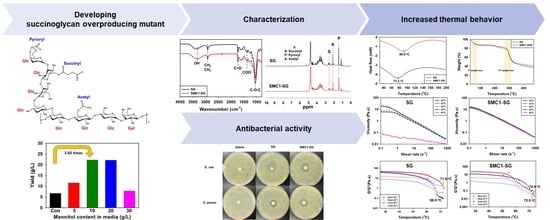
Physicochemical and Rheological Properties of Succinoglycan Overproduced by Sinorhizobium meliloti 1021 Mutant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Succinoglycan (SG)
2.3. N-Methyl-N′nitro-N-nitrosoguanidine (NTG) Mutagenesis
2.4. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
2.6. Differential Scanning Calorimetry (DSC)
2.7. Thermal Gravimetric Analysis (TGA)
2.8. Molecular Weight by Gel Permeation Chromatography (GPC)
2.9. Rheological Measurements
2.10. Gelation Test by Metal Cations
2.11. Antibacterial Test
3. Results and Discussion
3.1. Optimization of SG Yield
SG Yield Change Depending on Mannitol Content in Medium
3.2. Characterization of SG
3.2.1. Fourier Transform Infrared (FT-IR)
3.2.2. Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
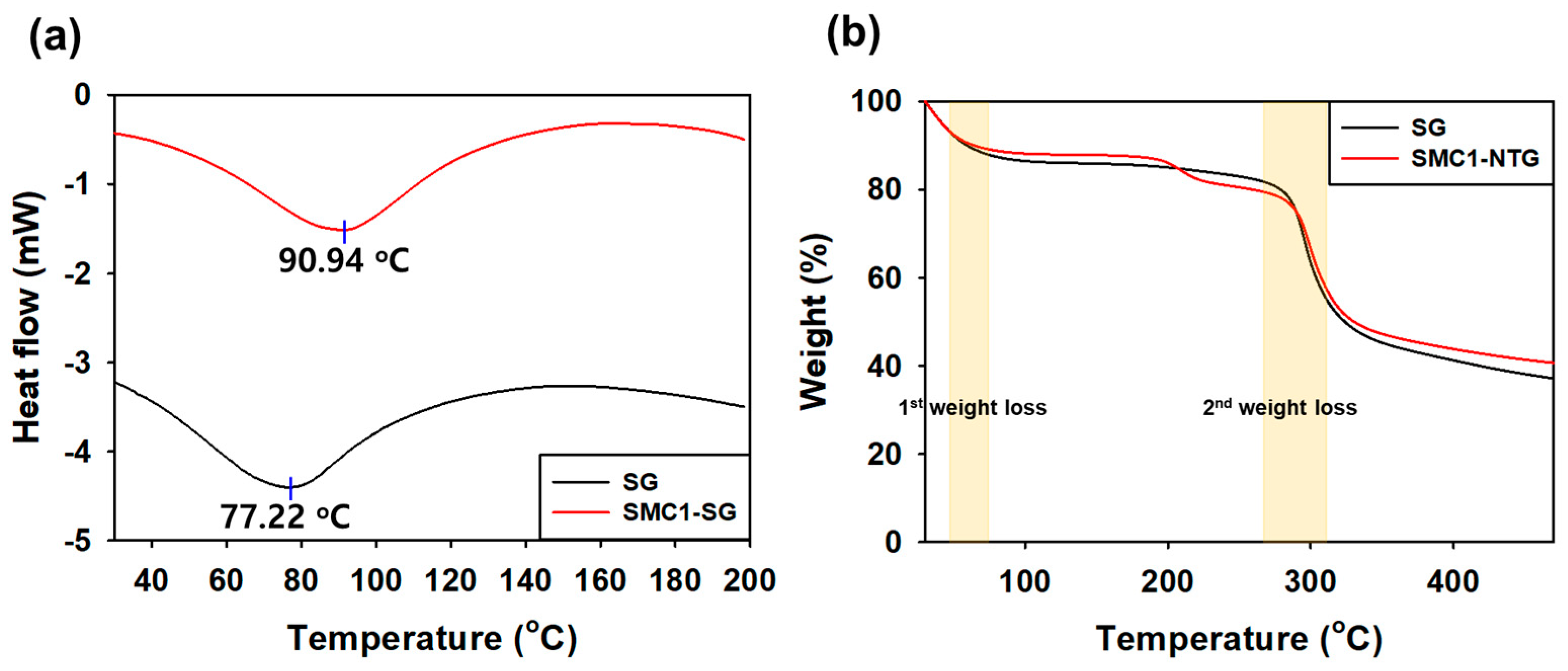
3.2.3. Differential Scanning Calorimetry (DSC)
3.2.4. Thermal Gravimetric Analysis (TGA)
3.2.5. Gel Permeation Chromatography (GPC)
3.3. Rheological Test of SG
3.3.1. Angular Frequency Test
3.3.2. Oscillation Test
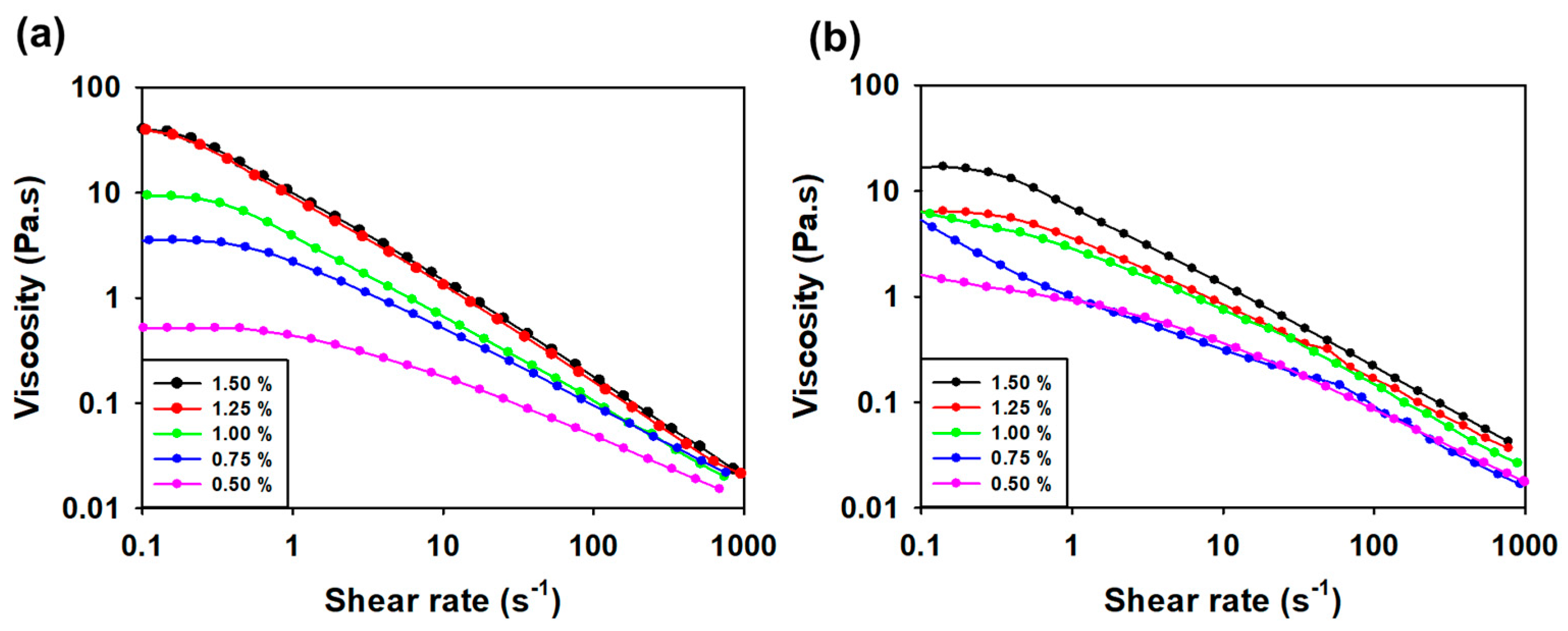
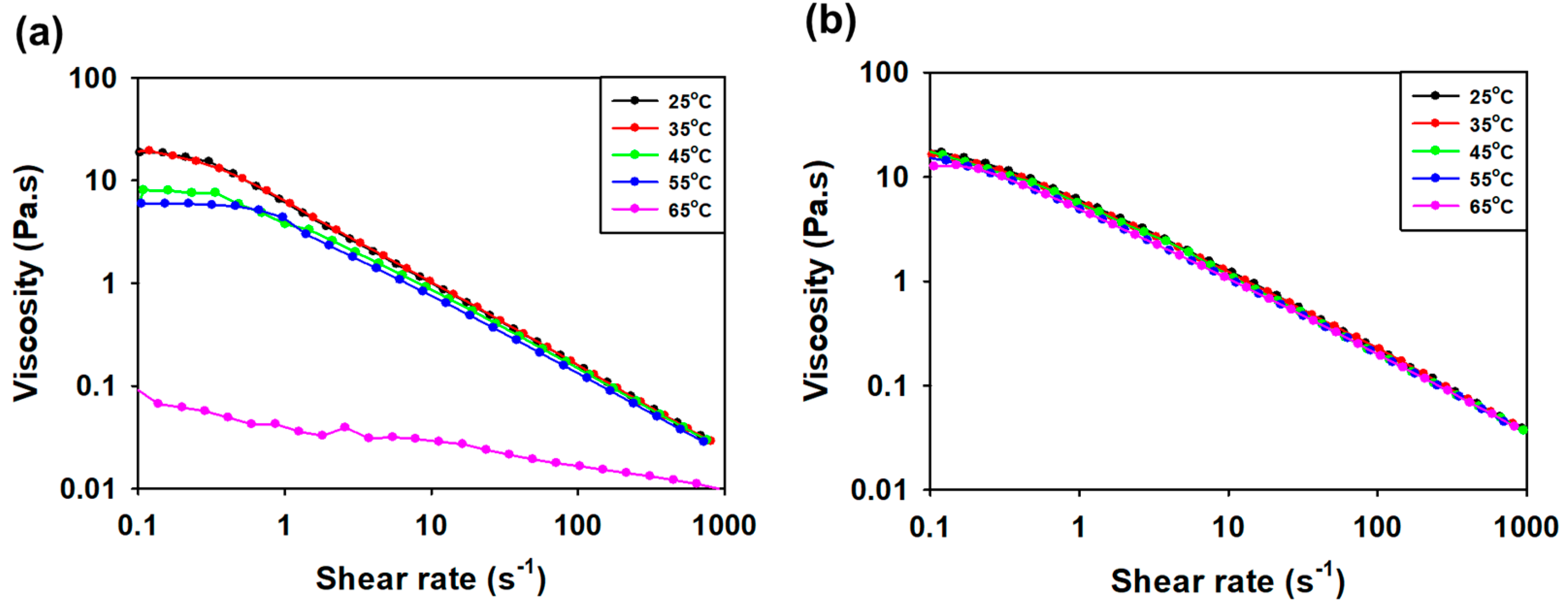
3.3.3. Viscosity Measurement according to Concentration and Temperature
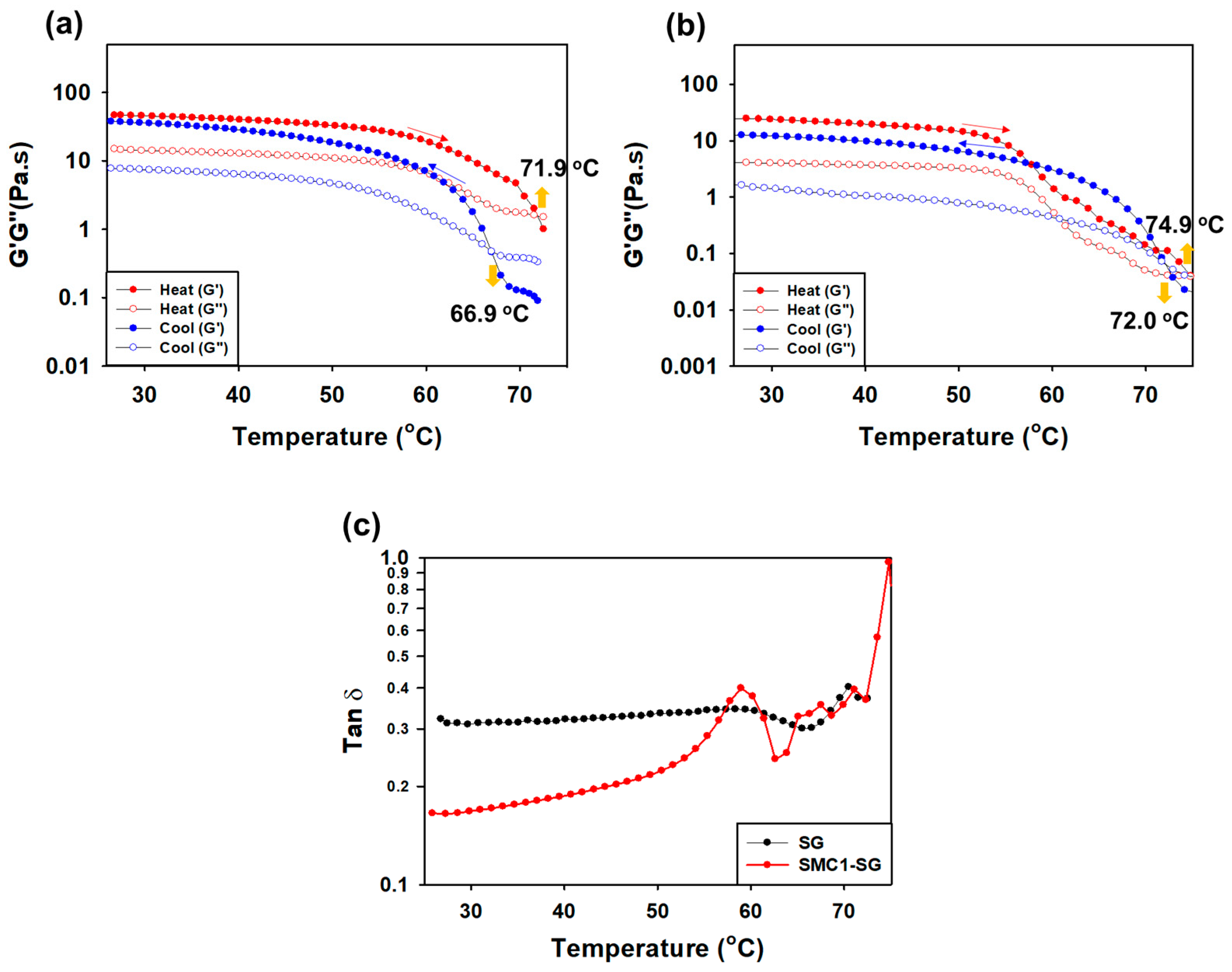
3.3.4. Measurement of G′ and G″ during Heating and Cooling
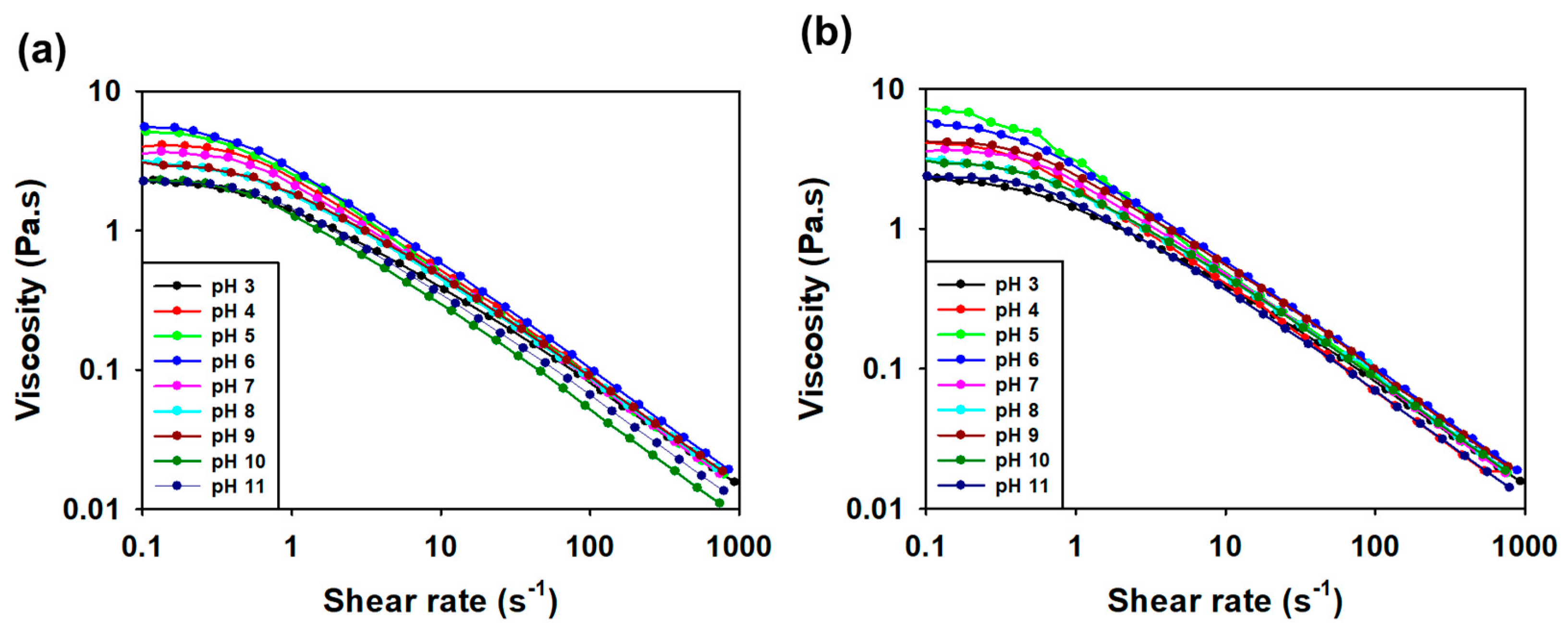
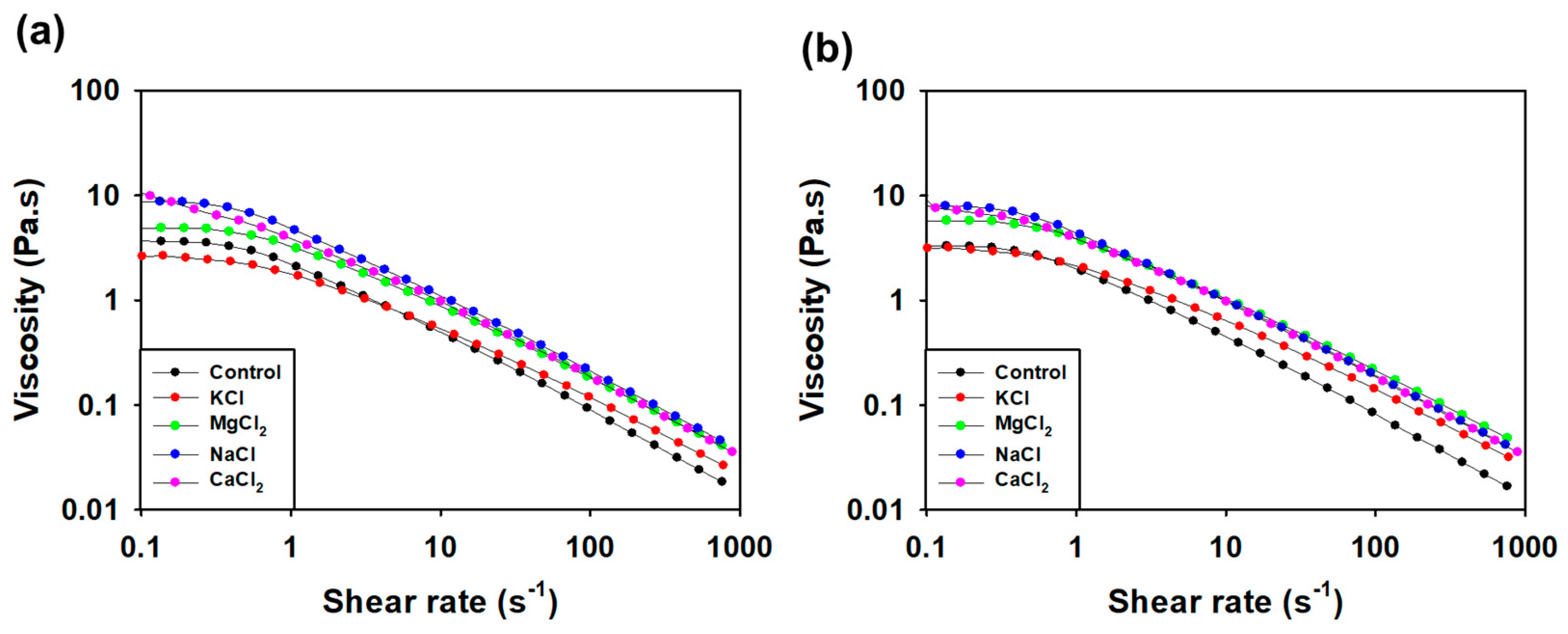
3.3.5. Viscosity Measurement according to pH and Salt Condition
3.4. Application Capabilities through Gelation and Antibacterial Test
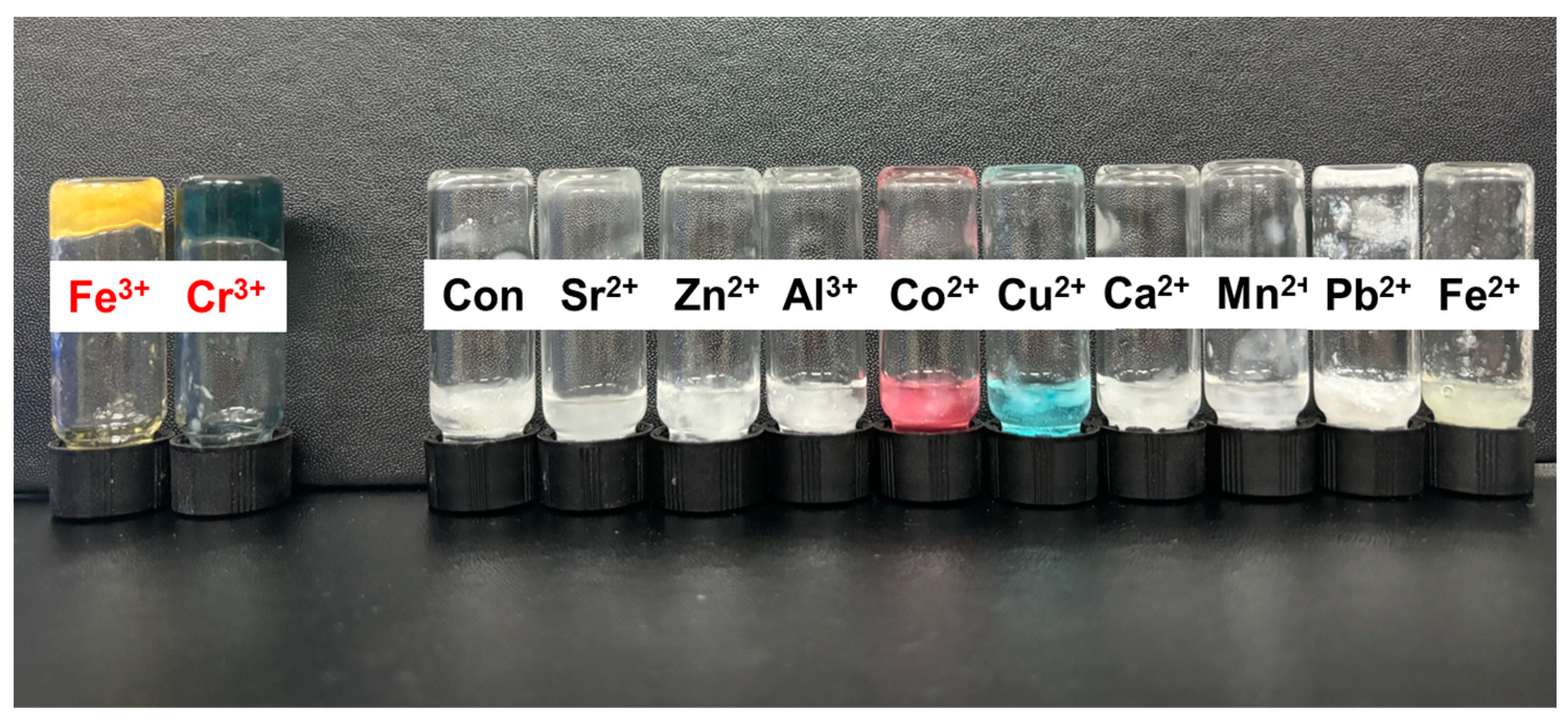
3.4.1. Metal Chelating Gelation by Metal Cation
3.4.2. Antibacterial Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushik, K.; Sharma, R.B.; Agarwal, S. Natural polymers and their applications. Int. J. Pharm. Sci. Rev. Res. 2016, 37, 30–36. [Google Scholar]
- Ahuja, V.; Bhatt, A.K.; Banu, J.R.; Kumar, V.; Kumar, G.; Yang, Y.-H.; Bhatia, S.K. Microbial Exopolysaccharide Composites in Biomedicine and Healthcare: Trends and Advances. Polymers 2023, 15, 1801. [Google Scholar] [CrossRef] [PubMed]
- Mende, S.; Peter, M.; Bartels, K.; Rohm, H.; Jaros, D. Addition of purified exopolysaccharide isolates from S. thermophilus to milk and their impact on the rheology of acid gels. Food Hydrocoll. 2013, 32, 178–185. [Google Scholar] [CrossRef]
- Jindal, N.; Khattar, J.S. Microbial polysaccharides in food industry. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–123. [Google Scholar]
- Sutherland, I.W. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998, 16, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Purohit, S.R. Microbial exopolysaccharide: Sources, stress conditions, properties and application in food and environment: A comprehensive review. Int. J. Biol. Macromol. 2023, 242, 124925. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, X.; Zhao, Y.; Ge, W.; Ding, Z.; Liu, J.; Wang, L.; Xu, X.; Zhang, J. Anti-tumor activity and immunogenicity of a succinoglycan riclin. Carbohydr. Polym. 2021, 255, 117370. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Zhang, Y.; Shao, R.; He, J.; Huang, W.; Liu, Z. Regulating effects of phytosterol esters-loaded emulsions stabilized with green tea polysaccharide conjugates and Tween on lipids in KKAy mice. Int. J. Biol. Macromol. 2023, 243, 125235. [Google Scholar] [CrossRef]
- Zevenhuizen, L. Succinoglycan and galactoglucan. Carbohydr. Polym. 1997, 33, 139–144. [Google Scholar] [CrossRef]
- Stredansky, M.; Conti, E.; Bertocchi, C.; Matulova, M.; Zanetti, F. Succinoglycan production by Agrobacterium tumefaciens. J. Ferment. Bioeng. 1998, 85, 398–403. [Google Scholar] [CrossRef]
- Simsek, S.; Wood, K.; Reuhs, B.L. Structural analysis of succinoglycan oligosaccharides from Sinorhizobium meliloti strains with different host compatibility phenotypes. J. Bacteriol. 2013, 195, 2032–2038. [Google Scholar] [CrossRef]
- Mendis, H.C.; Madzima, T.F.; Queiroux, C.; Jones, K.M. Function of succinoglycan polysaccharide in Sinorhizobium meliloti host plant invasion depends on succinylation, not molecular weight. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.A.; Walker, G.C. Exopolysaccharides of Rhizobium: Synthesis, regulation and symbiotic function. Trends Genet. 1994, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Matulová, M.; Toffanin, R.; Navarini, L.; Gilli, R.; Paoletti, S.; Cesàro, A. NMR analysis of succinoglycans from different microbial sources: Partial assignment of their 1H and 13C NMR spectra and location of the succinate and the acetate groups. Carbohydr. Res. 1994, 265, 167–179. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Singhal, R.; Kale, D. Effect of succinylation on the rheological profile of starch pastes. Carbohydr. Polym. 2002, 47, 365–371. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Carvalheira, M.; Costa, N.; Oliveira, R.; Reis, M.A. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr. Polym. 2009, 78, 549–556. [Google Scholar] [CrossRef]
- Andhare, P.; Delattre, C.; Pierre, G.; Michaud, P.; Pathak, H. Characterization and rheological behaviour analysis of the succinoglycan produced by Rhizobium radiobacter strain CAS from curd sample. Food Hydrocoll. 2017, 64, 1–8. [Google Scholar] [CrossRef]
- Alves, V.D.; Freitas, F.; Torres, C.A.; Cruz, M.; Marques, R.; Grandfils, C.; Gonçalves, M.; Oliveira, R.; Reis, M.A. Rheological and morphological characterization of the culture broth during exopolysaccharide production by Enterobacter sp. Carbohydr. Polym. 2010, 81, 758–764. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, Y.; Yang, Y.; Gao, Y.; Sun, X.; Wang, L.; Sun, Q.; Zhang, J.; Xu, X. In vitro and in vivo anti-Listeria effect of Succinoglycan Riclin through regulating MAPK/IL-6 axis and metabolic profiling. Int. J. Biol. Macromol. 2020, 150, 802–813. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Singhania, R.R.; Sabarinath, C.; Pandey, A. Fermentative production of gellan using Sphingomonas paucimobilis. Process Biochem. 2003, 38, 1513–1519. [Google Scholar] [CrossRef]
- Kamal, F.; Mehrgan, H.; Mazaheri Assadi, M.; Mortazavi, S.A. Mutagenesis of Xanthomonas campestris and selection of strains with enhanced xanthan production. Iran. Biomed. J. 2003, 7, 91–98. [Google Scholar]
- Chi, Z.; Zhao, S. Optimization of medium and cultivation conditions for pullulan production by a new pullulan-producing yeast strain. Enzym. Microb. Technol. 2003, 33, 206–211. [Google Scholar] [CrossRef]
- Poli, A.; Kazak, H.; Gürleyendağ, B.; Tommonaro, G.; Pieretti, G.; Öner, E.T.; Nicolaus, B. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr. Polym. 2009, 78, 651–657. [Google Scholar] [CrossRef]
- Saude, N.; Junter, G.-A. Production and molecular weight characteristics of alginate from free and immobilized-cell cultures of Azotobacter vinelandii. Process Biochem. 2002, 37, 895–900. [Google Scholar] [CrossRef]
- Andhare, P.; Goswami, D.; Delattre, C.; Pierre, G.; Michaud, P.; Pathak, H. Edifying the strategy for the finest extraction of succinoglycan from Rhizobium radiobacter strain CAS. Appl. Biol. Chem. 2017, 60, 339–348. [Google Scholar] [CrossRef]
- Jones, K.M. Increased production of the exopolysaccharide succinoglycan enhances Sinorhizobium meliloti 1021 symbiosis with the host plant Medicago truncatula. J. Bacteriol. 2012, 194, 4322–4331. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyari, M.; Moosavi-Nasab, M.; Askari, H. Optimization of succinoglycan hydrocolloid production by Agrobacterium radiobacter grown in sugar beet molasses and investigation of its physicochemical characteristics. Food Hydrocoll. 2015, 45, 18–29. [Google Scholar] [CrossRef]
- Guo, X.; Zou, X.; Sun, M. Optimization of extraction process by response surface methodology and preliminary characterization of polysaccharides from Phellinus igniarius. Carbohydr. Polym. 2010, 80, 344–349. [Google Scholar] [CrossRef]
- Yao, S.-Y.; Luo, L.; Har, K.J.; Becker, A.; Rüberg, S.; Yu, G.-Q.; Zhu, J.-B.; Cheng, H.-P. Sinorhizobium meliloti ExoR and ExoS proteins regulate both succinoglycan and flagellum production. J. Bacteriol. 2004, 186, 6042–6049. [Google Scholar] [CrossRef]
- Dantas, L.; Heyraud, A.; Courtois, B.; Courtois, J.; Milas, M. Physicochemical properties of ‘Exogel’exocellular β (1–4)-d-glucuronan from Rhizobium meliloti strain M5N1 CS (NCIMB 40472). Carbohydr. Polym. 1994, 24, 185–191. [Google Scholar] [CrossRef]
- Tsuda, H.; Hara, K.; Miyamoto, T. Binding of mutagens to exopolysaccharide produced by Lactobacillus plantarum mutant strain 301102S. J. Dairy Sci. 2008, 91, 2960–2966. [Google Scholar] [CrossRef]
- Cangelosi, G.; Hung, L.; Puvanesarajah, V.; Stacey, G.; Ozga, D.; Leigh, J.; Nester, E. Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interactions. J. Bacteriol. 1987, 169, 2086–2091. [Google Scholar] [CrossRef]
- Prombutara, P.; Allen, M. Flocculation-related gene identification by whole-genome sequencing of Thauera aminoaromatica MZ1T floc-defective mutants. Appl. Environ. Microbiol. 2016, 82, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, L.; Tian, J.; Huang, L.; Huang, D.; Zhang, W.; Xie, F.; Niu, Y.; Jin, M.; Jia, C. Characterization and rheological properties analysis of the succinoglycan produced by a high-yield mutant of Rhizobium radiobacter ATCC 19358. Int. J. Biol. Macromol. 2021, 166, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ananthalakshmy, V.K.; Gunasekaran, P. Isolation and characterization of mutants from levan-producing Zymomonas mobilis. J. Biosci. Bioeng. 1999, 87, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Bruchet, M.; Melman, A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange. Carbohydr. Polym. 2015, 131, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-based hydrogels prepared via ionic gelation: An experimental design approach to predict the crosslinking degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, M.-K.; Lee, I.-Y.; Yun, M.; Shin, J.E.N. Production and structural features of a water-soluble polysaccharide from a mutant strain of Agrobacterium sp. J. Ind. Eng. Chem. 2008, 14, 759–764. [Google Scholar] [CrossRef]
- Fujihara, S.; Yoneyama, T. Effects of pH and osmotic stress on cellular polyamine contents in the soybean rhizobia Rhizobium fredii P220 and Bradyrhizobium japonicum A1017. Appl. Environ. Microbiol. 1993, 59, 1104–1109. [Google Scholar] [CrossRef]
- Elsheikh, E.A.; Wood, M. Response of chickpea and soybean rhizobia to salt: Influence of carbon source, temperature and pH. Soil Biol. Biochem. 1989, 21, 883–887. [Google Scholar] [CrossRef]
- Breedveld, M.; Zevenhuizen, L.; Zehnder, A. Osmotically induced oligo-and polysaccharide synthesis by Rhizobium meliloti SU-47. Microbiology 1990, 136, 2511–2519. [Google Scholar] [CrossRef]
- Mandal, S.M.; Ray, B.; Dey, S.; Pati, B.R. Production and composition of extracellular polysaccharide synthesized by a Rhizobium isolate of Vigna mungo (L.) Hepper. Biotechnol. Lett. 2007, 29, 1271–1275. [Google Scholar] [CrossRef]
- Glucksmann, M.; Reuber, T.; Walker, G. Family of glycosyl transferases needed for the synthesis of succinoglycan by Rhizobium meliloti. J. Bacteriol. 1993, 175, 7033–7044. [Google Scholar] [CrossRef]
- Reinhold, B.B.; Chan, S.Y.; Reuber, T.L.; Marra, A.; Walker, G.C.; Reinhold, V.N. Detailed structural characterization of succinoglycan, the major exopolysaccharide of Rhizobium meliloti Rm1021. J. Bacteriol. 1994, 176, 1997–2002. [Google Scholar] [CrossRef]
- Simsek, S.; Mert, B.; Campanella, O.H.; Reuhs, B. Chemical and rheological properties of bacterial succinoglycan with distinct structural characteristics. Carbohydr. Polym. 2009, 76, 320–324. [Google Scholar] [CrossRef]
- He, D.; Lv, Y.; Tong, Q. Succinylation improves the thermal stability of egg white proteins. Molecules 2019, 24, 3783. [Google Scholar] [CrossRef]
- Ruiz, S.P.; Martinez, C.O.; Noce, A.S.; Sampaio, A.R.; Baesso, M.L.; Matioli, G. Biosynthesis of succinoglycan by Agrobacterium radiobacter NBRC 12665 immobilized on loofa sponge and cultivated in sugar cane molasses. Structural and rheological characterization of biopolymer. J. Mol. Catal. B Enzym. 2015, 122, 15–28. [Google Scholar] [CrossRef]
- Simsek, S.; Ojanen-Reuhs, T.; Stephens, S.B.; Reuhs, B.L. Strain-ecotype specificity in Sinorhizobium meliloti-Medicago truncatula symbiosis is correlated to succinoglycan oligosaccharide structure. J. Bacteriol. 2007, 189, 7733–7740. [Google Scholar] [CrossRef]
- Söyler, Z.; Onwukamike, K.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A. Sustainable succinylation of cellulose in a CO2-based switchable solvent and subsequent Passerini 3-CR and Ugi 4-CR modification. Green Chem. 2018, 20, 214–224. [Google Scholar] [CrossRef]
- Gurian, E.; Bellich, B.; Cesàro, A. Polysaccharide solutions and gels: Isothermal dehydration study by dynamic calorimetric experiments with DSC. Food Hydrocoll. 2016, 61, 163–171. [Google Scholar] [CrossRef]
- Ammar, Y.B.; Matsubara, T.; Ito, K.; Iizuka, M.; Limpaseni, T.; Pongsawasdi, P.; Minamiura, N. Characterization of a thermostable levansucrase from Bacillus sp. TH4-2 capable of producing high molecular weight levan at high temperature. J. Biotechnol. 2002, 99, 111–119. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Wang, D.; Zhang, R.; Liu, G.; Cheng, G. Thermogravimetric analysis of lignocellulosic biomass with ionic liquid pretreatment. Bioresour. Technol. 2014, 153, 379–382. [Google Scholar] [CrossRef]
- Zhang, F.; Ran, C.; Zheng, J.; Ding, Y.; Chen, G. Polysaccharides obtained from bamboo shoots (Chimonobambusa quadrangularis) processing by-products: New insight into ethanol precipitation and characterization. Int. J. Biol. Macromol. 2018, 112, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, F.; Chen, L.; Greenland, B.W.; Shaver, M.P. Installing multiple functional groups on biodegradable polyesters via post-polymerization olefin cross-metathesis. Macromolecules 2016, 49, 6826–6834. [Google Scholar] [CrossRef]
- Dobkowski, Z. Thermal analysis techniques for characterization of polymer materials. Polym. Degrad. Stab. 2006, 91, 488–493. [Google Scholar] [CrossRef]
- Sethi, S.; Saruchi; Kaith, B.S.; Kaur, M.; Sharma, N.; Kumar, V. Cross-linked xanthan gum–starch hydrogels as promising materials for controlled drug delivery. Cellulose 2020, 27, 4565–4589. [Google Scholar] [CrossRef]
- Blinov, P.A.; Dvoynikov, M.V. Rheological and Filtration Parameters of the Polymer Salt Drilling Fluids Based on Xanthan Gum. J. Eng. Appl. Sci. 2020, 15, 694–697. [Google Scholar]
- Wu, Y.; Guo, R.; Cao, N.; Sun, X.; Sui, Z.; Guo, Q. A systematical rheological study of polysaccharide from Sophora alopecuroides L. seeds. Carbohydr. Polym. 2018, 180, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Poslinski, A.; Ryan, M.; Gupta, R.; Seshadri, S.; Frechette, F. Rheological behavior of filled polymeric systems I. Yield stress and shear-thinning effects. J. Rheol. 1988, 32, 703–735. [Google Scholar] [CrossRef]
- Burrell, G.L.; Dunlop, N.F.; Separovic, F. Non-Newtonian viscous shear thinning in ionic liquids. Soft Matter 2010, 6, 2080–2086. [Google Scholar] [CrossRef]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244, 160–170. [Google Scholar] [CrossRef]
- Payan, Y.; Ohayon, J. Biomechanics of Living Organs: Hyperelastic Constitutive Laws for Finite Element Modeling; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Mead, D. Numerical interconversion of linear viscoelastic material functions. J. Rheol. 1994, 38, 1769–1795. [Google Scholar] [CrossRef]
- Phan-Thien, N.; Safari-Ardi, M. Linear viscoelastic properties of flour–water doughs at different water concentrations. J. Non-Newton. Fluid Mech. 1998, 74, 137–150. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Taherian, A.R.; Bakhtiyari, M.; Farahnaky, A.; Askari, H. Structural and rheological properties of succinoglycan biogums made from low-quality date syrup or sucrose using agrobacterium radiobacter inoculation. Food Bioprocess Technol. 2012, 5, 638–647. [Google Scholar] [CrossRef]
- Gravanis, G.; Milas, M.; Rinaudo, M.; Clarke-Sturman, A.J. Rheological behaviour of a succinoglycan polysaccharide in dilute and semi-dilute solutions. Int. J. Biol. Macromol. 1990, 12, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Burova, T.V.; Golubeva, I.A.; Grinberg, N.V.; Mashkevich, A.Y.; Grinberg, V.Y.; Usov, A.I.; Navarini, L.; Cesàro, A. Calorimetric study of the order-disorder conformational transition in succinoglycan. Biopolymers 1996, 39, 517–529. [Google Scholar] [CrossRef]
- Noda, S.; Funami, T.; Nakauma, M.; Asai, I.; Takahashi, R.; Al-Assaf, S.; Ikeda, S.; Nishinari, K.; Phillips, G.O. Molecular structures of gellan gum imaged with atomic force microscopy in relation to the rheological behavior in aqueous systems. 1. Gellan gum with various acyl contents in the presence and absence of potassium. Food Hydrocoll. 2008, 22, 1148–1159. [Google Scholar] [CrossRef]
- Swom, G.; Kerdavid, E. A comparison of the rheological properties of succinoglycan with xanthan gum. Gums Stabilisers Food Ind. 2012, 16, 89–97. [Google Scholar]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as antimicrobial agents: Mechanism and spectrum of activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Hu, J.; Zhang, Y.; Dai, Y.; Xia, F. Role of a high calcium ion content in extending the properties of alginate dual-crosslinked hydrogels. J. Mater. Chem. A 2020, 8, 25390–25401. [Google Scholar] [CrossRef]
- Simpson, N.E.; Stabler, C.L.; Simpson, C.P.; Sambanis, A.; Constantinidis, I. The role of the CaCl2–guluronic acid interaction on alginate encapsulated βTC3 cells. Biomaterials 2004, 25, 2603–2610. [Google Scholar] [CrossRef]
- Hu, Y.; Jeong, D.; Kim, Y.; Kim, S.; Jung, S. Preparation of succinoglycan hydrogel coordinated with Fe3+ ions for controlled drug delivery. Polymers 2020, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Purohit, A.S. Anti-Salmonella activity of pyruvic and succinic acid in combination with oregano essential oil. Food Control 2020, 110, 106960. [Google Scholar] [CrossRef]


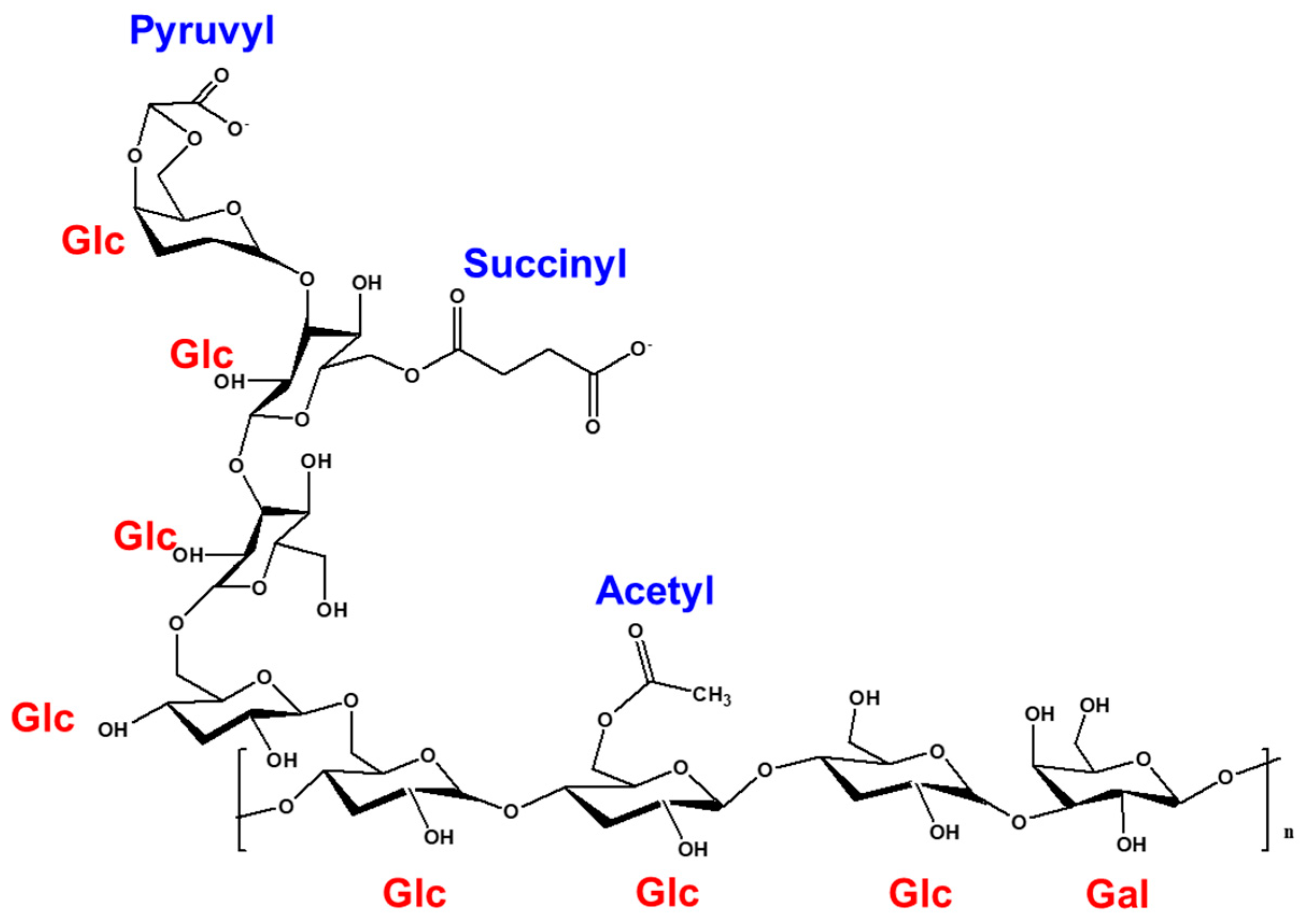



| Functional Group | Wavenumber (cm−1) | Characteristics |
|---|---|---|
| -OH | 3350 | Broad stretching vibration |
| -CH2, -CH3 | 2893 | Stretching vibration |
| C=O | 1723 | Stretching vibration |
| Asymmetric COO− | 1609 | Asymmetric stretching vibration |
| Symmetric COO− | 1371 | Symmetric stretching vibration |
| C-O-C | 1070 | Stretching vibration |
| β-glycosidic bond | 890 | Stretching vibration |
| Sample | Succinyl | Acetyl | Pyruvyl |
|---|---|---|---|
| SG | 1.28 | 0.85 | 1.00 |
| SMC1-SG | 1.34 | 0.89 | 1.00 |
| Angular Frequency (rad/s) | G′ (Pa) | G″ (Pa) | Tan (δ) | Step Time (s) |
|---|---|---|---|---|
| 0.1 | 1.245 ± 0.022 | 0.632 ± 0.019 | 0.508 ± 0.021 | 65.848 |
| 1 | 3.542 ± 0.008 | 2.168 ± 0.014 | 0.612 ± 0.011 | 180.618 |
| 10 | 8.442 ± 0.006 | 4.722 ± 0.025 | 0.559 ± 0.012 | 214.077 |
| 100 | 11.235 ± 0.192 | 8.805 ± 0.076 | 0.783 ± 0.144 | 246.779 |
| Angular Frequency (rad/s) | G′ (Pa) | G″ (Pa) | Tan (δ) | Step Time (s) |
|---|---|---|---|---|
| 0.1 | 1.460 ± 0.092 | 1.401 ± 0.137 | 0.959 ± 0.108 | 65.812 |
| 1 | 4.183 ± 0.043 | 2.529 ± 0.031 | 0.604 ± 0.037 | 180.396 |
| 10 | 9.269 ± 0.017 | 4.800 ± 0.172 | 0.517 ± 0.122 | 213.774 |
| 100 | 17.934 ± 0.061 | 8.212 ± 0.265 | 0.457 ± 0.142 | 247.009 |
| Oscillation Strain (%) | G′ (Pa) | G″ (Pa) | Tan δ | Step Time (s) |
|---|---|---|---|---|
| 0.1 | 2.006 ± 0.165 | 1.732 ± 0.021 | 0.863 ± 0.048 | 9.392 |
| 1 | 2.205 ± 0.113 | 1.932 ± 0.034 | 0.876 ± 0.042 | 58.582 |
| 10 | 2.203 ± 0.071 | 1.831 ± 0.028 | 0.831 ± 0.044 | 97.875 |
| 100 | 1.858 ± 0.068 | 2.012 ± 0.042 | 1.082 ± 0.04 | 147.119 |
| 1000 | 0.139 ± 0.001 | 0.260 ± 0.002 | 1.870 ± 0.001 | 245.546 |
| Oscillation Strain (%) | G′ (Pa) | G″ (Pa) | Tan δ | Step Time (s) |
|---|---|---|---|---|
| 0.1 | 1.920 ± 0.126 | 1.373 ± 0.011 | 0.715 ± 0.042 | 9.401 |
| 1 | 2.126 ± 0.141 | 1.393 ± 0.018 | 0.655 ± 0.057 | 58.689 |
| 10 | 1.984 ± 0.092 | 1.383 ± 0.021 | 0.697 ± 0.034 | 98.077 |
| 100 | 1.608 ± 0.051 | 1.254 ± 0.032 | 0.779 ± 0.037 | 147.27 |
| 1000 | 0.116 ± 0.002 | 0.432 ± 0.005 | 3.703 ± 0.002 | 245.663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jeong, J.-p.; Kim, Y.; Jung, S. Physicochemical and Rheological Properties of Succinoglycan Overproduced by Sinorhizobium meliloti 1021 Mutant. Polymers 2024, 16, 244. https://doi.org/10.3390/polym16020244
Kim J, Jeong J-p, Kim Y, Jung S. Physicochemical and Rheological Properties of Succinoglycan Overproduced by Sinorhizobium meliloti 1021 Mutant. Polymers. 2024; 16(2):244. https://doi.org/10.3390/polym16020244
Chicago/Turabian StyleKim, Jaeyul, Jae-pil Jeong, Yohan Kim, and Seunho Jung. 2024. "Physicochemical and Rheological Properties of Succinoglycan Overproduced by Sinorhizobium meliloti 1021 Mutant" Polymers 16, no. 2: 244. https://doi.org/10.3390/polym16020244
APA StyleKim, J., Jeong, J.-p., Kim, Y., & Jung, S. (2024). Physicochemical and Rheological Properties of Succinoglycan Overproduced by Sinorhizobium meliloti 1021 Mutant. Polymers, 16(2), 244. https://doi.org/10.3390/polym16020244