama, A.; Uchida, M.; Terao, K.; Yamaguchi, M.; Tamoto, K.; Uchimoto, Y. Nanorod Structuring of IrO x on a Unique Microstructure of Sb-Doped Tin Oxide to Dramatically Boost the Oxygen Evolution Reaction Activity for PEM Water Electrolysis. ACS Catal. 2023, 13, 12299–12309. [Google Scholar] [CrossRef]
- Yu, H.; Ke, J.; Shao, Q. Two Dimensional Ir-Based Catalysts for Acidic OER. Small 2023, 23, 04307. [Google Scholar] [CrossRef]
- Böhm, D.; Beetz, M.; Schuster, M.; Peters, K.; Hufnagel, A.G.; Döblinger, M.; Böller, B.; Bein, T.; Fattakhova-Rohlfing, D. Efficient OER catalyst with low Ir volume density obtained by homogeneous deposition of iridium oxide nanoparticles on macroporous antimony-doped tin oxide support. Adv. Funct. Mater. 2020, 30, 1906670. [Google Scholar] [CrossRef]
- Ma, C.; Sun, W.; Qamar Zaman, W.; Zhou, Z.; Zhang, H.; Shen, Q.; Cao, L.; Yang, J. Lanthanides regulated the amorphization–crystallization of IrO2 for outstanding OER performance. ACS Appl. Mater. Interfaces 2020, 12, 34980–34989. [Google Scholar] [CrossRef]
- Da Silva, G.C.; Fernandes, M.R.; Ticianelli, E.A. Activity and Stability of Pt/IrO2 Bifunctional Materials as Catalysts for the Oxygen Evolution/Reduction Reactions. ACS Catal. 2018, 8, 2081–2092. [Google Scholar] [CrossRef]
- Li, L.; Wang, B.; Zhang, G.; Yang, G.; Yang, T.; Yang, S.; Yang, S. Electrochemically Modifying the Electronic Structure of IrO2 Nanoparticles for Overall Electrochemical Water Splitting with Extensive Adaptability. Adv. Energy Mater. 2020, 10, 2001600. [Google Scholar] [CrossRef]
- Lee, J.K.; Anderson, G.; Tricker, A.W.; Babbe, F.; Madan, A.; Cullen, D.A.; Arregui-Mena, J.D.; Danilovic, N.; Mukundan, R.; Weber, A.Z.; et al. Ionomer-free and recyclable porous-transport electrode for high-performing proton-exchange-membrane water electrolysis. Nat. Commun. 2023, 14, 4592. [Google Scholar] [CrossRef]
- Ma, L.; Sui, S.; Zhai, Y. Investigations on high performance proton exchange membrane water electrolyzer. Int. J. Hydrog. Energy 2009, 34, 678–684. [Google Scholar] [CrossRef]
- Holzapfel, P.; Bühler, M.; Van Pham, C.; Hegge, F.; Böhm, T.; McLaughlin, D.; Breitwieser, M.; Thiele, S. Directly coated membrane electrode assemblies for proton exchange membrane water electrolysis. Electrochem. Commun. 2020, 110, 106640. [Google Scholar] [CrossRef]
- Khandavalli, S.; Park, J.H.; Kariuki, N.N.; Zaccarine, S.F.; Pylypenko, S.; Myers, D.J.; Ulsh, M.; Mauger, S.A. Investigation of the Microstructure and Rheology of Iridium Oxide Catalyst Inks for Low-Temperature Polymer Electrolyte Membrane Water Electrolyzers. ACS Appl. Mater. Interfaces 2019, 11, 45068–45079. [Google Scholar] [CrossRef]
- Wang, R.; Cao, J.; Cai, S.; Yan, X.; Li, J.; Yourey, W.M.; Tong, W.; Tang, H. MOF@Cellulose Derived Co–N–C Nanowire Network as an Advanced Reversible Oxygen Electrocatalyst for Rechargeable Zinc–Air Batteries. ACS Appl. Energy Mater. 2018, 1, 1060–1068. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, S.; Tan, H.; Wang, R.; Zhai, M.; Zhang, H.; Qin, H.; Tang, H. Construction of reliable ion-conducting channels based on the perfluorinated anion-exchange membrane for high-performance pure-water-fed electrolysis. Adv. Compos. Hybrid Mater. 2023, 6, 89. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Shi, Z.; Han, D.; Yang, J.; Wang, P.; Ni, J.; Xiao, M.; Liu, C.; Xing, W. Improving the Lattice Oxygen Reactivity of Rutile IrO2 via Partial Sn Substitution for Acidic Water Oxidation. J. Phys. Chem. C 2023, 127, 12541–12547. [Google Scholar] [CrossRef]
- Bernt, M.; Siebel, A.; Gasteiger, H.A. Analysis of Voltage Losses in PEM Water Electrolyzers with Low Platinum Group Metal Loadings. J. Electrochem. Soc. 2018, 165, F305–F314. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, T.; Zhao, X.; Jiang, W.-J.; Pan, H.; Gao, D.; Xu, C. Expediting in-situ electrochemical activation of two-dimensional metal–organic frameworks for enhanced OER intrinsic activity by iron incorporation. ACS Catal. 2019, 9, 7356–7364. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S.; Driess, M.; Menezes, P.W. The pitfalls of using potentiodynamic polarization curves for tafel analysis in electrocatalytic water splitting. ACS Energy Lett. 2021, 6, 1607–1611. [Google Scholar] [CrossRef]
- Berger, M.; Popa, I.M.; Negahdar, L.; Palkovits, S.; Kaufmann, B.; Pilaski, M.; Hoster, H.; Palkovits, R. Elucidating the Influence of Intercalated Anions in NiFe LDH on the Electrocatalytic Behavior of OER: A Kinetic Study. ChemElectroChem 2023, 10, e202300235. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, H.; Li, B.; Hu, Z.; Jiang D-e Yao, Q.; Wang, L.; Xie, J. Ligand effect on switching the rate-determining step of water oxidation in atomically precise metal nanoclusters. Nat. Commun. 2023, 14, 3374. [Google Scholar] [CrossRef]
- Li, S.-F.; Zheng, J.; Hu, L.; Ma, Y.; Yan, D. Facile surface defect engineering on perovskite oxides for enhanced OER performance. Dalton Trans. 2023, 52, 4207–4213. [Google Scholar] [CrossRef]
- Shabbir, B.; Drissi, N.; Jabbour, K.; Gassoumi, A.; Alharbi, F.; Manzoor, S.; Ashiq, M.F.; Alburaih, H.; Ehsan, M.F.; Ashiq, M.N. Development of Mn-MOF/CuO composites as platform for efficient electrocatalytic OER. Fuel 2023, 341, 127638. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.D.; Deng, L.; Li, W.Y.; Ren, Z.D.; Yang, M.; Yang, X.H.; Zhu, Y.C. Synthesis and characterization of an IrO2-Fe2O3 electrocatalyst for the hydrogen evolution reaction in acidic water electrolysis. RSC Adv. 2017, 7, 20252–20258. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Zhang, K.; Xie, Z.; Sun, K.; An, W.; Liang, X.; Zou, X. Advances and status of anode catalysts for proton exchange membrane water electrolysis technology. Mater. Chem. Front. 2023, 7, 1025–1045. [Google Scholar] [CrossRef]
- Yasutake, M.; Noda, Z.; Matsuda, J.; Lyth, S.M.; Nishihara, M.; Ito, K.; Hayashi, A.; Sasaki, K. Hybrid Anode Design of Polymer Electrolyte Membrane Water Electrolysis for the Reduction of Total Platinum Group Metal Loading; Electrochemical Society Meeting Abstracts 243; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2023; p. 2117. [Google Scholar]
- Kang, S.Y.; Park, J.E.; Jang, G.Y.; Choi, C.; Cho, Y.H.; Sung, Y.E. Directly Coated Iridium Nickel Oxide on Porous-Transport Layer as Anode for High-Performance Proton-Exchange Membrane Water Electrolyzers. Adv. Mater. Interfaces 2023, 22, 02406. [Google Scholar] [CrossRef]
- Hao, S.; Sheng, H.; Liu, M.; Huang, J.; Zheng, G.; Zhang, F.; Liu, X.; Su, Z.; Hu, J.; Qian, Y. Torsion strained iridium oxide for efficient acidic water oxidation in proton exchange membrane electrolyzers. Nat. Nanotechnol. 2021, 16, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Shin, K.; Park, Y.; Yun, Y.H.; Doo, G.; Jung, G.H.; Kim, M.; Cho, W.C.; Kim, C.H.; Lee, H.M. Catalyst-Support Interactions in Zr2ON2-Supported IrOx Electrocatalysts to Break the Trade-Off Relationship Between the Activity and Stability in the Acidic Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2301557. [Google Scholar] [CrossRef]
- Liu, C.; Luo, M.; Zeis, R.; Chuang, P.-Y.A.; Zhang, R.; Du, S.; Sui, P.-C. Fabrication of catalyst layer for proton exchange membrane water electrolyzer: I. Effects of dispersion on particle size distribution and rheological behavior. Int. J. Hydrog. Energy 2024, 52, 1143–1154. [Google Scholar] [CrossRef]
- Liu, G.; McLaughlin, D.; Thiele, S.; Van Pham, C. Correlating catalyst ink design and catalyst layer fabrication with electrochemical CO2 reduction performance. Chem. Eng. J. 2023, 460, 141757. [Google Scholar] [CrossRef]
- Li, T.; Chang, X.; Xin, Y.; Liu, Y.; Tian, H. Synergistic Strategy Using Doping and Polymeric Coating Enables High-Performance High-Nickel Layered Cathodes for Lithium-Ion Batteries. J. Phys. Chem. C 2023, 127, 8448–8461. [Google Scholar] [CrossRef]
- Dong, H.; Qi, S.; Wang, L.; Chen, X.; Xiao, Y.; Wang, Y.; Sun, B.; Wang, G.; Chen, S. Conductive Polymer Coated Layered Double Hydroxide as a Novel Sulfur Reservoir for Flexible Lithium-Sulfur Batteries. Small 2023, 23, 00843. [Google Scholar] [CrossRef]
- Reddy, K.R.; Jeong, H.M.; Lee, Y.; Raghu, A.V. Synthesis of MWCNTs-core/thiophene polymer-sheath composite nanocables by a cationic surfactant-assisted chemical oxidative polymerization and their structural properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1477–1484. [Google Scholar] [CrossRef]
- Zhao, C.; Yuan, S.; Cheng, X.; An, L.; Li, J.; Shen, S.; Yin, J.; Yan, X.; Zhang, J. Effect of perfluorosulfonic acid ionomer in anode catalyst layer on proton exchange membrane water electrolyzer performance. J. Power Sources 2023, 580, 233413. [Google Scholar] [CrossRef]
- Lu, J.; Zeng, Y.; Ma, X.; Wang, H.; Gao, L.; Zhong, H.; Meng, Q. Cobalt nanoparticles embedded into N-doped carbon from metal organic frameworks as highly active electrocatalyst for oxygen evolution reaction. Polymers 2019, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, S.; Wang, A.; Wang, M.; Guo, L.; Wei, Z. Blocking the sulfonate group in Nafion to unlock platinum’s activity in membrane electrode assemblies. Nat. Catal. 2023, 6, 392–401. [Google Scholar] [CrossRef]
- Choi, J.; Min, K.; Mo, Y.-H.; Han, S.-B.; Kim, T.-H. Understanding the Effect of Triazole on Crosslinked PPO–SEBS-Based Anion Exchange Membranes for Water Electrolysis. Polymers 2023, 15, 1736. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Park, J.H.; Kabir, S.; Neyerlin, K.C.; Kariuki, N.N.; Lv, H.; Stamenkovic, V.R.; Myers, D.J.; Ulsh, M.; Mauger, S.A. Impact of catalyst ink dispersing methodology on fuel cell performance using in-situ X-ray scattering. ACS Appl. Energy Mater. 2019, 2, 6417–6427. [Google Scholar] [CrossRef]
- Chabot, F.; Lee, J.; Vandenberghe, F.; Guetaz, L.; Gebel, G.; Lyonnard, S.; Porcar, L.; Rosini, S.; Morin, A. Detailed Catalyst Layer Structure of Proton Exchange Membrane Fuel Cells from Contrast Variation Small-Angle Neutron Scattering. ACS Appl. Energy Mater. 2023, 6, 1185–1196. [Google Scholar] [CrossRef]
- Choi, K.J.; Kim, S.-K. A Pt cathode with high mass activity for proton exchange membrane water electrolysis. Int. J. Hydrog. Energy 2023, 48, 849–863. [Google Scholar] [CrossRef]
- Piñeiro García, A.; Perivoliotis, D.; Wu, X.; Gracia-Espino, E. Benchmarking Molybdenum-Based Materials as Cathode Electrocatalysts for Proton Exchange Membrane Water Electrolysis: Can These Compete with Pt? ACS Sustain. Chem. Eng. 2023, 11, 7641–7654. [Google Scholar] [CrossRef]
- Pham, T.A.; Koo, S.; Park, H.; Luong, Q.T.; Kwon, O.J.; Jang, S.; Kim, S.M.; Kim, K. Investigation on the microscopic/macroscopic mechanical properties of a thermally annealed Nafion® membrane. Polymers 2021, 13, 4018. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.; Tian, T.; Wang, R.; Zhang, H.; Zhu, L.; Tang, H. Structure–Performance Correlation Inspired Platinum-Assisted Anode with a Homogeneous Ionomer Layer for Proton Exchange Membrane Water Electrolysis. Polymers 2024, 16, 237. https://doi.org/10.3390/polym16020237
Cheng F, Tian T, Wang R, Zhang H, Zhu L, Tang H. Structure–Performance Correlation Inspired Platinum-Assisted Anode with a Homogeneous Ionomer Layer for Proton Exchange Membrane Water Electrolysis. Polymers. 2024; 16(2):237. https://doi.org/10.3390/polym16020237
Chicago/Turabian StyleCheng, Feng, Tian Tian, Rui Wang, Hao Zhang, Liyan Zhu, and Haolin Tang. 2024. "Structure–Performance Correlation Inspired Platinum-Assisted Anode with a Homogeneous Ionomer Layer for Proton Exchange Membrane Water Electrolysis" Polymers 16, no. 2: 237. https://doi.org/10.3390/polym16020237
APA StyleCheng, F., Tian, T., Wang, R., Zhang, H., Zhu, L., & Tang, H. (2024). Structure–Performance Correlation Inspired Platinum-Assisted Anode with a Homogeneous Ionomer Layer for Proton Exchange Membrane Water Electrolysis. Polymers, 16(2), 237. https://doi.org/10.3390/polym16020237








